Abstract
DNA mismatch repair (MMR) is a DNA excision-resynthesis process that principally enhances replication fidelity. Highly conserved MutS (MSH) and MutL (MLH/PMS) homologs initiate MMR and in higher eukaryotes act as DNA damage sensors that can trigger apoptosis. MSH proteins recognize mismatched nucleotides, whereas the MLH/PMS proteins mediate multiple interactions associated with downstream MMR events including strand discrimination and strand-specific excision that are initiated at a significant distance from the mismatch. Remarkably, the biophysical functions of the MLH/PMS proteins have been elusive for decades. Here we consider recent observations that have helped to define the mechanics of MLH/PMS proteins and their role in choreographing MMR. We highlight the stochastic nature of DNA interactions that have been visualized by single molecule analysis as well as the plasticity of protein complexes that employ thermal diffusion to complete the progressions of MMR.
Graphics Abstract
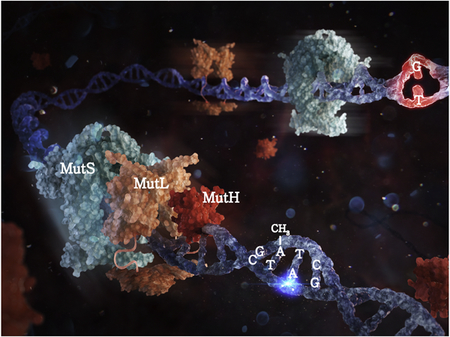
Introduction
DNA mismatch repair (MMR) plays a fundamental role in maintaining genomic stability [1–3]. Defects in the MMR genes increase mutation rates 100–1000 fold and are the cause of Lynch syndrome or hereditary non-polyposis colorectal cancer (LS/HNPCC) [4–7]. LS/HNPCC patients exhibit a high risk of developing colorectal cancer as well as endometrial, ovarian cancer and upper urinary tract cancers [4, 6, 7]. MMR defects have also been found to be associated with sporadic colorectal, endometrial and ovarian tumors [4–7]. The MMR proteins also participate in other cellular biological processes such as somatic hypermutation, class switch recombination, DNA damage signaling as well as inhibiting recombination between partially homologous sequences (homeologous recombination) [8–13]. Here, we focus exclusively on replication-coupled MMR, although the biophysical function(s) of the core MMR components are unlikely to be substantially different during these other biological processes.
The DNA replication machinery is good but not perfect and about every million nucleotides a misincorporation error (mismatch) is introduced into the DNA [1]. In order to maintain genomic continuity the offending nucleotide must be specifically removed from the newly replicated strand [1]. Post-replication MMR has evolved to perform this strand-specific excision [1]. An extraordinary feature of MMR is that excision may be initiated either 5’ or 3’ and up to 2 kb distant from the mismatch [1]. Moreover, the DNA excision process ultimately becomes directional extending from the distant initiation site to just past the mismatch [1]. The mechanics of communicating mismatch recognition to the distant site and then initiating and assembling the strand-specific excision components that uniquely remove the incorrect nucleotide has been historically enigmatic [1, 14–16].
Highly conserved MutS homologs (MSH) and MutL homologs (MLH/PMS) that initiate canonical strand-specific excision are unique to MMR and have been identified throughout terrestrial biology [1, 14, 17–19]. In addition to these core components most organisms utilize a 5’→3’ double-strand DNA (dsDNA) dependent single-stranded DNA (ssDNA) exonuclease (EXOI) that acts on duplex DNA starting at a strand break [1, 20–22]. A functionally conserved ssDNA binding protein protects the resulting excision tract [1] prior to resynthesis by the replicative polymerase machinery, which appears entirely independent of MMR excision [1].
It is likely that most organisms utilize persistent strand breaks associated with leading and lagging strand DNA synthesis as an entry site for the primary excision component EXOI [1]. However a subset of γ-proteobacteria, that includes the widely used model organism Escherichia coli, have recently evolved DNA adenine methylase (Dam) and MutH to introduce a scission into the newly replicated strand [19]. In these organisms Dam methylates GATC sequences within the DNA, while MutH protein cleaves hemimethylated GATC sites which only occur transiently following replication [23–26]. Most Dam/MutH-dependent γ-proteobacteria does not have EXOI and instead employ the UvrD helicase (Helicase II) that unwinds DNA from a strand break [1]. UvrD unwinding is combined with either a 5’- or 3’-ssDNA-dependent ssDNA exonuclease to perform strand-specific excision [1]. In addition, MMR is connected to the replication fork through the replication processivity factor β-clamp in bacteria and PCNA in archea and eukaryotes [1]. β-Clamp/PCNA also plays a regulatory role in activating an MLH/PMS endonuclease that is required for 3’-excision in most organisms (see below) [27]. A summary of MMR components and activities across several species can be found in Table 1 (see also Kim et al., this issue).
Table 1.
MMR Components Across Species
| E.coli | S.cerevisae | Human | Function |
|---|---|---|---|
| EcMutS | ScMsh2-ScMsh6 | HsMSH2-HsMSH6 | base-base/small IDL1 recognition; sliding clamp |
| ScMsh2-ScMsh3 | HsMSH2-HsMSH3 | small IDL1/large IDL1 (12 nt) recognition; sliding clamp | |
| EcMutL | ScMlh1-ScPms1 | HsMLH1-HsPMS2 | MSH-dependent sliding clamp; PCNA-activated endonuclease; MutH tether (E.coli) |
| EcMutH | - | - | Hemimethylated-GATC endonuclease |
| EcUvrD | - | - | 3’→5’ Helicase |
| EcExol, EcExoVII, EcExoX | - | - | ssDNA-dependent 3’→5’ exonuclease |
| EcExoVII, EcRecJ | - | - | ssDNA-dependent 5’→3’ exonuclease |
| - | ScExol | HsEXOI | dsDNA-dependent 3’→5’ ssDNA exonuclease |
| Ecβ-Clamp | ScPCNA | HsPCNA | Replication processivity factor; Polymerase tether |
Insertion-deletion loop type mismatch
One of the first clues that MMR relies on stochastic thermal diffusion to initiate excision came from studies of MSH1 proteins. The MSH proteins linked to MMR operate as a dimer or heterodimer and recognize mismatched nucleotides (Fig. 1a) [28–32]. Importantly, in the presence of ATP all MSH proteins undergo a conformational transition into a sliding clamp that randomly diffused along the DNA while in intermittent contact with the backbone similar to a washer on a string [33–40]. The biophysical progressions of ADP/ATP processing during mismatch recognition and sliding clamp formation have been detailed in numerous bulk and single molecule imaging studies (see also Kim et al., this issue) [33–40]. These observations were originally incorporated into a Molecular Switch/Sliding Clamp model for MMR [1, 41–44], which has been ardently debated for nearly two decades because of its fundamental reliance on stochastic mechanical events. However, a persistent puzzle with this model is how random bi-direction diffusion of an MSH sliding clamp could initiate a strand-specific excision process that is initiated at a distant site and proceeds toward the mismatch. This is particularly relevant to γ-proteobacteria where the MSH sliding clamp must first manage a strand-specific incision by the MutH protein, and then assemble the UvrD helicase-ssDNA exonuclease excision system at that distant strand scission site.
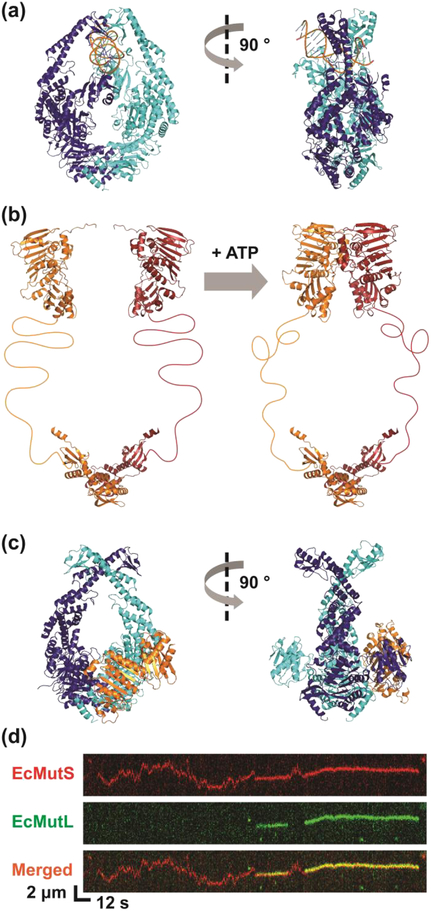
Figure 1. EcMutL Associates with ATP-bound EcMutS Sliding Clamp.
(a) Structure of EcMutS bound to a mismatch (PDB ID: 1E3M). (b) N- and C-terminus structure of EcMutL (PDB IDs: 1B63 and 1X9Z) joined by hypothetical flexible linkers. (c) Structure of EcMutLLN40 bound to EcMutSΔC800 (PDB ID: 5AKB). (d) Representative kymographs showing EcMutL loaded onto a mismatched DNA by an EcMutS sliding clamp. Binding of EcMutL (colocalization of red and green) alters the diffusion properties of EcMutS (red only). See text for description.
