Abstract
Marijuana is the most commonly abused illicit drug in the United States (US) and much of the Westernized World with a steadily increasing prevalence in usage and abuse over the past decade, especially among adolescents. Much of the available data on 9-tetrahydrocannabinol (THC), the main psychoactive ingredient in marijuana, relates to its neurological effects and anti-emetic properties, with very little on the cardiovascular (CV) effects of THC. Available literature shows that THC has three major effects on the CV and the peripheral vasculature in the form of “cannabis arteritis,” cannabis-induced vasospasms, and platelet aggregation, with an unknown verdict on the relationship between marijuana use and atherosclerosis progression. This manuscript reviews these effects and possible mechanisms of action. Moreover, limitations on current views of marijuana and indirect causes of CV toxicity will be investigated, such as concurrent drug use, lifestyle, and mental health. The effects of marijuana on the CV system are extremely worrisome and likely need more attention due to the growing legalization of cannabis for medicinal and recreational use across the US. As a result, awareness among health care professionals about potential side effects and toxicities associated with acute and chronic exposure of cannabis will increase in importance.
Introduction
According to the National Institute of Drug Abuse, marijuana is the most commonly used illicit drug in the US and the third most common cause of drug-related emergency department visits between 2004–2011. Table 1.1 A 2014 study by Terry-Mcelrath et al. demonstrated that marijuana use has steadily increased over the past decade among middle and high school students.2 Moreover, marijuana use will likely increase due to increasing legalization for medicinal and recreational use in the US. Although cannabinoids are the oldest and most widely used illicit drugs, the clinical and pharmacological effects of cannabinoids have only been recently studied, with much still unknown about the physiologic and pathologic effects of marijuana.
Table 1.
Weighted annual estimates of drug related emergency department visits for different types of illicit drug overdose according to the National Institute on Drug Abuse. Cannabinoids are the third most common cause of drug related ED visits in this country.
| Weighted Annual Estimates | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Estimates 2004 | Estimates 2005 | Estimates 2006 | Estimates 2007 | Estimates 2008 | Estimates 2009 | Estimates 2010 | Estimates 2011 | |
|
| ||||||||
| Non-alcohol illicits | 991,640 | 922,018 | 958,866 | 974,852 | 994,583 | 974,392 | 1,172,276 | 1,252,500 |
| Cocaine | 475,425 | 483,865 | 548,608 | 553,535 | 482,188 | 422,902 | 488,101 | 505,224 |
| Herion | 214,432 | 187,493 | 189,787 | 188,162 | 200,666 | 213,118 | 224,706 | 258,482 |
|
| ||||||||
| Cannabinoids | 281,619 | 279,668 | 290,568 | 308,547 | 374,443 | 376,492 | 461,028 | 455,668 |
|
| ||||||||
| Stimulants | 162,435 | 137,806 | 107,586 | 85,043 | 91,945 | 93,564 | 138,632 | 159,840 |
| Amphetamine | 34,085 | 35,083 | 32,251 | 21,545 | 31,534 | 37,431 | 52,388 | 70,831 |
| Methamphetamine | 132,576 | 109,655 | 79,924 | 67,954 | 66,308 | 64,117 | 94,929 | 102,961 |
Marijuana is derived from the hemp plant Cannabis sativa, with the biologically active ingredient being a group of cannabinoids and the main psychoactive constituent being 9-tetrahydrocannabinol (THC).2–4 The THC ligand binds to multiple receptors with especially high selectivity for the Cannabinoid 1 and 2 receptors (CB1 and CB2, respectively). CB1 receptors are predominantly expressed in the brain and peripheral tissues, including cardiac muscle, hepatic tissue, the gastrointestinal tract, and vascular endothelium,5, 6 while CB2 receptors are mainly expressed in immune cells. These CB2 receptors have recently been shown to play a role in inflammatory cytokine regulation.7
Research on the psychoactive effects of CB1 and CB2 receptor-induced activation by THC has been studied for decades, but the adverse physiological effects have not been studied in detail.
Pathological Effects of THC
CB1 receptors are mainly located in the cardiovascular (CV) system (CVS), the central nervous system (CNS), and peripheral vasculature. THC causes an acute, dose-dependent increase in blood pressure (BP) and heart rate (HR).8 Due to a quickly developing tolerance to the psychoactive effects of THC, higher doses and increasing usage frequency are commonly observed. There is evidence to suggest that increased frequency of marijuana use increases the risk of cardiac arrhythmias and myocardial infarction (MI).3, 9–11 Furthermore, chronic THC use has been associated with increased angina frequency, likely due to a decrease in the angina threshold, diminished sympathetic and parasympathetic nervous system signal transduction, serum aldosterone increases, central and peripheral vasoconstriction, and hypertension (HTN).12
For example, a study published in 2017 investigated the association between marijuana use and CV and cerebrovascular mortality.13 The design study was based on mortality follow up using linked participants aged 20 and above who responded to questions on marijuana usage during a 2005 US national health and nutrition examination survey to data from 2011 public use linked mortality file of the National Center for Health Statistics and Center for Disease Control and Prevention. Of the 1,213 participants, 72.5% were alive. Of the dead, adjusted hazard ratios for death from HTN among marijuana users compared to non-marijuana users was 3.42 (95% CI 1.20–9.79). Moreover, for each year of marijuana use, the hazard ratio for death related to HTN was 1.04 (95% CI 1–1.07) when comparing marijuana users to non-marijuana users. In summary, marijuana users had increased risk of HTN-associated mortality even if a prior diagnosis of HTN was treated. In addition, increased duration of marijuana use is associated with increased risk of death related to HTN.
