Abstract
Accurate and early detection of diverse HIV-1 subtypes using currently available p24 antigen assays have been a major challenge. We report the development of a sensitive time resolved fluorescence (TRF) europium nanoparticle immuno assay for cross subtype detection of p24 antigen using broadly cross-reactive antibodies. Several antibodies were tested for optimal reactivity with antigens of diverse HIV-1 subtypes and circulating recombinant forms. We tested HIV strains using this assay for sensitivity and quantification ability at the pico-gram per millilter level. We identified two broadly cross-reactive HIV-1 p24 antibodies C65690M and ANT-152, which detected all strains of HIV tested. These two antibodies also yielded a better signal to cutoff ratio for the same amount of antigen tested in comparison to a commercial assay. Using an appropriate combination of C65690M and ANT-152 p24 antibodies capable of detecting all HIV types and highly sensitive TRF-based europium nano particle assay platform, we developed a sensitive p24 antigen assay that can detect HIV infection of all HIV subtypes and may be useful in early detection.
Keywords: HIV, europium nanoparticle assay, CRFs, recombinants, time resolved fluorescence, ELISA
Introduction
The United Nations Program on HIV and AIDS (UNAIDS) has reported that 36.9 million people were living with the human immunodeficiency virus and around 2 million people were newly infected with HIV in 2014. There are ∼150 million children and adults in 129 low- and middle-income countries reportedly receiving HIV testing services in 2014, and this progress has been possible due to availability of rapid diagnostic tests that provide same-day test results.1 The multi-pronged approach to contain the global HIV/AIDS pandemic needs highly sensitive diagnostic assays that can detect all subtypes including emerging circulating recombinant forms (CRFs) at the earliest stage of infection. Early HIV diagnosis is critical to provide treatment to HIV-infected individuals and to prevent new HIV infections.
There is a significant reduction in new infection rates in recent times due to better testing and improved therapy resulting in reduction of AIDS deaths over the years. However, HIV infection continues to be a major problem due to emergence of new variants by mutation and recombination. HIV has two major types, HIV-1 and HIV-2, but HIV-1 continues to be the etiologic agent for most of AIDS cases and is prevalent worldwide. HIV-2 is primarily limited to West Africa.
In this study, we focused on HIV-1 detection because of its prevalence worldwide. The global spread of HIV-1 groups, subtypes, and recombinant forms pose a significant challenge to accurate diagnosis.1,2 No single available diagnostic test can accurately identify all the strains of HIV, in the very early stage of infection. There are four phylogenetically different HIV-1 groups, M (major), O (outlier), N (non-M-non-O), and P. HIV-1 Group M is further divided into nine different subtypes (A, B, C, D, F, G, H, J, and K) and to date 90 CRFs,3 some of which contribute substantially to the pandemic (such as CRF01_AE and CRF02_AG). The majority of individuals are infected with HIV-1 group M and global distribution of group M subtypes varies regionally.4 Subtype C largely circulates in sub-Saharan Africa and India, subtype A mostly circulates in Eastern Europe and Central Asia and subtype B mostly in Europe, the Americas, and Oceania. The recombinant forms CRF01_AE and CRF02_AG are frequently found in Southeast Asia and West Africa, respectively.
Elimination of HIV is the goal of all treatment and prevention strategies. And this requires early diagnosis and treatment. HIV diagnostics is one of the major approaches for HIV control and diagnostics has come a long way over the years. The latest immunoassays that are being widely used are fourth generation antigen (Ag)/antibody (Ab) combo assays that are continuously being improved because of novel detection technologies and use of better antibodies.
There is a need to capture and identify all the subtypes and recombinant forms in a single test.
Current fourth generation Ag/Ab combo assays are more sensitive than antibody only tests in detecting early HIV infection and can be adapted to point of care settings. It is common in many European countries and the United States to use fourth generation immunoassays for detection of HIV compared with HIV antibody-only tests as they have better detection of acute HIV cases.5 Here in our study we have identified two HIV p24 capture antibodies that can improve the fourth generation Ag/Ab combo assays.
