Introduction
The use of ultrasonography in the management of nephrolithiasis can be traced back to 1961, when Schlengel and colleagues first published on amplitude (A)-mode sonography for the intra-operative localization of renal stones.1 Though ultrasound (US) has continued to play a role in the management of stone disease, computed tomography (CT) has become the imaging study of choice due to its high sensitivity and specificity for stone detection.2,3 Recent concern about long-term effects of ionizing radiation exposure has given rise to renewed interest in US, which is already the preferred imaging study for children and pregnant patients with suspected nephrolithiasis.2 Some even have suggested that US should be the initial imaging modality for patients presenting with acute renal colic.4
Continued refinement of US technology has expanded its use in diagnosis and follow up, percutaneous access, minimally invasive renal surgery, and shockwave lithotripsy (SWL).5 Recent research in diagnostic US for stone detection and sizing may further enhance its role in the management of stone disease. US technology is also being applied to non-invasively move and break kidney stones. Such innovative developments have the potential to generate a future paradigm shift in the management of kidney stones.
Developments in Ultrasound-based Stone Imaging
Compared to CT, US has a low sensitivity and limited specificity for stone detection (24-70% and 88-94.4%, respectively).6–8 Moreover, stone sizing on US has poor accuracy, with average overestimation of 3.3 mm for stones ≤ 5 mm.9 As stone size has implications on the likelihood of spontaneous passage as well as determining the best surgical treatment option, clinical decision-making is predicated on this information. Management decisions made on US stone size alone result in mis-counseling in up to 22% of cases.10 Adequate stone detection and sizing accuracy therefore remain two of the primary challenges to more widespread use of US.
Improving Stone Detection
Renal stones have been traditionally identified on grayscale brightness (B)-mode US as echogenic foci that may be accompanied by a posterior acoustic shadow. However, some stones don’t have this classic appearance, and elude detection. Twinkling artifact was first described in 1996 and refers to a rapidly changing, heterogeneous distribution of colors around a stone on color-flow Doppler mode (Figure 1).11 Twinkling is present for 43-96% of stones on US, and has been proposed as a useful adjunct for stone detection.12–14 This artifact may highlight the presence of a stone not immediately evident on B-mode imaging.
Figure 1:
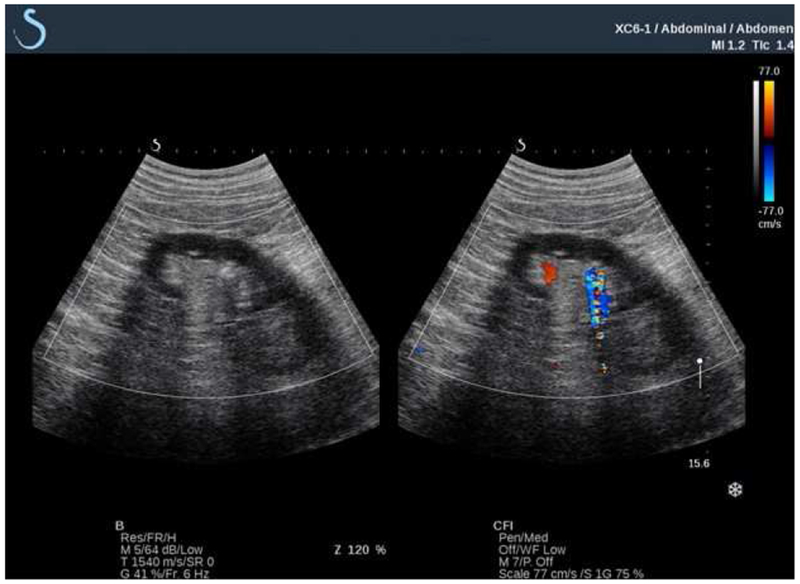
Example of stone twinkling (arrow). The same stone is imaged on grayscale sonography alone (left) and on color-flow Doppler (right). The flickering mosaic of color on color-flow doppler is called “twinkling.” Normal blood flow is also detected by Doppler (solid red) and can be readily distinguished from twinkling.
The prevailing explanation for twinkling is that small bubbles trapped in stone surface crevices oscillate and generate random backscatter when struck by incident Doppler pulses. These signals are interpreted and displayed as noise. Lu and colleagues provided evidence for this theory by demonstrating that twinkling could be extinguished with hyperbaric pressures and re-instated with reduced static pressure. Wetting stones with ethanol also reduced twinkling, presumably by influencing surface tension and bubble stabilization on the stone surface.15 Simon and colleagues captured the presence of microbubbles by exposing stones to lithotripter pulses and varying static pressures.16 Stones demonstrated reproducible twinkling signals, and bubble activity was directly visualized on the stone with lithotripter pulses.
Preclinical studies have assessed the potential utility of twinkling for stone detection. In phantom and sheep kidneys, twinkling was found to have higher contrast (with respect to background) than acoustic shadowing, suggesting that this may be more readily identifiable on US.17 In human studies, 85% of non-shadowing stones twinkled.18 Moreover, twinkling contrast was 37 times greater than the hyperechoic stone signal on grayscale US.19 Though the strength of the twinkling signal varies by stone composition, in vitro studies suggest that this may be more related to the stone’s structure.20,21 Twinkling strength may also be related to the location of the focal zone during a sonographic exam, likely because higher pressures at the focal zone cause greater microbubble oscillation.18
Clinical studies have evaluated the effect of twinkling on stone detection. Among patients with acute renal colic, twinkling signal demonstrated a sensitivity of 83% and positive predictive value (PPV) of 94%, compared to sensitivity of 80% and PPV of 65% for grayscale sonography alone. Considered together, detection sensitivity rose to 88% and PPV to 96%.22 In a clinic-based study of patients with CT-confirmed stones, twinkling alone had higher specificity than the hyperechoic stone signal on B-mode imaging (74% vs. 48%).12 In the acute setting, , twinkling was seen in 97.1% of patients with renal colic and nephrolithiasis, with a sensitivity of 97.2% and specificity of 99%.13
Others have found that twinkling is less reliable among patients without known nephrolithiasis. In this population, twinkling alone was 78% sensitive and 40% specific for stones. When additional sonographic features were considered (echogenic focus, posterior acoustic shadow, or both), sensitivity diminished to as low as 31% but specificity increased to as high as 95%.14 Thus, twinkling may be one of several sonographic features to indicate stone presence on US, but may be most useful among patients with a history of nephrolithiasis.