MutL Homologs
The MLH/PMS2 proteins also operate as a dimer or heterodimer and have been long known to play a central role in determining the biological outcome of MMR [45, 46]. These historical studies found that E. coli (Ec) EcMutL interacted with EcMutS, EcMutH, EcUvrD and Ecβ-Clamp [41, 47, 48]. The eukaryotic MLH/PMS have also been found to interact with the MSH, PCNA and EXOI proteins [27, 49–56]. These multiple physical interactions appeared consistent with the hypothesis that MLH/PMS proteins coordinated MSH mismatch recognition with downstream excision processes [1, 14–16, 18, 57]. However, distinct biophysical function(s) for MLH/PMS proteins during MMR has been elusive since their genetic discovery in 1960 [58].
EcMutL contains 615 amino acids with an N-terminal ATPase domain (amino acid 1–331) and a C-terminal domain that stably links the two EcMutL monomers (amino acid 432–615). Separate structures have been described for both the N-terminal and the C-terminal domains of other MLH/PMS proteins, although a full-length structure has not been resolved for a MLH/PMS from any organism [45, 59–64]. This is probably due to a disordered linker domain that connects the N- and C-terminal domains, which appears resistant to crystallography (Fig. 1b). The disordered linker displays no apparent sequence conservation, has been found to tolerate large amino acid substitutions, and varies between a few amino acids in Thermus aquaticus (Ta) TaMutL to approximately 800 amino acids in human HsMLH3 [65]. Taken as a whole these observations suggest the linker domain probably forms a random coil that is organism and/or function dependent [61, 66].
The N-terminal domain is a member of the highly conserved GHKL ATPase superfamily (Gyrase, Hsp90, histidine kinase and MutL) [62, 67]. Binding of ATP or its non-hydrolysable analog adenylyl-imidodiphosphate (AMP-PNP) has been shown to induce dimerization between separate N-terminal domains [60]. This conformational transition is assumed to be important since mutations that affect ATP-binding or ATPase activity lead to an MMR deficiency [60, 62, 68, 69].
The C-terminal domain of MLH/PMS proteins stably link dimer/heterodimer partners [61, 70, 71]. The C-terminal peptide sequences across biology are relatively divergent and likely evolved by selecting for compensatory changes between the interaction residues that maintain the dimer/heterodimer association [59, 61, 72, 73]. The MLH/PMS in organisms that do not utilize UvrD and a ssDNA exonuclease for excision maintain a conserved endonuclease domain that is just internal from the C-terminal interaction region. [27, 55, 56, 65, 70, 74, 75]. Mutations or truncations that disrupt the dimer/heterodimer interaction or endonuclease domains cause a mutator phenotype in vivo consistent with prominent functions in MMR [55, 61, 68, 70, 76].
MutS Sliding Clamps Provide a Platform to Recruit MutL onto the Mismatched DNA
Purified MLH/PMS proteins have been found to bind ssDNA in very low ionic conditions, which vanishes as the ionic strength approaches physiological conditions [45, 46, 77, 78]. Atomic force microscopy (AFM) studies appeared to suggest that ATP binding by MLH/PMS proteins resulted in collapse and presumed ordering of the linker region [79]. However, single molecule observations of EcMutL [80] strongly suggest that both these observations represented non-physiological settings, which errantly confounded the biophysical functions of these proteins in MMR.
Intriguingly, single-cysteine variants of EcMutS (EcMutSΔC800; C-terminal 53 amino acid residues deleted) were used to crosslink the N-terminal ATPase domain of EcMutL (EcMutLLN40) and crystalize the complex (Fig. 1c) [81]. A structure that was consistent with numerous earlier biochemical studies [1, 33, 34, 39, 41–44] emerged where the ATP-bound EcMutSΔC800 dimer had undergone a conformational change to form a clamp with a “donut hole” that placed the DNA in the center cavity. The peptide rearrangements that resulted in the EcMutSΔC800 sliding clamp produced a protein interface that positioned EcMutLLN40 onto the duplex DNA [41, 81]. Interestingly, surface plasmon resonance studies suggested that the EcMutSΔC800-EcMutLLN40 complex dissociated substantially slower from a DNA containing a mismatch in the presence of ATP than the EcMutSΔC800 alone [81]. This observation was used to argue that EcMutS-EcMutL formed a stable largely immobile complex on the DNA [81]. Importantly, mutation of the EcMutLLN40 residues that interacted with EcMutSΔC800 or the EcMutLLN40 residues that interacted with the DNA increased spontaneous mutation suggesting that these contacts contributed to efficient MMR [81].
Dynamic Interactions between MutS and MutL Alter their Diffusion Properties on the DNA
As discussed above, perhaps the singularly most unique biophysical challenge during MMR is communicating mismatch recognition to a distant excision-initiation site [1]. On the other end of the stochastic spectrum from the Molecular Switch/Sliding Clamp model, the two most prominent historical mechanisms for MMR have proposed the formation of a stable MSHMLH/PMS complex at the mismatch that initiates excision by either motoring to the distant site using the energy of ATP hydrolysis (Hydrolysis-Dependent Translocation Model) or capturing a lopping distant site (Static Transactivation Model) (see: https://www.nobelprize.org/nobel_prizes/chemistry/laureates/2015/fig_ke_en_15_mismatchrepair.pdf) [1]. However, the problem of initiating mismatch excision appears much more intricate than moving to, or colliding with, a distant site. For E.coli the first step after mismatch recognition is the detection of a hemimethylated GATC site by EcMutH, which has long been known to require activation by EcMutL [41]. But this progression suggests that EcMutH and EcMutL must together or separately engage in a DNA site search similar to other DNA recognition proteins [82].
While EcMutL alone does not bind DNA in physiological ionic conditions [78], real-time single molecule imaging showed it readily associated with randomly diffusing EcMutS sliding clamps (Fig. 1d) [80]. More significantly, the resulting EcMutS-EcMutL complex also randomly diffused bi-directionally along the mismatched DNA (Fig. 1d) [80]. This observation alone appeared to eliminate the Hydrolysis-Dependent Translocation and Static Transactivation MMR models as well as most other MMR mechanisms described in the literature [83–86]. While the EcMutS sliding clamps were always loaded onto the DNA at the mismatch the association of EcMutL with EcMutS sliding clamps occurred randomly along the entire DNA length (17 kb) [80]. Examining the ionic dependence of diffusion [87, 88] established that the interaction with EcMutL turned the EcMutS sliding clamp, that diffused randomly along the DNA in intermittent contact with the backbone, into a complex that still randomly diffused along the DNA but transitioned into continuous contact with the backbone [80]. Theoretical consideration of the DNA backbone as a rough diffusion landscape [89, 90] suggested that the EcMutS-EcMutL complex moves along the DNA by rotation-coupled diffusion similar to a nut on a screw [80]. Such a rotation-coupled diffusion along the backbone is generally considered the most efficient biophysical mechanism for a site-specific DNA search [82]. The reliance on stochastic interactions and random bi-directional diffusion along the DNA [80] appeared uniquely consistent with fundamental aspects of the Molecular Switch/Sliding Clamp MMR model [1, 41–44].
The lifetime of the initial EcMutS-EcMutL complex was on average 43 sec [80]. This was more than 10-fold longer than the association time of an MSH that is searching for a mismatch on duplex DNA by a similar rotation-coupled thermal diffusion (~3 sec) (see also Kim et al., this issue). However, since the diffusion coefficient of the MutS-MutL complex was approximately 10-fold slower (0.004 μm2/sec) than mismatch-searching MutS (0.034 μm2/sec), one can calculate that both configurations were capable of closely examining ~2 kb of duplex DNA. After the initial association there were four types of complex dissociation events (Fig. 2a). Perhaps the most uninteresting for MMR resulted in dissociation of both EcMutS and EcMutL (2% of the events) or dissociation of EcMutL that merely left the EcMutS starting component (22% of the events). Since both EcMutS and EcMutL are required to complete MMR, it is likely that these nearly one quarter of inaugural interaction events reflect the background of initial assembles that are non-productive; perhaps a measure of the local chaos that is the biology of MMR [91].