Fourth-generation (Ag/Ab) combo assays have made significant improvements to reduce the mean diagnostic window period by up to 4 days when compared with antibody assays. However, further optimization of assay performance is needed for several reasons.6 The combo assays have not replaced routine blood donor screening nucleic acid test (NAT) assays except for urgent donor screening due to reduced sensitivity. Further, the antigen-detection modules of Ag/Ab combo assays have shown lower and variable sensitivity for HIV-1 non-B subtypes, group O, and HIV-2. Although most p24 monoclonal antibodies are directed against conserved epitopes of p24 Ag,7 it has been reported that some EIA assays failed to correctly detect low-level antigen of HIV-1 non-B subtype strains in some cases.7,8 Finally, the fourth-generation Ag/Ab combo assays are used on two different test analytes that increase the risk of false positive results, due to nonspecific reactivity.
These limitations call for development of robust assays with broadly cross-reactive HIV p24 antibody that can detect all HIV-1 subtypes and HIV-2 to overcome challenges of lower sensitivity in these cases.
Neither traditional fourth-generation laboratory immunoassays nor HIV NATs are suitable for rapid point-of-care diagnosis in clinical settings as they are time consuming and require well trained personnel. Therefore, rapid point of care tests are required in resource limited settings and at the point of care where AHI (acute HIV infection) is common and the potential for identifying such cases is greatest. Rapid, point of care, sensitive fourth generation tests are needed for resource limited settings to detect all AHIs. For example, the sensitivity for p24 antigen assays detection varied widely in laboratory assessments of stored serum and plasma displaying lower sensitivity than other fourth-generation assays.9–16 The sensitivity reported for HIV Ag is also dependent on the HIV Ag reference panel used to evaluate them. Therefore, sensitivity claimed by many assays may not be comparable across strains as each standard may provide a different value.17 In one study none of the fourth generation assays showed optimal sensitivity for 31 Ag panels of HIV-1 subtypes analyzed. Such differences in sensitivity of HIV-1 subtypes have been observed in various studies that are mainly due to characteristics of monoclonal capture antibodies used.8,18 and different capture antibodies have varied avidity for different subtypes.
The comparative performance evaluation studies conducted by many researchers for fourth generation antigen and antibody combo assays have shown similar sensitivity for antibody detection for all genotypes of HIV-1 with a percentage from 99.8% to 100% accuracy. Although some assays have proved to be sensitive for large numbers of samples for HIV p24 detection, false negative results have been observed for some emerging strains as the HIV p24 capture antibodies are not broadly cross reactive to all strains.
HIV p24 antigen detection is an effective way to diagnose early infection as it is one of the earliest HIV infection markers to appear in the blood. The abundance of HIV p24 antigen early in infection makes it a promising marker for sensitive antigen assay development.
There are ∼3,000 HIV-1 p24 antigen molecules as opposed to two RNA copies per virus particle.19 Viral capsid P24 protein is the structural protein with highly conserved amino acid sequence present in large quantity early in infection compared with other viral proteins.20 Therefore, a commonly used marker for HIV replication is p24 Gag protein and is traditionally detected by enzyme-linked immunosorbent assay (ELISA21) but in recent years most fourth generation tests employ chemiluminescence method because of its higher sensitivity.
Some fourth-generation Ag and Ab combo assays do not distinguish signals from antigen and antibody and therefore have interpretation challenges. Assays that distinguish signals from antigen and antibody are expected to improve HIV testing in the future by identifying acute HIV cases.
There is an active and ongoing effort by researchers and manufacturers to develop sensitive assays for HIV-1 p24 to detect all strains. In this report, we describe development of a sensitive HIV-1 p24 assay that can detect all major subtypes and CRFs using a europium nanoparticle assay and combination of cross reactive antibodies.
Materials and Methods
HIV-1 isolates of various subtypes obtained from different research institutions are listed below:
-
1.
