Abstract
Background:
Pulmonary hypertension (PH) often presents with non-specific symptoms making early diagnosis difficult. Red cell distribution width (RDW) is a parameter routinely reported on an automated complete blood cell count that has been associated with numerous disease states. The purpose of this study was to further evaluate RDW as a biomarker for PH in at-risk populations.
Methods:
In a retrospective, cross-sectional analysis of patients seen at a PH center over 1 year, we examined both patients with PH and patients at risk for but without PH (e.g. systemic sclerosis, [SSc]). We also studied a group of age-and sex-matched, non-diseased controls. Relevant characteristics were compared among the 3 groups using one-way ANOVA. Similar comparisons were made across World Health Organization (WHO) PH groups 1–4.
Results:
RDW was highest in the PH patients (n=181), intermediate in the at-risk for PH patients (n=52), and lowest in matched controls (n=100) (15.9±2.8 vs 14.8±2.8 vs 14.2±1.1%, respectively; p<0.0001). There were no significant differences in RDW across WHO PH groups (p=0.50). SSc patients with PH had significantly higher RDW values compared to SSc patients without PH (16.0±2.2 vs 14.4±1.9%, respectively; p=0.03).
Conclusions:
RDW is significantly higher in PH patients, without regard to disease etiology, when compared to age- and sex-matched non-diseased controls. Importantly, RDW is also higher in PH patients compared to at-risk patients, particularly in the SSc cohort. The ease of obtaining RDW as a biomarker may help detect incident PH at earlier stages among patients who are at high risk for development of PH.
Keywords: Biomarker, high risk pulmonary hypertension populations, pulmonary arterial hypertension, red cell distribution width, systemic sclerosis
Summary at a Glance:
This study investigates the role of RDW as a biomarker for pulmonary hypertension in at risk populations. The use of an easily obtainable biomarker for detecting may help to detect pulmonary hypertension in patients in early stages allowing for earlier treatment, better outcomes and better quality of life.
Introduction
Pulmonary hypertension (PH) is a progressive and potentially mortal disease that often presents with non-specific symptoms leading to delayed diagnosis.1,2 Prompt diagnosis through the identification of risk factors and novel biomarkers holds the promise of earlier treatment and therefore a better prognosis. As PH symptoms such as dyspnea on exertion, shortness of breath, and fatigue are non-specific, early diagnosis can be difficult.2 The non-specific symptoms and strong benefit of early diagnosis and treatment underline the importance of risk factor identification and biomarkers that are widely available and discriminative for patients at risk for developing PH.2
Red cell distribution width (RDW), a measure of the variation in red blood cell size, is a routinely reported parameter on an automated complete blood cell count (CBC).3 Traditionally, RDW has been used to differentiate causes of anemia, but recent research has shown that elevations in RDW are associated with many disorders4 including heart failure5, chronic obstructive pulmonary disease6,7 and cancer.8 Additional studies have correlated elevated RDW with the risk of mortality in PH9, and with the risk for development of chronic thromboembolic PH (CTEPH) after an acute PE10. More recently, a study by Zhao et. al suggested that RDW may identify PH in patients with systemic sclerosis (SSc).11
The purpose of this study was to further examine the potential use of RDW as a biomarker for pulmonary hypertension. Specifically, we predicted that 1) RDW would be higher in patients with PH as compared to age- and sex-matched controls, 2) there would be a significant difference in RDW amongst different PH group classifications and 3) RDW would be higher in PH patients compared to those at high risk for developing PH, particularly in the SSc cohort.
