Abstract
The United States is currently in the midst of an opioid epidemic that has led to previously unforeseen opioid misuse, abuse, and death. A major emphasis is being placed on reducing the exposure patients have to opioids, whenever possible, in an effort to hopefully reduce the risk of dependency, addiction, and overdose. However, opioid alternatives must be chosen carefully to ensure efficacy, safety, and availability for patients within the community. Non-opioid medications and modalities can reliably be used to treat not only opioid naive patients, keeping them opioid naive if possible, but also patients with opioid use disorders. Unfortunately, robust randomized controlled trials comparing non-opioid alternatives to opioids are lacking. Therefore, this review provides recommendations for best practices regarding improving pain management via the use of novel alternatives in the emergency department, medical wards and outpatient setting.
Introduction
The United States is in the midst of a public health crisis. Drug overdose deaths have been on the rise over the past fifteen years with more than 64,000 drug related deaths reported in 2016 alone. Opioids play a major role in preventable drug overdose deaths, with prescription opioid related deaths claiming the lives of 19,354 Americans in 2016 according to the Center for Disease Control.1 Since leaving pain untreated is not an option, understanding the analgesic effectiveness of non-opioid modalities and medications for the management of acute and chronic pain is paramount to reducing opioid prescriptions.2 Interventions such as trigger point injection and topical analgesics are gaining interest in the management of musculoskeletal pain as they may treat underlying inflammation and spasm providing relief in lieu of prescription opioids.
This is the fourth essay in Missouri Medicine series originating from the Larry Lewis Symposium in August 2017 concerning the transdisciplinary responses to the current opioid epidemic. The vision of this series is to move beyond solely discussing the epidemic at large and to explore key innovations that can simultaneously support more responsible opioid prescribing while ensuring timely, definitive, and compassionate attention to patient’s pain or substance use disorder across a complex healthcare setting. This essay reviews two alternative treatments to opioids for pain with the goal to treat appropriate patients with non-opioid regimens in order to decrease opioid exposure. The logic is that decreased opioid exposures will be associated with a decrease in the risk of developing an opioid use disorder (OUD) with concomitant decreases in opioid overdoses and deaths.
Trigger Point Injections for Myofascial Pain
Musculoskeletal back pain is one of the leading causes of disability among working age Americans and a common presenting complaint in the emergency department and outpatient setting.3 A grossly under recognized cause of muscular pain is Myofascial Pain Syndrome (MPS).4,5 Patients with MPS may present with musculoskeletal pain that is worse with movement, and has a component of referred or regional pain. The vague presenting symptoms can make the accurate identification of trigger point(s) difficult as these findings can mimic other musculoskeletal sources of pain such as fractures, disc herniation, and neuropathy.6 Focal areas of hyperirritable muscle spasm, called trigger points, are a hallmark of this condition. Trigger points are small localized taut bands or nodules within skeletal muscle that are exquisitely painful when palpated, and fully reproduce the reported pain. They are largely due to repetitive strain, chronic musculoskeletal disorders, or acute myofascial injury.7 There are no standardized locations in which trigger points arise. Instead, trigger points can develop in any muscle group, but are most commonly identified in the upper, mid, and low back. 7
History is paramount for diagnosing MPS. Patients presenting with MPS will describe muscular pain exacerbated with movement leading to a decreased range of motion in the affected muscle group, as well as referred pain that does not follow a dermatomal or myotomal distribution.8 The majority of MPS patients report recent acute trauma or repetitive use of the affected area due to occupational demands. The most common muscles involved include the paraspinal cervical muscles of the neck, the upper trapezius muscles, rhomboids, quadratus luborum, and levator scapulae.5 Two important distinguishing features of trigger point pain include the history of pain with movement and the presence of very localized spasm that upon palpation feels like a nodule or taut band. Trigger points are not present in specific predetermined locations but rather develop spontaneously within skeletal muscle. A unique and pathognomonic characteristic of a trigger point includes a local twitch response with application of firm pressure or the insertion of a needle into the trigger point itself.10
MPS referred pain mimics conditions such as headache, radicular back pain, torticollis, temporomandibular joint pain and non-painful conditions such as tinnitus which can complicate or delay the correct diagnosis6. Performing a thorough physical exam is vital because trigger point identification as a root cause can significantly change management.12–14 When attempting to identify trigger points within a muscle it is important to communicate with the patient regarding localized areas of severe maximal pain. Although patients may report global muscle pain and spasm in the affected muscle group, trigger points present as focal taut bands or nodules that are significantly more painful upon palpation than the surrounding tissue.11 No high quality research (randomized controlled trials, meta-analysis) exist to support the accuracy or therapeutic impact of history and physical exam to identify trigger points, nor the effectiveness of injecting these sites in comparison to placebo, non-opioid, or opioid alternatives. In addition, no laboratory or imaging options exist to identify trigger points. In the essays and textbook chapters referenced, the diagnosis of a trigger point is based solely on the history and physical exam leaving open the possibility of multiple forms of diagnostic and therapeutic bias.