International Panel of 60 virus isolates were obtained from NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH representing the six major globally prevalent strains A, B, C, D, CRF01_AE, and CRF02_AG.22
-
2.
A diverse set of 106 recombinant HIV- 1 viruses from Cameroon were cultured in our lab and tested with a commercial kit for p24 antigen and genetically characterized for their recombinant type. They included CRF02_AG, URF, CRF06 cpx, F2, and G.
All monoclonal anti-HIV p24 antibodies were purchased from commercial vendors. ANT-152 from ProSpec-Tany TechnoGene Ltd. Polyclonal anti-HIV p24 antibodies were purchased from Perkin Elmer. Europium nanoparticles, 100 nm in diameter were obtained from Fisher. The preparation and characterization of Eu3+ NPs coated with streptavidin (SA) have been described previously.23 All monoclonal anti-HIV p24 antibodies were purchased from commercial vendors. Monoclonal antibodies C65690M, C65941M, and C65489M were purchased from Meridian Life Science, Inc., ANT-152 from ProSpec-Tany TechnoGene Ltd., NB500-473 from Novus Biologicals, and 012-A from Virogen.
Europium nanoparticle immuno assay
Although there are many commercially available ELISA format assays, the europium nanoparticle immuno assay (ENIA) avoids the use of enzymes and achieves high sensitivity, with significant improvements in detection of HIV-1 infection. Therefore, we evaluated other NPs to simplify the assay format and found that ENIA using Eu3+ NP is suitable for rapid and sensitive detection of HIV-1 p24. In the ENIA Eu3+ NPs modified with SA are bound to the HIV anti p24 and p24 antigen-antibody sandwiched complex, followed by the binding of biotinylated anti-SA antibody and SA-coated europium chelates (Perkin Elmer). Because each Eu3+ NP contains ∼30,000 europium ions, the Eu3+ NPs can produce intense long-lifetime fluorescence light similar to those in the dissociation-enhanced lanthanide fluoro-immunoassay (DELFIA) method and can be measured directly in the Victor Multi-label Counter (Perkin Elmer).24
The Nunc MaxiSorp plates (Thermo Fisher Scientific) are coated with HIV p24 antibody. The HIV p24 capture antibody is dissolved in carbonate-bicarbonate buffer (pH 9.6, 100 mM) to a concentration of 2 μg/mL and adding 55 μL of this mixture into each well of Nunc MaxiSorp plate (Thermo Fisher Scientific) to coat. The coated plate is then incubated at 4°C for 1 or 2 days for efficient coating of the capture antibody. Before performing the assay, the plate is washed five times with wash buffer (Perkin-Elmer) and 250 μL of blocking buffer is added (Starting Block T20 PBS Blocking Buffer from Pierce). Washed plates are kept in blocking buffer for 30 min and is then removed and different HIV strains diluted in blocking buffer containing 55 μL of 10% Triton-X-100 per milliliters are added to each well. The plate containing lysed HIV is incubated for 1 h at 37°C with shaking at 550 rpm. Plates are then washed five times with wash buffer and incubated with 100 μL of a biotinylated secondary polyclonal HIV p24 antibody solution (Perkin Elmer); added and incubated for 1 h at 37 C with shaking at 550 rpm. In the next step, plates are then washed five times with wash buffer and incubated with 100 μL of (1–2 × 109 NP/mL) SA conjugated europium nanoparticle for half an hour at 37°C with shaking. In the final step the plate is washed and read with the time resolved fluorescence (TRF) reader (Perkin Elmer Victor) with excitation at 340 nm and emission at 615 nm (decay time 0.4 ms, measurement window 0.4 ms). The assay includes controls and a blank well that does not contain antigen. Results are compared to the p24 standard ranging from a dynamic range of 1–500 pg/mL.