Methods
Patient Selection
Before beginning this study, approval was obtained from the LSU Institutional Review Board (LSUHSC IRB #9053). This study was a retrospective cross-sectional analysis of patients seen at a single PH Center from August 2014 to June 2015. Eligible patients were required to have at least one automated CBC in the electronic medical record (EMR). Pulmonary hypertension was defined as a mean pulmonary artery pressure mPAP≥25mmHg at rest on right heart catheterization. At-risk patients were defined as patients seen in our clinic without pulmonary hypertension but with one of the following conditions: SSc, mixed connective tissue disease (MCTD), systemic lupus erythematosus (SLE), or interstitial lung disease (ILD, e.g. idiopathic pulmonary fibrosis). Patients seen in a General Internal Medicine clinic who had an automated CBC but did not have either PH or conditions known to elevate RDW (i.e. malignancy12, anemia and hematological disorders13, history of blood transfusions14, heart failure15, or renal dysfunction16) were chosen as controls, age- and sex-matched to the PH patients.
Data Collection and Statistical Analysis
A database of clinical variables from the EMR was created using REDCap software. World Health Organization (WHO) functional class and PH group were obtained from physician notes (i.e. Group 1 -Pulmonary arterial hypertension, Group 2 - Pulmonary hypertension due to left heart disease, Group 3 -Pulmonary hypertension as a result of chronic lung disease/hypoxia, Group 4 - Chronic thromboembolic pulmonary hypertension (CTEPH), and Group 5 - Unclear multifactorial causes of pulmonary hypertension.17 RDW was collected from the first available CBC in the medical record during the time period of the study. RDW is calculated by taking the standard deviation of the red blood cell volume divided by the mean corpuscular volume then multiplied by 100, and is expressed as a percentage.
To assess for generalizability, baseline characteristics were compared between patients seen in the PH Center who had a CBC recorded in the medical record and those who did not have a CBC. Baseline characteristics, including RDW, were compared among 3 groups (PH, at-risk for PH, and controls) using either one-way ANOVA with a Tukey’s post-test or Chi square as appropriate. Similar comparisons were made across PH Groups 1–4. Patients classified as Group 5 were excluded from the analyses because the sample size was too small and the clinical heterogeneity of this subgroup too great to be meaningful. Multivariate analysis of RDW was conducted after adjusting for baseline differences between groups, including BMI, race, and smoking status; use of variance inflation factor analysis did not demonstrate co-linearity between these variables. Sensitivity and specificity were calculated and a receiver operator characteristics (ROC) curve was generated with area under the curve (AUC) analysis. The Youden index ([sensitivity + specificity]-1) was calculated to find the cutoff with the highest combination of sensitivity and specificity. For internal validation and to derive optimism-corrected estimates of AUC, bootstrapping with 1000 replacements was performed. Correlation analysis was conducted between RDW and markers of PH severity (WHO functional class, hemodynamics, and six minute walk test) using Pearson’s test. Patients who had systemic sclerosis, either with and without PH, were pre-defined as a primary subgroup of interest. All analyses were performed using STATA (version 13, College Station, TX) and Graph Pad Prism (version 5, La Jolla, CA), and a p value <0.05 was considered to be statistically significant.