In the CNS, THC has been shown to increase cerebral vasculature tone and central BP, which in turn has been shown to decrease cerebrovascular blood flow. This decrease in cerebrovascular blood flow has been associated with an increased risk of cerebral vascular accident (CVA) and transient ischemic attack (TIA). This has been reported in three studies,14 which suggested a correlation between TIAs and a reversible effect of cannabis on the cerebrovasculature attributed to transient vasospasm and/or increase in central BP decreasing cerebral flow.14 Furthermore, it has been suggested that cannabis use may increase the risk of CVA among patients with increased risk of CV disease (CVD) with or without anti-stroke medications, such as anti-platelet medication.15 It has also been reported that cocaine and/or marijuana use in young adults with an MI, is associated with worse all-cause and CVD mortality.16
Marijuana has been reported to have inflammatory effects on the peripheral vascular system. The pathophysiology is extremely similar to thromboangitis obliterans (Buerger’s disease), an inflammatory and thrombotic disease of the small and medium arteries and veins of the hands and feet associated with tobacco use.17–20 Additionally, cannabis usage has also been associated with claudication, Raynaud’s phenomenon, ischemic ulcers, and digital necrosis. Angiography of chronic cannabis users demonstrated atherosclerotic changes ranging from mild atherosclerotic plaques to total occlusion. Some users have even had improvement with marijuana smoking cessation.17, 21
With decades of research and an increase in the amount of marijuana usage among adults, as well as teenagers, the adverse effects of THC on the CVS still remains unclear. In this review, the current state of the literature on marijuana usage and adverse CVD events will be examined along with clarification on possible connection between marijuana and CV pathology.
Marijuana and the Cardiovascular System
There is a growing body of evidence that demonstrates an association between marijuana use and adverse CVD events. The current literature proposes that marijuana adversely affects the CVS through three different possible mechanisms – cannabis-induced arteritis, vasospasms, and platelet aggregation.22–24
Cannabis Arteritis
Cannabis arteritis was first described in the 1960s, with over 50 cases documented in the literature since then. Furthermore, numerous studies and case reports describe a thromboangitis obliterans-like disease that affects young adults who smoke marijuana.25 In 2013, Desbois et al. described the different arterial pathologies associated with cannabis smokers, including CVA, MI, and lower limb arteritis.26 In these cases, the onset of CVA and MI was usually within one hour of marijuana use. Moreover, compared with thromboangitis obliterans, individuals diagnosed with cannabis arteritis were younger, more often male, and had more frequent unilateral limb involvement.26 The mechanism of the vasculitis is currently not fully understood, but many reports suggest vasculopathy secondary to an arsenic-containing byproduct, which has been associated with endothelial inflammation.26
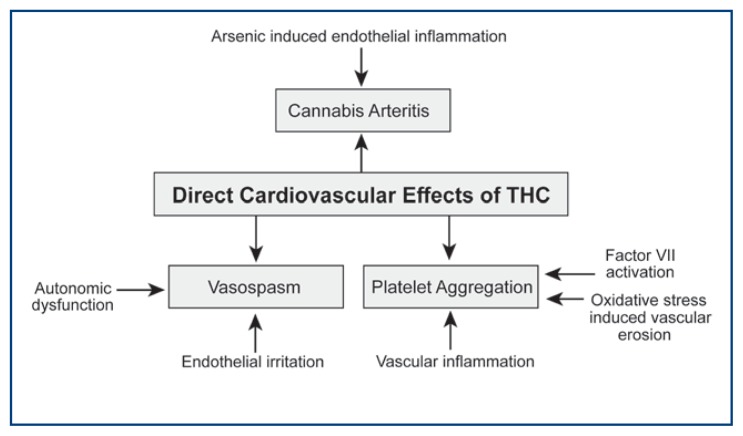
In 2005 and 2007, Combermale and Peyrot et al., respectively, reported cases of vascular inflammation among cannabis users.18,27 Combermale et al. reported a 38-year old chronic marijuana smoker who presented with dry necrotic lesions on the left big toe.27 Imaging revealed proximal arteriopathy of the lower limbs. Furthermore, the development of the arteritis paralleled the patient’s cannabis use. The necrotic lesions secondary to arterial insufficiency were successfully treated with hyperbaric oxygen therapy.27 Peyrot et al. reported of a 30-year-old habitual cannabis smoker without any significant medical history, including no vasculopathies, who presented with digital necrosis of the right toe.18 Arteriography revealed distal segmental arterial lesions that occluded the popliteal artery. After ruling out other causes, the vasculopathy was attributed to cannabis use.18 Figure 1.
Figure 1.
The direct effects of marijuana on the cardiovascular system.