Results
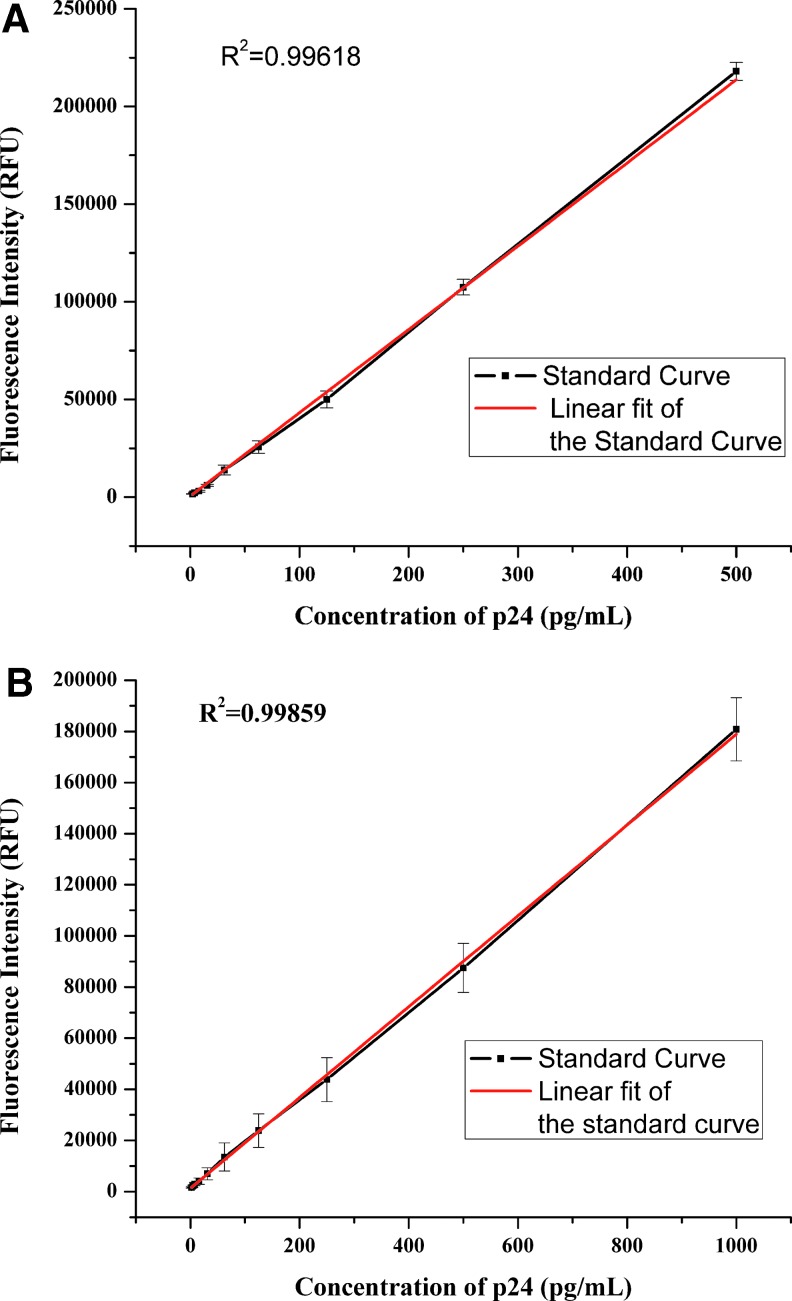
We screened several HIV-1 p24 antibodies for optimal reactivity with many different HIV-1 strains for HIV p24 detection and identified two broadly cross-reactive antibodies C65690M and ANT-152 that detected most strains of HIV tested. Using a combination of these two antibodies in a ratio 3:1, all HIV strains were detected. These two antibodies are not only broadly cross reactive but also showed better signal to cutoff ratios for the same amount antigen tested in comparison to a commercial assay. The two antibodies showed good linear dynamic range enabling quantification at high concentration. ANT-152 monoclonal antibody had a lower detection limit of 0.5 pg/mL with a linear dynamic range of 0.5–500 pg. as shown in Figure 1A and C65690M monoclonal antibody had a lower detection limit of 1 pg/mL with a linear dynamic range from 1 to 1,000 pg as shown in Figure 1B.