Results
Patient Selection and Baseline Characteristics
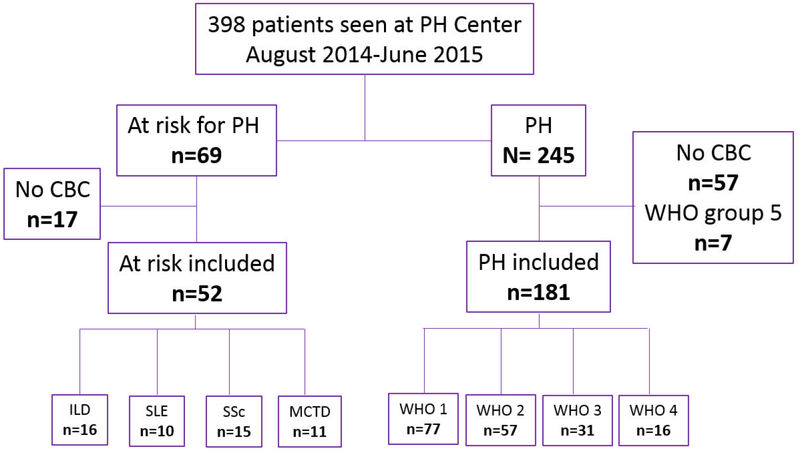
Three hundred ninety-eight separate patients were seen at our PH Center over a one-year period (Figure 1). Of these, 245 PH patients were screened and 64 were excluded (57 without a CBC in the EMR, 7 with WHO Group 5 PH etiology), leading to a total of 181 patients with pulmonary hypertension being included in the study. PH patients with CBCs were significantly younger than PH patients without CBCs (56±14 vs 62±15 years, respectively, p=0.008), but there were no differences in other demographic or hemodynamic parameters (Supplemental Table 1). The median time between the CBC used in the analysis and the defining RHC was 244 days (interquartile range 29, 945). These PH patients were further subdivided into WHO classification groups as follows: Group 1, n=77; Group 2, n=57; Group 3, n=31; and Group 4, n=16. PH patients were treated with prostanoids (n=21), endothelin receptor antagonists (n=58), phosphodiesterase-5 inhibitors (n=61), and soluble guanylate cyclase stimulators (n=10). Thirty-eight patients were on dual therapy and 18 patients were on triple therapy. Sixty-nine patients at risk for PH were also screened and 52 of these were included in the study after exclusion of 17 due to lack of a CBC. There were no significant differences in characteristics of at risk patients who had and did not have a CBC (data not shown).
Figure 1:
Patient selection for the analysis. One hundred non-diseased controls were age- and sex-matched to the PH patients. PH=pulmonary hypertension; CBC=complete blood count; ILD=interstitial lung disease; SLE=systemic lupus erythematosus; SSc=systemic sclerosis; MCTD=mixed connective tissue disease; WHO=World Health Organization PH classification
At-risk patients were significantly younger than PH patients or controls (p=0.046, Table 1). Compared to at-risk or PH patients, controls were more likely to be African-American (p<0.0001), more like to be current smokers (p<0.0001), and more likely to have a lower BMI (p=0.04). PH patients had the expected differences in hemodynamics on RHC compared to at-risk patients; specifically they had higher right atrial pressure, mPAP, pulmonary artery wedge pressure, and pulmonary vascular resistance. BNP values were also higher in the PH patients (123 [34, 356] vs 41 [20, 79] median and interquartile range, p<0.0001).
Table 1:
Baseline characteristics in PH patients, at risk for PH patients, and non-diseased age and sex matched controls
| Variable | PH (n=181) | At Risk (n=52) | Controls (n=100) | p-value | PH vs. At risk | PH vs. Controls | At Risk vs. Controls |
|---|---|---|---|---|---|---|---|
| Age (years) | 56±14 | 52±14 | 57±11 | 0.046 | NS | NS | NS |
| Sex (% female) | 75% | 79% | 63% | 0.06 | -- | -- | -- |
| Other | 7% | 8% | 0% | ||||
| Never smoker | 59% | 72% | 45% | ||||
| BMI (kg/m2) | 33±12 | 31±9 | 30±7 | 0.04 | NS | <0.05 | NS |
| BNP (pg/mL) median [IQR] | 123 [34, 356] | 41 [20, 79] | N/A | -- | <0.0001 | -- | -- |
| Hemoglobin (g/dL) | 12.9±2.0 | 13.3±1.4 | 12.9±1.5 | 0.13 | -- | -- | -- |
| RDW (%) | 15.9±2.8 | 14.8±2.8 | 14.2±1.1 | <0.0001 | <0.01 | <0.001 | NS |
| RA pressure (mmHg) | 10±6 | 4±3 | N/A | -- | 0.0002 | -- | -- |
| mPAP (mmHg) | 41±16 | 17±3 | N/A | -- | <0.0001 | -- | -- |
| PAWP (mmHg) | 16±9 | 9±3 | N/A | -- | 0.008 | -- | -- |
| CO (L/min) | 5.4±2.0 | 5.1±0.9 | N/A | -- | 0.70 | -- | -- |
| PVR (Wood units) | 5.1±4.4 | 1.7±0.8 | N/A | -- | 0.01 | -- | -- |