Trigger point management may be used in conjunction with a variety of systemic or topical analgesics, muscle relaxants, or varying non pharmacological modalities including ethyl chloride Spray and Stretch techniques, osteopathic manipulative therapy, ultrasonography, massage, or acupuncture. Unfortunately, high-quality effectiveness research quantifying the potential risks and benefits of these techniques is currently lacking.9,15 However, the authors’ experience suggests that trigger point injection provides targeted and immediate relief via direct mechanical inactivation of severe muscle spasm. The procedure is low risk so can be performed in most inpatient and outpatient settings with minimal supplies.5,11 Table 1.
Table 1.
| Adhesive Bandage | 21–25 gauge 1.5–2 inch needle (Use small gauge shorter needle for superficial trigger points and large gauge longer needle for larger trigger points) |
| 1–2 mL syringe | 1 mL local anesthetic |
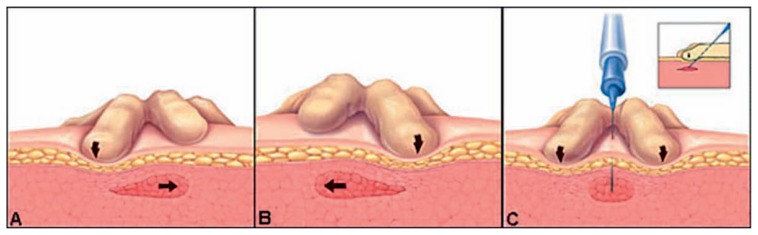
Trigger point injection is indicated in patients presenting with muscle spasm and referred pain who have a tender, well defined, taut band that with palpation fully reproduces pain. The success of the procedure depends on accurately identifying the presence and location of a trigger point. The majority of trigger points due to acute injuries will not require repeat injections.11 The two-part procedure (Figure 1) involves dry needling followed by a low volume injection of local anesthetic. Upon re-evaluation the patient should have a significant reduction in pain. If pain is not relieved, reinjection is not recommended. Discharge instruction should include stretching of the affected muscle groups, remaining active, and avoiding strenuous activity for approximately 72 hours.10,11
Figure 1. How to perform a trigger point injection.9,10.
The practitioner should hold firm pressure on either side or pinch the trigger point in between their fingers. The needle should then be inserted at a 30 degree angle deep enough to engage the trigger point. This is followed by withdrawing the needle slightly, almost all the way out of the skin, before redirecting it into the trigger point to inactivate different quadrants of the spasm. After breaking up all quadrants of the trigger point, local anesthetic should be infiltrated into the center and the needle withdrawn. When the procedure is completed an adhesive bandage should be applied.
The most common complication from TPI is vasovagal reaction,18 though a single case of necrotizing mediastinitis and one of intramuscular hematoma has been reported.19,20 Overlying cellulitis at the desired injection site and allergy to local anesthetic are the only absolute contraindications. Overall, the management of trigger point pain with a trigger point injection is a safe and relatively low risk procedure that can be easily performed in the inpatient and outpatient setting. Licensed physicians performing or directly supervising the trigger point injections can bill for the procedure using appropriate documentation, diagnosis, and CPT coding.