FIG. 1.
(A) Analytical sensitivity of HIV-1 p24 europium assay using Mab Ant 152. (B) Analytical Sensitivity of HIV-1 p24 europium assay using Mab C65690M.
Diverse HIV-1 viruses from Cameroon
We tested a total of 106 highly diverse viruses from Cameroon consisting of 39 CRF02_AG, 15 URFs, 1 CRF06 cpx, 1 G, 1 F2, and 26 unidentified strains of HIV for validation. We compared detection of these viruses using a commercial Perkin Elmer kit and the europium nanoparticle assay based on the two antibodies C65690M and ANT-152. The Perkin Elmer kit could detect 99 out of 106 recombinant forms whereas the europium nanoparticle assay could detect all HIV strains with high signal to cutoff ratio using both capture antibodies.
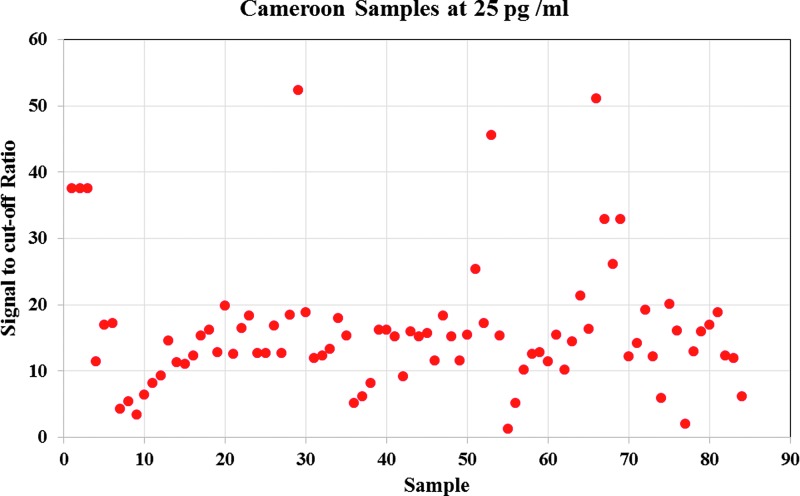
The europium nanoparticle in-house assay showed better performance compared with the commercial assay quantifying antigen several folds higher in most cases with good signal to cutoff ratio. We also diluted the Cameroon HIV isolates to 25 pg/mL using the reference value and tested them using the europium assay based on Mab ANT-152. We could detect all Cameroon HIV isolates at this 25 pg/mL level and obtained better signal to cutoff values for all samples as shown in Figure 2 and Supplementary Table S1, demonstrating that the europium assay could detect all subtypes at lower levels of HIV 24 antigen concentration.
FIG. 2.
Sensitivity testing of Cameroon HIV-1 p24 europium assay at 25 pg/mL.
The summary of testing results is shown in the Table 1. For the 106 Cameroon samples tested, the nanoparticle assay with ANT-152 quantified 101 samples higher than with the C65690M antibody and the commercial Perkin Elmer assay. The nanoparticle assay using C65690M antibody quantified 76 samples better than the commercial Perkin Elmer assay for all the 106 viruses. The results for the subset of 15 URFs showed that ANT-152 quantified 13 samples higher than the commercial Perkin Elmer and 14 compared to C65690M, whereas C65690M quantified 12 samples higher than the commercial Perkin Elmer. The details of the comparative assay data for each Cameroon strains with two antibodies ANT-152 and C65690M tested are shown in Supplementary Table S2.
Table 1.
Summary Results of All the HIV-I Panels Tested
| Testing of diverse HIV-1 strains | ||
|---|---|---|
| HIV panels | No. of tested | ENIA positive |
| Cameroon recombinants | 106 | All positive |
| NIH international panels | 60 | All positive |
ENIA, europium nanoparticle immuno assay.
