Abstract
Purpose:
To investigate the association between metabolic syndrome (MetS) and risk of breast cancer mortality by menopausal status, obesity, and subtype.
Methods:
Data from 94,555 women free of cancer at baseline in the National Institute of Health-American Association of Retired Persons Diet and Health Study cohort (NIH-AARP) was used to investigate the prospective associations of baseline MetS and components with risk of breast cancer mortality using Cox proportional hazard regression models adjusted for baseline behavioral and demographic covariates.
Results:
During a mean follow-up duration of 14 years, 607 women in the cohort died of breast cancer. Overall, MetS was associated with a 73% increased risk of breast cancer mortality (HR: 1.73; 95% CI: 1.09– 2.75), but the association was significant among post-menopausal women overall (HR: 2.07, 95% CI: 1.32, 3.25), and those with overweight/obesity (HR: 1.15, 95% CI: 0.81, 1.64). MetS was associated with increased risk of breast cancer mortality for ER+/PR+ (HR: 1.28, 95% CI: 0.52, 3.16) and lower risk for ER-/PR- (HR: 0.44, 95% CI: 0.11, 1.75) subtypes; however, the associations were not statistically significant. Of the individual MetS components, high waist circumference (HR: 1.32, 95% CI: 1.03, 1.70), high cholesterol (HR: 1.24, 95% CI: 1.05, 1.46), and hypertension (HR: 1.24, 95% CI: 1.05, 1.46) were independently associated with increased risk of breast cancer mortality.
Conclusions:
MetS was associated with increased risk of breast cancer mortality, especially among post-menopausal women. Further studies with larger sample sizes are needed to definitively determine the extent to which these associations vary by breast cancer subtype.
Keywords: Metabolic syndrome, breast cancer mortality, menopause, obesity, hormone-receptor subtypes
INTRODUCTION
MetS is a constellation of metabolic dysfunctions that include central obesity, dyslipidemia, hypertension and insulin resistance, and significantly increases the risk of chronic diseases such as coronary heart disease, stroke, type-2 diabetes, and cancer[1–4]. Individual components of MetS are associated with increased risk of cancer [5–10]; however, studies on the association of MetS with breast cancer mortality are limited. Of the eight published studies evaluating breast cancer outcomes till date [11–18], three observed increased risk of breast cancer mortality among women with MetS [11,15,18] , and four studies reported significant associations between MetS and other breast cancer outcomes—including distant metastasis [12] and aggressive phenotypes [11,13,14]. Of the three studies that directly examined the association between MetS and breast cancer mortality, two were limited by small sample sizes [15, 18], and one was conducted among European populations, limiting generalizability [15]. Moreover, while breast cancer hormonal subtypes are established prognostic indicators for breast cancer [19], results from the only two studies that evaluated the association between MetS and breast cancer subtypes were inconclusive [17,20] , and none of the previous studies simultaneously investigated whether the associations between MetS and breast cancer mortality varied by menopausal status and BMI.
It remains unclear whether MetS is a risk factor for breast cancer mortality overall and by subtype, and whether these associations vary by BMI, especially given the limited number of cohort studies and strong potential for residual confounding by BMI in cross-sectional studies. While obesity, a major component of MetS, is recognized as an established risk factor for breast cancer among post-menopausal women [21], obesity is also a risk factor for breast cancer mortality among women of all ages [22,23]. Additionally, most of the other risk factors for breast cancer outcomes also vary by menopausal status, including the distribution of hormone-receptor subtype [19], suggesting that the association between MetS and breast cancer mortality may vary by menopausal status and subtype[20]. It is also unclear whether the individual MetS components—including hypertension, high cholesterol, and high fasting glucose—show independent or synergistic associations with breast cancer mortality across these categories.
The aim of this study was to examine the association between MetS, individual components and breast cancer mortality in a large prospective cohort, and to evaluate these associations across menopausal status, BMI and subtype. We also examined the association between different combinations of MetS components and risk of breast cancer mortality by menopausal status to identify which combination was most strongly associated with mortality risk.
MATERIALS AND METHODS
Data from 94,555 women in the National Institute of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study were analyzed to investigate the prospective association between MetS and risk of breast cancer mortality. Over half a million NIH-AARP members were recruited to the NIH-AARP Diet and Health Study in1995–1996, and baseline data on demographics, behavioral and other participant characteristics were collected. The NIH-AARP Diet and Health Study has been described in detail elsewhere [24]. Participants were followed up prospectively to ascertain cancer incidence and mortality outcomes [25]. After excluding male participants (n=325,171), those with cancer diagnosis (n=23,971) or mortality (n=1,375) at baseline, poor general health (n=3,407), proxy respondents (n=15,760), those who did not return Risk Factor Questionnaire (n=58,374) and those with missing data for the main study variables (n=43,501), there were 94,555 women included in the present analysis. There were significant differences in the distribution of missing values by menopausal status, race, age, and education (p-values<0.0001), but no difference by BMI (p-value=0.117).