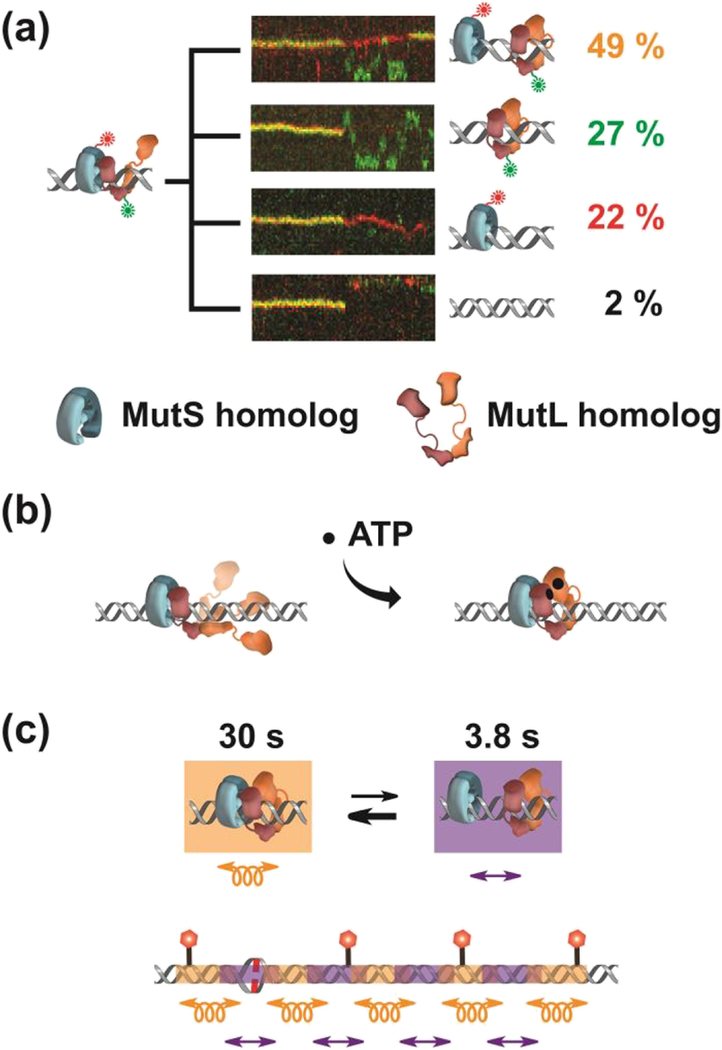
Figure 2. The Formation of an EcMutL Sliding Clamp Leads to an Oscillating EcMutSEcMutL Search Complex.
(a) Representative kymographs and illustrations showing the different types (colored letters) of EcMutS-EcMutL complex dissociations. The frequency (%) of each dissociation type is shown (right, colored numbers). (b) Illustration of a possible 3D diffusion mechanism for forming an EcMutL sliding clamp. (c) An illustration of a segmented search by an oscillating EcMutS-EcMutL complex. EcMutS-EcMutL complex diffuses in continuous DNA contact (left) while free EcMutS and EcMutL clamps diffuse in intermittent DNA contact (right).
Another one quarter of the initial EcMutS-EcMutL binding events (27%) resulted in dissociation of EcMutS leaving EcMutL particles alone on the mismatched DNA (Fig. 2a) [80]. Astonishingly, these previously unknown structures were incredibly stable on the DNA with an average lifetime of 14 min [80]. Moreover, ionic-dependence analysis demonstrated that they diffused along the entire length of the 17 kb mismatched DNA while in intermittent contact with the backbone, sometimes across the entire DNA length within one frame (0.1 sec) [80]. The formation of these particles was contingent on the prior existence of EcMutS sliding clamps and absolutely required ATP binding by EcMutL [80, 92]. These and other observations were consistent with a unifying structural paradigm in which one of the N-terminal EcMutL ATPase domains initially associated with an EcMutS sliding clamp [81], resulting in a complex that moved along the DNA by rotation-coupled diffusion (Fig. 2b) [80]. Those random rotation-coupled motions of the EcMutS-EcMutL complex were imagined to result in a stochastic wrapping of the remaining EcMutL peptide domains around the DNA triggering ATP binding-dependent dimerization by the two N-terminal domains (Fig. 2b) [59]. The net result would be the formation of a ring-like EcMutL sliding clamp on the DNA [80]. In support of this model the EcMutL(R95F) mutation that is incapable of binding ATP, initially associates with ATP-bound EcMutS siding clamps but never transitions to a EcMutL sliding clamp [80]. Taken as a whole these observations clearly indicated that the fundamental role of ATP binding by EcMutL is to form a clamp on the mismatched DNA.
Approximately half (49%) of the initial EcMutS-EcMutL association events resulted in both EcMutS and EcMutL sliding clamps becoming stably associated with the mismatched DNA (Fig. 2a) [80]. Surprisingly, these sliding clamps oscillated between the MutS-MutL complex and free EcMutS and EcMutL sliding clamps (Fig. 2c, top). The re-associated EcMutS-EcMutL complexes displayed an average lifetime of 30 sec and randomly moved along the DNA by rotation-coupled diffusion with a diffusion coefficient that was identical to the initial association complex (0.004 μm2/sec). The two sliding clamps were separated on average for 3.8 sec and moved along the DNA in intermittent contact with the backbone (Fig. 2c, top) [80]. An oscillating association-dissociation that elicits differing diffusion properties appears unique in biology. Combined with accompanying diffusion coefficients this observation suggests that these two stable sliding clamps may search the DNA bi-directionally by rotation-coupled diffusion for 30 sec interrogating ~2 kb, then move ~2 kb while in intermittent DNA contact, followed by another rotation-coupled DNA search. During the limiting lifetime of the EcMutS sliding clamps (3 min) this oscillating search mechanics was calculated to randomly interrogate ~12 kb of naked DNA surrounding the mismatch (Fig. 2c, bottom) [80]; significantly more than the 0.25–2 kb range of distances between Dam GATC sites within the E.coli genome [93]. The conservation of the ATP binding domain of MSH and MLH/PMS, even to the exclusion of the other peptide domains within these proteins, strongly suggests similarly conserved cascading sliding clamp and controlled diffusion function(s) throughout biology. However, the EcMutSEcMutL complex does not appear capable of recognizing anything that would be relevant to MMR, such as a strand scission or a hemimethylated GATC site.
Forming a MutS-MutL-MutH Search Complex to find a Hemimethylated GATC Site
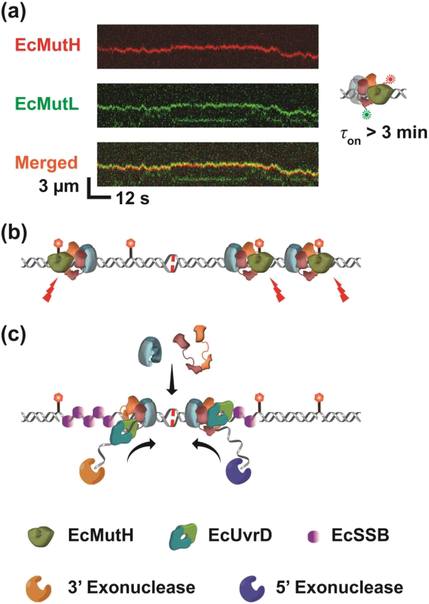
The addition of EcMutH uncovered one application of the EcMutS-EcMutL diffusion mechanics [80]. The EcMutH protein does not bind fully methylated GATC sequences and displays rather weak binding to unmethylated (KD = 3.5 μM) and hemimethylated (KD = 0.9 μM) GATC sites. These binding kinetics are well above the nM cellular concentration of the MutH protein [94, 95]. Single molecule images showed that EcMutH rapidly associated with EcMutL sliding clamps on the mismatched DNA (Fig. 3a) [80]. The lifetime of this complex was ~3 min, which theoretically increases the KD of MutH on a mismatched DNA by at least 1000-fold [80]. The EcMutS and the EcMutL-EcMutH complex oscillated between the free and EcMutS-EcMutL-EcMutH condensed states similar to EcMutS and EcMutL alone [80]. However, only the EcMutS-EcMutL-EcMutH complex moved along the DNA in continuous contact with the backbone consistent with a rotation-coupled diffusion DNA search. These studies did not directly demonstrate incision of hemimethylated GATC sites (see below). But the implication appears clear. It is the assembly of the EcMutS-EcMutL-EcMutH stochastic search complex that requires the progressive formation of two extremely stable ATP-bound sliding clamps, which is required to introduce the strand-specific DNA break that ultimately initiates MMR in E.coli (Fig. 3b).
Figure 3. Methyl-Direct E. coli MMR.
(a) A Representative kymograph of EcMutH (red) colocalization and diffusion with EcMutL (green) loaded with unlabeled EcMutS on a single mismatched DNA. Association time of EcMutH on DNA (τon) is shown on right. (b) An illustration of multiple incisions randomly introduced by a long-lived EcMutS-EcMutL-EcMutH complex. (c) A model showing how redundant EcMutS and EcMutL sliding clamps on the DNA might direct excision towards the mismatch. In the presence of a mismatch, cascading EcMutS and EcMutL sliding clamps may be continuously loaded onto the mismatched DNA. ATP-bound EcMutL associates with EcUvrD to initiate the unwinding in the 3’- or 5’-direction. ssDNA-dependent ssDNA exonucleases degrade the displaced ssDNA of the newly replicated strand. EcSSB binds the exposed ssDNA in the template strand.