NIH international panel
The NIH international panel of 60 virus samples was tested using the europium nanoparticle assay that had the two antibodies as capture antibodies for HIV p24 and results were compared with the known values provided. The europium assay with ANT-152 quantified 46 viruses higher than the known values and MAb C65690M europium assay quantified all 60 viruses higher than the given reference value. The results are shown in the Supplementary Table S3.
Subtype sensitivity testing
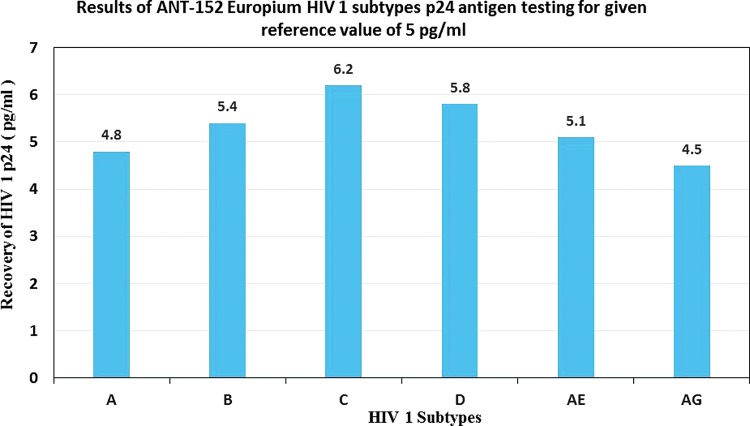
We tested six different HIV-1 subtypes spiked in base matrix to mimic the actual patient sample and tested using the europium assay and compared results with the given reference p24 value from the provider. The Europium assay detected all subtypes at 5 pg/mL level and quantified them close to the reference value of 5 pg/mL. The results are shown in the Figure 3.
FIG. 3.
Results of ANT-152 ENIA HIV-1 subtype p24 sensitivity testing for the original given reference value at 5 pg/mL.
Our results demonstrate that europium nanoparticles in combination with appropriate capture antibodies improve the sensitivity of detection of diverse HIV subtypes. The ENIA yielded higher viral load in samples by using an appropriate combination of p24 antibodies and a highly sensitive TRF-based europium nano particles. The combination of ANT-152 and C65690M in the ratio 3:1 gave significantly higher signals in our Europium-based assay compared to any single antibody as we have shown in our earlier publication. In this study we tested four different sets of samples to evaluate sensitivity and specificity of the antibodies and these two antibodies showed excellent reactivity to HIV-1 24 antigens from different strains.
Discussion
Most commercial assays can detect HIV- 1 subtype B p24 antigen but only a few commercially available assays can detect all major diverse non B strains with optimal sensitivity.17 In our previous study we screened several p24 monoclonal antibodies for HIV-1 group O detection and we continued our efforts in this direction to identify HIV-1 p24 monoclonal antibodies that can detect all strains of HIV. The high sensitivity and specificity of p24 Ag detection observed in our study using this assay may be helpful in providing new ways to enhance sensitivity of antigen portion of the Ag/Ab combo assays for detection of diverse HIV strains in future.8,18 HIV Ag/Ab combo assays along with viral load may be useful for patient counselling and clinical management to determine patient prognosis.25 Also, new HIV Ag/Ab assays with improved specificity and sensitivity may be more affordable in resource limited settings for blood donor screening as compared to NAT that is skill oriented, costly, and laborious. The use of highly sensitive HIV combo assays may also be useful in testing organ donations particularly, in urgent care emergency situations.
Sensitive and specific HIV-1 antigen assays may be useful in diagnosing mother-to-child transmission (MTCT). As MTCT remains the primary cause of HIV infection in infants in developing countries, early HIV detection and ART initiation in HIV-infected mothers can effectively prevent MTCT.26 Many infants born to women with HIV are undiagnosed owing to unavailability of nucleic acid amplification tests in many under developed countries.27 Antibody-only assays are not useful in infant diagnosis as these infants born to HIV-infected mothers have high levels of maternal HIV antibodies for the first 18 months regardless of their HIV status.