Topical Analgesics in the ED
Topical analgesics serve as a viable therapeutic modality for managing acute and chronic painful conditions including neuropathic pain (herpetic and post-herpetic neuralgia), musculoskeletal pain (sprain, strain, overuse injuries, contusions, tendinopathies), burns and flares of rheumatoid arthritics (RA) and osteoarthritis (OA)21. The advantages favoring topical analgesics include painless and efficient medication delivery, minimal side effects, and improved patients’ adherence and acceptance. Furthermore, their application is associated with direct access to target site, avoidance of first-pass metabolism, short-lived adverse effects and the ease of dose termination. Lastly, these analgesics possess comparative analgesic efficacy to their oral or parenteral equivalents but without systemic adverse effects.22,23 However, while systemic absorption for many of these can be low, patients can become toxic from using too many patches or creams containing methyl salicylate (develop salicylism similar to an aspirin overdose) or camphor. Applying them with heat or to areas with breaks in the skin will increase the amount that is systemically absorbed.
Based on one review the best candidates for pain management with topical analgesic would include patients with cardiovascular and gastrointestinal risk factors that limit the use of oral nonsteroidal anti-inflammatory drugs (NSAID’s), patients with renal or hepatic dysfunction (impaired systemic drug metabolism and clearance), patient with more than one chronic condition (limits potential for systemic drug–drug interactions), and older patients with multiple comorbidities and potential for severe adverse effects from systemic analgesic administration.22
The commonly used topical analgesics can be divided into 4 groups based on their mechanism of action: topical NSAIDs that reversibly inhibit cyclooxygenase enzyme (COX-1,2); topical rubefacients (counter-irritants) that dilate local blood vessels upon application, cause local erythema and warmth, and subsequently decrease painful sensation; topical capsaicin that activates TRPV1 receptors and provide analgesia by causing a reversible degeneration of nerve terminals; and local anesthetics that alleviate pain through blockade of voltage-gated sodium channels.
There are several factors clinicians should consider prior to prescribing topical analgesics for various acute and chronic pain, including patients’ age, comorbidities, and concomitant medications, as well as pain type, duration, skin sensitivity, allergies, and medication cost.22 As an example, for patients suffering from acute strains and sprains, topical NSAIDs or topical rubefacients are reasonable options; for pain related to osteoarthritis or rheumatoid arthritis topical NSAIDs, topical rubefacients, and low-concentration topical capsaicin could be considered; and for neuropathic pain, topical local anaesthetic (lidocaine, for example) or high-concentration topical capsaicin are options.21
The commonly used topical analgesics with their generic names, dosing, indications and contraindications, adverse effects and cost are presented in Tables 2 and 3. In addition, a number needed to treat (NNT) defined as an achievement of at least 50% pain relief at 7 days in comparison to placebo for acute pain and for 6–12 weeks for chronic pain; and a number needed to harm (NNH) defined as a development of a harmful adverse events (local and systemic), and particularly serious adverse events in comparison to placebo at 7 days for acute pain and for up to 12 weeks for chronic pain are included.
Table 2.