BMI=body mass index; BNP=B-type natriuretic peptide; RA=right atrial; mPAP=mean pulmonary artery pressure; PAWP=pulmonary artery wedge pressure; CO=cardiac output; PVR=pulmonary vascular resistance; NS=not significant; N/A=not applicable
RDW
RDW values were significantly different between PH patients and controls, as well as between PH patients and at-risk patients (15.9±2.8 vs 14.8±2.8 vs 14.2±1.1% for PH, at-risk, and controls, respectively, p<0.0001; Figure 2A). Differences in RDW between PH patients and controls persisted after multivariate adjustment for race, smoking status, and BMI (p<0.0001). There were no significant differences in RDW values when comparing across WHO Classification of Groups 1–4 (p=0.50, Figure 2B). SSc patients with PH (n=21) had significantly higher RDW values compared to SSc patients without PH (n=15) (16.0±2.2 vs 14.4±1.9% respectively, p=0.03; Figure 3A). The ROC AUC was 0.76 (95% CI 0.55 to 0.97, p=0.02, Figure 3B) to differentiate SSc patients into those with and without PH by using RDW. The optimal cut-point determined by the Youden index was 13.25%, with a 100% sensitivity and 40% specificity to discriminate SSc patients with and without PH. Bootstrapping for internal validation revealed a similar AUC for RDW in SSc patients of 0.73 (95% CI 0.53 to 0.93). The AUC for BNP to discriminate SSc patients with and without PH was 0.80 (95% CI 0.61 to 0.98, p value for comparison to RDW AUC=0.52). Combining BNP and RDW led to an increase in the ROC AUC to 0.82 (95% CI 0.61 to 1.00).
Figure 2:
(A) Red cell distribution width (RDW) values for the pulmonary hypertension (PH) patients (n=181), at-risk for PH patients (n=52), and non-disease matched controls (n=100). (B) RDW values for the PH patients broken down by WHO group classification (group 1=77, group 2=57, group 3=31, group 4=16). Displayed as mean ± SD.
Figure 3:
(A) Red cell distribution width (RDW) values for systemic sclerosis (SSc) patients with (n=21) and without pulmonary hypertension (PH, n=15). Displayed as mean ± SD. (B) ROC curve analysis for RDW to discriminate SSc patients with and without PH. The highest combination of sensitivity and specificity occurred as a cut-off of 13.25.
Other CBC parameters are listed in Supplemental Table 2.
Correlations between RDW and prognostic factors for the pulmonary hypertension patients:
In the PH cohort, there was no significant difference in RDW between patients who had a functional class of 1 or 2 (15.6±2.7%, n=81) and those who had a functional class of 3 or 4 (16.4±3.0%, n=88, p=0.10). There were significant, but weak, correlations between RDW and 6-minute walk distance (r= −0.34, p=0.0004) and between RDW and log BNP (r=0.32, p<0.0001). No significant correlations existed between RDW and mPAP, C-reactive protein (CRP), or diffusion capacity for carbon monoxide (DLCO).
Discussion:
In this cross-sectional study we have demonstrated that RDW is significantly higher in PH patients compared to age- and sex-matched, non-diseased controls. We discovered that RDW is higher in PH patients compared to a diverse group of patients without PH but who are at high risk for disease development. While the ability to differentiate healthy controls from PH patients using RDW is of relatively low clinical value, the fact that a simple, commonly available CBC parameter may be helpful to identify which patients among those with a risk factor for PH (e.g. SSc, MCTD, SLE, ILD) that actually have this devastating complication may be quite clinically relevant. Our study also demonstrates that RDW is elevated in PH across the WHO Groups, without regard to the etiology of pulmonary hypertension.