Twinkling has also been correlated to duration of renal colic symptoms, pain, and difficulty with guidewire passage at time of endoscopic intervention.23 Though this may have potential clinical implications by providing prognostic information at the time of stone diagnosis, further research validating these findings is warranted. Current understanding of the twinkling signal remains limited by single-institution studies and variability in imaging techniques. Optimization and standardization of twinkling, which was once perceived an artifact, might provide greater utility in improving the identification of renal stones.
Optimizing Stone Sizing Accuracy
Improving stone sizing on US has been another area of active research. The degree and wide variability of size overestimation limits the use of US for clinical decision making, particularly in the acute setting where it has been promoted as first-line imaging for suspected nephrolithiasis.4,24,25 Techniques to optimize the accuracy of stone sizing are therefore paramount.
Several US system-specific factors have been found to influence stone sizing. The degree of size overestimation appears to be correlated with greater stone depth and gain.26 At increasing depths beyond the focus, US rays diverge, decreasing spatial resolution. Placing the focus at the stone therefore minimizes beam spread and maximizes stone resolution. In vitro, high gain settings increased measured stone size by 18%.26 High gain can saturate the US image, decrease stone contrast and detectability, and make identification of stone edges more difficult. The greatest size overestimation is seen with spatial compounding, as averaging of multiple images generates a smoother overall image but also blurs the stone and shadow borders. In vitro, harmonic imaging has been found to minimize stone size overestimation.27
The posterior acoustic shadow has also been suggested as an adjunct to improve the accuracy of stone sizing (Figure 2). In stone phantoms, measuring shadow width decreased average overestimation error to 0.5 mm, regardless of imaging depth or modality. Shadow size was significantly more accurate than measured stone size on US images and reduced misclassification of stone size in clinically relevant size categories (>5 mm vs. ≤5mm) from 50% to 15%. Using this technique, 78% of stones had a <1 mm size error. Notably, 53% of stones <5 mm did not demonstrate a shadow, suggesting that the absence of a shadow may indicate a small stone.27
Figure 2:
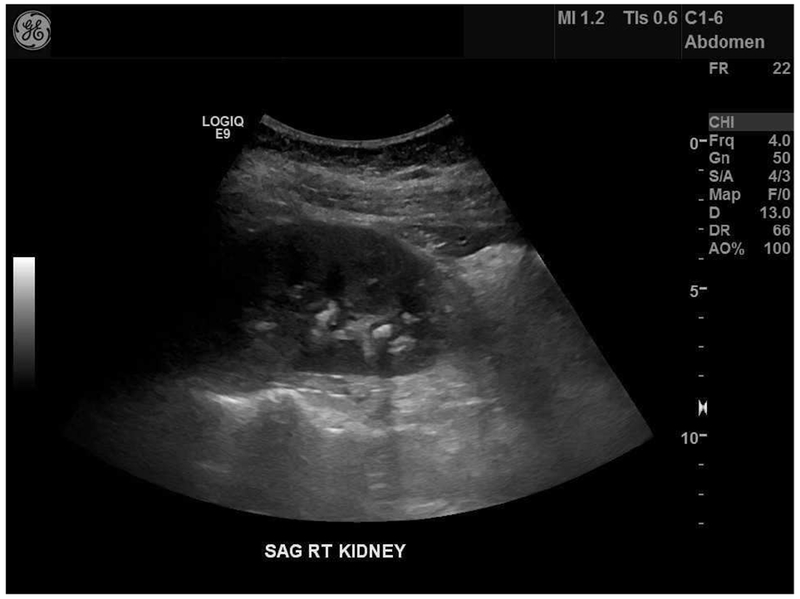
Example of difference in stone size based on measurement of the echogenic stone signal and the posterior acoustic shadow width. Dashed guide-lines indicate the borders of the stone shadow. This stone (arrow) measured 5 mm on CT, 9.7 mm on US, and 6.8 mm±3 mm by shadow measurement among 26 novice reviewers. From Dai JC, Dunmire B, Chen T, et al. Clinical Outcomes in Pediatric Patients with Ureteral Sontes Are Correlated with The Posterior Acoustic Shadow Measurement on Ultrasound: A Pilot Study. In: Societies for Pediatric Urology, Pediatric Urology Fall Congress. ; 2018, with permission.
Studies in human subjects support these findings. On a research US system, 55% of imaged stones demonstrated a shadow width within 1 mm of corresponding CT size. Average shadow size overestimation was 1.1 mm for stones ≤ 10 mm.28 Evaluation of clinical US images from a commercial system demonstrated that shadow measurements resulted in <1 mm sizing error for 42% of stones, with respect to CT.29 Additionally, up to 83% of non-shadowing stones measured <5 mm on CT, further suggesting that non-visualization of a shadow on US indicates a smaller stone.29 Even among pediatric patients, shadow sizes were significantly smaller than reported US stone sizes (mean difference 2.9 mm, p<0.001) and resulted in down-sizing of 68% of stones by at least 1 size category (p<0.001).30 Availability of such information prospectively may potentially influence provider decisions regarding patient management.
As the shadow sizing technique requires no additional hardware or software modification, it was thought to be easily adoptable in clinical practice. A single-institution study examined the uptake of this technique among clinicians. Providers familiar but inexperienced with the training technique demonstrated that shadow measurements on clinical US images were no more accurate than reported US sizes (with respect to CT) at initial adoption. However, after a brief training module, there was significant improvement in overestimation bias and more stones with 1 mm concordance between shadow and CT size. No improvements were seen with repeated practice alone.31 Techniques reviewed in the training module included careful identification of the shadow, evaluation for confounding artifacts, tracing the entire shadow path using guide-lines to project the shadow back to the stone, and measuring the shadow perpendicular to the direction of the US beam.31
Future Directions in Stone Imaging
The development of stone-specific algorithms to optimize stone imaging is an area of active research. One such research system, coined “S-mode,” maximizes stone contrast and highlights the posterior acoustic shadow by avoiding speckle reduction and spatial compounding.32 It uses a higher frequency transducer and higher scanning line density to improve resolution. Side-by-side simultaneous imaging can be performed with grayscale and color-flow Doppler (Figure 3). A custom Doppler mode uses lower frequency to enhance the twinkling signal and suppress blood flow signal. In human subjects, sensitivity for stones was 84%, with 44% of stone sizes demonstrating 1 mm concordance with corresponding CT measurements.28 Additional refinement of this system may further enhance stone detection capabilities on US.19
Figure 3:
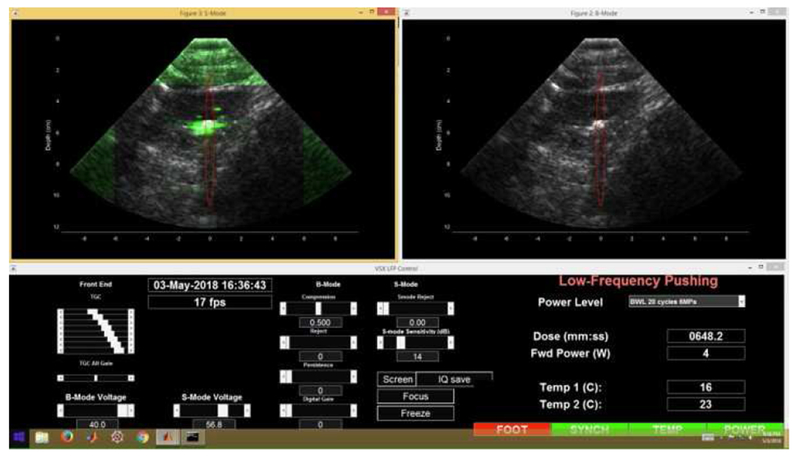
Example of stone imaging output platform on customized “S-mode” imager. Color-flow Doppler mode is visualized on the left, with twinkling signal displayed in green.