Commonly Used Topical NSAIDs
Non-steroidal anti-inflammatory drugs21,23–32
| |||
| Generic (Brand name) | Dosing (initial) | Dosing (maximum/24h) | NNT/NNH (when available) |
| Diclofenac sodium solution (150ml) 1.5%, 2 % with/w/o topical solution (Pennsaid) Cost: $41–78 with Pennsaid $43–97 without |
1.5% solution: 40 drops QID 2% solution: 40 mg BID |
1.5% solution: 40 drops QID 2% solution: 40 mg BID |
NNT = 7 to reduce acute pain by ≥50% within 1-week |
| Diclofenac 1% gel (100g) (Voltaren) Cost: $23–42 |
2 g QID | Osteoarthritis:8g/24h for single joint upper extremity 16g/24h for single joint lower extremity Other: 2–4 g/24h |
NNT = 2 to reduce acute pain by ≥50% within 1-week NNT = 5 to reduce chronic pain of less than 6 weeks’ duration within 6–12 weeks NNT = 10 to reduce chronic pain of greater than 6 weeks’ duration within 6–12 weeks NNH = 51 |
| Diclofenac epolamine 1.3% patch (Flector patch) (30 patches) Cost: $337 |
1 patch (180mg) BID | 1 patch (180mg) BID | NNT = 5 to reduce acute pain by ≥50% within 1-week |
| Etofenamate 5–10% cream (50g) Cost: $22 |
5% cream: 5 cm ribbon TID (1.7 g) 10% cream: 5–10 cm ribbon TID (3.3g) |
9 g (450 mg) | |
| Ibuprofen 5% gel (40g) Cost: $13 Ibuprofen 10% cream (50g), Cost: $14 |
1–4 cm ribbon TID | 4 cm ribbon TID | NNT = 4 to reduce acute pain by ≥50% within 1-week NNT = 6 to reduce acute pain by ≥50% within 1-week |
| Ketoprofen 2.5 % gel Cost: $ |
5–10 cm (2–4 g) ribbon BID-TID | 10cm ribbon (4g) TID | NNT = 3 to reduce acute pain by ≥50% within 1-week NNT = 7 to reduce chronic pain within 6–12 weeks |
| Piroxicam 0.5% cream Cost: $22–55 |
3cm ribbon (1g) TID | 3cm ribbon (1g) TID | NNT = 4 to reduce acute pain by ≥50% within 1-week |
Table 3.
Commonly Used Topical Rubefacients, Capsaicin and Local Anesthetics
Topical Rubefacients21
| |||
|
| |||
| Methyl salicylate 10%, 18% + Menthol 3%, 7.5% cream, gel, ointment, patch Cost: $5–12 |
2–4 cm ribbon QID | 4 cm ribbon QID | NNH = 26 |
|
| |||
Capsaicin21,33–35
| |||
|
| |||
| Capsaicin 0.025% to 0.15% OTC (gels, creams, liquids, lotions, and transdermal patches) Cost: $15 for 60g |
4–6 cm ribbon up to QID | 4–6 cm ribbon QID | NNT = 11 to reduce chronic pain within 6–12 weeks |
| Capsaicin 8% patch (by prescription only) Cost: $800 (per patch) |
NNH = 8 | ||
| Capsaicin and Camphor/Menthol OTC cream Cost: $5–15 |
|||
|
| |||
Topical Anesthetics21,36—40
| |||
|
| |||
| Lidocaine 5% Patch Cost: (6) $72–150 |
1–3 patches daily with 12 h free period | 3 patches daily with 12 h free period | |
| Lidocaine 4% Patch Cost: (6) $8–12 |
|||
Conclusion
An increasing array of non-opioid medications and modalities now exist for the management of acute pain. One size pain management does not fit all. As practitioners work to minimize opioid exposure and potential harms maximizing the use of alternatives such as trigger point injections and topical analgesics is paramount. Expanding physician’s awareness of multi-modal analgesia with an understanding of regional anesthesia techniques this population can receive adequate analgesia without opioids in many cases.
Footnotes
Alexis M. LaPietra, DO, (above), is Medical Director of Emergency Medicine Pain Management, Department of Emergency Medicine, St. Joseph’s Health Wayne Hospital, Paterson, New Jersey. Sergey Motov, MD, is Associate Research Director and Attending Physician, Department of Emergency Medicine, Maimonides Hospital, Brooklyn, New York.
Contact: lapietra@sjhmc.org
Disclosure
None reported.
References
- 1. [Accessed on September 17, 2018]. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
- 2.Lewis L, Carpenter CR, Schwarz ES, Jotte RS, Waller C, Winograd R, Williams R, Stenger S, Rehder H, Governick, Giuffra L. The opioid crisis in Missouri: A call to action for physicians, legislators, and society. Missouri Med. 2017;114:440–446. [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Orthopaedic Surgeons. One in two Americans have a musculoskeletal condition: New report outlines the prevalence, scope, cost and projected growth of musculoskeletal disorders in the U.S. Science Daily. 2016. Mar 1, Retrieved May 14, 2018 from www.sciencedaily.com/releases/2016/03/160301114116.htm.