Red cell distribution width is a routinely reported CBC parameter that measures variation in red blood cell size (i.e. anisocytosis). Elevations in RDW indicate that red blood cells vary in size which may be due to increased red blood cell destruction, ineffective red blood cell production (e.g. B12 deficiency), or blood transfusion5. Numerous disease states such as cancer12, heart failure18, atherosclerosis19, and chronic kidney disease20 have been shown to be associated with increased RDW. However, the mechanisms of RDW elevation in these diseases have not been established.
Some suggest that inflammation, a key factor in many chronic diseases, may affect erythropoiesis ultimately leading to increased RDW. A study by Lippi et. al showed a strong graded association between high sensitivity CRP, erythrocyte sedimentation rate, and RDW in a cohort of unselected adult outpatients21. However, we did not find a significant correlation between RDW and CRP in our patient cohort, perhaps because of the relatively small sample size for this comparison and the time lags between RDW and CRP measurement. Another possible explanation for increased RDW is that oxidative stress negatively impacts red cell survival thus increasing RDW. One study showed that superoxide dismutase (SOD-1) knockout mice had decreased red blood cell survival, which was then increased following antioxidant administration22. Finally, RDW elevations may be caused by endothelial dysfunction and higher RDW was associated with decreased flow mediated dilation23. It is likely that the pathophysiology of RDW elevation in PH is multifactorial since the pathogenesis of PH is related to inflammation, oxidative stress, and endothelial dysfunction, which adds plausibility of RDW as a biomarker of PH.
Approximately 8–12% of patients with SSc will develop PH24–25 and it is one of the leading causes of death in this condition26. Despite extensive efforts at the early identification of PH in SSc patients, this population continues to have PH detected at an advanced stage27. An earlier study by Farkas et al reported that RDW was higher in patients with SSc, particularly those with diffuse skin disease and those with anti-topoisomerase antibodies28. Although these investigators found an association between high RDW and SSc, the low number of patients with PH limited their conclusions regarding this manifestation. The findings of our study corroborate those of Zhao et al, who demonstrated that RDW was elevated in patients with SSc-PAH.11 They reported that the best RDW cut off point was 14.3% to discriminate SSc patients without PH from SSc-PAH patients, with a sensitivity of 78.6% and specificity of 69.9%. Using this cutoff in our population, we found a 71.4% sensitivity and 60% specificity. Our study found an optimal cutoff of 13.25% to maximize sensitivity and specificity. As this would ideally be used as a screening test for SSc-PH, this high degree of sensitivity would be useful to identify patients who may benefit from further testing.
Our study has limitations that must be acknowledged. Due to its retrospective nature, it was not possible to standardize at what point testing was performed in the natural history of disease. We did, however, show that age was the only parameter that was significantly different between PH patients who did and did not have an RDW in the medical record. Therefore, we believe that our results should be generalizable. Although the control patients were aged-matched to the PH group, the at-risk for PH group appeared to be younger, although this was not statistically significant. We did encounter some missing variables due to the retrospective design, but all patients included had an RDW measured and were characterized in detail. Although our overall sample size was relatively large for a rare disease like PH, our pre-defined subgroup analyses were somewhat limited by small numbers of patients in each group, particularly WHO Groups 3 and 4 PH. Lastly, this was a single center study which limits external validity and therefore requires confirmation in a different patient cohort.
In conclusion, the results of our study seem to indicate that RDW is elevated in patients with PH, but was independent of WHO Group classification. Our study also further supports the evidence that RDW is elevated in patients with SSc and PH as compared to patients with SSc without PH. We speculate that the ease of obtaining this biomarker in SSc patients may help to detect PH at earlier stages, leading to better outcomes and better quality of life. The validity of using RDW in a screening strategy to diagnose SSc-PH earlier should now be validated in a large prospective study. Since we demonstrated that RDW was elevated in our group of PH patients compared to patients at high risk for PH (rather than just healthy controls), this RDW-based screening strategy should also be studied in other at-risk populations (MCTD, ILD, HIV, etc), with larger samples sizes.
Supplementary Material
Highlights.
Red cell distribution width (RDW), a routinely reported parameter on complete blood count, is higher in patients with pulmonary hypertension (PH) compared to those with a high-risk disease or healthy controls.