Metabolic syndrome:
MetS was defined following the harmonized criteria [26] based on the presence of three of the following components: 1) High waist circumference (WC): >88 cm; 2) Dyslipidemia or self-reported history of high cholesterol level; 3) High blood pressure or self-reported history of hypertension; 4) High glucose or self-reported history of diabetes. The NIH-AARP dataset did not include data on high-density lipoprotein cholesterol (HDL) and triglycerides; therefore, the current analysis is based on 4 out of the 5 MetS components. Women with races other than Black or Non-Hispanic White had substantial missing values for the main covariates, thus the present study was limited to women who self-reported Non-Hispanic White or Black race.
Breast cancer mortality:
Breast cancer incidence was ascertained from cancer registries in 6 US states including California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania, and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan) with complete case ascertainment, and from registries in three additional states (Arizona, Nevada, and Texas) where participants relocated to during the follow up period [27]. Breast cancer mortality was determined based on data from the National Death Index (NDI), Social Security Administration Death Master File (SSADMF), Cancer Registry Returns, and Follow-up Questionnaire Responses.
Covariates:
Baseline demographic covariates in this study included age (categorical), BMI derived from weight and height (normal BMI: 18.5 to <25 kg/m2; overweight/obese: ≥25 kg/m2), region (Mid-West, North East, South, West), race (Black or White), and marital status (married, widowed, divorced, separated, or never married). Baseline behavioral covariates included physical activity (never, rarely, 1–3 times/month, 1–2 times/week, 3–4 times/week or ≥5 times/week), and smoking status (yes/no). Breast cancer hormonal receptor subtypes (estrogen receptor (ER), progesterone receptor (PR)) were identified from cancer registry data according to established protocols[19]. However, human epidermal growth factor receptor 2 (HER2) status was not assessed in the majority of women and was not included in the analysis.
Statistical analysis:
Differences in baseline characteristics by breast cancer mortality status were assessed using Chi-squared (χ2) tests for categorical variables. To estimate the HR of breast cancer mortality, a series of Cox proportional hazards regression models were fitted with MetS as the exposure—comparing women with MetS with those without MetS. The primary outcome of interest was risk of breast cancer specific mortality assessed from time of entry into the cohort. The proportional hazard assumption was tested using cumulative sum of martingale residuals and Kolmogorov-type supremum test [28]. In separate models, each individual MetS component and number of MetS components present were evaluated in relation to breast cancer mortality. Each model was adjusted for age, race, education, region, smoking, physical activity, marital status, and BMI (in models not stratified by BMI), and in sensitivity analysis, BMI stratified models were further adjusted for continuous measures of BMI to address residual confounding by BMI. These associations were also evaluated by BMI (normal weight and overweight/obese), menopausal status (pre- and post-menopause), and hormone receptor subtype (ER+/PR+, ER+/PR− and ER−/PR−) analysis. Effect modification in the stratified models were assessed using the Breslow-Day-Tarone test [29] and likelihood ratio test for interaction terms. Individuals were censored at the time of death due to breast cancer, other causes, loss to follow-up, or December 31, 2011, whichever happened first. Results are presented as adjusted HRs and corresponding 95% Confidence Intervals (CIs); p values ≤0.05 were considered statistically significant, and for interaction terms, p values ≤0.1 were considered statistically significant. All statistical analyses were conducted using SAS version 9.4, SAS Institute Inc., Cary, NC, USA.