Cannabis-Induced Vasospasms
Reversible arterial vasospasm is considered the most common cause of marijuana-induced vascular events.22 While, the underlying mechanisms for this are unclear, numerous case reports have associated THC with coronary vasospasm-induced cardiomyopathy.28–31
Basnet et al. reported a healthy 17-year-old adolescent who presented to the emergency room complaining of burning chest pain radiating to the jaw that awoke him from sleep.30 He admitted to marijuana use and denied cocaine use. The Electrocardiogram (ECG) demonstrated ST-segment elevation in the lateral leads followed by a blood troponin of 0.23 U/l (normal, 0.00–0.04 U/l). Echocardiogram revealed a hypokinetic apex. Cardiac magnetic resonance imaging was performed, and it was suggested that symptoms and objective cardiac findings were secondary to transient myocardial ischemia. This report was one of the first to describe coronary vasospasms in an adolescent likely from marijuana abuse. Another case reported by Gunawardena et al. described a 29-year-old male who presented with acute coronary syndrome following consumption of marijuana. He had dynamic ST-segment elevation in different leads and a markedly elevated troponin.28 Coronary angiography did not elucidate any occlusive atherosclerotic disease. There was evidence of slow flow in the left anterior descending artery, which improved with intracoronary nitrate therapy. A diagnosis of coronary vasospasm was made, and it was inferred that cannabis consumption was the primary cause. In both reports, coronary angiography did not demonstrate occlusive atherosclerotic disease.
Mendiabal et al. reported that chronic marijuana use may result in autonomic nervous system (ANS) dysfunction, leading to cycles of vasoconstriction and vasodilation independent of skeletal muscle activity.32 It was suggested that baseline ANS dysfunction coupled with THC-induced irritation of the vascular endothelium could possibly explain the increased risk of vasospasm in chronic marijuana users.32
Cannabis-Induced Platelet Aggregation
While cannabis has been shown to be pro-thrombotic, little is known on the mechanism of THC-induced platelet aggregation.22 In 2004, Deusch et al. demonstrated the presence of CB1 and CB2 receptors on platelet cell membranes.33 Furthermore, it was also shown that the expression of glycoprotein IIb-IIIa and P-selectin on platelet membranes increases during CB1 activation in a dose-dependent manner.33 These results suggest that THC may act directly on platelets and may activate the clotting cascade initiating the formation of a thrombus. In fact, there are many published case reports describing young adults with non-atherosclerotic dependent thrombi, most likely secondary to chronic marijuana use.22, 34, 35 For example, in 2012, Dahdouh et al. published a report of a 20-year-old cannabis user with no CVD history who presented with acute MI.23 Coronary angiography revealed a large, occlusive thrombus in the left anterior descending artery which was attributed to THC use.23
Research has shown that CB1 and CB2 receptors exist on platelet membranes and that high concentrations of cannabinoids can induce non-reversible platelet aggregation.36 One possible mechanism for platelet aggregation by cannabinoids is an indirect action closely associated with the vascular wall. Once cannabinoids enter the bloodstream, an inhibition of parasympathetic activity possibly induces an inflammatory effect in the arterial wall which through oxidative stress causes endothelial erosion, factor VII activation, which ultimately leads to thrombus formation.34, 37–40
Cannabis and Atherosclerosis
Stimulation of the CB1 and CB2 receptors has also been shown to modulate the function of cytoskeletal elements in the vessel wall, which may elicit an inflammatory cascade, resulting in atheroma formation.41 Because pulmonary and CVS are populated with CB1 and CB2 receptors, marijuana use has been hypothesized to be involved in the progression of atherosclerotic disease.
Ironically, CB1 and CB2 receptors may play antagonistic roles to one another in CV atherogenesis. CB1 has pro-inflammatory effects on macrophages and monocytes and a pro-atherogenic relationship with the endothelium. CB2 receptors demonstrate anti-inflammatory and anti-atherogenic capabilities along with promoting lipid homeostasis. Interestingly, both play a role in decreasing the area of necrosis in ischemia-reperfusion injury to the heart.42 This antagonistic interaction between the CB1 and CB2 receptor is known as the marijuana paradox-smoking marijuana may decrease the angina threshold and precipitate coronary artery events, but animal models suggest marijuana could modulate atherogenesis in appropriate doses. 42
Marijuana is Not Just THC
Marijuana contains almost 500 compounds, including 70 cannabinoids which provide the psychoactive effect.43,44 When a user smokes marijuana, they do not solely ingest THC but rather a host of other chemicals.45 From the combustion of marijuana, chemicals, such as acetaldehyde, ammonia, benzene, carbon monoxide, hydrogen cyanide, and polycyclic hydrocarbons, are inhaled.45 For example, Groternhermen et al. found that there are numerous adulterants in marijuana, and these compounds could also contribute to CVD processes.45 For instance, during the marijuana plant cultivation, farmers may spray chemicals and fertilizers that contain N-nitrosamines compounds that persist for years.46 Moreover, other chemicals, such as sulfur, copper sulfate, and Ceresan M (a mercury containing compound), are also present on some forms of marijuana.45 Even arsenic has been found on marijuana, in the concentration of 2 mg of arsenic per 100 grams of marijuana, which is considered “a medical dose.”47
