Abstract
Objective
The aim of this study was to translate the Chronic Otitis Media Questionnaire–12 (COMQ-12) into Turkish, evaluate the internal consistency of the test and test-retest reliability, and validate the adaptation for further use in Turkish studies.
Methods
A total of 50 healthy subjects and 50 patients with chronic otitis media (COM) have completed a translated Turkish version of the COMQ-12. Healthy subjects were asked to complete the test twice. A statistical analysis was performed to evaluate the validity and test-retest reliability of the questionnaire. Patients were divided into three groups. Group 1 were patients with COM; Group 2 represented the first test of the control group; and Group 3 represented the second test of the control group. Cronbach’s test was performed to test internal consistency, and Spearman’s test was performed to evaluate test-retest validity.
Results
The average score was 30.64 for Group 1, 3.60 for Group 2, and 3.66 for Group 3. The COMQ-12 score of the patient group was significantly higher than the score of the control group (p<0.001). The area under the receiver operating characteristics curve value was calculated as 0.992, which showed a strong diagnostic accuracy, and the cut-off point was defined as 9. A Cronbach’s alpha value of 0.810 was found. Spearman’s rank correlation coefficient value (Spearman’s rho) was calculated as 0.920.
Conclusion
The Turkish adaptation of the COMQ-12 is a consistent and valid test with high sensitivity and specificity that can be used in Turkish for further studies instead of the original questionnaire.
Keywords: Otitis media, health surveys, quality of life
Introduction
Chronic otitis media (COM) is a common disease that affects approximately 2% of the population worldwide (1). However, the prevalence of the disease may range from <1% in high-income countries to up to 4% in developing countries (2). COM may profoundly affect the quality of life of patients due to symptoms such as chronic ear pain, malodorous ear drainage, hearing loss, and vertigo. Furthermore, it might cause serious complications such as meningitis, brain abscess, and total hearing loss; therefore, customized treatment of this disease in patients is vital.
Measuring the Health-Related Quality of Life (HRQoL) scores of patients using the Patient-Reported Outcome Measures is an increasingly used method for clinical evaluation and personalized treatment choice (3, 4). The Chronic Otitis Media Questionnaire–12 (COMQ-12) was developed by Phillips et al. (5) who used three older HROoQL questionnaires developed for COM: Chronic Ear Survey (1), Chronic Otitis Media Outcome Test–15 (6), and Chronic Otitis Media–5 (7) questionnaires.
The aim of this study was to translate the COMQ-12 questionnaire into Turkish, evaluate the internal consistency of the test, test-retest reliability, and validate the adaptation for further use into studies in Turkish.
Methods
The Ethics Committee Approval for the study was taken from the İstanbul University School of Medicine Ethics Committee for Scientific Research with file number 2018/998.
Questionnaire
The COMQ-12 consists of 12 questions that evaluate the severity and frequency of the symptoms and their impact on the quality of life of the patients on a scale from 0 to 5. The first seven questions investigate the intensity of the symptoms, and Questions 8 and 9 evaluate the frequency of symptoms affecting patients’ life at home and work. Questions 10 and 11 assess the impact of the disease on public health, and the last question evaluates the general impact of the disease on patients’ general life (Appendix 1).
Translation
The COMQ-12 was translated into Turkish by the first author and checked by the senior authors. After corrections were made, the Turkish questions were re-translated into English by a native English speaker. No differences were observed between the two versions of the questionnaire; hence the adaptation was verified.
Subjects
Informed consents were obtained from the patients prior to filling out the questionnaire.
A total of 100 (50 healthy and 50 with COM) Turkish-speaking individuals between 18 and 65 years of age completed the Turkish version of the COMQ-12 questionnaire. Three groups were formed for further evaluation and statistical analysis. Group 1 included 50 patients with COM, Group 2 represented the first test of the control group, and Group 3 represented the retest of the control group.
Fifty patients with COM were asked to fill out the questionnaire on their preoperative visit one day prior to their surgery (Group 1). Patients who were candidates for myringoplasty with or without ossicular chain reconstruction or mastoidectomy (primary or revision) were included in the study and divided into three subgroups: dry ear, draining ear, and visible cholesteatoma. For the Group 1, only patients with a history of more than two ear drainages that lasted for more than two weeks without medical therapy during past 12 months were included in the study. Patients who had prior successful tympanoplasty or mastoidectomy were excluded from the study.