Simultaneous grayscale imaging is shown on the right for the same stone. Red oval represents focal zone for ultrasonic propulsion therapy that can be delivered using the same probe, discussed below.
Others have explored advanced beamforming techniques, such as plane wave synthetic focusing, short-lag spatial coherence imaging, mid-lag spatial coherence imaging with incoherent compounding, and aperture domain model image reconstruction. When Tierney and colleagues compared stone size and contrast on each mode to traditional B-mode imaging in vitro, stone sizing error was minimized with plane wave synthetic focusing (0.3±2.9 mm vs. 1.2±1.1 mm).33 Further work to compare and integrate these techniques into existing imaging platforms remains.
3-dimensional (3D) US is another area of emerging research, as it provides unique information about stone morphology. 3D US imaging with surface rendering of stones has been shown to be feasible and has been used in pregnant patients.34 A transrectal 3D US approach has been proposed to evaluate the success of SWL for distal ureteral stones.35,36 This technology may also offer radiation-free opportunities for improved percutaneous stone treatment planning and enhanced trainee and patient learning.37–39 Reconstruction of the pelvicalyceal system from 3D US images has been shown to be feasible and anatomically comparable to casts of the collecting system.40 This may ultimately help optimize stone treatment outcomes.
Movement of Stones with Ultrasound
Non-invasive movement of kidney stones and stone fragments has generated significant interest over the past decade. Specifically, the conundrum of how to clear residual fragments following stone treatment has spurred much research. Inversion therapy, mechanical percussion, and diuresis have been shown to help, but are non-specific and can be labor intensive.41,42 The “Lithecbole,” a novel mechanical percussion device, has also been shown to significantly improve stone-free rates and expedite passage of stone fragments following SWL,43,44 as well as symptomatic distal ureteral stones.45
Recent application of US technology to this space has led to novel methods of non-invasive stone manipulation. The movement of kidney stones using US energy has been termed “ultrasonic propulsion.” This innovative technology is currently in clinical trials under an investigational device exemption from the Food and Drug Administration (FDA).
Ultrasonic Propulsion: Development and Evolution
Ultrasonic propulsion was first described in 2010 by Shah and colleagues at the University of Washington.46 This technology is based on the acoustic radiation force resulting from the transfer of acoustic wave momentum to a visualized stone. In the initial prototype, an annular array of elements was incorporated into a handheld probe, yielding a focused acoustic beam. Combined with a coaxial imaging probe, this could target stones visualized on US for real-time pushing.46
Since the initial feasibility study, several refinements have been made to improve device efficacy. The evolution of this technology and treatment details are summarized in Table 1. Following the original research prototype in 2010, a second-generation probe was developed in 2013, which integrated imaging and treatment capabilities into a single commercial US probe. This system utilized lower peak pressures and a shorter 1-second burst of push pulses.47 The device was modified again in 2014 to improve stone targeting by integrating a touch-screen to both visualize stone targets and activate push pulses. Push burst duration was also decreased to limit the amount of ineffective, off-target energy delivered to the treatment focus after the stone moves out of the focal field.48,49
Table 1:
Evolution of ultrasonic propulsion technology and effectiveness over time, from initial feasibility studies to the first human clinical study.