There is no difference in RDW values among the different etiologies of PH.
RDW can accurately discriminate systemic sclerosis patients with and without PH.
Acknowledgements:
Matthew R. Lammi is the guarantor of the content of the manuscript and takes responsibility for data and analysis.
Funding Information: This work was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health (COBRE Pilot Program grant number 1P306M106392–01A1 to [MRL] and 1 U54 GM104940, which funds the Louisiana Clinical and Translational Science Center to [MRL]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements: No conflicts of interests exist for any above authors.
Notification of prior Presentation: Part of this research was presented as a poster at the 2016 American Thoracic Society International Conference (San Francisco, CA).
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
☐ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References:
- 1.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Langleben D, Manes A, Satoh T, Torres F, wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. Journal of the American College of Cardiology. 2013; 62(25):D42–D50. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Rubin LJ, Hoeper MM, Jansa P, Al-Hiti H, Meyer G, Chiossi E, Kusic-Pajic A, Simmonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomized controlled trial. The Lancet. 2008; 371(9630): 2093–2100. [DOI] [PubMed] [Google Scholar]
- 3.Sarma PR. Red Cell Indices In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990:720–723. [PubMed] [Google Scholar]
- 4.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Critical Reviews in Clinical Laboratory Sciences. 2014; 52(2): 86–105. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, Swedberg, Wang D, Yusuf S, Michelson EL, Granger CB, CHARM investigators. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM program and the duke databank. Journal of the American College of Cardiology. 2007; 50(1): 40–47. [DOI] [PubMed] [Google Scholar]
- 6.Ozgul G, Seyhan EC, Ozgul MA, Gunluoglu MZ. Red blood cell distribution width in patients with chronic obstructive pulmonary disease and healthy subjects. Archivos de bronconeumologia 2017; 53(3): 107–113. [DOI] [PubMed] [Google Scholar]
- 7.Sincer I, Zorlu A, Yilmaz MB, Dogan OT, Ege MR, Amioglu, Aydin G, Ardic I, Tandogan. Relationship between red cell distribution width and right ventricular dysfunction in patients with chronic obstructive pulmonary disease. Heart & Lung. 2012; 41(3):238–243. [DOI] [PubMed] [Google Scholar]
- 8.Montagnana M, Danese E. Red cell distribution width and cancer. Annals of Translational Medicine. 2016; 4(20): 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. 2009; 104(6): 868–872. [DOI] [PubMed] [Google Scholar]
- 10.Abul Y, Ozsu S, Korkmaz A, Bulbul Y, Orem A, Ozlu T. Red cell distribution width: A new predictor for chronic thromboembolic pulmonary hypertension after pulmonary embolism. Chronic Respiratory Disease. 2014; 11(2):73–81. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Mo H, Guo X, Wang Q, Xu D, Hou Y, Tian Z, Liu Y, Wang H, Lai J, Li M, Zeng X. Red blood cell distribution width as a related factor of pulmonary arterial hypertension in patients with systemic sclerosis. Clinical Rheumatology. 2017; 37(4): 979–985. [DOI] [PubMed] [Google Scholar]
- 12.Hu L, Li M, Ding Y, Pu L, Liu J, Xie J, Cabanero M, Li J, Xiang R, Xiong S. Prognostic value of RDW in cancers: as systematic review and meta-analysis. Oncotarget. 2017; 8(9): 16027–16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park K, Kim KY. Clinical Evaluation of Red Cell Volume Distribution Width. Yonsei Medical Journal. 1987; 28(4): 282–290. [DOI] [PubMed] [Google Scholar]
- 14.Agapian JV, Camelo M, Love B, Kriger D, Lee H, Ludi D, Hendra W, Tabuenca A. Effects of allogenic blood transfusion on red cell distribution width (RDW) as a predictor of mortality and massive blood transfusion in trauma patients. Journal of American College of Surgeons. 2017; 225(4): 174–175. [Google Scholar]
