Abstract
Catastrophizing, a persistent negative mental set characterized by helplessness, rumination, and magnification of pain sensations, has a potent effect on pain report and clinical outcomes. Previous studies have documented an association between cognitive factors and central sensitization. The current analysis sought to test the potential modulating effect of pain catastrophizing on the association between capsaicin pain and the region of secondary hyperalgesia. Thirty-eight healthy individuals (50% women, mean age = 25.7, SD = 5.3) completed the Pain Catastrophizing Scale (PCS), then underwent topical application of 10% capsaicin, which was covered by a thermode maintained at 40°C for 90-min. Following removal of the capsaicin, the region of secondary hyperalgesia was determined. Hayes’ PROCESS macro was employed to examine catastrophizing’s potential moderating effect, which did not reveal a significant association between capsaicin pain ratings and the region of secondary hyperalgesia (β = 15.1, p = .06). Though PCS was not associated with area of secondary hyperalgesia (β = 23.9, p = .29), a significant interaction was present between PCS and capsaicin pain ratings (β = 3.7, p = .0004). Specifically, those endorsing higher catastrophizing levels and higher pain ratings experienced the greatest areas of secondary hyperalgesia. The Johnson-Neyman technique was used to determine the regional effect of the moderation, which indicated that when PCS scores were ≥10.6, capsaicin pain significantly moderated the association between pain and area of secondary hyperalgesia. These results suggest that catastrophizing plays an important role in the area of secondary hyperalgesia, and potentially central sensitization, warranting further testing in future research.
1 | INTRODUCTION
Pain catastrophizing—a set of negative emotional/cognitive processes involving rumination and pessimism, perceptions of helplessness, and magnification of pain-related symptoms—has a broad and substantial impact on pain perception and a variety of pain-related outcomes (Quartana, Campbell, & Edwards, 2009). A large body of literature supports that catastrophizing is associated with increases in pain and poorer pain treatment outcomes (Edwards, Campbell, Jamison, & Wiech, 2009). While the temporal relationship between catastrophizing and pain remains in question, emerging clinical and experimental data suggest that alterations (increases or decreases) in catastrophizing precede, and potentially induce, the subsequent increases or decreases in pain (Campbell, Quartana, Buenaver, Haythornthwaite, & Edwards, 2010; Campbell et al., 2012).
Although the underlying mechanisms of catastrophizing’s effect on pain have yet to be fully elucidated, catastrophizing has a notable effect on pain perception. Secondary hyperalgesia, increased pain sensitivity beyond the borders of the area of injury, may be a valid proxy for central sensitization in the laboratory setting (Latremoliere & Woolf, 2009; Magerl, Wilk, & Treede, 1998; Woolf, 2011; Ziegler, Magerl, Meyer, & Treede, 1999). Central sensitization is an integral component of our current understanding of pain, particularly chronic pain (Latremoliere & Woolf, 2009). These animal studies demonstrate amplification of neural signals in response to nociceptive input and that this central sensitization contributes to hyperalgesia and allodynia. By exciting an activity-dependent set of neurons with an initial nociceptive stimulus, pain can then be reported after termination of the stimulus in response to both previously painful (hyperalgesia) and previously non-painful (allodynia) inputs. (Woolf, 2011). The concept of central sensitization has contributed to the development of medications whose primary mode of action is the central nervous system and has shed light on confusing clinical syndromes such as fibromyalgia (Woolf, 2011). A recent study in healthy individuals, examining the association among heat pain sensation, area of secondary hyperalgesia and central sensitization, found that repetitive heat pain stimulation induced large areas of secondary hyperalgesia to pinprick stimuli (Jurgens, Sawatzki, Henrich, Magerl, & May, 2014). This work highlights similar results found in a number of other human studies, in which secondary hyperalgesia was induced via a variety of primary stimuli ranging from capsaicin injection to electric shocks (Ali, Meyer, & Campbell, 1996; Klede, Handwerker, & Schmelz, 2003). The measurement of pain catastrophizing has advanced in recent years to include both trait (Sullivan, Bishop, & Pivik, 1995) and situational (Campbell, Kronfli, et al., 2010; Campbell, Quartana, et al., 2010) assessments. In patients with sickle cell disease, those with greater levels of laboratory measured central sensitization reported significantly greater situational catastrophizing during laboratory pain testing, but not greater trait catastrophizing (Campbell et al., 2016). Another recent study examined the impact of a brief cognitive-behavioral training (CBT) on laboratory pain intensity and an index of central sensitization in healthy participants (Salomons, Moayedi, Erpelding, & Davis, 2014). The cognitive intervention had an effect on the affective dimension of pain, reducing ratings for pain unpleasantness, and reduced the zone of secondary hyperalgesia to a series of painful thermal stimuli, an index of central sensitization used in human studies. Interestingly, although this brief CBT intervention did not significantly reduce trait catastrophizing in these healthy subjects, these investigators found that the magnitude of the reduction in trait catastrophizing in the intervention group positively correlated with the reduction in the area of heat-induced secondary hyperalgesia.