Marijuana that is grown “naturally” rarely arrives to the consumer in one step and is generally passed down from person to person because it is sold on the black market.45 Marijuana that is sold in the black market has been shown to contain a wide variety of insecticides and fungicides containing toxins, such as aldicarb, carbaryl, Diazinon, malathion, maneb, parathion, and zineb.48 Moreover, drug dealers may adulterate marijuana with other psychotropic substances, such as paraquat, Lysergic acid diethylamide (LSD), or opium, thus raising the perceptive potency but increasing the pathologic side effects.49 Bell analyzed a sample of hashish, a concentrated form of marijuana, which contained 25% opium and camphor. Lastly, Johnson et al. analyzed over 8,000 different samples of marijuana and found adulterants, which included tobacco, phencyclidine, catnip, heroin, methamphetamine, LSD, peyote, mescaline, belladonna, and numerous other poisons.50 Also, black market “drug dealers” might also purposely adulterate marijuana to increase the weight of the substance therefore selling less marijuana for the same price.45
Man-made and natural formed toxins are not the only impurities that exist on the marijuana plant. Because marijuana is a naturally growing crop, many biologically active organisms also grow on the plant.45 It is well known and well written that spores of fungi infest cannabis.45, 51–54 One of the main fungal species that exist on marijuana is the Aspergillus species, more specifically Aspergillus fumigatus.55 The Aspergillus species is known to produce aflatoxins, which could have serious acute effects on the CVS, including vascular erosion and degradation, disruption of protein synthesis in cardiac myocytes, and mitochondrial disruption in heart tissue.56 57 The aflatoxins themselves can survive for years on the marijuana plant, in marijuana cigarettes, and more importantly in the water that is used for smoking marijuana through water pipes (“bongs”).45 These toxins generally do not produce reactions in healthy young subjects, but can produce life threatening reactions in older adults and especially in immunocompromised patients.58–60
Fungal spores are not the only organisms that can survive on the marijuana plant; bacteria have also been shown to grow, survive, and reproduce on marijuana.61 Ungerleider et al. identified contaminants on marijuana cigarettes supplied by the National Institute of Drugs Abuse, which included Klebsiella pneumoniae, Enterobacter cloacae, group D Streptococcus, and some Bacillus species.61 Moreover, Taylor et al. isolated Salmonella muenchen62 and Kurup et al. isolated some Thermoactinomyces species.63
Medical marijuana obtained from dispensaries are similar in form to recreational marijuana which is often purchased by the patients undergoing chemotherapy to control nausea/vomiting, to control pain or neuropathy or to stimulate appetite. Thompson et al. obtained twenty cannabis samples from different dispensaries in northern California for microbiome analysis.64 Upon analysis, many of the samples consisted of multiple pathogens that would probably be attributed to hospital-acquired infection in addition to gram-negative bacilli and fungal pathogens which can pose grave risk to immunocompromised patients.64
These publications illustrate that when a user smokes marijuana, numerous other chemicals, poisons, and toxin producing fungi and bacteria enter the bloodstream along with THC. All of these contaminants in marijuana could also play a role in the development of life threatening CVD episodes.
Concomitant Drug Use and Lifestyle
Marijuana has been known as a “gateway drug” for decades, and its regular use in adolescents is clearly associated with an increased risk for abuse and dependence on other illicit drugs.65, 66 Cannabis use might be a marker for illicit behavior which can result in the advancement to “harder” illicit drugs once available to the user.66, 67 Patton et al, reported that cannabis smokers who used weekly were eight times more likely to use tobacco and progress to nicotine dependence.68
In 2017, Jin et al. performed a follow-up study on 712 Danish teenagers aged between 15–19 who smoked marijuana, and utilized a questionnaire to study changes in body mass index (BMI), lifestyle, socioeconomic status, alcohol, and tobacco use among other parameters.69 This publication reported that cannabis use was associated with significant increases in cigarette smoking and alcohol intake, and inversely associated with physical activity levels.69 However, there was no association between cannabis use and changes in BMI or long-term weight change into midlife. Some limitations to this study, however were that the data was gathered via questionnaire and adolescents might not have been fully honest with weight history or dietary intake.
The result of this study is similar to other publications investigating the direct correlation between marijuana use and other illicit drug use. In 2000, Gledhill-Hoyt et al. released a study that reported that college students who used marijuana within the past 30 days also had increased rates of tobacco usage, binge alcohol drinking, and other illicit drugs, except for LSD.70 Moreover, they reported that out of the students who tried marijuana for the first time, 34% started to use marijuana regularly along with engaging in increased binge drinking behavior.70
Smit et al. examined dietary intakes and nutritional status of marijuana users and non-current marijuana uses in US persons aged 20–59 years of age using date from the third National Health and Nutrition Examination Studies.71 They found that marijuana users had higher intake of energy and nutrients compared to their non-current marijuana counterparts, although there was no significant change in BMI. Also, the authors found that current marijuana users smoked more cigarettes and consumed higher amount of sodas and alcohol, when compared to their counter cohort. It was also reported that marijuana smokers had less folate and carotenoids in their blood possibly secondary to increased oxidation from both marijuana and tobacco smoke inhalation.
From these studies, it seems reasonable to suggest that marijuana use does lead to additional drug use, especially tobacco and alcohol, the latter being a known cardiotoxin. Moreover, marijuana users have a lifestyle that is low in healthy nutritional intake and exercise and high in fat and non-nutritional calories from “junk food” and beer, all of which can have deleterious effects on the CVS.