Strand-Specific Excision
While there are at least fourteen DNA helicases in E. coli, only EcUvrD seems to function in MMR [96, 97]. There are two historical observations that are germane when considering the EcUvrD mechanics during the strand-specific MMR excision process: 1) the EcUvrD helicase unwinds DNA exclusively in the 3’→5’ direction [98], and 2) while MMR can be initiated from either side of the mismatch (bidirectional) the excision tract invariably extends from the distant strand break to just past the mismatch [99]. How the stochastic motions of EcMutS and EcMutL are translated into this directional excision tract is unknown.
The strand break produced by EcMutH has been proposed to act as entry sites for EcUvrD [100, 101]. DNA unwinding by EcUvrD can then hypothetically generate a displaced DNA strand that is a substrate for one of four ssDNA exonucleases that are required for MMR (Table 1) [102]. Purified EcUvrD has indeed been shown to initiate unwinding from a DNA strand break [103–105]. However, the processivity is very low (40–50 bp), even under the enhanced DNA binding conditions associated with extremely low (non-physiological) ionic strength [106–108]. This modest helicase activity appears sufficient for the UV lesion-specific excision system where EcUvrD must merely unwind 12–13 nucleotides between incisions introduced by the EcUvrABC complex [109]. But it would appear inadequate to support production of the long excision tracts observed in E. coli MMR [99, 110, 111].
Several studies have shown that EcMutL interacts with and stimulates the EcUvrD helicase [48, 100, 112]. Crosslinking studies seemed to suggest that both the N- and C-terminus of EcMutL interacted with EcUvrD, which was originally used to support the Static Transactivation Model for MMR [61]. Because EcUvrD is essential for both 3’- and 5’-directed MMR, the helicase must be loaded on the correct DNA strand at the site of the strand break to exploit its uniquely directional 3’→5’ unwinding activity. Functionally, that would mean loading EcUvrD on the contiguous strand when the scission is 5’ of the mismatch or conversely on the strand containing the scission when it is 3’ of the mismatch. Taken as a whole, these observations appear consistent with the notion that EcMutL may assist in the loading of EcUvrD onto the mismatched DNA, although the mechanics remain a mystery.
Preliminary single molecule imaging has been unable to detect a stable EcMutL-EcUvrD complex, similar to the EcMutL-EcMutH complex, that might arises prior to the initiation of unwinding from a strand scission (unpublished observations) [80]. Moreover, there is no visual evidence that the EcMutS-EcMutL complex or EcMutL alone is capable of recognizing a strand scission prior to loading EcUvrD [113, 114]. The available images (at a 100 msec resolution) seem to indicate a 3D collision between EcMutL and EcUvrD at the site of the strand break. Moreover, the direction of unwinding appears to correlate with which side of the strand scission the EcMutL clamp was sliding upon prior to the interaction with EcUvrD. These observations exposed a number of unanswered mechanical questions, but appear consistent with a model in which the excision direction is determined by a stochastic asymmetry (sidedness) in the EcMutL diffusion encounters with a strand break that ultimately secures EcUvrD (Fig. 3c).
This sidedness concept and the idea that MMR component interactions might be labile were built into the stochastic nature of the Molecular Switch/Sliding Clamp model [1, 41–44] Thus, any single MMR excision tract may be created from several smaller excision tracts produced by multiple labile MMR complexes that progressively dissociate and are reassembled from new or remaining components at the emerging excision site. Termination of the excision tract just past the mismatch was envisioned to be stochastic, with a contributing factor that once the mismatch is excised there would be no additional EcMutS or EcMutL sliding clamps loaded onto the DNA that are the ultimate drivers of excision. It is likely that the reconstitution of the complete excision reaction on single DNA molecules will be required to unequivocally determine whether this model or some additional/different mechanics may account for MMR.
Connecting Mismatch Repair to the Replication Fork
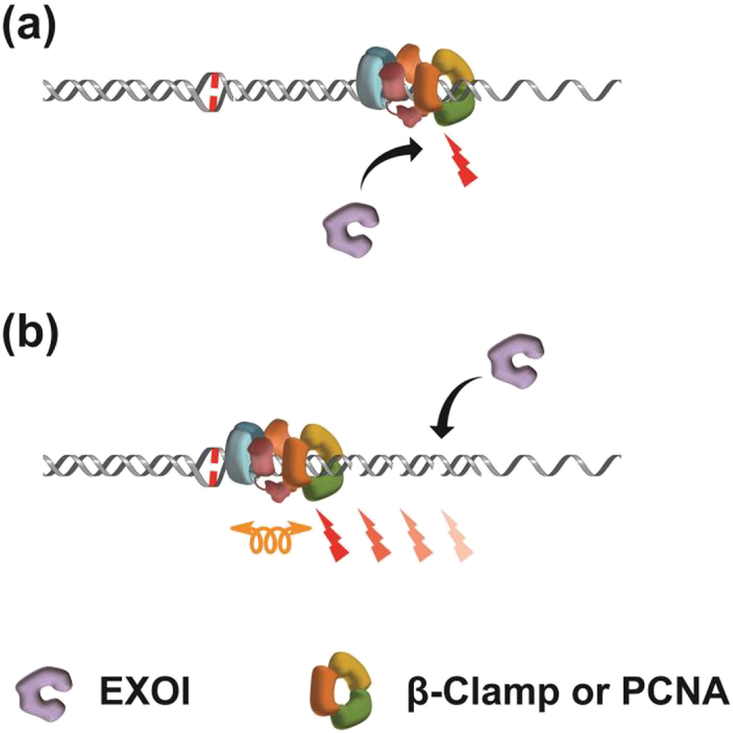
There is substantial evidence that the MMR machinery is localized at or near the multi-protein complex that makes up the replication fork where most mismatched nucleotides arise [115]. Localization has been ascribed to interactions between the core MSH and MLH/PMS proteins and β-clamp/PCNA [116–118]. The β-clamp/PCNA is loaded onto the 3’-end of the DNA as a ring-like structure by replication-specific clamp loaders [119, 120]. The β-clamp/PCNA then tethers the DNA polymerase to the replication fork and slides along the DNA to dramatically increase replication processivity [121–123]. Both MSH and MLH/PMS proteins have been found to directly bind to β-clamp/PCNA [118, 124–128]. The role of these interactions remains enigmatic. However, in organisms that do not utilize the Dam/MutH system, β-clamp/PCNA interacts with and activates an intrinsic MLH/PMS endonuclease activity [27, 55, 56, 65, 74, 129–131]. While the MLH/PMS endonuclease is essential for MMR excision from a 3’-strand scission (3’-MMR), excision from a 5’-strand scission (5’-MMR) does not require MLH/PMS in vitro [27, 132–134]. In addition to apparently different mechanics for 3’-MMR and 5’-MMR, the utilization of a β-clamp/PCNA-activated MLH/PMS endonuclease highlights a significant difference between Dam/MutH MMR and the rest of biology. Importantly, when activated the MLH/PMS endonuclease introduces episodic scissions into the 3’-strand, which have been posited to provide an entry site for the EXOI 5’→3’ exonuclease during 3’-MMR (Fig. 4a) [27]. The human (Hs) 5’-MMR excision process has been reconstituted on single DNA molecules with HsMSH2-HsMSH6, HsEXOI and HsRPA [135]. However, single molecule imaging of 3’-MMR excision has not been reported for any organism. Consequently how and when β-clamp/PCNA stimulates the MLH/PMS endonuclease remains speculative but almost certainly takes advantage of the controlled diffusion mechanics that are likely conserved by the core MMR components (Fig. 4b) (see also Kim et al., this issue).
Figure 4. Incorporating MMR Core Component Diffusion Mechanics into MutL homolog-dependent Excision.
(a) β-Clamp/PCNA located at the dsDNA-ssDNA replication fork junction positions the MutL homolog (MLH/PMS) on the DNA strand containing the misincorporation error. Strand breaks are then generated by the endonuclease activity of the MLH/PMS. EXOI may then be recruited to the strand breaks and DNA is excised from 5’→3’. (b) Hypothetical movement of a MutS homolog (MSH)-MLH/PMS-β-Clamp/PCNA complex by rotation-coupled diffusion along DNA to introduce specific breaks on the DNA strand containing the misincorporation error.
Plasticity during Mismatch Repair
The concept of plasticity appears to have been first considered in an 1890 volume by the psychologist William James while studying human behavioral habits [136]. A few years later neurobiologists expanded on the idea to include synaptic and cortical changes associated with learning and memory [137]. A similar terminology was coined in the early 20th century by materials scientists, although the fundamental concepts of elasticity that underpin materials plasticity may go back as far as Leonardo De Vince [138]. In all cases, plasticity refers to adaptations associated with changes in the environment.
Biochemical plasticity might be anticipated in multi-protein complexes made up of components with locally intrinsic association-dissociation rates. In theory, the “ideal” multi-protein complex contains all of the individual components folded and interacting flawlessly. However, such complexes as well as the individual components are continuously exposed to thermal motions that may alter these ideal interactions [139]. Biochemical plasticity implies that a range of these non-perfections does not fully disrupt function, and that the biological task continues albeit not at the peak rate [139].