Although studies have examined the effect of cognitive interventions on catastrophizing, pain severity, and secondary hyperalgesia, no known study has looked at the potential effect of catastrophizing as modulating the association between pain and secondary hyperalgesia. The current analyses sought to examine the potential moderating role of pain catastrophizing on the association between pain and secondary hyperalgesia in a laboratory study of healthy individuals.
2 | METHODS
We conducted a secondary data analysis of healthy subjects without current pain that participated in a larger, recently completed randomized, double blind, placebo-controlled distraction analgesia experimental study. All study- related procedures and materials were approved by the Johns Hopkins Institutional Review Board. Participants were recruited via flyers posted around the community, a recruitment website for clinical studies on the Johns Hopkins Bayview Medical Center campus, and by word-of-mouth. Following telephone screening for initial eligibility, subjects were scheduled for an in-person screening session. Written informed consent was obtained upon participant arrival to the screening session. If eligible, participants completed four testing sessions, lasting up to 2 hr each, which involved the application of capsaicin under non-distracted (two sessions) and distracted (two sessions; via video game play) conditions. The two sessions under each condition were split into one naloxone and one placebo session. In each of the four sessions, capsaicin was applied (as described below) and rated every 5 min. After 25 min, naloxone (0.1 mg/kg), or placebo (saline) was administered similar to previous studies (Anderson, Sheth, Bencherif, Frost, & Campbell, 2002). Only the pain alone (non-distracted), saline session was evaluated in the current secondary analyses and is described in detail below.
2.1 | Participants
A total of 38 healthy individuals (50% female) participated in the study (see Table 1 for demographic information). Only those participants who identified their racial/ethnic background as either non-Hispanic black or non-Hispanic white were included in the study. Eligibility criteria included reporting no pain or medical/psychiatric disorders, current alcohol or drug abuse problems, or use of narcotics, antidepressants, anticonvulsants, and muscle relaxants. Subjects were excluded if they were unable to perceive or tolerate capsaicin procedures during the screening session.
TABLE 1.
Demographic, psychosocial, and session characteristics
| Variables of interest | Participants (n = 38) |
|---|---|
| Gender (% women) | 50 |
| Race (% African American/Black) | 47.4 |
| Age Mean (SD), Range | 25.7 (5.3), 20–42 |
| BMI Mean (SD) | 24.5 (4.1) |
| Education (%) | |
| High School/GED | 7.9 |
| Some College/Tech School | 31.5 |
| College/Post College Education | 60.5 |
| Marital status (% Single) | 79 |
| Pain Catastrophizing Scale Mean (SD) | 10.3 (8.5) |
| Capsaicin Pain Rating Mean (SD) | 45.7 (25.4) |
| Flare Mean in mm2 (SD) | 2,405.7 (1,028.9) |
| Secondary Hyperalgesia Mean in mm2 (SD) | 1,976.9 (1,376.4) |
SD, standard deviation.