- 15.Huang YL, Hu ZD, Liu SJ. Prognostic value of red blood cell distribution width for patients with heart failure: a systematic review and meta-analysis of cohort studies. PLoS ONE. 2014; 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relationship between red blood cell distribution width and kidney function tests in a large cohot of unselected outpatients. Scandanavian Journal of Clinical Laboratory Investigation. 2008; 68(8): 745–748. [DOI] [PubMed] [Google Scholar]
- 17.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez-Sanchez MA, Krishna K, Landzberg M, Machado R, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2013;62(25): D34–D41. [DOI] [PubMed] [Google Scholar]
- 18.Girish R, Narayanan BK, Gupta AK. Red cell distribution width in heart failure. Indian Heart Journal. 2016; 67(1): S102 [Google Scholar]
- 19.Wonnerth A, Krychtiuk KA, Mayer FJ, Minar E, Wojta J, Schillinger M, Koppensteiner R, Hoke M. Red cell distribution width and mortality in carotid atherosclerosis. European Journal of clinical Investigation. 2015. 36(2): 198–204. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Fan P, Lee C, Wu VC, Tian YC, Yang CW, Chen YC, Chang CH. Red cell distribution width associated with adverse cardiovascular outcomes in patients with chronic kidney disease. BMC Nephrology. 2017;18 (361). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippi G, Filippozzi L, Montagnana M, Salvagno GL, Franchini M, Guidi GC, Targher G. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clinical Chemistry and Laboratory Medicine. 2009; 47(3): 353–357. [DOI] [PubMed] [Google Scholar]
- 22.Friedman JS, Lopez MF, Fleming MD, Rivera A, Martin FM, Welsh ML, Boyd A, Doctrow SR, Burakoff SJ. SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response and antioxidant responsiveness. Blood. 2004: 104(8): 2565–2573. [DOI] [PubMed] [Google Scholar]
- 23.Solak Y, Yilmaz MI, Saglam M, Caglar K, Verim S, Unal HU, Gok M, Demirkaya E, Gaipov A, Kayrak M, Centikaya H, Eyileten T,Turk S, Vural A. Red cell distribution width is independently related to endothelial dysfunction in patients with chronic kidney disease. American Journal of Medical Science. 2014; 347(2): 118–124. [DOI] [PubMed] [Google Scholar]
- 24.Chung L, Domsic RT, Lingala B, Alkassab F, Bolster M, Csuka ME, Derk C, Fischer A, Frech T, Fürst DE, Gomberg-Maitland M, Hinchcliff M, Hsu V, Hummers LK, Khanna D, Medsger TA, Molitor JA, Preston IR, Schiopu E, Shapiro L, Silver R, Simms R, Varga J, Gordon JK, Steen VD. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care & Research. 2013; 66(3): 489–495. [DOI] [PubMed] [Google Scholar]
- 25.Morrisroe K, Stevens W, Sahhar J, Rabusa C, Nikpour M, Proudman S. Epidemiology and disease characteristics of systemic sclerosis related pulmonary arterial hypertension: results from a real-life screening programme. Arthritis Research &Therapy. 2017; 19(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenavandeh S, Naseri R. Assessment of hospitalization and mortality o scleroderma in-patients: a thirteen-year study. Reumatologia 2017; 55(4) 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao Y, Thakkar V, Stevens W, Morrisroe K, prior D, Rabusa C, Youssef P, Gabbay E, Roddy J, Walker J, Zochling J, Sahhar J, Nash P, Lester S, Rischmueller M, Proudman SM, Nikpour M. A comparison of the predictive accuracy of three screening models for pulmonary arterial hypertension in systemic sclerosis. Arthritis Research and Therapy. 2015; 17 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farkas N, Szabo A, Lorand V, Sarlos DP, Minier T, Prohaszka Z, Czirjak L, Varju C. Clinical Usefulness of measuring red blood cell distribution width in patients with systemic sclerosis. Rheumatology. 2014; 53(8) 1439–1445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.