Marijuana and Mental Health
As mentioned above, the increased usage of marijuana can have toxic effects on the CVS directly, but similar to the physiologic effects of marijuana, very little is known about the indirect cardiotoxic nature of marijuana through psychological disease.72 The acute effects of marijuana can exacerbate underlying psychosis and psychotic disorders 73, 74 and chronic use can cause anxiety, depression, and decreased motivation.75 For example, a survey by Thomas et al. reported that cannabis use and anxiety were directly associated, and females were more likely to have an increased level of baseline anxiety after THC administration.76 Moreover, questionnaires have also shown that chronic users have increased depressive episodes in both male and female adolescents and adults without concurrent usage of other illicit drugs.77, 78 The current state of the literature does not propose a mechanism that connects marijuana administration and anxiety or depression.73, 79
Chronic anxiety, panic disorder, and phobic anxiety have been linked to increased CVD morbidity.80, 81 In 1994, Kawachi et al. released two studies which reported that increased anxiety, whether phobic or acute, have direct association with coronary heart disease.80,81 Chronic use of marijuana can cause chronic anxiety and this over time could be an indirect contributor towards CVD mortality.
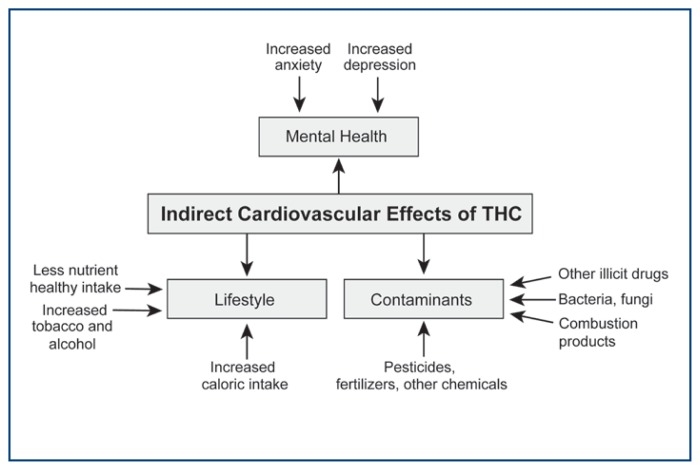
Numerous studies have also shown various physiologic mechanisms linking depression and CVD.82 One possible mechanism uses stress as a possible factor. Patients with depression are generally under a constant state of stress with chronic upregulation of the hypothalamic-pituitary-adrenal (HPA) system and the sympathetic nervous system, leading to excess adrenergic and serotonergic activity.83, 84 Chronic upregulation of the HPA and sympathetic nervous systems cause abnormal physiologic responses, such as unregulated platelet function, autonomic tone, inflammation, and endothelial function.82 As mentioned previously, abnormal platelet function, arrhythmia, and endothelial dysfunction and inflammation are all factors that connect marijuana usage with increased CVD episodes. Figure 2.
Figure 2.
The indirect effects of marijuana on the cardiovascular system
Conclusion
Marijuana is the most common illicit substance used in the US and the rate of usage is only increasing. Most of the research into THC is mainly focused on combating the side effects of anti-cancer chemotherapeutics. However, research is starting to focus on the systemic and CVD adverse effects induced by THC. This brief review has organized three main pathologies associated with marijuana usage and the CVS – peripheral arteritis, coronary vasospasms, and platelet aggregation; however, many of the acute and, more so, long-term potentially toxic effects of marijuana and THC still remain unknown. As more and more states legalize the use of marijuana for recreation and medicinal use, more research will be needed to explore the safety and therapeutic profile of THC. Since fewer and fewer people are users of inhaled combustible marijuana smoke, and almost all studied risks of cannabis are based on users of such inhaled combustible smoke, the risks related to the cannabis use also need to be updated.85 In addition, increased collaboration among cardiologists, drug users, and addiction experts to further understand the potential health consequences of increased cannabis use is very important.85
Footnotes
Venkat N. Subramaniam, MD, MS, (left) was previously a medical student, and Arthur R. Menezes, MD, and Alban DeSchutter, MD, were previously cardiovascular fellows at Ochsner, and Carl J. Lavie, MD, (right), currently works in the Department of Cardiovascular Diseases, John Ochsner Heart and Vascular Institute, Ochsner Clinical School-The University of Queensland School of Medicine, New Orleans, Louisiana. Dr. Subramaniam is currently affiliated with the Banner University Medical Center, Phoenix, Arizona.
Contact: clavie@ochsner.org
Disclosure
None reported.
Editor’s Note
See “Marijuana is More Dangerous Than You Think” on page 88 of this issue.
References
- 1.NIDA. Drug-Related Hospital Emergency Room Visits. 2011. https://www.drugabuse.gov/publications/drugfacts/drug-related-hospital-emergency-room-visits.