2.2 | Capsaicin procedure
The use of topical capsaicin with skin temperature control to induce tonic pain has been previously described (Anderson et al., 2002). In brief, a piece of thick, non-porous adhesive dressing with a 10.08 cm2 opening cut into it (used to standardize the area of capsaicin cream application) was applied to the skin on the dorsum of the non- dominant hand. Approximately 0.35 g of 10% topical capsaicin cream were measured and applied inside the adhesive opening and evenly spread on the skin using a small spatula. The area was then covered by Tegaderm transparent dressing (3M Health Care, St. Paul, MN, USA). Since topical capsaicin-induced pain varies strongly as a function of skin temperature (Dirks, Petersen, & Dahl, 2003), a Peltier-device heating element (Medoc US, Minneapolis, MN, USA) was strapped on top of the 10.08 cm2 area with Velcro wrist straps. This device was held at a constant temperature of 40°C during the session. This methodology produces pain that is rated, on average, as moderate in intensity, and which peaks at 15–25 min post-application and plateaus for approximately 1 h afterward (Anderson et al., 2002; Bencherif et al., 2002). This capsaicin plus heat procedure was conducted for 90 min on each participant. Participants provided verbal pain intensity ratings on a 0–100 scale (0 = no pain, 10 = most intense pain imaginable) every 5 min for the duration of the task. As capsaicin pain increases gradually over 25 min and then plateaus, pain ratings obtained from 30 to 90 min were averaged for analyses. Following completion of the session, the capsaicin cream was removed from the skin with an alcohol prep pad and the area of skin flare and secondary hyperalgesia were marked (as described below).
2.3 | Skin flare/Secondary hyperalgesia
2.3.1 | Flare
Following removal of the capsaicin, the area of redness, or “flare,” initiated by the capsaicin procedure was traced from the back of the hand on to a sheet of acetate paper and measured using a digital planimeter (Planix 10S). A flare is the neurogenic inflammatory response (axon reflex vasodilation) associated with capsaicin and is commonly identified as the area of primary hyperalgesia (Carter, 1991; Dray, 1992).
2.3.2 | Secondary hyperalgesia
Assessment of secondary hyperalgesia was performed at and around the site of thermode/capsaicin application. The area of secondary hyperalgesia was quantified with a normally non-painful mechanical probe (32 mN force; mechanical hyperalgesia) by first stimulating the skin distant from the treated area and then gradually moving inward until the participant indicated the stimulus had become noxious or until the subject reported a definite change in sensation (i.e., burning, tenderness, more intense pricking). This marking was done along 8 radial arms (rostral-caudal, lateral-medial, etc.) in steps of 5 mm at intervals of approximately 1 s, and the borders were marked with a felt pen. The distances were traced to a sheet of acetate paper for later measurement and calculation of surface area similar to flare procedures described above (Frymoyer, Rowbotham, & Petersen, 2007; Mathiesen, Imbimbo, Hilsted, Fabbri, & Dahl, 2006).
2.4 | Questionnaires
The Pain Catastrophizing Scale (PCS) consists of 13 items rated on a 5-point scale ranging from 0 (not at all) to 4 (all the time). Participants are instructed to indicate the degree to which they have specific thoughts and feelings when experiencing pain. This was completed following consent procedures, prior to undergoing any testing. The measure assesses three dimensions of catastrophizing: rumination, magnification, and helplessness. The PCS has been validated for both clinical and nonclinical samples (Osman et al., 2000; Sullivan et al., 1995). The total score was used in all analyses.
The Situational Catastrophizing Questionnaire is an adaptation of the Pain Catastrophizing Scale (Sullivan et al., 1995) modified to assess catastrophizing in response to laboratory pain. It is a six item questionnaire; the scale has been described more fully by Edwards and colleagues (Edwards, Smith, Stonerock, & Haythornthwaite, 2006) and it’s psychometric properties have been documented (Campbell et al., 2008). Participants were instructed to reference the pain they were experiencing in their hand while completing the questionnaire at each time point. Participants completed the catastrophizing questionnaire at multiple points during capsaicin testing; these were averaged to create one situational catastrophizing score.