| Year | Publication | Probe | Treatment parameters | Study Type | Experimental Set-up | Outcomes |
|---|---|---|---|---|---|---|
| 2010 | Shah, et al46 |
Therapy: 6 cm annular probe, 8 elements, 2 MHz, 4.5-8.5 cm focal depth Imaging: Philips 5000 HDI imaging system, P4-2 transducer |
5 W to 40 W; 2-5 second pulse duration; 50% duty cycle | In vitro | glass beads (2.5-4 mm) and human COM, COD or CaP stones (3-8 mm) implanted in kidney phantom | Stones or beads repositioned from lower pole to renal pelvis |
| 2012 | Shah, et al50 |
Therapy: 6 cm annular probe, 8 elements, 2 MHz, 4.5-8.5 cm focal depth Imaging: Philips 5000 HDI imaging system, P4-2 transducer |
325W/cm2 average treatment exposure; 1-4 second pulse duration; 50% duty cycle | Animal model | glass/metal beads (3-5 mm) and human cystine, COM, or CaP stones (1-8 mm) implanted endoscopically or percutaneously into lower or interpolar calyces of 6 anesthetized pigs | Stones or beads repositioned from mid or lower pole calyces to renal pelvis and UPJ in all 6 pigs within 10 minutes |
| 2013 | Harper, et al47 | Integrated imaging and therapy probe: HDI ATL C5-2 or Philips P4-2; Verasonics imaging system | 0-1 second push burst duration (250 pulses of 0.1 millisecond duration per 1 second burst); 3% duty cycle | Animal model | 26 COM stones or beads (2-8 mm) endoscopically implanted in 12 kidneys (interpolar or lower pole calyces) of 8 anesthetized pigs | 17 stones (65%) successfully relocated from calyx to renal pelvis, UPJ, or ureter; 2 moved out of calyx but did not reach renal pelvis; 7 stones moved within calyx. Average displacement time 14.2 ± 7.9 minutes using a mean of 23 ± 16 push bursts Average displacement 5.6±2.7 linear cm |
| 2014 | Harper, et al48 | HDI ATL C5-2 probe; Verasonics imaging system; integrated touchscreen monitor | 50 or 90 V settings; 50 millisecond push burst duration; 73% duty cycle | Animal model | 1) 6 COM stones (2-5 mm) endoscopically implanted into right lower pole calyces of 5 anesthetized pigs 2) de novo stones in 3 pigs in a diet-induced hyperoxaluria model; 2 < 3 mm stones identified in 6 renal units. |
1) 6 stones (100%) successfully repositioned from lower pole to UPJ or proximal ureter. Average displacement time 14±8 minutes using a mean of 13±6 bursts. 2) 2 stones (100%) repositioned to collecting system. Average displacement time 20±13 minutes using a mean 10±8 push bursts. |
| 2016 | Harper, et al 53 | HDI ATL C5-2 probe, Verasonics imaging system | 50 or 90 V settings; 50 millisecond push duration; 73% duty cycle; maximum of 40 pushes | Human subjects | 1) 6 patients with < 5 mm residual fragments following lithotripsy 2) 3 patients with <5 mm de novo stones 3) 4 patients with ≥ 5 mm de novo stones 4) 2 patients with ≥ 5 mm de novo stones undergoing ureteroscopy |
1) 4 of 6 patients passed stone fragments; 47% moved <3 mm and 18% moved ≥3 mm. Mean of 39 push bursts 2) 0 stones passed; 25% moved < 3 mm. Mean of 39 push bursts. 3) 19% moved <3 mm. Mean of 23 push bursts. 4) 30% moved < 3 mm, 12% moved ≥3 mm. Mean of 28 push bursts. |
COM = calcium oxalate monohydrate, COD = calcium oxalate dihydrate, CaP = calcium phosphate, UPJ = ureteropelvic junction
The first human clinical study was performed in 2016. Ultrasonic propulsion was applied to 13 awake subjects in clinic and 2 anesthetized subjects at the time of endoscopic stone treatment. Of the awake subjects, 6 had residual fragments after lithotripsy <5 mm, 3 had <5 mm de novo stones, and 4 had ≥ 5 mm de novo stones. Stone compositions included calcium oxalate monohydrate (COM), calcium oxalate dihydrate (COD), apatite, and brushite. Stone motion was achieved at 50 and 90 V power settings. Four of 6 patients passed post-lithotripsy fragments. Notably, there was symptomatic pain relief in 1 subject with a 10 mm ureteropelvic junction stone. Ultrasonic propulsion provided additional diagnostic information in 4 subjects by dispersing a collection of small fragments thought to be a larger stone on pre-treatment imaging.
Safety
Multiple safety studies of ultrasonic propulsion in porcine models have demonstrated no histologic evidence of injury to the kidney.47,48,50 Pre-clinical animal studies demonstrated that even at maximal treatment settings of 90 V, no histologic injury was induced with transcutaneous or direct organ treatment. Survival studies in pigs demonstrated no adverse events or deaths at moderate or high dose treatment settings, with normal hematologic and urinary parameters at time of necropsy after 1 week.48
Though treatment-level settings have not been shown to cause tissue injury, power settings in excess of typical treatment parameters can still induce tissue injury. Thermal coagulation injury was caused in 6 of 7 porcine kidneys treated at excessive exposures (1900 W/cm2), with a maximal injury size of 1 cm. In contrast, treatment-level exposure was only 325 W/cm2.50 Connors and colleagues further demonstrated that at high power settings (240 W), no renal injury occurred with transcutaneous treatment, but direct treatment of the kidney resulted in hemorrhagic injury similar to that of SWL; thermal coagulation injury occurred at lower power treatments.51 The resulting area of injury was still less than one-third that caused by SWL (0.46% vs 1.56% of total renal volume).
Further work to determine more precise tissue injury thresholds was conducted by Wang and colleagues, who demonstrated that a spatial peak intensity of 16,620 W/cm2 was needed to cause significant kidney injury with direct treatment. In comparison, the maximum spatial peak intensity generated during transcutaneous treatment in animal models was nearly 7 times lower.52 As this work transitioned to human trials, spatial peak intensity remained over 330 times lower than this injury threshold.49,53
In the first human clinical study, none of the 13 awake subjects reported any sensation with a 50 V push burst. All reported warming of the skin at the transducer interface at 90 V, and 2 subjects reported a brief “internal sensation,” which was not considered painful. There were no unanticipated or serious adverse events.53
Clinical Use
Ultrasonic propulsion has many potential clinical applications. These include facilitating passage of post-lithotripsy fragments, re-positioning stones pre-operatively or intra-operatively, dislodging obstructing stones, and providing endpoint detection for SWL or other transcutaneous lithotripsy modalities. Combined with treatments such as diuresis, inversion therapy, or mechanical percussion, this technology may optimize residual stone passage after stone treatment, particularly for lower pole stones.42
Ultrasonic propulsion is envisioned as part of a clinic-based, non-invasive approach to kidney stone management. However, provider education might be necessary to facilitate its adoption among urologists. Hsi and colleagues developed and piloted a training curriculum on the fundamentals of renal US and ultrasonic propulsion among 10 board-certified urologists. After completing the curriculum, all participants successfully moved lower pole stones within a phantom model, and 90% successfully repositioned stones into the renal pelvis with a mean of 15.7 pushes and a mean time of 4.5 minutes.54
Optimization and Future Work
To better optimize clearance of stone fragments, further modifications to the ultrasonic propulsion system have been made since the first human trial. A custom probe with an integrated imaging transducer and water-circulating coupling head was developed, with less probe heating (Figure 4). This incorporated a longer focal beam and burst duration (3 seconds vs. 50 milliseconds). At 4.5 and 9.5 cm depths, 1-2 mm fragments, 3-4 mm fragments, and a 4 × 7 mm stone were successfully expelled out of a phantom calyx.55 In a 7-day porcine survival study, there were no adverse clinical, laboratory, or histologic findings with treatments. Successful movement of larger 8-12 mm stones of varying compositions (COM, ammonium acid urate, calcium phosphate, and struvite) out of a phantom calyx model was achieved in 95% of cases.56
Figure 4:
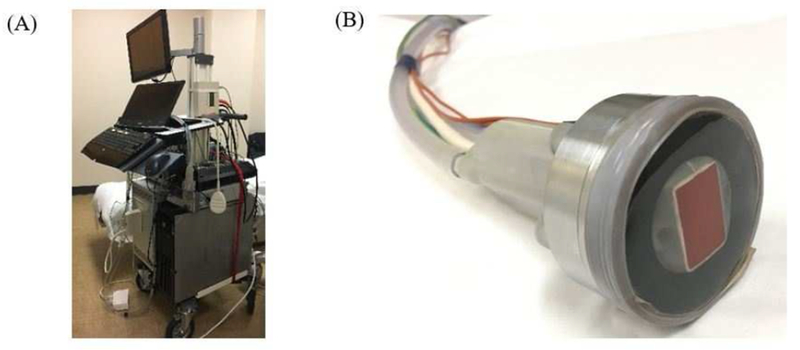
The current ultrasonic propulsion system (A) and custom therapy probe (B). The annular therapy probe is visible in silver and the coaxial imaging probe is seen in red. A water-circulating coupling head minimizes overheating of the device.