- 2.Terry-McElrath YM, O’Malley PM, Johnston LD. Alcohol and marijuana use patterns associated with unsafe driving among U.S. high school seniors: high use frequency, concurrent use, and simultaneous use. Journal of studies on alcohol and drugs. 2014;75:378–389. doi: 10.15288/jsad.2014.75.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prakash R, Aronow WS, Warren M, Laverty W, Gottschalk LA. Effects of marihuana and placebo marihuana smoking on hemodynamics in coronary disease. Clin Pharmacol Ther. 1975;18:90–95. doi: 10.1002/cpt197518190. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 5.Steffens S, Mach F. Cannabinoid receptors in atherosclerosis. Curr Opin Lipidol. 2006;17:519–526. doi: 10.1097/01.mol.0000245257.17764.b2. [DOI] [PubMed] [Google Scholar]
- 6.Larrinaga G, Sanz B, Pérez I, et al. Cannabinoid CB (1) Receptor Is Downregulated in Clear Cell Renal Cell Carcinoma. J Histochem Cytochem. 2010;58:1129–1134. doi: 10.1369/jhc.2010.957126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galiegue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 8.Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–2809. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Rosenberg J, Rogers W, Bachman J, Jones RT. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin Pharmacol Ther. 1979;25:440–446. doi: 10.1002/cpt1979254440. [DOI] [PubMed] [Google Scholar]
- 10.Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–765. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- 11.Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav. 1998;60:777–784. doi: 10.1016/s0091-3057(97)00386-9. [DOI] [PubMed] [Google Scholar]
- 12.Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58s–63s. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 13.Yankey BA, Rothenberg R, Strasser S, Ramsey-White K, Okosun IS. Effect of marijuana use on cardiovascular and cerebrovascular mortality: A study using the National Health and Nutrition Examination Survey linked mortality file. European journal of preventive cardiology. 2017 doi: 10.1177/2047487317723212. 2047487317723212. [DOI] [PubMed] [Google Scholar]
- 14.Mouzak A, Agathos P, Kerezoudi E, Mantas A, Vourdeli-Yiannakoura E. Transient ischemic attack in heavy cannabis smokers--how ‘safe’ is it? Eur Neurol. 2000;44:42–44. doi: 10.1159/000008191. [DOI] [PubMed] [Google Scholar]
- 15.Wolff V, Lauer V, Rouyer O, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke. 2011;42:1778–1780. doi: 10.1161/STROKEAHA.110.610915. [DOI] [PubMed] [Google Scholar]
- 16.DeFilippis EM, Singh A, Divakaran S, et al. Cocaine and Marijuana Use Among Young Adults With Myocardial Infarction. Journal of the American College of Cardiology. 2018;71:2540–2551. doi: 10.1016/j.jacc.2018.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noel B, Ruf I, Panizzon RG. Cannabis arteritis. J Am Acad Dermatol. 2008;58:S65–67. doi: 10.1016/j.jaad.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Peyrot I, Garsaud AM, Saint-Cyr I, Quitman O, Sanchez B, Quist D. Cannabis arteritis: a new case report and a review of literature. J Eur Acad Dermatol Venereol. 2007;21:388–391. doi: 10.1111/j.1468-3083.2006.01947.x. [DOI] [PubMed] [Google Scholar]
- 19.Tennstedt D, Saint-Remy A. Cannabis and skin diseases. Eur J Dermatol. 2011;21:5–11. doi: 10.1684/ejd.2011.1212. [DOI] [PubMed] [Google Scholar]
- 20.Grotenhermen F. Cannabis-associated arteritis. Vasa. 2010;39:43–53. doi: 10.1024/0301-1526/a000004. [DOI] [PubMed] [Google Scholar]
- 21.Duchene C, Olindo S, Chausson N, Jeannin S, Cohen-Tenoudji P, Smadja D. [Cannabis-induced cerebral and myocardial infarction in a young woman]. Rev Neurol (Paris) 2010;166:438–442. doi: 10.1016/j.neurol.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Hodcroft CJ, Rossiter MC, Buch AN. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J Emerg Med. 2014;47:277–281. doi: 10.1016/j.jemermed.2013.11.077. [DOI] [PubMed] [Google Scholar]
- 23.Dahdouh Z, Roule V, Lognone T, Sabatier R, Grollier G. Cannabis and coronary thrombosis: What is the role of platelets? Platelets. 2012;23:243–245. doi: 10.3109/09537104.2011.601824. [DOI] [PubMed] [Google Scholar]
- 24.Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:3091–3101. doi: 10.1093/brain/awm256. [DOI] [PubMed] [Google Scholar]
- 25.Cottencin O, Karila L, Lambert M, et al. Cannabis arteritis: review of the literature. J Addict Med. 2010;4:191–196. doi: 10.1097/ADM.0b013e3181beb022. [DOI] [PubMed] [Google Scholar]
- 26.Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996–1005. doi: 10.1016/j.avsg.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Combemale P, Consort T, Denis-Thelis L, Estival JL, Dupin M, Kanitakis J. Cannabis arteritis. Br J Dermatol. 2005;152:166–169. doi: 10.1111/j.1365-2133.2005.06340.x. [DOI] [PubMed] [Google Scholar]
- 28.Gunawardena MD, Rajapakse S, Herath J, Amarasena N. Myocardial infarction following cannabis induced coronary vasospasm. BMJ Case Rep. 20142014 doi: 10.1136/bcr-2014-207020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casier I, Vanduynhoven P, Haine S, Vrints C, Jorens PG. Is recent cannabis use associated with acute coronary syndromes? An illustrative case series. Acta cardiologica. 2014;69:131–136. doi: 10.1080/ac.69.2.3017293. [DOI] [PubMed] [Google Scholar]