2.5 | Statistical analyses
Demographic and psychosocial measures were computed, as were average pain over the session (from 30 to 90 min), area of flare, and area of secondary hyperalgesia. Pearson product-moment correlations were computed to examine the association between psychosocial measures, pain, flare and secondary hyperalgesia. Any demographic variable associated with secondary hyperalgesia was then controlled in the moderation analysis. Hayes’ (2012) PROCESS macro was employed to examine the potential moderating effect of catastrophizing on the association between capsaicin pain and secondary hyperalgesia. An ordinary least squares or logistic regression-based path analytical framework is employed in this macro to analyze statistical models. The program automatically examines conditional effects of the independent variable (pain) on the dependent variable (secondary hyperalgesia) at three levels of the moderator (catastrophizing)- the mean and plus/minus one standard deviation from the mean. Model 1, for simple moderation with mean centering was used in the current analyses. All analyses were conducted using SPSS 23.
3 | RESULTS
Demographic, psychosocial and session characteristics are summarized in Table 1. Correlations are presented in Table 2 for the variables of interest. Age (ranging from 20 to 42 in this sample) was significantly associated with the region of secondary hyperalgesia (r = .39, p = .03) and was thus included as a covariate in the moderation analysis.
TABLE 2.
Pearson bivariate correlation matrix for relevant variables
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
| 1. SH | 1.0 | ||||
| 2. Flare | 0.25 | 1.0 | |||
| 3. Pain rating | 0.26 | 0.01 | 1.0 | ||
| 4. PCS | −0.01 | −0.17 | −0.37* | 1.0 | |
| 5. Situational PCS | 0.38* | −0.04 | 0.53* | 0.20 | 1.0 |
| 6. Gender | 0.03 | −0.13 | 0.10 | −0.01 | −0.09 |
| 7. Race | −0.02 | 0.22 | −0.31 | 0.03 | −0.26 |
| 8. Age | 0.39* | 0.001 | 0.22 | 0.01 | 0.35* |
| 9. BMI | 0.23 | −0.12 | −0.001 | −0.28 | 0.10 |
| 10. Education | −0.14 | 0.17 | −0.05 | −0.13 | −0.22 |
| 11. Marital status | −0.09 | −0.14 | 0.04 | 0.01 | −0.01 |
SH, secondary hyperalgesia; PCS, Pain Catastrophizing Scale; BMI, body mass index.
p < .05.
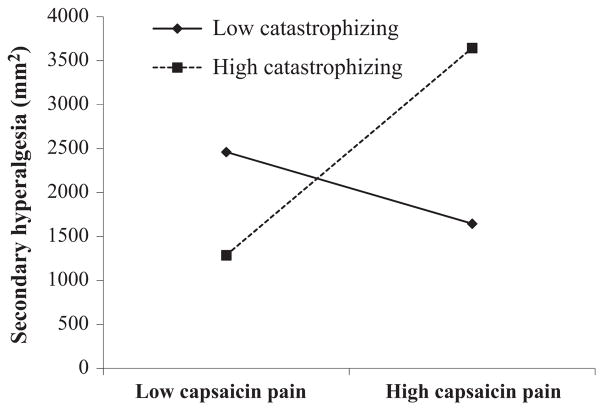
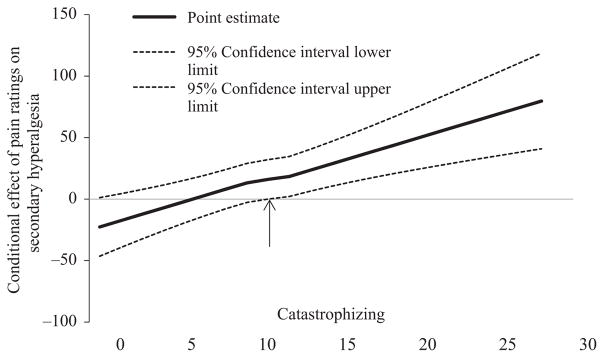
Capsaicin pain report was associated with secondary hyperalgesia (β = 17.6, p = .03; however, this was rendered non-significant once age was included in the model (β = 15.1, p = .06). While catastrophizing was not associated with secondary hyperalgesia (β = 23.9, p = .29), a significant interaction was found between catastrophizing and capsaicin pain (β = 3.7, p = .0004). This interaction is represented graphically in Figure 1, and depicts the association of secondary hyperalgesia for low and high catastrophizing. Of note, we included race as an additional covariate; this did not alter the direction or strength of the interaction. Simple slopes were tested across low, medium, and high levels of capsaicin pain and only those with lower or higher pain report revealed a significant association between catastrophizing and secondary hyperalgesia (lower pain: β = −68.73, p = .03; higher pain: β = 25.2, p = .002). We probed the interaction further by use of the Johnson-Neyman technique (Hayes, 2012) to evaluate the regions of significance of the conditional effect. This allows for visualization of the range of values within the moderator where the interaction is significant. Figure 2 plots the conditional effect of capsaicin pain rating on secondary hyperalgesia across values of catastrophizing. The region of significance lies where the confidence interval does not include 0. Thus, capsaicin pain is associated with secondary hyperalgesia when catastrophizing scores are ≥10.6. Those with catastrophizing scores ≥10.6 accounted for 44.7% of the sample. Situational catastrophizing was not a significant moderator of the interaction (p = .06). Additionally, when flare was denoted as the dependent variable, the moderation was not significant.