Recent clinical studies utilizing the custom probe were performed during ureteroscopic stone treatment. Ultrasonic propulsion treatment was applied simultaneously with direct endoscopic visualization of stone movement (Figure 5). Stone targets ranged in size from dust to 7 mm. Blinded review of endoscopic videos demonstrated target movement > 3 mm in 14 of 15 kidneys. Ultrasonic propulsion obviated the need for stone basketing in 2 cases by repositioning stones to a more favorable intra-renal location. There were no serious or unanticipated adverse events.57 A randomized clinic-based trial using ultrasonic propulsion to facilitate clearance of residual fragments and an Emergency Department-based trial of ultrasonic propulsion to move obstructing ureteral stones are ongoing at the University of Washington.
Figure 5:
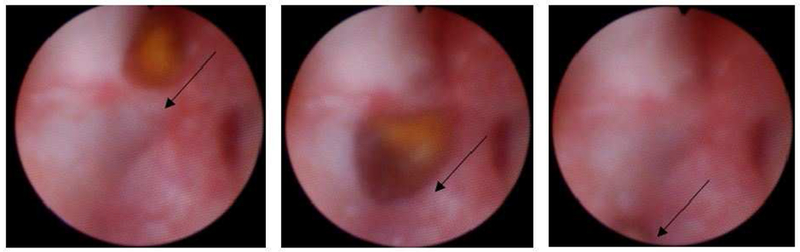
Endoscopic visualization of a 5 mm stone being repositioned using ultrasonic propulsion (panels progress in time from left to right). Arrows indicate direction of stone movement. The stone traveled about 1 cm in under 1 second.
Acoustic tractor beam technology is yet another emerging area of research in US-based stone movement. This may enable directed capture and navigation of a stone through the calyceal system.58 In recent years, tractor beam technology has been shown to have the capability to move targets > 1 cm in size.59 Integration with current ultrasonic propulsion technology may allow for more directed, non-invasive stone movements. However, its potential application in this space remains to be explored.
Ultrasonic Stone Fragmentation: Burst wave Lithotripsy
SWL has greatly evolved since the development of the Dornier HM1 in 1980. Better understanding of the role of stone density, skin-to-stone distance, coupling, shockwave delivery rate, and power ramping have influenced treatment delivery.60,61 Further technological developments such as wider focal zones and tandem or dual head lithotripters have also been suggested to improve treatment success. However, with continued advances in ureteroscopic technology, the role of SWL has been questioned.62 Burst wave lithotripsy (BWL) is an emerging, US-based approach to extracorporeal lithotripsy that holds promise as a novel option for non-invasive stone treatment.
Proof of concept
Current SWL machines typically employ single-cycle pulses at a slow rate (≤ 2 Hz) and high peak pressures (30-100 MPa). In contrast, BWL utilizes short bursts of focused, sinusoidal US pulses. These are hypothesized to minimize the accumulation of cavitation bubbles that shield acoustic wave propagation to the stone, resulting in more effective stone comminution.63 BWL is administered transcutaneously under US guidance using a hand-held probe at higher rates (< 200Hz) and lower peak pressures (<12 MPa) than SWL. Lower pressures are hypothesized to make BWL safer and more tolerable to awake patients.
Initial in vitro experiments by Maxwell and colleagues demonstrated the potential of this technology. Using a 170, 285, or 800 KHz transducer, fragmentation of artificial Begostones occurred above peak pressures of 2.3 MPa. At peak pressures of 6.5 MPa, stones of varying compositions were successfully comminuted to < 4 mm fragments. Uric acid stones were treated most rapidly (0.17-1.40 minutes), followed by struvite (0.07-2.02 minutes), COM (8.0-18.1 minutes), and cystine stones (10.3-21.3 minutes).63 Finer fragments were generated by higher frequency treatment.
The efficacy of BWL has also been studied in animal models. Five COM stones 5-7 mm in size were surgically implanted into 3 pig kidneys and treated transcutaneously for 30-minutes with a 350 kHz transducer. Peak negative focal pressures were 6.5-7 MPa. Eighty-two percent of treated stone mass was fragmented to < 2 mm. Three of 5 stones were entirely comminuted, and in all cases ≥ 58% of the stone was fragmented. Figure 6 shows an example of treatment effect. Gross examination of treated kidneys revealed only minor petechial injury to the urothelium where the stone was targeted, with no effects on the overlying skin or parenchyma.64
Figure 6:

Example of stone comminution effect with BWL on a COM stone.
Safety
Tissue injury can occur with BWL, and this has been demonstrated on real-time US imaging. Using 170 kHz and 335 kHz transducers, May and colleagues treated 10 porcine kidneys at exposures of 5.8-8.1 MPa. Cavitation during treatment was observed as echogenicity on US. Treatment was deliberately continued to purposefully induce injury, which was seen in 10 of 21 treated sites. No injury > 0.1% of renal volume was seen with the 335 kHz transducer, but larger areas of injury < 5.2% of renal volume were generated with the 170 kHz transducer.65 Histologic analysis demonstrated intraparenchymal hemorrhage, focal tubular injury and focal necrosis, similar to SWL-related injury.66 Cavitation on US predicted BWL-related renal injury with 100% sensitivity and specificity.65 Thus, US imaging feedback may allow adjustment of treatment parameters in real time to avoid renal injury during BWL treatment.