- 30.Basnet S, Mander G, Nicolas R. Coronary vasospasm in an adolescent resulting from marijuana use. Pediatric cardiology. 2009;30:543–545. doi: 10.1007/s00246-009-9384-7. [DOI] [PubMed] [Google Scholar]
- 31.El Menyar AA. Drug-induced myocardial infarction secondary to coronary artery spasm in teenagers and young adults. J Postgrad Med. 2006;52:51–56. [PubMed] [Google Scholar]
- 32.Mendizabal VE, Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: a tale of passions and illusions. Br J Pharmacol. 2007;151:427–440. doi: 10.1038/sj.bjp.0707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deusch E, Kress HG, Kraft B, Kozek-Langenecker SA. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth Analg. 2004;99:1127–1130. doi: 10.1213/01.ANE.0000131505.03006.74. table of contents. [DOI] [PubMed] [Google Scholar]
- 34.Dwivedi S, Kumar V, Aggarwal A. Cannabis smoking and acute coronary syndrome: two illustrative cases. International journal of cardiology. 2008;128:e54–57. doi: 10.1016/j.ijcard.2007.04.167. [DOI] [PubMed] [Google Scholar]
- 35.Ghannem M, Belhadj I, Tritar A, et al. [Cannabis and acute coronary syndrome with ST segment elevation]. Ann Cardiol Angeiol (Paris) 2013;62:424–428. doi: 10.1016/j.ancard.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Levy R, Schurr A, Nathan I, Dvilanski A, Livne A. Impairment of ADP-induced platelet aggregation by hashish components. Thrombosis and haemostasis. 1976;36:634–640. [PubMed] [Google Scholar]
- 37.Tatli E, Yilmaztepe M, Altun G, Altun A. Cannabis-induced coronary artery thrombosis and acute anterior myocardial infarction in a young man. International journal of cardiology. 2007;120:420–422. doi: 10.1016/j.ijcard.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Cappelli F, Lazzeri C, Gensini GF, Valente S. Cannabis: a trigger for acute myocardial infarction? A case report. Journal of cardiovascular medicine (Hagerstown, Md) 2008;9:725–728. doi: 10.2459/JCM.0b013e3282f2cd0d. [DOI] [PubMed] [Google Scholar]
- 39.Bailly C, Merceron O, Hammoudi N, Dorent R, Michel PL. Cannabis induced acute coronary syndrome in a young female. International journal of cardiology. 2010;143:e4–6. doi: 10.1016/j.ijcard.2008.11.200. [DOI] [PubMed] [Google Scholar]
- 40.Kocabay G, Yildiz M, Duran NE, Ozkan M. Acute inferior myocardial infarction due to cannabis smoking in a young man. Journal of cardiovascular medicine (Hagerstown, Md) 2009;10:669–670. doi: 10.2459/JCM.0b013e32832bcfbe. [DOI] [PubMed] [Google Scholar]
- 41.Singla S, Sachdeva R, Mehta JL. Cannabinoids and atherosclerotic coronary heart disease. Clin Cardiol. 2012;35:329–335. doi: 10.1002/clc.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durst R, Lotan C. The Potential for Clinical Use of Cannabinoids in Treatment of Cardiovascular Diseases. Cardiovascular Therapeutics. 2011;29:17–22. doi: 10.1111/j.1755-5922.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- 43.Nahas G, Latour C. The human toxicity of marijuana. The Medical journal of Australia. 1992;156:495–497. [PubMed] [Google Scholar]
- 44.Ashton CH. Pharmacology and effects of cannabis: a brief review. The British journal of psychiatry: the journal of mental science. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- 45.Grotenhermen F. Cannabis and Cannabinoids: pharmacology, toxicology, and therapeutic potential. Birmingham, NY: The Haworth Integrated healing process; 2002. [Google Scholar]
- 46.Farnsworth NR, Cordell GA. New Potential Hazard Regarding Use of Marijuana – Treatment of Plants with Liquid Fertilizers. Journal of Psychedelic Drugs. 1976;8:151–155. [Google Scholar]
- 47.Cherian M. Pests of ganja. Madras Agricultural journal. 1932:259–265. [Google Scholar]
- 48.Rosenthal E. Marijuana Grower’s Handbook: The Indoor High Yield Guide: Quick American Archives. 1998. [Google Scholar]
- 49.Classics revisited. On the haschisch or Cannabis indica. By John Bell, 1857. Journal of substance abuse treatment. 1985;2:239–243. [PubMed] [Google Scholar]
- 50.Johnson DW, Gunn JW., Jr Dangerous drugs: adulterants, diluents, and deception in street samples. Journal of forensic sciences. 1972;17:629–639. [PubMed] [Google Scholar]
- 51.DuToit BM. Continuity and change in cannabis use by Africans in South Africa. Journal of Asian and African studies. 1976;11:203–208. [PubMed] [Google Scholar]
- 52.Margolis JS, Clorfene R. A Child’s Garden of Grass: The Official Handbook for Marijuana Users: Pocket Books. 1970. [Google Scholar]
- 53.McPartland J. Fungal pathogens of cannabis sativa in Illinois. Phytopathology. 1984;797 [Google Scholar]
- 54.McPartland J. Common names for diseases of Cannabis sativa L. Plant Diseases. 1991:226–227. [Google Scholar]
- 55.Chusid MJ, Gelfand JA, Nutter C, Fauci AS. Letter: Pulmonary aspergillosis, inhalation of contaminated marijuana smoke, chronic granulomatous disease. Annals of internal medicine. 1975;82:682–683. doi: 10.7326/0003-4819-82-5-682. [DOI] [PubMed] [Google Scholar]
- 56.Dubey NK. Natural Products in Plant Pest Management. CABI; 2011. [Google Scholar]
- 57.Bbosa GS, Lubega A, Kyegombe DB, Kitya D, Ogwal-Okeng J, Anokbonggo WW. Review of the Biological and Health Effects of Aflatoxins on Body Organs and Body Systems. INTECH Open Access Publisher; 2013. [Google Scholar]