Subjects in the control group were chosen among medical students, hospital staff, and families of the authors. Every subject was examined by the first two authors prior to filling out the questionnaire. Subjects who had pathological findings on their examination were excluded from the study along with subjects who had previous ear surgery or a history of recurrent (more than once) acute otitis media. Subjects in the control group were asked to complete the questionnaire for a second time one week after the initial test. The data of the first tests were determined as Group 2 and the data of retest as Group 3.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) version 21 (IBM Corp., Armonk, NY, USA) was used to perform the statistical analysis. A p<0.05 was accepted as statistically significant. Six different tests were used:
The Shapiro–Wilk test was used to evaluate the normality of the data distribution of the investigated groups and subgroups.
The interpretation of the validity of the questionnaire was tested by comparing the overall scores of the healthy (Group 2) and patient (Group 1) groups using the non-parametric independent-samples Mann–Whitney U test.
The one-way analysis of variance (ANOVA) test was used to evaluate the difference between the overall scores of the Group 1 subgroups.
The diagnostic value of the COMQ-12 was assessed using a receiver operating characteristics (ROC) curve. A ROC curve was drawn and the area under the ROC curve (AROC) was calculated. The cut-off score was chosen for the diagnosis of COM according to the best specificity and sensitivity values.
Cronbach’s alpha was calculated to evaluate the inter-item correlation and the internal consistency of the questionnaire in the patient group (Group 1).
Finally, Spearman’s rank correlation coefficient value (Spearman’s rho) was measured to show the test-retest reliability. Correlation coefficients varied between 0 (no reliability) and 1 (perfect reliability). In general, a value of >0.7 is adopted as acceptable reliability in the literature (8).
The intraclass correlation coefficient (ICC) was obtained using the average measures, absolute agreement two-way mixed effects model to show the agreement between the repeated measurements.
Results
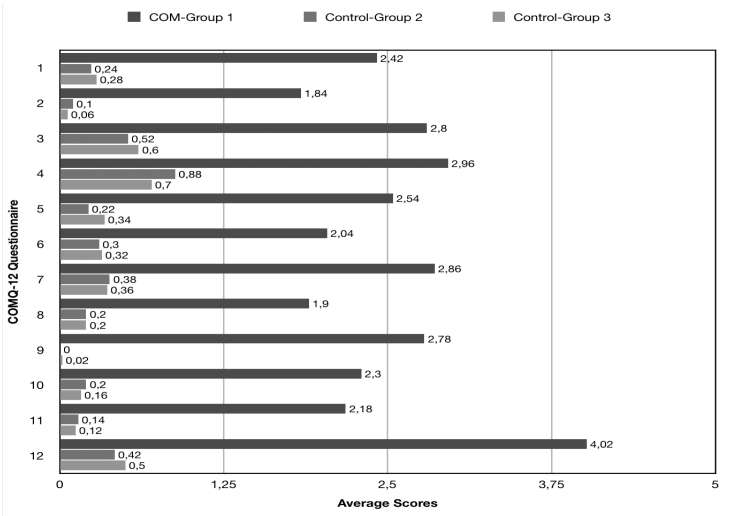
Fifty patients (28 females, 22 males) with COM were included in the study. The average age+standard deviation was 39.3±12.7 years (range: 18–64), and the average score for the COMQ-12 test was 30.6±11.8. Detailed patient information is listed in Table 1 and Figure 1.
Table 1.
Overall scores and demographic distribution of Groups 1, 2, and 3
| Overall Score | COM Group 1 (n=50) |
Control-Group 2 (n=50) | Control-Group 3 (n=50) |
|---|---|---|---|
| Mean | 30.640 | 3.600 | 3.660 |
| SD | 11.764 | 3.653 | 3.863 |
| SE | 1.664 | 0.5167 | 0.5463 |
| Median | 32.5 | 3 | 2 |
| Minimum | 8 | 0 | 0 |
| Maximum | 55 | 14 | 14 |
| Range | 47 | 14 | 14 |
| Age | |||
| Mean | 39.300 | 36.580 | 36.580 |
| SD | 12.706 | 13.200 | 13.200 |
| Minimum | 18 | 20 | 20.0 |
| Maximum | 64 | 63 | 63 |
| Sex | |||
| Male | 22 | 23 | 23 |
| Female | 28 | 27 | 27 |
COM: chronic otitis media; n: number; SD: standard deviation; SE: standard error
Figure 1.