Preliminary porcine studies provide further evidence for the safety of this technology under treatment conditions. Six pigs were treated at exposures of 7 MPa for 30 minutes. There were 4 untreated controls and histopathologic evaluation of kidneys was performed 1 week later. There were no chemistry abnormalities and no gross or histologic findings of injury.67 These results have been submitted to the FDA for approval for an investigational clinical trial.
Future Directions
Recent in vitro studies have examined the combined efficacy of ultrasonic propulsion and BWL for stone fragmentation. Dispersion of comminuted stone fragments from the target with ultrasonic propulsion was hypothesized to increase BWL efficiency. When both technologies were used together, fragmentation was increased for artificial crystalline calcite stones, Begostones, and human COM stones. The most pronounced effect was noted when push pulses were interweaved with BWL pulses.68 This study suggests that integration of these two technologies during a single treatment may optimize the utility of both.
Remaining challenges include optimizing targeting in vivo, determining treatment endpoints, and defining optimal treatment parameters. Moreover, the ideal stone and patient characteristics for this technology are unknown. Ultimately, treatment parameters may be potentially adjusted in real-time and guided by advanced imaging feedback to tailor treatment sessions to the individual patient. Future studies assessing the efficacy and safety of this therapeutic technology in humans remain to be completed.
Conclusions
US has significantly evolved as a diagnostic and therapeutic modality for kidney stones since over the past 60 years. Identification and optimization of sonographic features such as the twinkling signal and the posterior acoustic shadow may help improve stone detection and sizing. Novel beam-forming techniques and 3D US are future areas for research. Novel therapeutic US technologies have also been developed. Ultrasonic propulsion has been shown to be safe and feasible in human subjects, and its clinical impact is beginning to be explored. BWL development is ongoing and progressing toward human trials. For both, there remain many unknowns regarding the optimal candidates, treatment parameters, and ultimate adoption of into clinical practice. As future research continues, such innovations in US technology may open up new avenues for stone management and treatment.
KEY POINTS.
Twinkling signal improves the detection of kidney stones on ultrasound.
Posterior acoustic shadow measurements improve the accuracy of stone sizing on ultrasound.
Ultrasonic propulsion allows non-invasive movement of stones in awake patients. This has many potential applications, including dislodging obstructing stones and mobilizing residual stone fragments after surgery.
Burst wave lithotripsy is a promising ultrasound-based technology for transcutaneous stone fragmentation. Effectiveness and safety have been shown in animals.
Ultrasonic propulsion and burst wave lithotripsy may be integrated and combined or used separately.
SYNOPSIS.
This article reviews new advances in ultrasound (US) technology for urinary stone disease. Recent research to facilitate the diagnosis of nephrolithiasis, including use of the twinkling signal and the posterior acoustic shadow, have helped to improve the use of US for detecting renal stones. New therapeutic applications of ultrasound technology for stone disease have also emerged. These include ultrasonic propulsion to reposition stones and burst wave lithotripsy to fragment stones non-invasively. The safety, efficacy, and evolution of these technologies in phantom, animal, and human studies are reviewed herein. New developments in these rapidly growing areas of ultrasound research are also highlighted.
Acknowledgements
The authors gratefully acknowledge funding support from the National Institutes of Health NIDDK through grant P01 DK043881. This material is the result of work supported by resources from the Veterans Affairs Puget Sound Health Care System, Seattle, Washington. We thank our colleagues at the Center for Industrial and Medical Ultrasound, Department of Urology at the University of Washington, and within NIH Program Project DK043881 for their help in reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Dr. Bailey, and Dr. Sorensen have equity in and consult for SonoMotion, Inc. Drs. Dai and Harper have nothing to disclose.
References
- 1.Schlegel J, Diggdon P, Cuellar J. The use of ultrasound for localizing renal calculi. J Urol 1961;(86):367–369. [DOI] [PubMed] [Google Scholar]
- 2.Fulgham PF, Assimos DG, Pearle MS, et al. Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol 2013;189(4):1203–1213. [DOI] [PubMed] [Google Scholar]
- 3.Fwu CW, Eggers PW, Kimmel PL,et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int 2013. doi: 10.1038/ki.2012.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wisely Choosing. American College of Emergency PHysicians. http://www.choosingwisely.org/wp-content/uploads/2015/02/ACEP-Choosing-Wisely-List.pdf Published 2015. Accessed April 13, 2018.
- 5.Tzou DT, Usawachintachit M, Taguchi K, et al. Ultrasound Use in Urinary Stones: Adapting Old Technology for a Modern-Day Disease. J Endourol 2017. doi: 10.1089/end.2016.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler K a B, Locken JA, Duchesne JH, et al. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology. 2002;222(1):109–113. [DOI] [PubMed] [Google Scholar]
- 7.Ray AA, Ghiculete D, Pace KT, H et al. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010;76(2):295–300. [DOI] [PubMed] [Google Scholar]
- 8.Kanno T, Kubota M, Sakamoto H, et al. The efficacy of ultrasonography for the detection of renal stone. Urology. 2014;84(2):285–288. [DOI] [PubMed] [Google Scholar]
- 9.Sternberg KM, Eisner B, Larson T, H et al. Ultrasonography Significantly Overestimates Stone Size When Compared to Low-dose, Noncontrast Computed Tomography. Urology. 2016;95:67–71. [DOI] [PubMed] [Google Scholar]
- 10.Ganesan V, De S, Greene D, et al. Accuracy of ultrasonography for renal stone detection and size determination: is it good enough for management decisions? BJU Int 2017;119(3):464–469. [DOI] [PubMed] [Google Scholar]