The excellent sensitivity of our ENIA at 0.5 pg/mL and ability to detect various subtypes makes it suitable for diagnosis of early HIV-1 infection in worldwide settings where diverse subtypes are prevalent.24 These two antibodies also showed higher avidity for HIV-1 p24 antigen compared with the other antibodies tested and thus provided higher quantification of antigen concentration compared with the commercial assay that used other antibodies.
We have also demonstrated in previous studies on HIV-1 group O that this assay can be adapted to a microchip microfluidic platform where in a 4.5-fold reduction in sample/reagent consumption and a twofold reduction in assay time were achieved with the microchip compared to conventional microtiter plate assay.28
In conclusion, we have developed a sensitive TRF based ENIA for detection of diverse HIV-1 subtype p24 antigen. The high sensitivity of the assay is due to the unique properties of highly sensitive TRF europium nanoparticles that improve analytical sensitivity combined with the two monoclonal antibodies C65690M and ANT-152 that bind more strongly to HIV-1 p24 antigen as compared to other antibodies tested.
Supplementary Material
Acknowledgments
This work was supported by the NHLBI Nano IAA-A-HL-12-001 and NIAID IAA-AAI17023. The authors are thankful to the NIH AIDS Reagent Program for providing HIV-1 strains.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Food and Drug Administration, U.S. Department of Health and Human Services.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Toro C, Amor A, Soriano V: [Diagnosis of HIV-1 non-B subtypes and HIV-2]. Enferm Infecc Microbiol Clin 2008;26 Suppl 13:66–70. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 2. UNAIDS: AIDS by the numbers 2015, 2015. www.unaids.org/en/resources/documents/2015/AIDS_by_the_numbers_2015
- 3. Brian Foley TL, Apetrei C, Hahn B, et al. (eds.): HIV Sequence Compendium 2015. Los Alamos National Laboratory, Los Alamos, New Mexico, 2015 [Google Scholar]
- 4. Taylor BS, Hammer SM: The challenge of HIV-1 subtype diversity. N Engl J Med 2008;359:1965–1966 [DOI] [PubMed] [Google Scholar]
- 5. Jurriaans S, Back NK, Wolthers KC: Ten years of HIV testing with fourth generation assays: The Amsterdam experience. J Clin Virol 2011;52 Suppl 1:S67–S69 [DOI] [PubMed] [Google Scholar]
- 6. Vallefuoco L, Mazzarella C, Portella G: Fourth generation assays for HIV testing. Expert Rev Mol Diagn 2016;16:723–732 [DOI] [PubMed] [Google Scholar]
- 7. Weber B, Fall EH, Berger A, Doerr HW: Reduction of diagnostic window by new fourth-generation human immunodeficiency virus screening assays. J Clin Microbiol 1998;36:2235–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber B, Berger A, Rabenau H, Doerr HW: Evaluation of a new combined antigen and antibody human immunodeficiency virus screening assay, VIDAS HIV DUO Ultra. J Clin Microbiol 2002;40:1420–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pilcher CD, Louie B, Facente S, et al. : Performance of rapid point-of-care and laboratory tests for acute and established HIV infection in San Francisco. PLoS One 2013;8:e80629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faraoni S, Rocchetti A, Gotta F, et al. : Evaluation of a rapid antigen and antibody combination test in acute HIV infection. J Clin Virol 2013;57:84–87 [DOI] [PubMed] [Google Scholar]
- 11. Masciotra S, Luo W, Youngpairoj AS, et al. : Performance of the Alere Determine HIV-1/2 Ag/Ab Combo Rapid Test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. J Clin Virol 2013;58 Suppl 1:e54–e58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brauer M, De Villiers JC, Mayaphi SH: Evaluation of the Determine fourth generation HIV rapid assay. J Virol Methods 2013;189:180–183 [DOI] [PubMed] [Google Scholar]