The Molecular Switch/Sliding Clamp model proposes that MMR has solved the problem of localized thermal interference and biochemical plasticity by introducing redundancy as well as converting 3D interactions into stable 1D progressions capable of regulating diffusion mechanics. Redundancy is a natural consequence of randomly diffusing MSH and MLH/PMS sliding clamps [34, 42, 43]. Once an MSH recognizes and slides off a mismatch, additional MSH proteins may interact and be loaded as sliding clamps onto the DNA. The oscillating behavior of MSH-MLH/PMS sliding clamps theoretically provides windows for the MSH to load additional MLH/PMS sliding clamps. The net effect would be to amplify the mismatch recognition signal to insure successful repair. In addition to providing plasticity in MMR such redundancy is likely to overcome the approximately 25% of initial interactions that are unproductive (Fig. 2a).
A fundamental mechanical problem associated with MMR involves connecting mismatch recognition to the assembly of strand-specific excision components at a distant site from the mismatch. It is well known that site-specific searches by DNA binding proteins frequently involve short 1D interrogation events along the DNA [82, 140]. This is certainly the case for MSH proteins during mismatch recognition (see also Kim et al., this issue) [35]. There is evidence that MSH proteins do not actually distinguish the eight possible mismatches, but instead recognize localized configuration flexibility in normally smooth DNA backbone that is introduced by poorly paired and/or poorly stacked nucleotides [141]. However, the MSH mismatch search on DNA lasts for ~3 sec and mismatch binding for perhaps 30 sec (absence of ATP) [35]. These short time scales would mean that the ensuing complex formation as well as the subsequent search for a distant excision initiation site would need to be extraordinarily efficient and substantially resistant to thermal disruption. MMR solved these time and chaos issues by linking two long-lived clamps onto the DNA. This effectively converted critical 3D association-dissociation events into stable 1D progressions that form complexes capable of searching the DNA as well as assembling distant excision complexes. Importantly, stably linking essential components to the DNA as clamps ensures rapid 1D re-association of active complexes should there be thermal disruption.
Perspective
The identification of cascading MSH and MLH/PMS sliding clamps resolved a number of enigmatic MMR questions and solidified several stochastic aspects of the Molecular Switch/Sliding Clamp Model [1, 41–44]. At the same time these observations uncovered additional mechanical uncertainties. For example, how is the strand-specific excision complex assembled and what happens with component plasticity during this process? What are the mechanics of the EcMutS-EcMutL-EcMutH strand scission at a hemimethylated GATC site? How is EcUvrD captured at a strand scission and then loaded onto the appropriate strand for 3’- or 5’-MMR? Is there a role for β-clamp in E.coli MMR where activation of the EcMutL endonuclease is not utilized? How does the MLH/PMS endonuclease introduce strand scissions uniquely into one DNA strand? These and other MMR problems will most likely only be determined by single molecule imaging methods.
Finally, the diffusion properties and lifetime of the EcMutS-EcMutL-EcMutH complex appears random, suggesting that the EcMutH incision does not necessarily occur at the nearest hemimethylated GATC site. Indeed, incision efficiency was found to be independent of the distance between the mismatch and the GATC site [142]. Furthermore, dual hemimethylated GATC sites were processively incised suggesting multiple incisions may be introduced into the DNA during a single MMR event [142]. These results appear to harmonize with the observation that multiple GATC sites increase the MMR efficiency both in vitro and in vivo [142–144]. Taken as a whole it seems possible that stochastic excision between two adjacent GATC scissions may occur in E.coli [145]; an idea that appears to have been dismissed in the early days of MMR biochemistry [146, 147]. In a mechanism where excision occurs between adjacent GATC incision sites, for MMR to be completed the correcting excision event would have to be between two GATC sites that also included the mismatch. Obviously the localized excision of multiple GATC→GATC segment, some not containing the mismatch, would introduce an additional stochastic character to E.coli MMR. We expect that future single molecule imaging studies will further determine the degree of stochasticity and plasticity between the protein complexes that appears common in MMR.
Highlights.
We review the history of mismatch repair, the development of models describing mismatch repair mechanisms and recent single molecule studies that have contributed to our understanding of these models/mechanisms.
Acknowledgements
We would like to thank our laboratory colleagues for many helpful insights and discussions. This work was supported by NRF of Korea Grant No. 2017K1A1A2013241 (J.-B.L) and NIH grant CA67007 (R.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
MSH refers to all MutS homologs. Bacterial and Archeal MSH proteins form a functional homodimer. Eucaryotic MSH proteins exist as heterodimers (e.g. MSH2-MSH6; see Table 1) that likely arose from gene duplication, which ultimately evolved to solidify separation-of-function activities between mismatch recognition and the control of ATP binding (see Ref. 1).
MLH/PMS refers to all MutL homologs. The PMS designation signifies a Post Meiotic Segregation gene-designation that resulted from unrepaired mismatched nucleotides generated by homologous recombination. The first of these in S.cerevisae (scPMS1) was subsequently found to be a MutL homolog. The closest human (Hs) homolog to ScPMS1 was erroneously designated HsPMS2 with a second relative designated HsPMS1 that appears in retrospect to be a homolog of ScMLH2. In Bacteria and Archea MLH proteins form a functional homodimer. In Eucaryotes MLH proteins exist as heterodimers (e.g. ScMLH1-ScPMS1 or HsMLH1-HsPMS2; see Table 1) that likely arose from gene duplications, which ultimately evolved to solidify separation-of-function for MSH interaction and control of ATP binding (see Ref. 1).
REFERENCES
- [1].Fishel R. Mismatch repair. J Biol Chem. 2015;290:26395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kolodner R. Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev. 1996;10:1433–42. [DOI] [PubMed] [Google Scholar]
- [3].Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem. 1996;65:101–33. [DOI] [PubMed] [Google Scholar]
- [4].Wheeler JM, Bodmer WF, Mortensen NJ. DNA mismatch repair genes and colorectal cancer. Gut. 2000;47:148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jager AC, Rasmussen M, Bisgaard HC, Singh KK, Nielsen FC, Rasmussen LJ. HNPCC mutations in the human DNA mismatch repair gene hMLH1 influence assembly of hMutLalpha and hMLH1-hEXO1 complexes. Oncogene. 2001;20:3590–5. [DOI] [PubMed] [Google Scholar]
- [6].Muller A, Fishel R. Mismatch repair and the hereditary non-polyposis colorectal cancer syndrome (HNPCC). Cancer Invest. 2002;20:102–9. [DOI] [PubMed] [Google Scholar]
- [7].Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015;15:181–94. [DOI] [PubMed] [Google Scholar]
- [8].Buermeyer AB, Deschenes SM, Baker SM, Liskay RM. Mammalian DNA mismatch repair. Annu Rev Genet. 1999;33:533–64. [DOI] [PubMed] [Google Scholar]
- [9].Peltomaki P Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21:1174–9. [DOI] [PubMed] [Google Scholar]
- [10].Spies M, Fishel R. Mismatch repair during homologous and homeologous recombination. Cold Spring Harb Perspect Biol. 2015;7:a022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tham KC, Kanaar R, Lebbink JH. Mismatch repair and homeologous recombination. DNA Repair (Amst). 2016;38:75–83. [DOI] [PubMed] [Google Scholar]
- [12].Li GM. The role of mismatch repair in DNA damage-induced apoptosis. Oncol Res. 1999;11:393–400. [PubMed] [Google Scholar]
- [13].Chahwan R, Edelmann W, Scharff MD, Roa S. Mismatch-mediated error prone repair at the immunoglobulin genes. Biomed Pharmacother. 2011;65:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: functions and mechanisms. Chem Rev. 2006;106:302–23. [DOI] [PubMed] [Google Scholar]