RESULTS
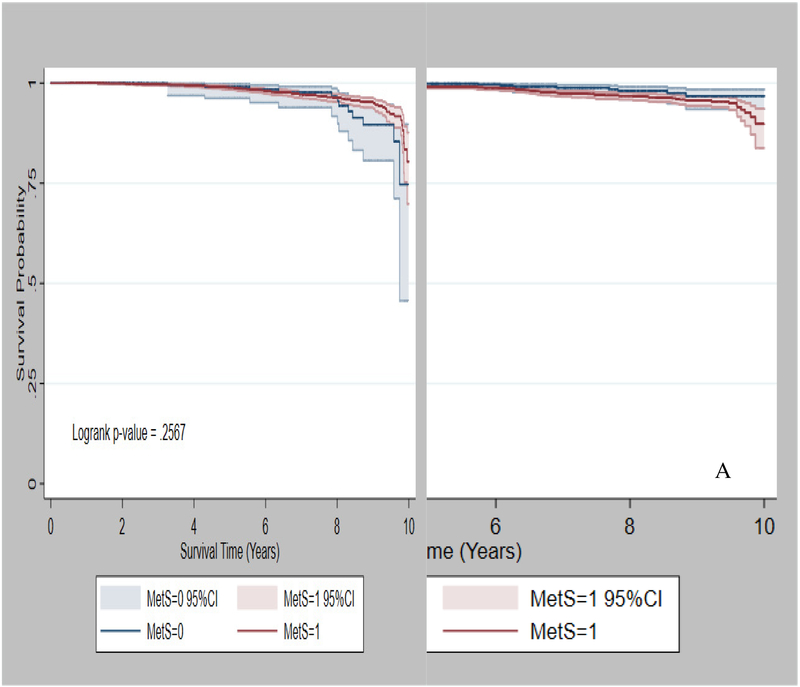
During a mean follow-up of 14 years (SD: 3.6), 6,631 women in the NIH-AARP cohort developed breast cancer, and 607 (9%) died due to breast cancer. Compared with women without MetS, those with MetS were more likely to be overweight /obese (91% vs. 52%), have high waist circumference (96% vs. 30%), and were less likely to be current hormone therapy users (40% vs. 47%), all p-values < 0.05 (Table 1). Among women with normal BMI, those with MetS had relatively lower 10-year survival probability than those without MetS (Fig 1A), but there was no significant difference in 10-year survival probability by MetS among women with overweight/obesity (Fig 1B).
Table 1.
Baseline characteristics of women in the NIH-AARP cohort by baseline MetS (N=94,555) a
| Overall | MetS | P-values | ||
|---|---|---|---|---|
| Yes | No | |||
| N (%)b | N (col %) | N (col %) | ||
| Sample size | 94,555 | 4,956 (5.24) | 89,599 (94.76) | |
| Race/Ethnicity | ||||
| White | 90,753 (95.98) | 4,630 (93.42) | 86,123 (96.12) | <0.0001 |
| Black | 3,802 (4.02) | 326 (6.58) | 3,476 (3.88) | |
| Age at entry (years) | ||||
| 50–59 | 35,805 (37.87) | 1,579 (31.86) | 34,226 (38.20) | <0.0001 |
| 60–69 | 55,638 (58.84) | 3,178 (64.12) | 52,460 (58.55) | |
| 70–79 | 3,112 (3.29) | 199 (4.02) | 2,913 (3.25) | |
| Education | ||||
| < High school | 4,058 (4.29) | 255 (5.15) | 3,803 (4.24) | <0.0001 |
| High school or GED | 33,226 (35.14) | 1,934 (39.02) | 31,292 (34.92) | |
| Some college | 24,563 (25.98) | 1,294 (26.11) | 23,269 (25.97) | |
| >= College | 32,708 (34.59) | 1,473 (29.72) | 31,235 (34.86) | |
| Menopausal status | ||||
| Pre-menopausal | 3,693 (3.91) | 164 (3.31) | 3,529 (3.95) | 0.025 |
| Post-menopausal | 90,662 (96.09) | 4,785 (96.69) | 85,877 (96.05 | |
| BMI (kg/m2) | ||||
| Normal BMI | 43,546 (46.05) | 449 (9.06) | 43,097 (48.10) | <0.0001 |
| Overweight or obese | 51,009 (53.95) | 4,507 (90.94) | 46,502 (51.90) | |
| Current hormone therapy | ||||
| Yes (%) | 44,387 (46.94) | 1,999 (40.33) | 42,388 (47.31) | <0.0001 |
| Family history of breast cancer | ||||
| Yes (%) | 11,949 (12.64) | 617 (12.45) | 11,332 (12.65) | 0.700 |
| Hormone receptors | ||||
| ER+/PR+ | 2,995 (71.29) | 167 (75.23) | 2,828 (70.03) | 0.258 |
| ER+/PR− | 587 (13.97) | 21 (9.46 ) | 566 (14.02) | |
| ER−/PR− | 619 (14.73) | 31 (13.96) | 588 (14.56) | |
| High WC (%) | 22,919 (34.87) | 4,776 (96.37) | 18,143 (29.86) | <0.0001 |
| High cholesterol (%) | 51,324 (54.28) | 3,870 (78.09) | 47,454 (52.96) | <0.0001 |
| High blood pressure (%) | 33,518 (35.45) | 4,624 (93.30) | 28,894 (32.25) | <0.0001 |
| High fasting glucose (%) | 5,819 (6.15) | 2,072 (41.81) | 3,747 (4.18) | <0.0001 |
| Smoking (%) | 51,476 (54.44) | 2,577 (52.00) | 48,899 (54.58) | 0.0004 |
P values are from Chi-square test or Fisher exact test. Abbreviations: BMI, body mass index; GED, general education development; Ref, reference; WC, waist circumference
N=sample size.
