With the high rate of cardiovascular disease and cardiovascular co-morbidities, the burden on the healthcare system in the USA has reached $329.7 billion dollars per year1. As developing countries become more industrialized, they also become susceptible to entering a phase in which cardiovascular disease (a non-communicable disease) begins to outrank communicable diseases such as malaria, tuberculosis and HIV/AIDS. However, the rate of novel drug discovery dropped 33% between the years 2000 – 2009 as compared to the prior decade while, the price of bringing a new drug to market cost over $50 billion in the USA in 20082. Consequently, great effort has been made to identify new therapeutics yet, few therapeutics have been found due to the limitations of current techniques.
Fortunately, cardiomyocytes are one of the most readily produced and distinguishable cell types that can be differentiated from pluripotent stem cells (PSC)3. Human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs), while immature, have the machinery at hand to express all the electrophysiological ion channels found in an adult human cardiomyocyte, along with their critical sarcomere structures. These repeating sarcomeres, along with the sarcolemma-binding costamere structures, provide cardiomyocytes with their mechanotransductive properties. Due to the indispensable nature of these protein complexes, mutations affecting sarcomere and costamere protein-coding genes lead to severe and often lethal diseases. Additionally, gene-editing technologies such as CRISPR/Cas9 have progressed alongside improved hPSC culture and patient-derived somatic cell reprogramming methods to enable controlled in vitro studies of these diseases. With these combined tools researchers can now identify specific drugs that may aid these patients in a precision medicine approach. This new approach to human cardiac disease modeling has now reached a point where drugs can be screened for off-target cardiac toxicity, pro-arrhythmic events, and patient-specific cardiac diseases.
As advances in automation, high-content imaging, gene-editing, stem cell technologies, and sequencing enable researchers to model diseases at increasingly large scales, there are still several challenges that prevent researchers from fully recapitulating disease phenotypes in vitro. Specifically, hPSC-cardiomyocytes continue to exhibit fetal-like phenotypes in the transcriptional, protein, and functional space4. The sarcomere structures that form in hPSC-CMs exhibit a misaligned, often rounded morphology, unlike the uniformly-oriented, consistently-spaced structures that are observed in primary tissues5. Several approaches have been implemented to improve this sarcomere structure, to push the hPSC-CMs toward a more mature-like state. Thus, there is a high demand for methods to quantify sarcomere structure in a dynamic culture, as they would allow researchers to screen for both hPSC-CM disease phenotypes, treatment corrections, as well as maturation factors for protocol optimization.
Currently in the field, it has been a challenge to obtain dynamic assessment of contraction parameters in beating hPSC-CMs. The current gold standard in determining adult cardiomyocyte contractile properties is the IonOptix system6. In this system, a region of interest is drawn over a horizontally-aligned myocyte, from which a Fast Fourier Transform (FFT) is derived to achieve sarcomere spacing. However, this system is best utilized with single cell adult cardiomyocytes, as a clear contractile axis is required for the imaging software. Since adult cardiomyocytes are not available from humans and current techniques to mature hPSC-CMs are not yet able to achieve full adult-like structure, other single cell assays have been developed to quantify sarcomere structure. Higher throughput means of determining relative displacement of monolayers of cardiomyocytes includes correlation-based correction quantification (CCQ)7 and FFT to directly monitor changes in sarcomere length8. There are additional methods that more directly quantify the forces applied by cardiomyocytes on their surroundings, such as micro-post platforms9 and traction force microscopy10. Both techniques examine contractile function by measuring the deformations of materials that hPSC-CMs are seeded on, rather than observing the sarcomeres themselves.
In this issue of Circulation Research, Toepfer et al. generated a new computational pipeline, coined SarcTrack, which uses wavelet transforms to non-invasively determine the contractile properties of hPSC-CMs in a robust manner11. What is exciting about the SarcTrack software is it can robustly identify multiple sarcomere units at varying directionalities and distances to asses many sarcomeres in a given field of view from multiple cells. As a result, the resolution is much greater than other techniques as it is at the individual sarcomere level.
Like FFTs, wavelet transforms are often used in image processing for de-noising, compression, and object detection. These transforms are similar in that they both convert a signal or image into the frequency domain, allowing a researcher to quickly quantify any repeating pattern that is present in a signal (such as a specific audio pitch), or a repeating pattern that is present in an image (such as a striated myofibril). The advantage of wavelets is that they provide both the frequency of a repeating pattern within an image, along with the location of that repeating structure. In contrast, FFT only contains frequency information, and thus is more effective if the entire analyzed region contains a uniform, unidirectional repeating structure.