- 11.Rahmouni A, Bargoin R, Herment A, et al. Color Doppler twinkling artifact in hyperechoic regions. Radiology. 1996. doi: 10.1148/radiology.199.1.8633158. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen MD, Harper JD, Hsi RS, et al. B-mode Ultrasound Versus Color Doppler Twinkling Artifact in Detecting Kidney Stones. J Endourol 2013;27(2):149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Gawad M, Kadasne RD, Elsobky E, et al. A Prospective Comparative Study of Color Doppler Ultrasound with Twinkling and Noncontrast Computerized Tomography for the Evaluation of Acute Renal Colic. J Urol 2016;196(3):757–762. [DOI] [PubMed] [Google Scholar]
- 14.Masch WR, Cohan RH, Ellis JH, et al. Clinical effectiveness of prospectively reported sonographic twinkling artifact for the diagnosis of renal calculus in patients without known urolithiasis. Am J Roentgenol 2016. doi: 10.2214/AJR.15.14998. [DOI] [PubMed] [Google Scholar]
- 15.Lu W, Sapozhnikov OA, Bailey MR, et al. Evidence for trapped surface bubbles as the cause for the twinkling artifact in ultrasound imaging. Ultrasound Med Biol 2013;39(6):1026–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon JC, Sapozhnikov OA, Kreider W, et al. The role of trapped bubbles in kidney stone detection with the color Doppler ultrasound twinkling artifact. Phys Med Biol 2018;63(2):25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabana W, Bude RO, Rubin JM. Comparison between color Doppler twinkling artifact and acoustic shadowing for renal calculus detection: an in vitro study. Ultrasound Med Biol 2009;35(2):339–350. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Kim SH, Cho JY, et al. Color and power doppler twinkling artifacts from urinary stones: Clinical observations and phantom studies. Am J Roentgenol 2001. doi: 10.2214/ajr.176.6.1761441. [DOI] [PubMed] [Google Scholar]
- 19.Cunitz BW, Harper JD, Sorensen MD, et al. Quantification of Renal Stone Contrast with Ultrasound in Human Subjects. J Endourol 2017. doi: 10.1089/end.2017.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chelfouh N, Grenier N, Higueret D, et al. Characterization of urinary calculi: In vitro study of “twinkling artifact” revealed by color-flow sonography. Am J Roentgenol 1998. doi: 10.2214/ajr.171.4.9762996. [DOI] [PubMed] [Google Scholar]
- 21.Shang M, Sun X, Liu Q, et al. Quantitative evaluation of the effects of urinary stone composition and size on color doppler twinkling artifact: A phantom study. J Ultrasound Med 2017. doi: 10.7863/ultra.16.01039. [DOI] [PubMed] [Google Scholar]
- 22.Kielar AZ, Shabana W, Vakili M, et al. Prospective evaluation of Doppler sonography to detect the twinkling artifact versus unenhanced computed tomography for identifying urinary tract calculi. J Ultrasound Med 2012;31(10):1619–1625. [DOI] [PubMed] [Google Scholar]
- 23.Sharma G, Sharma A. Clinical implications and applications of the twinkling sign in ureteral calculus: A preliminary study. J Urol 2013. doi: 10.1016/j.juro.2012.11.176. [DOI] [PubMed] [Google Scholar]
- 24.Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography vs Computed Tomography for Suspected Nephrolithiasis. N Engl J Med 2014;371(12):1100–1110. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg KM, Littenberg B. Trends in imaging use for the evaluation and follow-up of kidney stone disease: A single center experience. J Urol 2017;198(2):383–388. [DOI] [PubMed] [Google Scholar]
- 26.Dunmire B, Lee FC, Hsi RS, et al. Tools to Improve the Accuracy of Kidney Stone Sizing with Ultrasound. J Endourol 2015;29(2):147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunmire B, Harper JD, Cunitz BW, et al. Use of the Acoustic Shadow Width to Determine Kidney Stone Size with Ultrasound. J Urol 2016;195(1):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May P, Haider Y, Dunmire B, et al. Stone-Mode Ultrasound for Determining Renal Stone Size. J Endo 2016;30(9):958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai JC, Dunmire B, Sternberg KM, et al. Retrospective comparison of measured stone size and posterior acoustic shadow width in clinical ultrasound images. World J Urol December 2017. doi: 10.1007/s00345-017-2156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai JC, Dunmire B, Chen T, et al. Clinical Outcomes in Pediatric Patients with Ureteral Sontes Are Correlated with The Posterior Acoustic Shadow Measurement on Ultrasound: A Pilot Study. In: Societies for Pediatric Urology, Pediatric Urology Fall Congress ; 2018. [Google Scholar]
- 31.Dai J, Dunmire B, Liu Z, et al. Measurement of Posterior Acoustic Stone Shadow on Ultrasound is a Learnable Skill for Inexperienced Users to Improve Accuracy of Stone Sizing. In: Engineering and Urology Society, 33rd Annual Meeting 18 May ; 2018:33, Abstract #20. http://engineering-urology.org/am/33EUS_2018.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunitz B, Dunmire B, Paun M, et al. Improved detection of kidney stones using an optimized Doppler imaging sequence. In: IEEE International Ultrasonics Symposium, IUS ; 2014. doi: 10.1109/ULTSYM.2014.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tierney JE, Schlunk SG, Jones R, et al. In vitro feasibility of next generation non-linear beamforming ultrasound methods to characterize and size kidney stones. Urolithiasis. 2018. doi: 10.1007/s00240-018-1036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shukunami KI, Nishijima K, Miyazaki M, et al. Visualization of renal stone using 3-D ultrasound with surface rendering in a pregnant woman [1]. Eur J Obstet Gynecol Reprod Biol 2006. doi: 10.1016/j.ejogrb.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Volkmer BG, Nesslauer T, Kuefer R, et al. Visualization of urinary stones by 3-D ultrasound with surface rendering. Ultrasound Med Biol 2002. doi: 10.1016/S0301-5629(01)00493-8. [DOI] [PubMed] [Google Scholar]
- 36.Volkmer BG, Nesslauer T, Kuefer R, et al. Evaluation of disintegration in prevesical ureteral calculi by 3-dimensional endo-ultrasound with surface rendering. J Urol 2002. doi: 10.1016/S0022-5347(05)64656-3. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Chen Y, Liu C, et al. Construction of a three-dimensional model of renal stones: Comprehensive planning for percutaneous nephrolithotomy and assistance in surgery. World J Urol 2013. doi: 10.1007/s00345-012-0998-7. [DOI] [PubMed] [Google Scholar]
- 38.Atalay HA, Ülker V, Alkan İ, et al. Impact of Three-Dimensional Printed Pelvicaliceal System Models on Residents’ Understanding of Pelvicaliceal System Anatomy Before Percutaneous Nephrolithotripsy Surgery: A Pilot Study. J Endourol 2016. doi: 10.1089/end.2016.0307. [DOI] [PubMed] [Google Scholar]