- 4.Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20(3):165–169. doi: 10.1053/ajem.2002.32643. [DOI] [PubMed] [Google Scholar]
- 5.Roldan CJ, Hu N. Myofascial Pain Syndromes in the Emergency Department: What Are We Missing? J Emerg Med. 2015;49(6):1004–1010. doi: 10.1016/j.jemermed.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Facco E, Ceccherelli F. Myofascial pain mimicking radicular syndromes. Acta neurochir. 2005;92(Suppl):147–50. doi: 10.1007/3-211-27458-8_32. [DOI] [PubMed] [Google Scholar]
- 7.Fogelman Y, Kent J. Efficacy of dry needling for treatment of myofascial pain syndrome. J Back Musculoskelet Rehabil. 2015;28(1):173–179. doi: 10.3233/BMR-140547. [DOI] [PubMed] [Google Scholar]
- 8.Vadivelu N, Urman RD, Hines RL. Essentials of Pain Management. Springer Science & Business Media; 2011. [Google Scholar]
- 9.Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653–660. [PubMed] [Google Scholar]
- 10.Hopwood MB, Abram SE. Factors associated with failure of trigger point injections. Clin J Pain. 1994;10(3):227–234. doi: 10.1097/00002508-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Simons DG, Travell JG, Simons LS. The Trigger Point Manual. Williams & Wilkins; 1999. Travell & Simons’ Myofascial Pain and Dysfunction. [Google Scholar]
- 12.Dommerholt J. Persistent myalgia following whiplash. Curr Pain Headache Rep. 2005;9(5):326–330. doi: 10.1007/s11916-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez de las peñas C, Cuadrado ML, Gerwin RD, Pareja JA. Referred pain from the trochlear region in tension-type headache: a myofascial trigger point from the superior oblique muscle. Headache. 2005;45(6):731–737. doi: 10.1111/j.1526-4610.2005.05140.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumbhare DA, Elzibak AH, Noseworthy MD. Assessment of Myofascial Trigger Points Using Ultrasound. Am J Phys Med Rehabil. 2016;95(1):72–80. doi: 10.1097/PHM.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 15.Fishman S, Ballantyne J, Rathmell JP. Bonica’s Management of Pain. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 16.Ay S, Evcik D, Tur BS. Comparison of injection methods in myofascial pain syndrome: a randomized controlled trial. Clin Rheumatol. 2010;29(1):19–23. doi: 10.1007/s10067-009-1307-8. [DOI] [PubMed] [Google Scholar]
- 17.Iwama H, Ohmori S, Kaneko T, Watanabe K. Water-diluted local anesthetic for trigger-point injection in chronic myofascial pain syndrome: evaluation of types of local anesthetic and concentrations in water. Reg Anesth Pain Med. 2001;26(4):333–336. doi: 10.1053/rapm.2001.24672. [DOI] [PubMed] [Google Scholar]
- 18.Carr CM, Plastaras CT, Pingree MJ, et al. Immediate Adverse Events in Interventional Pain Procedures: A Multi-Institutional Study. Pain Med. 2016;17(12):2155–2161. doi: 10.1093/pm/pnw051. [DOI] [PubMed] [Google Scholar]
- 19.Kim SG, Shim KS, Lee DW, et al. Intramuscular hematoma with motor weakness after trigger point injection: A case report. Medicine (Baltimore) 2017;96(39):e8135. doi: 10.1097/MD.0000000000008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choe JY, Kim JK, Lee DE, et al. Descending necrotizing mediastinitis after a trigger point injection. Clin Exp Emerg Med. 2017;4(3):182–185. doi: 10.15441/ceem.16.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derry S, Wiffen PJ, Kalso EA, Bell RF, et al. Topical analgesics for acute and chronic pain in adults - an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017 May 12;5:CD008609. doi: 10.1002/14651858.CD008609.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarberg B, D’Arcy Y. Options in topical therapies in the management of patients with acute pain. Postgrad Med. 2013;125(4 suppl):19–24. doi: 10.1080/00325481.2013.1110567011. [DOI] [PubMed] [Google Scholar]
- 23.Zempsky W. Topical analgesics in treating neuropathic and musculoskeletal pain. Pain Medicine News. 2013;11(10):7–10. [Google Scholar]