FIGURE 1.
Interaction of catastrophizing and capsaicin pain (each depicted as −/+ one standard deviation from the mean) predicting secondary hyperalgesia
FIGURE 2.
Conditional effect of capsaicin pain ratings on secondary hyperalgesia across values of catastrophizing. The region of significance lies where the confidence interval does not include 0. Thus, average capsaicin pain rating is associated with secondary hyperalgesia when catastrophizing is >10.6
4 | DISCUSSION
This study sought to investigate whether catastrophizing moderates the known association between pain perception and secondary hyperalgesia using the laboratory capsaicin model. We did not find a direct association between catastrophizing and the area of secondary hyperalgesia. We did find that age correlated with the region of secondary hyperalgesia and, in our multivariate model controlling for age, we found support for the moderating effect of catastrophizing on the association between capsaicin pain intensity and the area of secondary hyperalgesia. These findings extend our understanding of the contribution of catastrophizing to the experience of pain and may have implications for increasing central sensitization.
Our most important finding is that trait catastrophizing did have a modulating effect on the association between pain intensity and area of secondary hyperalgesia, measured as pain in response to stimulation outside the zone of primary hyperalgesia and flare elicited by capsaicin. Among participants with higher catastrophizing scores (+1 standard deviation), this region nearly tripled in size for those reporting higher pain scores as compared to those reporting lower pain scores in response to capsaicin. In the same comparison among participants with low catastrophizing scores, there was no significant difference in the size of this region. These data are consistent with growing interest linking cognitive and affective factors with central sensitization (Lumley et al., 2011; Yunus, 2007). As measured in this study, catastrophizing is a trait that people report as their typical or general cognitive/affective response to the experience of pain. This finding of moderation is consistent with a diathesis-stress model in which high levels of pain catastrophizing are a diathesis that is activated by the experience of high levels of pain in response to capsaicin, a stressor. Only under these conditions of combined high catastrophizing and high pain (and not the condition of high catastrophizing and low capsaicin pain) are the largest zones of secondary hyperalgesia observed (see Figure 1). Situational catastrophizing was not a significant moderator; however, a trend towards significance was observed.
Also consistent with the diathesis-stress model is our finding that a certain level, or threshold, of catastrophizing incurs risk for secondary hyperalgesia. Specifically we find that the conditional effect of catastrophizing occurred only in participants with scores exceeding 10.6 on the PCS. This score is well below the threshold for clinically significant catastrophizing (PCS ≥ 30, 75th percentile (Sullivan, 2009)) yet is common in studies of healthy adults (i.e., those not reporting chronic pain) and constituted 45% of our sample. This raises the important possibility that studies primarily investigating pain catastrophizing in healthy adults using laboratory stimuli likely need to set minimum criteria for study inclusion, or specifically test the moderating role for catastrophizing, in order to adequately test study hypotheses about the impact of pain catastrophizing on laboratory pain. Further support for the value of this approach in the current study is the lack of a direct association between pain catastrophizing and area of secondary hyperalgesia.