- 13. Laperche S, Leballais L, Ly TD, Plantier JC: Failures in the detection of HIV p24 antigen with the Determine HIV-1/2 Ag/Ab Combo rapid test. J Infect Dis 2012;206:1946–1947; author reply:1949–1950 [DOI] [PubMed] [Google Scholar]
- 14. Fox J, Dunn H, O'Shea S: Low rates of p24 antigen detection using a fourth-generation point of care HIV test. Sex Transm Infect 2011;87:178–179 [DOI] [PubMed] [Google Scholar]
- 15. Beelaert G, Fransen K: Evaluation of a rapid and simple fourth-generation HIV screening assay for qualitative detection of HIV p24 antigen and/or antibodies to HIV-1 and HIV-2. J Virol Methods 2010;168:218–222 [DOI] [PubMed] [Google Scholar]
- 16. Kilembe W, Keeling M, Karita E, et al. : Failure of a novel, rapid antigen and antibody combination test to detect antigen-positive HIV infection in African adults with early HIV infection. PLoS One 2012;7:e37154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ly TD, Ebel A, Faucher V, Fihman V, Laperche S: Could the new HIV combined p24 antigen and antibody assays replace p24 antigen specific assays? J Virol Methods 2007;143:86–94 [DOI] [PubMed] [Google Scholar]
- 18. Sickinger E, Stieler M, Kaufman B, et al. : Multicenter evaluation of a new, automated enzyme-linked immunoassay for detection of human immunodeficiency virus-specific antibodies and antigen. J Clin Microbiol 2004;42:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barletta JM, Edelman DC, Constantine NT: Lowering the detection limits of HIV-1 viral load using real-time immuno-PCR for HIV-1 p24 antigen. Am J Clin Pathol 2004;122:20–27 [DOI] [PubMed] [Google Scholar]
- 20. Summers MF, Henderson LE, Chance MR, et al. : Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci 1992;1:563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patton JC, Sherman GG, Coovadia AH, Stevens WS, Meyers TM: Ultrasensitive human immunodeficiency virus type 1 p24 antigen assay modified for use on dried whole-blood spots as a reliable, affordable test for infant diagnosis. Clin Vaccine Immunol 2006;13:152–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jagodzinski LL, Wiggins DL, McManis JL, et al. : Use of calibrated viral load standards for group M subtypes of human immunodeficiency virus type 1 to assess the performance of viral RNA quantitation tests. J Clin Microbiol 2000;38:1247–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Du B, Zhang P, et al. : Development of a microchip Europium nanoparticle immunoassay for sensitive point-of-care HIV detection. Biosens Bioelectron 2014;61:177–183 [DOI] [PubMed] [Google Scholar]
- 24. Tang S, Hewlett I: Nanoparticle-based immunoassays for sensitive and early detection of HIV-1 capsid (p24) antigen. J Infect Dis 2010;201 Suppl 1:S59–S64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berrey MM, Schacker T, Collier AC, et al. : Treatment of primary human immunodeficiency virus type 1 infection with potent antiretroviral therapy reduces frequency of rapid progression to AIDS. J Infect Dis 2001;183:1466–1475 [DOI] [PubMed] [Google Scholar]
- 26. Shafiee H, Wang S, Inci F, et al. : Emerging technologies for point-of-care management of HIV infection. Annu Rev Med 2015;66:387–405 [DOI] [PubMed] [Google Scholar]
- 27. Creek TL, Sherman GG, Nkengasong J, et al. : Infant human immunodeficiency virus diagnosis in resource-limited settings: Issues, technologies, and country experiences. Am J Obstet Gynecol 2007;197:S64–S71 [DOI] [PubMed] [Google Scholar]
- 28. Haleyur Giri Setty MK, Liu J, Mahtani P, et al. : Novel time-resolved fluorescence europium nanoparticle immunoassay for detection of human immunodeficiency virus-1 group O viruses using microplate and microchip platforms. AIDS Res Hum Retroviruses 2016;32:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.