Average scores of Groups 1, 2, and 3 for each question
While 27 patients had dry tympanic membrane perforations (TMP), 10 of them had draining ear, and 13 had visible cholesteatoma accompanying the TMP at the time of completing the survey. From this point on, we will discuss the results for three groups separately.
Out of 27 patients with dry TMP, 16 of the patients were female and 11 were male. Their average age was 39.1 years (range: 18–56). The overall average score for COMQ-12 was 28.3±12.2 (range: 8–51) (Table 2).
Table 2.
Overall scores and demographic distribution of Group 1 subgroups
| Overall Score | Visible Cholesteatoma (n=13) | Draining Ear (n=10) | Dry Tm Perforation (n=27) |
|---|---|---|---|
| Mean | 31.692 | 35.5 | 28.333 |
| SD | 8.390 | 13.607 | 12.209 |
| Minimum | 15 | 8 | 8 |
| Maximum | 45 | 55 | 51 |
| Range | 30 | 47 | 43 |
| Median | 32 | 39.5 | 27 |
| Age | |||
| Mean | 37.69 | 41.9 | 39.11 |
| SD | 14.65 | 14.87 | 42.675 |
| Minimum | 18 | 22 | 18 |
| Maximum | 64 | 62 | 56 |
| Sex | |||
| Male | 7 | 4 | 11 |
| Female | 6 | 6 | 16 |
Tm: tympanic membrane; n: number; SD: standard deviation
Out of 10 patients with an active drainage TMP, 6 were female and 4 were male. Their average age was 41.9 years (range: 22–62). The overall average score for COMQ-12 was 35.5±13.6 (range: 8–55) (Table 2).
Out of 13 patients with TMP with visible cholesteatoma, 6 were female and 7 were male. Their average age was 37.7 years (range: 18–64). The overall average score for COMQ-12 was 31.7±8.4 (range: 15–45) (Table 2).
The control group consisted of 50 subjects (27 females, 23 males), and the average age of the group was 36.6±13.2 years (range: 20–63). As explained in detail in the Methods section, each subject in the control group completed the questionnaire two times with one week of interval. The overall average results for the first test and the retest were 3.60 and 3.66, respectively.
The Shapiro–Wilk test was used to evaluate the normality of the data. In the patient group, the p-value for the overall scores was 0.482, and the p-value for age was 0.166. Since both of the values were >0.05, the distribution of the patient group was evaluated as normal. In Groups 2 and 3, the p-values for the overall scores and the age were <0.05, therefore, the distribution was not evaluated as normal. Because the distribution was abnormal in Groups 2 and 3, non-parametric statistical tests were used for analysis.
As mentioned above, we divided Group 1 into three different subgroups: dry TMP, draining ear, and TMP with visible cholesteatoma. The normality of the data of these subgroups was also checked with the Shapiro-Wilk test. All subgroup data were found normal (p=0.944, 0.584, and 0.501, respectively). Therefore, the one-way ANOVA test comes forth as the only parametric test used in our study.
Independent-samples Mann–Whitney U test was used to compare the overall scores of Group 1 and Group 2. The COMQ-12 score of the patient group was significantly higher than the control group (<0.001).
The one-way ANOVA test showed that there was no significant difference between the overall scores of the subgroups of the Group 1 (p=0.245).
A ROC curve was created to show the diagnostic characteristics of the COMQ-12 questionnaire. Overall scores were determined as test variable, and groups (Group 1 and Group 2) as state variable. The Aroc value was calculated as 0.992, which stated the strong diagnostic accuracy of the COMQ-12 questionnaire, as well as its ability to differentiate the COM from the normal ear (Figure 2). Using the ROC curve, a cut-off point of 9 was defined to distinguish COM from healthy ear achieving the best-balanced sensitivity and specificity values (0.96 and 0.92, respectively).
Figure 2.