Similarly, we previously reported no elevated level of trait catastrophizing in sickle cell patients high in an index of laboratory measured central sensitization (Campbell et al., 2016), although we did not test a moderating effect of pain catastrophizing in that study. Since we did find an association between situational catastrophizing (measured during pain testing) and greater central sensitization in these sickle cell patients, catastrophizing seems to have a differential association with central sensitization depending on the conditions under which it is measured. Further complicating our knowledge of the association between catastrophizing and central sensitization is the finding that among a group of healthy individuals exposed to a brief CBT intervention, those who showed the greatest reduction in an index of central sensitization also reported the largest reductions in trait catastrophizing (Salomons et al., 2014). While the Salomons study did not find a significant effect of this brief cognitive intervention on trait catastrophizing in these healthy adults when compared to a control intervention, the substantial literature demonstrating reductions in catastrophizing with cognitive-behavioral interventions (Riddle et al., 2011; Terry, Thompson, & Rhudy, 2015; Turner et al., 2016) suggests that more prolonged interventions in clinical samples may show even further reductions in indices of central sensitization such as secondary hyperalgesia following CBT treatment.
Initially, capsaicin pain and secondary hyperalgesia were significantly associated. Surprisingly, once age was included in the model, this became non-significant. This may suggest that age, and potentially other factors, could be contributing to the pain-central sensitization association. Additionally, our finding that pain catastrophizing moderates the association between capsaicin pain and the area of secondary hyperalgesia may in part account for some inconsistencies across studies. Overall, our findings suggest that if a study with an adequate number of participants below the threshold level of catastrophizing, a significant association between pain and area of secondary hyperal-gesia might not exist. However, if participants score above the threshold, the participants may be more vulnerable to the effects of the pain stimulus inducing central sensitization, perhaps leading to an increase in the area of secondary hyperalgesia. Our threshold finding needs to be replicated and the cut-point further validated in future work.
An association between age and area of secondary hyperalgesia was noted in our analyses, such that our older subjects showed a greater area of secondary hyperalgesia, though the oldest study participant was 42 years. Previous studies have theorized a mechanism for catastrophizing through the endogenous opioid pathway (Campbell & Edwards, 2009), while other studies have observed an age-dependent effect on the expression of opioid receptors, the internalization of those receptors, and an effect on opioid tolerance (Zhao, Xin, Xie, Palmer, & Huang, 2012). A hypothesis that endogenous opioid function either moderates or mediates either the association between age and secondary hyperalgesia or between age and pain catastrophizing merits further investigation.
There are several limitations to our methodology and findings. First, our sample of 38 young, healthy participants was fairly small and findings may not generalize to a clinical population. While we did find significant effects with this relatively small sample, these findings require replication and our insignificant findings should be interpreted with caution as our power was limited to at least moderate effect sizes. Studies have indicated that the area of capsaicin-induced secondary hyperalgesia is greater in patients with chronic pain disorders and that it is reflective of the abnormal sensory activity seen in the clinical setting among these patients (Morris, Cruwys, & Kidd, 1997, 1998). Additionally, these are secondary data analyses and, because the goal was not to recruit participants that would represent a wide variety of catastrophizing levels, may artificially bias the level and variability of catastrophizing observed in participants.
In summary, we found that pain catastrophizing moderated the association between capsaicin pain and the area of secondary hyperalgesia. Further analysis of the region of significance for this effect indicated that moderation only occurred above a subclinical threshold (of 10.6 or more on the PCS) in this group of healthy adults. This finding should be replicated in other groups of healthy adults and validated in clinical settings, which may indicate a different threshold for central sensitization for clinical populations. For example, clinical studies should investigate whether individuals reporting high levels of pain catastrophizing, who then experience high levels of acute pain, are at increased risk to experience central sensitization, including secondary hyperalgesia. Ideally, these studies are longitudinal and determine whether this pattern contributes to long-term chronicity of pain.