- 58.Denning DW, Follansbee SE, Scolaro M, Norris S, Edelstein H, Stevens DA. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. The New England journal of medicine. 1991;324:654–662. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 59.Hamadeh R, Ardehali A, Locksley RM, York MK. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient. Chest. 1988;94:432–433. doi: 10.1378/chest.94.2.432. [DOI] [PubMed] [Google Scholar]
- 60.Marks WH, Florence L, Lieberman J, et al. Successfully treated invasive pulmonary aspergillosis associated with smoking marijuana in a renal transplant recipient. Transplantation. 1996;61:1771–1774. doi: 10.1097/00007890-199606270-00018. [DOI] [PubMed] [Google Scholar]
- 61.Ungerleider JT, Andrysiak T, Tashkin DP, Gale RP. Contamination of marihuana cigarettes with pathogenic bacteria--possible source of infection in cancer patients. Cancer treatment reports. 1982;66:589–591. [PubMed] [Google Scholar]
- 62.Taylor DN, Wachsmuth IK, Shangkuan YH, et al. Salmonellosis associated with marijuana: a multistate outbreak traced by plasmid fingerprinting. The New England journal of medicine. 1982;306:1249–1253. doi: 10.1056/NEJM198205273062101. [DOI] [PubMed] [Google Scholar]
- 63.Kurup VP, Resnick A, Kagen SL, Cohen SH, Fink JN. Allergenic fungi and actinomycetes in smoking materials and their health implications. Mycopathologia. 1983;82:61–64. doi: 10.1007/BF00436948. [DOI] [PubMed] [Google Scholar]
- 64.Thompson GR, 3rd, Tuscano JM, Dennis M, et al. A microbiome assessment of medical marijuana. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2017;23:269–270. doi: 10.1016/j.cmi.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Hall W, Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Current opinion in psychiatry. 2007;20:393–397. doi: 10.1097/YCO.0b013e32812144cc. [DOI] [PubMed] [Google Scholar]
- 66.Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clinic proceedings. 2012;87:172–186. doi: 10.1016/j.mayocp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raphael B, Wooding S, Stevens G, Connor J. Comorbidity: cannabis and complexity. Journal of psychiatric practice. 2005;11:161–176. doi: 10.1097/00131746-200505000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction (Abingdon, England) 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- 69.Jin LZ, Rangan A, Mehlsen J, Andersen LB, Larsen SC, Heitmann BL. Association Between Use of Cannabis in Adolescence and Weight Change into Midlife. PloS one. 2017;12:e0168897. doi: 10.1371/journal.pone.0168897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gledhill-Hoyt J, Lee H, Strote J, Wechsler H. Increased use of marijuana and other illicit drugs at US colleges in the 1990s: results of three national surveys. Addiction (Abingdon, England) 2000;95:1655–1667. doi: 10.1046/j.1360-0443.2000.951116556.x. [DOI] [PubMed] [Google Scholar]
- 71.Smit E, Crespo CJ. Dietary intake and nutritional status of US adult marijuana users: results from the Third National Health and Nutrition Examination Survey. Public health nutrition. 2001;4:781–786. doi: 10.1079/phn2000114. [DOI] [PubMed] [Google Scholar]
- 72.Strang J, Witton J, Hall W. Improving the quality of the cannabis debate: defining the different domains. BMJ (Clinical research ed) 2000;320:108–110. doi: 10.1136/bmj.320.7227.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Archives of general psychiatry. 1994;51:273–279. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- 74.Hall W. Cannabis use and psychosis. Drug and alcohol review. 1998;17:433–444. doi: 10.1080/09595239800187271. [DOI] [PubMed] [Google Scholar]
- 75.Reilly D, Didcott P, Swift W, Hall W. Long-term cannabis use: characteristics of users in an Australian rural area. Addiction (Abingdon, England) 1998;93:837–846. doi: 10.1046/j.1360-0443.1998.9368375.x. [DOI] [PubMed] [Google Scholar]
- 76.Thomas H. A community survey of adverse effects of cannabis use. Drug and alcohol dependence. 1996;42:201–207. doi: 10.1016/s0376-8716(96)01277-x. [DOI] [PubMed] [Google Scholar]
- 77.Rey JM, Sawyer MG, Clark JJ, Baghurst PA. Depression among Australian adolescents. The Medical journal of Australia. 2001;175:19–23. doi: 10.5694/j.1326-5377.2001.tb143505.x. [DOI] [PubMed] [Google Scholar]
- 78.Troisi A, Pasini A, Saracco M, Spalletta G. Psychiatric symptoms in male cannabis users not using other illicit drugs. Addiction (Abingdon, England) 1998;93:487–492. doi: 10.1046/j.1360-0443.1998.9344874.x. [DOI] [PubMed] [Google Scholar]
- 79.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ (Clinical research ed) 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study Circulation. 1994;90:2225–2229. doi: 10.1161/01.cir.90.5.2225. [DOI] [PubMed] [Google Scholar]
- 81.Kawachi I, Colditz GA, Ascherio A, et al. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89:1992–1997. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- 82.Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation. 2005;111:250–253. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 83.Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Molecular psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 84.Elhwuegi AS. Central monoamines and their role in major depression. Progress in neuro-psychopharmacology & biological psychiatry. 2004;28:435–451. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 85.Lee JD, Schatz D, Hochman J. Cannabis and Heart Disease Journal of the American College of Cardiology. 2018;71:2552. doi: 10.1016/j.jacc.2018.03.010. [DOI] [PubMed] [Google Scholar]