- [15].Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18:85–98. [DOI] [PubMed] [Google Scholar]
- [16].Fukui K DNA mismatch repair in eukaryotes and bacteria. J Nucleic Acids. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fishel R, Wilson T. MutS homologs in mammalian cells. [Review] [84 refs]. Curr Opin Genet Dev. 1997;7:105–13. [DOI] [PubMed] [Google Scholar]
- [18].Marinus MG. DNA Mismatch Repair. EcoSal Plus. 2012;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Putnam CD. Evolution of the methyl directed mismatch repair system in Escherichia coli. DNA Repair (Amst). 2016;38:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schmutte C, Marinescu RC, Sadoff MM, Guerrette S, Overhauser J, Fishel R. Human exonuclease I interacts with the mismatch repair protein hMSH2. Cancer Research. 1998;58:4537–42. [PubMed] [Google Scholar]
- [21].Tishkoff DX, Boerger AL, Bertrand P, Filosi N, Gaida GM, Kane MF, et al. Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wilson DM 3rd, Carney JP, Coleman MA, Adamson AW, Christensen M, Lamerdin JE. Hex1: a new human Rad2 nuclease family member with homology to yeast exonuclease 1. Nucleic Acids Res. 1998;26:3762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barras F, Marinus MG. The great GATC: DNA methylation in E. coli. Trends Genet. 1989;5:139–43. [DOI] [PubMed] [Google Scholar]
- [24].Campbell JL, Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990;62:967–79. [DOI] [PubMed] [Google Scholar]
- [25].Lahue RS, Su SS, Modrich P. Requirement for d(GATC) sequences in Escherichia coli mutHLS mismatch correction. Proc Natl Acad Sci U S A. 1987;84:1482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Welsh KM, Lu AL, Clark S, Modrich P. Isolation and characterization of the Escherichia coli mutH gene product. J Biol Chem. 1987;262:15624–9. [PubMed] [Google Scholar]
- [27].Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126:297–308. [DOI] [PubMed] [Google Scholar]
- [28].Su S-S, Modrich P. Escherichia coli mutS-encoded protein binds to mismatched DNA base pairs. Proc Natl Acad Sci U S A. 1986;83:5057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lamers MH, Perrakis A, Enzlin JH, Winterwerp HH, de Wind N, Sixma TK. The crystal structure of DNA mismatch repair protein MutS binding to a G x T mismatch. Nature. 2000;407:711–7. [DOI] [PubMed] [Google Scholar]
- [30].Obmolova G, Ban C, Hsieh P, Yang W. Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature. 2000;407:703–10. [DOI] [PubMed] [Google Scholar]
- [31].Sixma TK. DNA mismatch repair: MutS structures bound to mismatches. Curr Opin Struct Biol. 2001;11:47–52. [DOI] [PubMed] [Google Scholar]
- [32].Natrajan G, Lamers MH, Enzlin JH, Winterwerp HH, Perrakis A, Sixma TK. Structures of Escherichia coli DNA mismatch repair enzyme MutS in complex with different mismatches: a common recognition mode for diverse substrates. Nucleic Acids Res. 2003;31:4814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gradia S, Acharya S, Fishel R. The human mismatch recognition complex hMSH2-hMSH6 functions as a novel molecular switch. Cell. 1997;91:995–1005. [DOI] [PubMed] [Google Scholar]
- [34].Gradia S, Subramanian D, Wilson T, Acharya S, Makhov A, Griffith J, et al. hMSH2-hMSH6 forms a hydrolysis-independent sliding clamp on mismatched DNA. Mol Cell. 1999;3:255–61. [DOI] [PubMed] [Google Scholar]
- [35].Jeong C, Cho WK, Song KM, Cook C, Yoon TY, Ban C, et al. MutS switches between two fundamentally distinct clamps during mismatch repair. Nat Struct Mol Biol. 2011;18:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cho WK, Jeong C, Kim D, Chang M, Song KM, Hanne J, et al. ATP alters the diffusion mechanics of MutS on mismatched DNA. Structure. 2012;20:1264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lamers MH, Winterwerp HH, Sixma TK. The alternating ATPase domains of MutS control DNA mismatch repair. EMBO J. 2003;22:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mazur DJ, Mendillo ML, Kolodner RD. Inhibition of Msh6 ATPase activity by mispaired DNA induces a Msh2(ATP)-Msh6(ATP) state capable of hydrolysis-independent movement along DNA. Mol Cell. 2006;22:39–49. [DOI] [PubMed] [Google Scholar]
- [39].Snowden T, Acharya S, Butz C, Berardini M, Fishel R. hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell. 2004;15:437–51. [DOI] [PubMed] [Google Scholar]
- [40].Wilson T, Guerrette S, Fishel R. Dissociation of mismatch recognition and ATPase activity by hMSH2-hMSH3. J Biol Chem. 1999;274:21659–44. [DOI] [PubMed] [Google Scholar]
- [41].Acharya S, Foster PL, Brooks P, Fishel R. The coordinated functions of the E. coli MutS and MutL proteins in mismatch repair. Mol Cell. 2003;12:233–46. [DOI] [PubMed] [Google Scholar]
- [42].Fishel R Mismatch repair, molecular switches, and signal transduction. [Review] [56 refs]. Genes & Development. 1998;12:2096–101. [DOI] [PubMed] [Google Scholar]
- [43].Fishel R Signaling mismatch repair in cancer. Nature Medicine. 1999;5:1239–41. [DOI] [PubMed] [Google Scholar]
- [44].Fishel R, Acharya S, Berardini M, Bocker T, Charbonneau N, Cranston A, et al. Signaling mismatch repair: the mechanics of an adenosine-nucleotide molecular switch. Cold Spring Harb Symp Quant Biol. 2000;65:217–24. [DOI] [PubMed] [Google Scholar]
- [45].Guarne A The functions of MutL in mismatch repair: the power of multitasking. Prog Mol Biol Transl Sci. 2012;110:41–70. [DOI] [PubMed] [Google Scholar]
- [46].Guarne A, Charbonnier JB. Insights from a decade of biophysical studies on MutL: Roles in strand discrimination and mismatch removal. Prog Biophys Mol Biol. 2015;117:149–56. [DOI] [PubMed] [Google Scholar]
- [47].Friedhoff P, Li P, Gotthardt J. Protein-protein interactions in DNA mismatch repair. DNA Repair (Amst). 2016;38:50–7. [DOI] [PubMed] [Google Scholar]
- [48].Hall MC, Jordan JR, Matson SW. Evidence for a physical interaction between the Escherichia coli methyl-directed mismatch repair proteins MutL and UvrD. EMBO J. 1998;17:1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mendillo ML, Hargreaves VV, Jamison JW, Mo AO, Li S, Putnam CD, et al. A conserved MutS homolog connector domain interface interacts with MutL homologs. Proc Natl Acad Sci U S A. 2009;106:22223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mendillo ML, Mazur DJ, Kolodner RD. Analysis of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 and MLH1-PMS1 complexes with DNA using a reversible DNA end-blocking system. J Biol Chem. 2005;280:22245–57. [DOI] [PubMed] [Google Scholar]
- [51].Plotz G, Raedle J, Brieger A, Trojan J, Zeuzem S. N-terminus of hMLH1 confers interaction of hMutLalpha and hMutLbeta with hMutSalpha. Nucleic Acids Res. 2003;31:3217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Plotz G, Welsch C, Giron-Monzon L, Friedhoff P, Albrecht M, Piiper A, et al. Mutations in the MutSalpha interaction interface of MLH1 can abolish DNA mismatch repair. Nucleic Acids Res. 2006;34:6574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schmutte C, Sadoff MM, Shim KS, Acharya S, Fishel R. The interaction of DNA mismatch repair proteins with human exonuclease I. J Biol Chem. 2001;276:33011–8. [DOI] [PubMed] [Google Scholar]
- [54].Nielsen FC, Jager AC, Lutzen A, Bundgaard JR, Rasmussen LJ. Characterization of human exonuclease 1 in complex with mismatch repair proteins, subcellular localization and association with PCNA. Oncogene. 2004;23:1457–68. [DOI] [PubMed] [Google Scholar]
- [55].Pillon MC, Lorenowicz JJ, Uckelmann M, Klocko AD, Mitchell RR, Chung YS, et al. Structure of the endonuclease domain of MutL: unlicensed to cut. Mol Cell. 2010;39:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pluciennik A, Dzantiev L, Iyer RR, Constantin N, Kadyrov FA, Modrich P. PCNA function in the activation and strand direction of MutLalpha endonuclease in mismatch repair. Proc Natl Acad Sci U S A. 2010;107:16066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. [DOI] [PubMed] [Google Scholar]
- [58].Miyake T Mutator factor in Salmonella typhimurium. Genetics. 1960;45:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ban C, Yang W. Crystal structure and ATPase activity of MutL: implications for DNA repair and mutagenesis. Cell. 1998;95:541–52. [DOI] [PubMed] [Google Scholar]
- [60].Ban C, Junop M, Yang W. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell. 1999;97:85–97. [DOI] [PubMed] [Google Scholar]