To use SarcTrack, a user provides a discrete set of distances and angles that could define the relative distances of any two neighboring Z-disks/M-bands. The algorithm then generates pairs of standard Mortlet mother wavelets, and transforms these pairs based on the possible list of distances and angles provided. This creates a bank of small images that resemble “ideal sarcomeres” under a variety of distances and rotations. The algorithm then convolutes these wavelet pairs frame-by-frame over a video of contracting cells. The convolution algorithm finds regions within the image that closely resemble any of the defined wavelet pairs, labeling that region as a sarcomere of known rotation and distance. These wavelet pairs allow quantification of distances and angles between Z-lines and M-lines at the single sarcomere scale, with no requirement that myofibrils be unidirectional and have uniform sarcomere lengths.
One of the interesting features of this analysis technique is the ability to track various alignments of sarcomeres in hPSC-CMs. Since global tracking of various sarcomeres in a cell can be performed, a diseased cell or a drug that results in malformation/disassembly of sarcomeres, will manifest as a large distribution in analyzed values. Immature hPSC-CMs have poor arrangement of myofibrils. While many of the dominant myofibrils in an hPSC-CM will probably be parallel to the long axis of the cardiomyocyte, there will be many other myofibrils randomly distributed. Consequently, these other myofibrils with random orientations to the long axis or the axis of contraction will most likely have different contractile properties compared to the main myofibrils in the cell. As a result, there will be a great deal of variation found within the assessed sarcomere analysis. It is impressive that we now have tools that have the resolution to measure so many myofibrils in the cell. It would be exciting to combine this imaging technology with current cell patterning strategies to generate well aligned myocytes and/or other maturation techniques to obtain mature and aligned sarcomeres for analysis7. This will help to reduce the amount of noise generated in the data and be well suited to disease modeling and drug discovery studies.
The current software tool is restricted to contractile measurements. However, this imaging modality could be coupled with other engineered cell lines that express calcium or voltage sensitive fluorophores. This multiplexing feature would broaden the field of view for the screening potential performed as both contractile and electrophysiological properties could be assessed at once. The requirement of user-defined wavelet kernels spanning a discrete set of distances and angles does allow for misaligned sarcomere detection, however at a significant cost of computational power. Additionally, this forces the analyst to balance between detection resolution and speed of analysis. A first-pass script to automatically determine sarcomere directionality and distance, even coarsely, may help to address this challenge, as well as reduce the likelihood of user error when handling the script.
Finally, there has been a great deal of effort to find novel ways to mature hPSC-CMs to a more adult like state. Tissue engineering is a prominent technique used to recapitulate the three-dimensional structure of the native myocardium and to introduce multiple cell types that would help promote a more mature cardiomyocyte12. To assess force of contraction in bulk tissues, the constructs are tethered to deflectable posts that can be used to determine force of contraction of the whole tissue13, 14. While single cell force of contraction and sarcomere dynamics can not be assessed in this paradigm, bulk tissue assessment to drugs or maturation cues can be achieved.
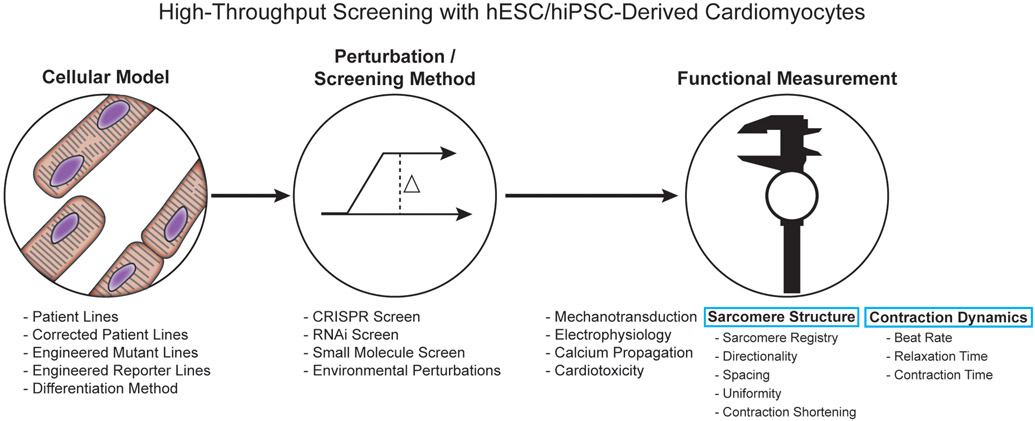
In order to capitalize upon hPSC-CMs, complementary technology needs to be generated to assess the functional state of the hPSC-CMs during screens: genetic, small molecule and pharmacological (Figure 1). These technologies need to be non-invasive, non-destructive, provide a robust assessment of cardiac function and be easily scalable to efficiently assess many cells and merge into the pharmaceutical pipeline. Developing tools such as SarcTrack helps to push the utility of hPSC-CMs as a model to study and find novel interventions of cardiac disease. Further development of such tools and multiplexing tools to assess multiple concurrent cardiomyocyte functional outputs will help generate robust in vitro platforms for rapid, reliable and cost-effective drug discovery.