- 24.Stanos S. Topical agents for the management of musculoskeletal pain. J Pain Symptom Manage. 2007;33(3):342–355. doi: 10.1016/j.jpainsymman.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Heyneman C, Lawless-Liday C, Wall G. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60(3):555–574. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 26.Barking RL. Topical Nonsteroidal Anti-Inflammatory Drugs: The Importance of Drug, Delivery, and Therapeutic Outcome. Am J Ther. 2015 Sep-Oct;22(5):388–407. doi: 10.1097/MJT.0b013e3182459abd. [DOI] [PubMed] [Google Scholar]
- 27.Predel HG, Giannetti B, Seigfried B, Novellini R, et al. A randomized, double-blind, placebo-controlled multicentre study to evaluate the efficacy and safety of diclofenac 4% spray gel in the treatment of acute uncomplicated ankle sprain. J Int Med Res. 2013 Aug;41(4):1187–1202. doi: 10.1177/0300060513487639. [DOI] [PubMed] [Google Scholar]
- 28.Mason L, Moore RA, Edwards JE, Derry S, et al. Topical NSAIDs for acute pain: a meta-analysis. BMC Fam Pract. 2004 May 17;5:10. doi: 10.1186/1471-2296-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derry S, Moore RA, Gaskell H, McIntyre M, Wiffen PJ. Topical NSAIDs for acute musculoskeletal pain in adults. Cochrane Database Syst Rev. 2015 Jun 11;6:CD007402. doi: 10.1002/14651858.CD007402.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed SU, Zhang Y, Chen L, Cohen A, St Hillary K, Vo T, Houghton M, Mao J. Effect of 1.5% Topical Diclofenac on Clinical Neuropathic Pain. Anesthesiology. 2015 Jul;123(1):191–198. doi: 10.1097/ALN.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 31.Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskelet Disord. 2004 Aug 19;5:28. doi: 10.1186/1471-2474-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derry S, Conaghan P, Da Silva JA, Wiffen PJ, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016 Apr 22;4:CD007400. doi: 10.1002/14651858.CD007400.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galer BS, Rowbotham M, Perander J, Devers A, et al. Topical diclofenac patch relieves minor sports injury pain: results of a multicenter controlled clinical trial. J Pain Symptom Manage. 2000 Apr;19(4):287–294. doi: 10.1016/s0885-3924(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 34.Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011 Oct;107(4):490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004 Apr 24;328(7446):991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin PL, Fan SZ, Huang CH, Huang HH, et al. Analgesic effect of lidocaine patch 5% in the treatment of acute herpes zoster: a double-blind and vehicle-controlled study. Reg Anesth Pain Med. 2008 Jul-Aug;33(4):320–325. doi: 10.1016/j.rapm.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Gimbel J, Linn R, Hale M, Nicholson B. Lidocaine patch treatment in patients with low back pain: results of an open-label, nonrandomized pilot study. Am J Ther. 2005 Jul-Aug;12(4):311–319. doi: 10.1097/01.mjt.0000164828.57392.ba. [DOI] [PubMed] [Google Scholar]
- 38.Garnock-Jones KP, Keating GM. Lidocaine 5% medicated plaster: a review of its use in postherpetic neuralgia. Drugs. 2009 Oct 22;69(15):2149–2165. doi: 10.2165/11203220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of 5% lidocaine (lignocaine) medicated plaster in comparison with pregabalin in patients with postherpetic neuralgia and diabetic polyneuropathy: interim analysis from an open-label, two-stage adaptive, randomized, controlled trial. Clin Drug Investig. 2009;29(4):231–241. doi: 10.2165/00044011-200929040-00002. [DOI] [PubMed] [Google Scholar]
- 40.Devers A, Galer BS. Topical lidocaine patch relieves a variety of neuropathic pain conditions: an open-label study. Clin J Pain. 2000 Sep;16(3):205–208. doi: 10.1097/00002508-200009000-00005. [DOI] [PubMed] [Google Scholar]