- 39.Atalay HA, Canat HL, Ülker V, et al. Impact of personalized three-dimensional (3D) printed pelvicalyceal system models on patient information in percutaneous nephrolithotripsy surgery: A pilot study. Int Braz J Urol 2017. doi: 10.1590/S1677-5538.IBJU.2016.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghani KR, Pilcher J, Patel U, et al. Three-dimensional ultrasound reconstruction of the pelvicaliceal system: An in-vitro study. World J Urol 2008. doi: 10.1007/s00345-008-0276-x. [DOI] [PubMed] [Google Scholar]
- 41.Pace KT, Tariq N, Dyer SJ, et al. Mechanical percussion, inversion and diuresis for residual lower pole fragments after shock wave lithotripsy: A prospective, single blind, randomized controlled trial. J Urol 2001. doi: 10.1016/S0022-5347(05)65507-3. [DOI] [PubMed] [Google Scholar]
- 42.Liu LR, Li QJ, Wei Q, et al. Percussion, diuresis, and inversion therapy for the passage of lower pole kidney stones following shock wave lithotripsy. Cochrane Database Syst Rev 2013. doi: 10.1002/14651858.CD008569.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Long Q, Zhang J, Xu Z, et al. A Prospective Randomized Controlled Trial of the Efficacy of External Physical Vibration Lithecbole after Extracorporeal Shock Wave Lithotripsy for a Lower Pole Renal Stone Less Than 2 cm. J Urol 2016. doi: 10.1016/j.juro.2015.10.174. [DOI] [PubMed] [Google Scholar]
- 44.Wu W, Yang Z, Xu C, et al. External Physical Vibration Lithecbole Promotes the Clearance of Upper Urinary Stones after Retrograde Intrarenal Surgery: A Prospective, Multicenter, Randomized Controlled Trial. J Urol 2017. doi: 10.1016/j.juro.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, Cheng Y, Wu W, et al. Treatment of Distal Ureteral Calculi Using Extracorporeal Physical Vibrational Lithecbole Combined with Tamsulosin: A New Option to Speed Up Obstruction Relief. J Endourol 2018. doi: 10.1089/end.2017.0560. [DOI] [PubMed] [Google Scholar]
- 46.Shah A, Owen NR, Lu W, et al. Novel ultrasound method to reposition kidney stones. Urol Res 2010;38(6):491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harper JD, Sorensen MD, Cunitz BW, et al. Focused ultrasound to expel calculi from the kidney: Safety and efficacy of a clinical prototype device. J Urol 2013;190(3):1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harper JD, Dunmire B, Wang Y-N, et al. Preclinical Safety and Effectiveness Studies of Ultrasonic Propulsion of Kidney Stones. Urology. 2014;84(2):484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunitz BW, Dunmire B, Bailey MR. Characterizing the Acoustic Output of an Ultrasonic Propulsion Device for Urinary Stones. IEEE Trans Ultrason Ferroelectr Freq Control. 2017. doi: 10.1109/TUFFC.2017.2758647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah A, Harper JD, Cunitz BW, et al. Focused ultrasound to expel calculi from the kidney. J Urol 2012;187(2):739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Connors BA, Evan AP, Blomgren PM, et al. Comparison of tissue injury from focused ultrasonic propulsion of kidney stones versus extracorporeal shock wave lithotripsy. J Urol 2014;191(1):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YN, Simon JC, Cunitz BW, et al. Focused ultrasound to displace renal calculi: Threshold for tissue injury. J Ther Ultrasound. 2014;2(1). doi: 10.1186/2050-5736-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harper JD, Cunitz BW, Dunmire B, et al. First in Human Clinical Trial of Ultrasonic Propulsion of Kidney Stones. J Urol 2016;195(4):956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsi RS, Dunmire B, Cunitz BW, et al. Content and face validation of a curriculum for ultrasonic propulsion of calculi in a human renal model. J Endourol 2014;28(4):459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen KM, Brand TC, Cunitz BW, et al. Safety and Effectiveness of a Longer Focal Beam and Burst Duration in Ultrasonic Propulsion for Repositioning Urinary Stones and Fragments. J Endourol 2017;31(8):793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janssen KM, Brand TC, Bailey MR, et al. Effect of Stone Size and Composition on Ultrasonic Propulsion Ex Vivo. Urology. 2018;111:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harper J, Dai JC, Chang H, et al. Quantitative assessment of effectiveness of ultrasonic propulsion of kidney stones In: World Congress Endourology. ; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghanem MA, Maxwell AD’, Krieder W, et al. Field Characterization and Compensation of Vibrational Non-uniformity for a 256-element Focused Ultrasound Phased Array. IEEE Trans Ultrason Ferroelectr Freq Control. 2018. doi: 10.1109/TUFFC.2018.2851188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Démoré CEM, Dahl PM, Yang Z, et al. Acoustic tractor beam. Phys Rev Lett 2014. doi: 10.1103/PhysRevLett.112.174302. [DOI] [PubMed] [Google Scholar]
- 60.Lingéman JE, McAteer JA, Gnessin E, et al. Shock wave lithotripsy: Advances in technology and technique. Nat Rev Urol 2009. doi: 10.1038/nrurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhojani N, Lingeman JE. Shockwave Lithotripsy-New Concepts and Optimizing Treatment Parameters. Urol Clin North Am 2013. doi: 10.1016/j.ucl.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Vicentini F In the era of flexible ureteroscopy is there still a place for Shock-wave lithotripsy? Int Braz J Urol 2015;41(2):203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maxwell AD, Cunitz BW, Kreider W, et al. Fragmentation of Urinary Calculi In Vitro by Burst Wave Lithotripsy. J Urol 2015;193(1):338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y-N, Krieder W, Hunter C, et al. Burst Wave Lithotripsy: An in vivo demonstration of efficacy and acute safety using a porcine model. In: 176th Meeting of the Acoustical Society of America ; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.May PC, Kreider W, Maxwell AD, et al. Detection and Evaluation of Renal Injury in Burst Wave Lithotripsy Using Ultrasound and Magnetic Resonance Imaging. J Endourol 2017. doi: 10.1089/end.2017.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matlaga BR, McAteer JA, Connors BA, et al. Potential for Cavitation-Mediated Tissue Damage in Shockwave Lithotripsy. J Endourol 2008. doi: 10.1089/end.2007.9852. [DOI] [PubMed] [Google Scholar]
- 67.Sorensen MD, Wang Y-N, Kreider W, et al. Preclinical safety and effectiveness of burst wave lithotripsy In: World Congress Endourology. ; 2018. [Google Scholar]
- 68.Zwaschka TA, Ahn JS, Cunitz BW, et al. Combined Burst Wave Lithotripsy and Ultrasonic Propulsion for Improved Urinary Stone Fragmentation. J Endourol 2018. doi: 10.1089/end.2017.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]