Components to consider when performing a high-throughput screen on human pluripotent stem cell-derived cardiomyocytes.
Modern techniques allow for a plethora of different parameters to consider when setting out to perform a high throughput screen including: cellular model, type of screen and endpoint functional measurements. Choosing the optimal conditions will depend on the disease/biological perturbation being studied which, will dictate the desired functional measurement to record and whether the cell line will require modifications to accommodate the functional measurement.
Acknowledgments
Sources of funding
Dr. Kim reports grant support from the National Institutes of Health (R01HL135143 and UG3EB028094) and the American Heart Association.
Footnotes
Disclosures
Dr. Kim is named as coinventor on pending and issued patents held by the University of Washington relating to functional assays for drug-induced cardiotoxicity screening and cardiac disease modeling. Dr. Kim is a co-founder and scientific advisor of NanoSurface Biomedical Inc. Both Dr. Miklas and Dr. Salick report no conflicts.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, et al. American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Fordyce CB, Roe MT, Ahmad T, Libby P, Borer JS, Hiatt WR, Bristow MR, Packer M, Wasserman SM, Braunstein N, Pitt B, DeMets DL, Cooper-Arnold K, Armstrong PW, Berkowitz SD, Scott R, Prats J, Galis ZS, Stockbridge N, Peterson ED and Califf RM. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol. 2015;65:1567–82. [DOI] [PubMed] [Google Scholar]
- 3.Burridge PW, Keller G, Gold JD and Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mummery CL. Perspectives on the Use of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Biomedical Research. Stem cell reports. 2018;11:1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasqualini FS, Sheehy SP, Agarwal A, Aratyn-Schaus Y and Parker KK. Structural phenotyping of stem cell-derived cardiomyocytes. Stem cell reports. 2015;4:340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorski PA, Kho C and Oh JG. Measuring Cardiomyocyte Contractility and Calcium Handling In Vitro. Methods in molecular biology. 2018;1816:93–104. [DOI] [PubMed] [Google Scholar]
- 7.Macadangdang J, Miklas JW, Smith AST, Choi E, Leung W, Wang Y, Guan X, Lee S, Salick MR, Regnier M, Mack D, Childers MK, Ruohola-Baker H and Kim D-H. Engineered developmental niche enables predictive phenotypic screening in human dystrophic cardiomyopathy. bioRxiv. 2018:456301. [Google Scholar]
- 8.Pasqualin C, Gannier F, Yu A, Malecot CO, Bredeloux P and Maupoil V. SarcOptiM for ImageJ: high-frequency online sarcomere length computing on stimulated cardiomyocytes. Am J Physiol Cell Physiol. 2016;311:C277–83. [DOI] [PubMed] [Google Scholar]
- 9.Beussman KM, Rodriguez ML, Leonard A, Taparia N, Thompson CR and Sniadecki NJ. Micropost arrays for measuring stem cell-derived cardiomyocyte contractility. Methods. 2016;94:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kijlstra JD, Hu D, Mittal N, Kausel E, van der Meer P, Garakani A and Domian IJ. Integrated Analysis of Contractile Kinetics, Force Generation, and Electrical Activity in Single Human Stem Cell-Derived Cardiomyocytes. Stem cell reports. 2015;5:1226–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toepfer CN, Sharma A, Cicconet M, Garfinkel AC, Mucke M, Neyazi M, Willcox JA, Agarwal R, Schmid M, Rao J, Ewoldt JK, Pourquie O, Chopra A, Chen C, Seidman JG and Seidman CE. SarcTrack: An Adaptable Software Tool for Efficient Large-Scale Analysis of Sarcomere Function in hiPSC-Cardiomyocytes. Circ Res. 2019. [Google Scholar]
- 12.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M and Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB and Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue engineering Part A. 2012;18:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, Pahnke A, Protze S, Lee JH, Davenport Huyer L, Jekic D, et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell. 2019;176:913–927 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]