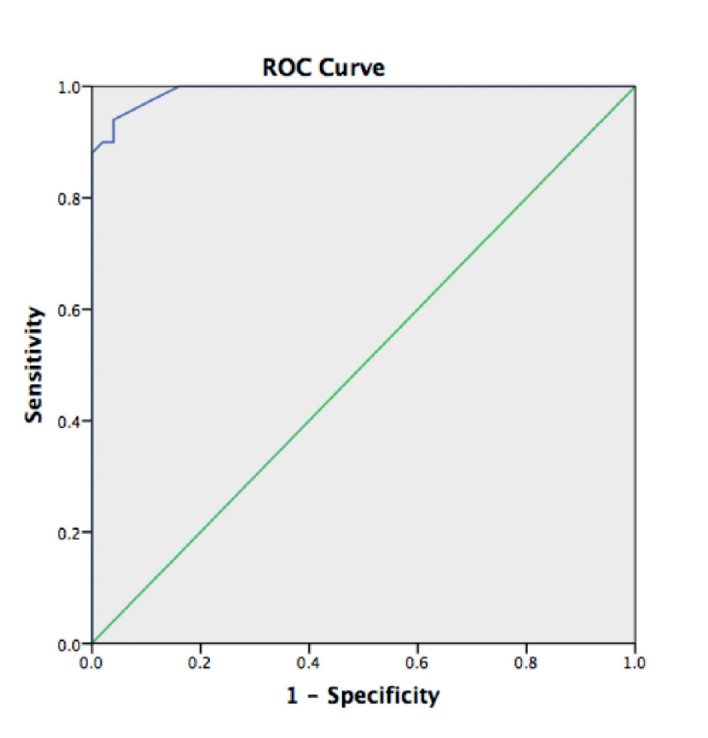
The questionnaire ROC curve. A threshold score of 9 was estimated to distinguish between chronic otitis media and a healthy ear
Cronbach’s alpha test was performed in the patient group. An alpha value of 0.810 was found. The score was higher than 0.70 and close to 1, thus it can be stated that the inter-item correlation and the internal consistency of the questionnaire is high (9).
The Spearman’s rank correlation coefficient value (Spearman’s rho) was calculated as 0.920. The correlation of the test is significant at 0.01 level. This shows that the test and retest are correlated, and the test-retest reliability is safe.
The average measured ICC was 0.918 (with a 95% confidence interval ranging from 0.856 to 0.954), which means a “good” to an “excellent” level of test-retest reliability (10).
Discussion
Patient-based evaluation of the symptoms and targeted treatment based on the patient’s symptoms are getting more significant every day (3); thereby, questionnaires that evaluate symptoms and quality of life are getting more important. The COMQ-12 is a newly created questionnaire that has combined three older tests that evaluate patients with COM. To date, the COMQ-12 has been translated into Dutch (11), the Kannada (12) language, and Portuguese (13).
The patient group had an overall average score of 30.64+11.76 (range: 8–55), which is similar to the results reported by Fonseca et al. (13) (mean: 29). The mean total scores for the control groups ranged from 0 to 14 for both Group 2 and Group 3 with an average of 3.60 and 3.66, respectively. Because of the high scores observed in the control group, we investigated the symptoms and questions that have led to this unexpected result. Most of the high results come from the high scores of the question about either hearing loss or tinnitus. Similar results have also been indicated by van Dinther et al. (11) in the Dutch adaptation and validation of the COMQ-12.
In our study, our secondary gain was to evaluate if there was a significant difference between the subgroups of COM patients by using COMQ-12 scores. Although the mean value of the patients with dry TMP (28.3) was slightly lower than with visible cholesteatoma (31.7) and draining ear (35.5), there was no statistically significant difference. Therefore, we cannot suggest the use of COMQ-12 to discriminate between the forms of COM. However, further studies with more subjects should be designed to evaluate the strength of the COMQ-12 in discriminating between the COM subgroups.
After the ROC curve analysis and the calculation of the area under the curve, the cut-off value was set as 9, with a sensitivity of 0.96 and a specificity of 0.92. Although the cut-off limit is higher in our study than the cut-off limits set by Philipps et al. (14) and in the Dutch version (11), the sensitivity and the specificity of the value are higher.
Studies on the Dutch (11) and the Serbian (15) versions measured the ICC to show test-retest reliability. Their ICC values were 0.859 and 0.985, respectively. Like the other versions, the test-retest reliability was acceptable based on the Spearman’s rho value of 0.920 in our study.
Furthermore, the Cronbach’s alpha value of 0.810 was found for the test. The result is similar to the previous results from the original English study (0.889) (5), the Serbian version (0.84) (15), and the Portuguese (0.85) (13) version. Thus, the inter-item correlation and internal consistency of the questionnaire are high.