Acknowledgments
Funding information
Foundation for the National Institutes of Health, Grant/Award Number: K23 DA029609 and K23 NS070933; American Pain Society; The Blaustein Pain Foundation, Grant/Award Number: K23 NS070933 and K23 DA029609; The JHBMC Clinical Research Unit
This research was supported by grants K23 NS070933, K23 DA029609, The Blaustein Pain Foundation, & The JHBMC Clinical Research Unit.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
References
- Ali Z, Meyer RA, Campbell JN. Secondary hyperalgesia to mechanical but not heat stimuli following a capsaicin injection in hairy skin. Pain. 1996;68:401–411. doi: 10.1016/s0304-3959(96)03199-5. [DOI] [PubMed] [Google Scholar]
- Anderson WS, Sheth RN, Bencherif B, Frost JJ, Campbell JN. Naloxone increases pain induced by topical capsaicin in healthy human volunteers. Pain. 2002;99:207–216. doi: 10.1016/s0304-3959(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Fuchs PN, Sheth R, Dannals RF, Campbell JN, Frost JJ. Pain activation of human supraspinal opioid pathways as demonstrated by [11C]-carfentanil and positron emission tomography (PET) Pain. 2002;99:589–598. doi: 10.1016/S0304-3959(02)00266-X. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Edwards RR. Mind-body interactions in pain: The neurophysiology of anxious and catastrophic pain-related thoughts. Translational Research. 2009;153:97–101. doi: 10.1016/j.trsl.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CM, Kronfli T, Buenaver LF, Haythornthwaite JA, Smith MT, Edwards RR. In vivo vs. standard catastrophizing in multiple pain measures among healthy, TMD and arthritis patients. The Journal of Pain. 2008;9(4):56. Ref Type: Abstract. [Google Scholar]
- Campbell CM, Kronfli T, Buenaver LF, Smith MT, Berna C, Haythornthwaite JA, Edwards RR. Situational versus dispositional measurement of catastrophizing: Associations with pain responses in multiple samples. The Journal of Pain. 2010;11:443–453. doi: 10.1016/j.jpain.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CM, McCauley L, Bounds SC, Mathur VA, Conn L, Simango M, … Fontaine KR. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: Cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Research & Therapy. 2012;14:R231. doi: 10.1186/ar4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CM, Moscou-Jackson G, Carroll CP, Kiley K, Haywood C, Jr, Lanzkron S, … Haythornthwaite JA. An evaluation of central sensitization in patients with sickle cell disease. The Journal of Pain. 2016;17:617–627. doi: 10.1016/j.jpain.2016.01.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CM, Quartana PJ, Buenaver LF, Haythornthwaite JA, Edwards RR. Changes in situation-specific pain catastrophizing precede changes in pain report during capsaicin pain: A cross-lagged panel analysis among healthy, pain-free participants. The Journal of Pain. 2010;11:876–884. doi: 10.1016/j.jpain.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Carter RB. Topical capsaicin in the treatment of cutaneous disorders. Drug Development Research. 1991;22:109–123. [Google Scholar]
- Dirks J, Petersen KL, Dahl JB. The heat/capsaicin sensitization model: A methodologic study. The Journal of Pain. 2003;4:122–128. doi: 10.1054/jpai.2003.10. [DOI] [PubMed] [Google Scholar]
- Dray A. Neuropharmacological mechanisms of capsaicin and related substances. Biochemical Pharmacology. 1992;44:611–615. doi: 10.1016/0006-2952(92)90393-w. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Campbell CM, Jamison RN, Wiech K. The neurobiological underpinnings of coping with pain. Current Directions in Psychological Science. 2009;18:237–241. [Google Scholar]
- Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clinical Journal of Pain. 2006;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- Frymoyer AR, Rowbotham MC, Petersen KL. Placebo-controlled comparison of a morphine/dextromethorphan combination with morphine on experimental pain and hyperalgesia in healthy volunteers. The Journal of Pain. 2007;8:19–25. doi: 10.1016/j.jpain.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper] 2012 Retrieved from http://www.afhayes.com [On-line]