- [61].Guarne A, Ramon-Maiques S, Wolff EM, Ghirlando R, Hu X, Miller JH, et al. Structure of the MutL C-terminal domain: a model of intact MutL and its roles in mismatch repair. EMBO J. 2004;23:4134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Guarne A, Junop MS, Yang W. Structure and function of the N-terminal 40 kDa fragment of human PMS2: a monomeric GHL ATPase. EMBO J. 2001;20:5521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Arana ME, Holmes SF, Fortune JM, Moon AF, Pedersen LC, Kunkel TA. Functional residues on the surface of the N-terminal domain of yeast Pms1. DNA Repair (Amst). 2010;9:448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wu H, Zeng H, Lam R, Tempel W, Kerr ID, Min J. Structure of the human MLH1 N-terminus: implications for predisposition to Lynch syndrome. Acta Crystallogr F Struct Biol Commun. 2015;71:981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fukui K, Baba S, Kumasaka T, Yano T. Structural Features and Functional Dependency on beta-Clamp Define Distinct Subfamilies of Bacterial Mismatch Repair Endonuclease MutL. J Biol Chem. 2016;291:16990–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Argueso JL, Kijas AW, Sarin S, Heck J, Waase M, Alani E. Systematic mutagenesis of the Saccharomyces cerevisiae MLH1 gene reveals distinct roles for Mlh1p in meiotic crossing over and in vegetative and meiotic mismatch repair. Mol Cell Biol. 2003;23:873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dutta R, Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci. 2000;25:24–8. [DOI] [PubMed] [Google Scholar]
- [68].Aronshtam A, Marinus MG. Dominant negative mutator mutations in the mutL gene of Escherichia coli. Nucleic Acids Res. 1996;24:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Junop MS, Yang W, Funchain P, Clendenin W, Miller JH. In vitro and in vivo studies of MutS, MutL and MutH mutants: correlation of mismatch repair and DNA recombination. DNA Repair (Amst). 2003;2:387–405. [DOI] [PubMed] [Google Scholar]
- [70].Gueneau E, Dherin C, Legrand P, Tellier-Lebegue C, Gilquin B, Bonnesoeur P, et al. Structure of the MutLalpha C-terminal domain reveals how Mlh1 contributes to Pms1 endonuclease site. Nat Struct Mol Biol. 2013;20:461–8. [DOI] [PubMed] [Google Scholar]
- [71].Guerrette S, Acharya S, Fishel R. The interaction of the human MutL homologues in hereditary nonpolyposis colon cancer. J Biol Chem. 1999;274:6336–41. [DOI] [PubMed] [Google Scholar]
- [72].Wu X, Platt JL, Cascalho M. Dimerization of MLH1 and PMS2 limits nuclear localization of MutLalpha. Mol Cell Biol. 2003;23:3320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kosinski J, Steindorf I, Bujnicki JM, Giron-Monzon L, Friedhoff P. Analysis of the quaternary structure of the MutL C-terminal domain. J Mol Biol. 2005;351:895–909. [DOI] [PubMed] [Google Scholar]
- [74].Kadyrov FA, Holmes SF, Arana ME, Lukianova OA, O’Donnell M, Kunkel TA, et al. Saccharomyces cerevisiae MutLalpha is a mismatch repair endonuclease. J Biol Chem. 2007;282:37181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Correa EM, De Tullio L, Velez PS, Martina MA, Argarana CE, Barra JL. Analysis of DNA structure and sequence requirements for Pseudomonas aeruginosa MutL endonuclease activity. J Biochem. 2013;154:505–11. [DOI] [PubMed] [Google Scholar]
- [76].Chao EC, Velasquez JL, Witherspoon MS, Rozek LS, Peel D, Ng P, et al. Accurate classification of MLH1/MSH2 missense variants with multivariate analysis of protein polymorphisms-mismatch repair (MAPP-MMR). Hum Mutat. 2008;29:852–60. [DOI] [PubMed] [Google Scholar]
- [77].Drotschmann K, Hall MC, Shcherbakova PV, Wang H, Erie DA, Brownewell FR, et al. DNA binding properties of the yeast Msh2-Msh6 and Mlh1-Pms1 heterodimers. Biol Chem. 2002;383:969–75. [DOI] [PubMed] [Google Scholar]
- [78].Park J, Jeon Y, In D, Fishel R, Ban C, Lee JB. Single-molecule analysis reveals the kinetics and physiological relevance of MutL-ssDNA binding. PLoS One. 2010;5:e15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sacho EJ, Kadyrov FA, Modrich P, Kunkel TA, Erie DA. Direct Visualization of Asymmetric Adenine Nucleotide-Induced Conformational Changes in MutLalpha. Mol Cell. 2008;29:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu J, Hanne J, Britton BM, Bennett J, Kim D, Lee JB, et al. Cascading MutS and MutL sliding clamps control DNA diffusion to activate mismatch repair. Nature. 2016;539:583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Groothuizen FS, Winkler I, Cristovao M, Fish A, Winterwerp HH, Reumer A, et al. MutS/MutL crystal structure reveals that the MutS sliding clamp loads MutL onto DNA. Elife. 2015;4:e06744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mirny L, Slutsky M, Wunderlich Z, Tafvizi A, Leith J, Kosmrlj A. How a protein searches for its site on DNA: the mechanism of facilitated diffusion. J Phys A: Math Theor. 2009;42:434013. [Google Scholar]
- [83].Junop MS, Obmolova G, Rausch K, Hsieh P, Yang W. Composite active site of an ABC ATPase: MutS uses ATP to verify mismatch recognition and authorize DNA repair. Mol Cell. 2001;7:1–12. [DOI] [PubMed] [Google Scholar]
- [84].Kunkel TA. Celebrating DNA’s Repair Crew. Cell. 2015;163:1301–3. [DOI] [PubMed] [Google Scholar]
- [85].Modrich P. Mechanisms and biological effects of mismatch repair. [Review] [143 refs]. Annu Rev Genet. 1991;25:229–53. [DOI] [PubMed] [Google Scholar]
- [86].Qiu R, Sakato M, Sacho EJ, Wilkins H, Zhang X, Modrich P, et al. MutL traps MutS at a DNA mismatch. Proc Natl Acad Sci U S A. 2015;112:10914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Berg OG, von Hippel PH. Diffusion-controlled macromolecular interactions. [Review] [96 refs]. Annual Review of Biophysics & Biophysical Chemistry. 1985;14:131–60. [DOI] [PubMed] [Google Scholar]
- [88].Berg OG, von Hippel PH. Selection of DNA binding sites by regulatory proteins. Statistical-mechanical theory and application to operators and promoters. Journal of Molecular Biology. 1987;193:723–50. [DOI] [PubMed] [Google Scholar]
- [89].Bagchi B, Blainey PC, Xie XS. Diffusion constant of a nonspecifically bound protein undergoing curvilinear motion along DNA. J Phys Chem B. 2008;112:6282–4. [DOI] [PubMed] [Google Scholar]
- [90].Blainey PC, Luo G, Kou SC, Mangel WF, Verdine GL, Bagchi B, et al. Nonspecifically bound proteins spin while diffusing along DNA. Nat Struct Mol Biol. 2009;16:1224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Thormann V, Borschiwer M, Meijsing SH. Transcription Regulation: when 1+1≠2 In: Rogato A, Zazzu V., Guarracino M., editor. Dynamics of Mathematical Models in Biology. Switzerland: Springer International Publishing; 2016. p. 1–16. [Google Scholar]
- [92].Spampinato C, Modrich P. The MutL ATPase is required for mismatch repair. J Biol Chem. 2000;275:9863–9. [DOI] [PubMed] [Google Scholar]
- [93].Barras F, Marinus MG. Arrangement of Dam methylation sites (GATC) in the Escherichia coli chromosome. Nucleic Acids Res. 1988;16:9821–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Feng G, Tsui HC, Winkler ME. Depletion of the cellular amounts of the MutS and MutH methyl-directed mismatch repair proteins in stationary-phase Escherichia coli K-12 cells. J Bacteriol. 1996;178:2388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lee JY, Chang J, Joseph N, Ghirlando R, Rao DN, Yang W. MutH complexed with hemi- and unmethylated DNAs: coupling base recognition and DNA cleavage. Mol Cell. 2005;20:155–66. [DOI] [PubMed] [Google Scholar]
- [96].Matson SW. DNA helicases of Escherichia coli. Prog Nucleic Acid Res Mol Biol. 1991;40:289–326. [DOI] [PubMed] [Google Scholar]
- [97].Lohman TM. Escherichia coli DNA helicases: mechanisms of DNA unwinding. Mol Microbiol. 1992;6:5–14. [DOI] [PubMed] [Google Scholar]
- [98].Matson SW. Escherichia coli helicase II (urvD gene product) translocates unidirectionally in a 3’ to 5’ direction. J Biol Chem. 1986;261:10169–75. [PubMed] [Google Scholar]
- [99].Su SS, Grilley M, Thresher R, Griffith J, Modrich P. Gap formation is associated with methyl-directed mismatch correction under conditions of restricted DNA synthesis. Genome. 1989;31:104–11. [DOI] [PubMed] [Google Scholar]
- [100].Dao V, Modrich P. Mismatch-, MutS-, MutL-, and helicase II-dependent unwinding from the single-strand break of an incised heteroduplex. J Biol Chem. 1998;273:9202–7. [DOI] [PubMed] [Google Scholar]
- [101].Mechanic LE, Frankel BA, Matson SW. Escherichia coli MutL loads DNA helicase II onto DNA. Journal of Biological Chemistry. 2000;275:38337–46. [DOI] [PubMed] [Google Scholar]