The COMQ-12 is a newly developed HRQoL questionnaire for the assessment of active COM, and a valid test that can be used in both preoperative and postoperative evaluations of patients. The cut-off limits and high sensitivity and specificity of the test presented by the previous studies, as well as in our study, show that the questionnaire can be used as a valuable tool in the evaluation period of patients with COM.
Conclusion
The Turkish adaptation of the COMQ-12 is a consistent and valid test with high sensitivity and specificity that can be used in further studies.
Appendix 1
Footnotes
You can reach the appendix table of this article at 10.5152/tao.2019.3693.
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of İstanbul University School of Medicine (2018/998).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.D., M.Ç., H.K., B.P., Y.G., K.S.O.; Design - C.D., M.Ç., H.K., B.P., Y.G., K.S.O.; Supervision - C.D., M.Ç., H.K., B.P., Y.G., K.S.O.; Resource - B.P., Y.G., K.S.O.; Data Collection and/or Processing - C.D., M.Ç., H.K.; Analysis and/or Interpretation - B.P., Y.G., K.S.O.; Literature Search - C.D., M.Ç., H.K.; Writing - C.D., M.Ç., H.K., B.P., Y.G., K.S.O.; Critical Reviews - C.D., M.Ç., H.K., B.P., Y.G., K.S.O.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Nadol JB, Staecker H, Gliklich RE. Outcomes assessment for chronic otitis media: the Chronic Ear Survey. Laryngoscope. 2000;110:32–5. doi: 10.1097/00005537-200003002-00009. [DOI] [PubMed] [Google Scholar]
- 2.Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167. doi: 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- 4.Marshall S, Haywood K, Fitzpatrick R. Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract. 2006;12:559–68. doi: 10.1111/j.1365-2753.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- 5.Phillips JS, Haggard M, Yung M. A new health-related quality of life measure for active chronic otitis media (COMQ-12): development and initial validation. Otol Neurotol. 2014;35:454–8. doi: 10.1097/MAO.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 6.Baumann I, Kurpiers B, Plinkert PK, Praetorius M. Development and validation of the Chronic Otitis Media Outcome Test 15 (COMOT-15). Measurement of health-related quality of life in patients with chronic otitis media. HNO. 2009;57:889–95. doi: 10.1007/s00106-008-1870-3. [DOI] [PubMed] [Google Scholar]
- 7.Vlastos IM, Kandiloros D, Manolopoulos L, Ferekidis E, Yiotakis I. Quality of life in children with chronic suppurative otitis media with or without cholesteatoma. Int J Pediatr Otorhinolaryngol. 2009;73:363–9. doi: 10.1016/j.ijporl.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 9.Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–5. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo TK, Li MY. A Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dinther J, Droessaert V, Camp S, Vanspauwen R, Maryn Y, Zarowski A, et al. Validity and test-retest reliability of the Dutch version of the Chronic Otitis Media Questionnaire 12 (COMQ-12) J Int Adv Otol. 2015;11:248–52. doi: 10.5152/iao.2015.1701. [DOI] [PubMed] [Google Scholar]
- 12.Prabhu P, Chandrashekar A, Jose A, Ganeshan A, Kiruthika L. Development and administration of Chronic Suppurative Otitis Media Questionnaire-12 (COMQ-12) and Chronic Otitis Media Outcome Test-15 (COMOT-15) in Kannada. Int Arch Otorhinolaryngol. 2018;22:108–12. doi: 10.1055/s-0037-1603644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonseca ACO, Ramos P, Balsalobre FA, Freitas EL, Phillips JS, Yung MW, et al. Validation of a Portuguese version of the health-related quality of life measure for active chronic otitis media (COMQ-12) Braz J Otorhinolaryngol. 2018;84:708–12. doi: 10.1016/j.bjorl.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips JS, Yung MW. COMQ-12 scores in adult patients without chronic middle ear disease. Clin Otolaryngol. 2014;39:362–7. doi: 10.1111/coa.12306. [DOI] [PubMed] [Google Scholar]
- 15.Bukurov B, Arsovic N, Grujicic SS, Haggard M, Spencer H, Marinkovic JE. Psychometric characteristics of the chronic otitis media questionnaire 12 (COMQ–12): stability of factor structure and replicability shown by the Serbian version. Health Qual Life Outcomes. 2017;15:207. doi: 10.1186/s12955-017-0782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]