- Jurgens TP, Sawatzki A, Henrich F, Magerl W, May A. An improved model of heat-induced hyperalgesia – repetitive phasic heat pain causing primary hyperalgesia to heat and secondary hyperalgesia to pinprick and light touch. PLoS One. 2014;9:e99507. doi: 10.1371/journal.pone.0099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klede M, Handwerker HO, Schmelz M. Central origin of secondary mechanical hyperalgesia. Journal of Neurophysiology. 2003;90:353–359. doi: 10.1152/jn.01136.2002. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. The Journal of Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, … Keefe FJ. Pain and emotion: A biopsychosocial review of recent research. Journal of Clinical Psychology. 2011;67:942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain. 1998;74:257–268. doi: 10.1016/s0304-3959(97)00177-2. [DOI] [PubMed] [Google Scholar]
- Mathiesen O, Imbimbo BP, Hilsted KL, Fabbri L, Dahl JB. CHF3381, a N-methyl-D-aspartate receptor antagonist and monoamine oxidase-A inhibitor, attenuates secondary hyperalgesia in a human pain model. The Journal of Pain. 2006;7:565–574. doi: 10.1016/j.jpain.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Morris VH, Cruwys SC, Kidd BL. Characterisation of capsaicin-induced mechanical hyperalgesia as a marker for altered nociceptive processing in patients with rheumatoid arthritis. Pain. 1997;71:179–186. doi: 10.1016/s0304-3959(97)03361-7. [DOI] [PubMed] [Google Scholar]
- Morris V, Cruwys S, Kidd B. Increased capsaicin-induced secondary hyperalgesia as a marker of abnormal sensory activity in patients with fibromyalgia. Neuroscience Letters. 1998;250:205–207. doi: 10.1016/s0304-3940(98)00443-1. [DOI] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: Further psychometric evaluation with adult samples. Journal of Behavioral Medicine. 2000;23:351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: A critical review. Expert Review of Neurotherapeutics. 2009;9:745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Keefe FJ, Nay WT, McKee D, Attarian DE, Jensen MP. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: A quasi-experimental study. Archives of Physical Medicine and Rehabilitation. 2011;92:859–865. doi: 10.1016/j.apmr.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Moayedi M, Erpelding N, Davis KD. A brief cognitive-behavioural intervention for pain reduces secondary hyperalgesia. Pain. 2014;155:1446–1452. doi: 10.1016/j.pain.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ. The Pain Catastrophizing Scale. User manual. 5th. 2009 http://sullivan-painresearch.mcgill.ca/pdf/pcs/PCSManual_English.pdf. Ref Type: Serial (Book, Monograph)
- Sullivan MJ, Bishop S, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- Terry EL, Thompson KA, Rhudy JL. Experimental reduction of pain catastrophizing modulates pain report but not spinal nociception as verified by mediation analyses. Pain. 2015;156:1477–1488. doi: 10.1097/j.pain.0000000000000192. [DOI] [PubMed] [Google Scholar]
- Turner JA, Anderson ML, Balderson BH, Cook AJ, Sherman KJ, Cherkin DC. Mindfulness-based stress reduction and cognitive behavioral therapy for chronic low back pain: Similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. Pain 2016. 2016;157(11):2434–2444. doi: 10.1097/j.pain.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus MB. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Seminars in Arthritis and Rheumatism. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Zhao J, Xin X, Xie GX, Palmer PP, Huang YG. Molecular and cellular mechanisms of the age-dependency of opioid analgesia and tolerance. Molecular Pain. 2012;8:38. doi: 10.1186/1744-8069-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122(Pt 12):2245–2257. doi: 10.1093/brain/122.12.2245. [DOI] [PubMed] [Google Scholar]