- [102].Viswanathan M, Lovett ST. Single-strand DNA-specific exonucleases in Escherichia coli - roles in repair and mutation avoidance. Genetics. 1998;149:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Runyon GT, Lohman TM. Escherichia coli helicase II (uvrD) protein can completely unwind fully duplex linear and nicked circular DNA. J Biol Chem. 1989;264:17502–12. [PubMed] [Google Scholar]
- [104].Runyon GT, Bear DG, Lohman TM. Escherichia coli helicase II (UvrD) protein initiates DNA unwinding at nicks and blunt ends. Proc Natl Acad Sci U S A. 1990;87:6383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Runyon GT, Lohman TM. Kinetics of Escherichia coli helicase II-catalyzed unwinding of fully duplex and nicked circular DNA. Biochemistry. 1993;32:4128–38. [DOI] [PubMed] [Google Scholar]
- [106].Ali JA, Lohman TM. Kinetic measurement of the step size of DNA unwinding by Escherichia coli UvrD helicase. Science. 1997;275:377–80. [DOI] [PubMed] [Google Scholar]
- [107].Dessinges MN, Lionnet T, Xi XG, Bensimon D, Croquette V. Single-molecule assay reveals strand switching and enhanced processivity of UvrD. Proc Natl Acad Sci U S A. 2004;101:6439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Comstock MJ, Whitley KD, Jia H, Sokoloski J, Lohman TM, Ha T, et al. Protein structure. Direct observation of structure-function relationship in a nucleic acid-processing enzyme. Science. 2015;348:352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Truglio JJ, Croteau DL, Van Houten B, Kisker C. Prokaryotic nucleotide excision repair: the UvrABC system. Chem Rev. 2006;106:233–52. [DOI] [PubMed] [Google Scholar]
- [110].Grilley M, Griffith J, Modrich P. Bidirectional excision in methyl-directed mismatch repair. J Biol Chem. 1993;268:11830–7. [PubMed] [Google Scholar]
- [111].Lahue RS, Au KG, Modrich P. DNA mismatch correction in a defined system. Science. 1989;245:160–4. [DOI] [PubMed] [Google Scholar]
- [112].Yamaguchi M, Dao V, Modrich P. MutS and MutL activate DNA helicase II in a mismatch-dependent manner. J Biol Chem. 1998;273:9197–201. [DOI] [PubMed] [Google Scholar]
- [113].Mechanic LE, Frankel BA, Matson SW. Escherichia coli MutL loads DNA helicase II onto DNA. J Biol Chem. 2000;275:38337–46. [DOI] [PubMed] [Google Scholar]
- [114].Matson SW, Robertson AB. The UvrD helicase and its modulation by the mismatch repair protein MutL. Nucleic Acids Res. 2006;34:4089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hombauer H, Campbell CS, Smith CE, Desai A, Kolodner RD. Visualization of eukaryotic DNA mismatch repair reveals distinct recognition and repair intermediates. Cell. 2011;147:1040–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chen C, Kolodner RD. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat Genet. 1999;23:81–5. [DOI] [PubMed] [Google Scholar]
- [117].Shell SS, Putnam CD, Kolodner RD. The N terminus of Saccharomyces cerevisiae Msh6 is an unstructured tether to PCNA. Mol Cell. 2007;26:565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Umar A, Buermeyer AB, Simon JA, Thomas DC, Clark AB, Liskay RM, et al. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996;87:65–73. [DOI] [PubMed] [Google Scholar]
- [119].Kuriyan J, O’Donnell M. Sliding clamps of DNA polymerases. [Review] [70 refs]. J Mol Biol. 1993;234:915–25. [DOI] [PubMed] [Google Scholar]
- [120].O’Donnell M, Kuriyan J. Clamp loaders and replication initiation. Curr Opin Struct Biol. 2006;16:35–41. [DOI] [PubMed] [Google Scholar]
- [121].Tan CK, Castillo C, So AG, Downey KM. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986;261:12310–6. [PubMed] [Google Scholar]
- [122].Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326:515–7. [DOI] [PubMed] [Google Scholar]
- [123].Prelich G, Tan CK, Kostura M, Mathews MB, So AG, Downey KM, et al. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987;326:517–20. [DOI] [PubMed] [Google Scholar]
- [124].Clark AB, Valle F, Drotschmann K, Gary RK, Kunkel TA. Functional interaction of proliferating cell nuclear antigen with MSH2-MSH6 and MSH2-MSH3 complexes. J Biol Chem. 2000;275:36498–501. [DOI] [PubMed] [Google Scholar]
- [125].Flores-Rozas H, Clark D, Kolodner RD. Proliferating cell nuclear antigen and Msh2p-Msh6p interact to form an active mispair recognition complex. Nat Genet. 2000;26:375–8. [DOI] [PubMed] [Google Scholar]
- [126].Gu L, Hong Y, McCulloch S, Watanabe H, Li GM. ATP-dependent interaction of human mismatch repair proteins and dual role of PCNA in mismatch repair. Nucleic Acids Res. 1998;26:1173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Johnson RE, Kovvali GK, Guzder SN, Amin NS, Holm C, Habraken Y, et al. Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J Biol Chem. 1996;271:27987–90. [DOI] [PubMed] [Google Scholar]
- [128].Lopez de Saro FJ, Marinus MG, Modrich P, O’Donnell M. The beta sliding clamp binds to multiple sites within MutL and MutS. J Biol Chem. 2006;281:14340–9. [DOI] [PubMed] [Google Scholar]
- [129].Pillon MC, Miller JH, Guarne A. The endonuclease domain of MutL interacts with the beta sliding clamp. DNA Repair (Amst). 2011;10:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Pillon MC, Babu VM, Randall JR, Cai J, Simmons LA, Sutton MD, et al. The sliding clamp tethers the endonuclease domain of MutL to DNA. Nucleic Acids Res. 2015;43:10746–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Yamamoto T, Iino H, Kim K, Kuramitsu S, Fukui K. Evidence for ATP-dependent structural rearrangement of nuclease catalytic site in DNA mismatch repair endonuclease MutL. J Biol Chem. 2011;286:42337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Bowen N, Smith CE, Srivatsan A, Willcox S, Griffith JD, Kolodner RD. Reconstitution of long and short patch mismatch repair reactions using Saccharomyces cerevisiae proteins. Proc Natl Acad Sci U S A. 2013;110:18472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Genschel J, Modrich P. Mechanism of 5’-directed excision in human mismatch repair. Mol Cell. 2003;12:1077–86. [DOI] [PubMed] [Google Scholar]
- [134].Zhang Y, Yuan F, Presnell SR, Tian K, Gao Y, Tomkinson AE, et al. Reconstitution of 5’-directed human mismatch repair in a purified system. Cell. 2005;122:693–705. [DOI] [PubMed] [Google Scholar]
- [135].Jeon Y, Kim D, Martin-Lopez JV, Lee R, Oh J, Hanne J, et al. Dynamic control of strand excision during human DNA mismatch repair. Proc Natl Acad Sci U S A. 2016;113:3281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].James W. Principles of Psychology. London, UK: MacMillan; 1890. [Google Scholar]
- [137].Berlucchi G, Buchtel HA. Neuronal plasticity: historical roots and evolution of meaning. Exp Brain Res. 2009;192:307–19. [DOI] [PubMed] [Google Scholar]
- [138].Osakada K History of plasticity and metal forming analysis. J Mater Process Tech. 2010;210:1436–54. [Google Scholar]
- [139].Min W, Xie XS, Bagchi B. Two-dimensional reaction free energy surfaces of catalytic reaction: effects of protein conformational dynamics on enzyme catalysis. J Phys Chem B. 2008;112:454–66. [DOI] [PubMed] [Google Scholar]
- [140].Berg OG, Winter RB, von Hippel PH. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry. 1981;20:6929–48. [DOI] [PubMed] [Google Scholar]
- [141].Mazurek A, Johnson CN, Germann MW, Fishel R. Sequence context effect for hMSH2-hMSH6 mismatch-dependent activation. Proc Natl Acad Sci U S A. 2009;106:4177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hermans N, Laffeber C, Cristovao M, Artola-Boran M, Mardenborough Y, Ikpa P, et al. Dual daughter strand incision is processive and increases the efficiency of DNA mismatch repair. Nucleic Acids Res. 2016;44:6770–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lu AL. Influence of GATC sequences on Escherichia coli DNA mismatch repair in vitro. J Bacteriol. 1987;169:1254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Bruni R, Martin D, Jiricny J. d(GATC) sequences influence Escherichia coli mismatch repair in a distance-dependent manner from positions both upstream and downstream of the mismatch. Nucleic Acids Res. 1988;16:4875–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Radman M, Wagner R. Mismatch repair in Escherichia coli. Ann Rev Genet. 1986;20:523–38. [DOI] [PubMed] [Google Scholar]
- [146].Modrich P DNA mismatch correction. Ann Rev Biochem. 1987;56:435–66. [DOI] [PubMed] [Google Scholar]
- [147].Modrich P Methyl-directed DNA mismatch correction. J Biol Chem. 1989;264:6597–600. [PubMed] [Google Scholar]