N (%)=count (percent).
Fig 1.
Ten-year survival probability by MetS status. A) Normal BMI B) Overweight /Obese
In Cox regression models (Table 2), MetS was associated with a 2-fold increased risk of breast cancer mortality in crude age-adjusted models (HR: 2.12, 95% CI: 1.35, 3.31) and a 73% increased risk of breast cancer mortality in the fully adjusted model (HR: 1.73, 95% CI: 1.09, 2.75). High WC (HR: 1.32, 95% CI: 1.03, 1.70), high cholesterol (HR: 1.24, 95% CI: 1.05, 1.46) and high blood pressure (HR: 1.24, 95% CI: 1.05, 1.46) were each associated with increased risk of breast cancer mortality overall in multivariable adjusted models. Furthermore, compared to individuals without any component of MetS present, the risk of breast cancer mortality increased steeply as the number of MetS components increased. For one component, the risk was 44% higher (HR: 1.44, 95% CI: 1.02, 2.04), for two by 87% (HR: 1.87, 95% CI: 1.29, 2.71), and for three or more the risk doubled (HR: 2.02, 95% CI: 1.29, 3.17). Risk of breast cancer mortality increased by 24% for every additional component of MetS present (HR: 1.24, 95% CI: 1.09, 1.41, p-trend=0.001). When stratified by stage, MetS was associated with 29% increased risk of cancer mortality for early stage (stage I-II) diagnoses (HR: HR: 1.29, 95% CI: 0.60, 2.77), and over a 2-fold increased risk for late stage diagnoses (HR: 2.59, 95% CI: 1.28, 5.24).
Table 2.
Hazard ratios (HRs)a and 95% confidence intervals for the association between MetS and breast cancer mortality by BMI
| All | Normal BMI | Overweight /obese | |
|---|---|---|---|
| N (Deaths)b | 94,555 (607) | 43,546 (231) | 51,009 (376) |
| MetSδ | |||
| Crude | 2.12(1.35, 3.31) | 1.44 (0.34, 6.09) | 1.54 (0.85, 2.82) |
| Adjusted§ | 1.73 (1.09, 2.75) | 1.28 (0.30, 5.45) | 1.48(0.81, 2.72) |
| Components | |||
| High WC | 1.32 (1.03, 1.70) | 1.10 (0.65, 1.84) | 1.40 (1.04, 1.89) |
| High cholesterol | 1.24 (1.05, 1.46) | 1.39 (1.05, 1.83) | 1.16 (0.95, 1.42) |
| High blood pressure | 1.24 (1.05, 1.46) | 1.17 (0.88, 1.57) | 1.26 (1.03, 1.58) |
| High fasting glucose | 1.31 (0.97, 1.76) | 1.36 (0.64, 2.90) | 1.30 (0.94, 1.80) |
| Number of MetS Components | |||
| 0(Ref) | 1.00 | 1.00 | 1.00 |
| 1 | 1.44 (1.02, 2.04) | 1.73 (1.12, 2.69) | 1.02 (0.58, 1.79) |
| 2 | 1.87 (1.29, 2.71) | 1.91 (1.11, 3.29) | 1.51 (0.88, 2.59) |
| 3+ | 2.02 (1.29, 3.17) | 1.31 (0.31, 5.55) | 1.49 (0.82, 2.73) |
Models are adjusted for age (crude model), plus BMI(in model not stratified by BMI only), region,race,physical activity,smoking, and marital status.
Death=number of breast cancer deaths; N= Number of observations.
The reference category was women without any components of MetS.
Bold indicates statistically significant at α= 0.05.
Abbreviation: BMI, body mass index; MetS, metabolic syndrome; NE, non-estimable; Ref, reference; WC, waist circumference.
MetS was associated with a 28% (HR: 1.28, 95% CI: 0.30, 5.45) and 48% (HR: 1.48, 95% CI: 0.81, 2.72) increased risk of breast cancer mortality among women with normal BMI and overweight/obesity, respectively, Table 2. However, these associations were not statistically significant, and the formal test of effect modification by BMI was not statistically significant (Breslow-Day-Test P=0.716; P-interaction = 0.147). High cholesterol was associated with a 39% increased risk of breast cancer mortality among women with normal BMI (HR: 1.39, 95% CI: 1.05, 1.83), while high waist circumference (HR: 1.40, 95% CI: 1.04, 1.89) and high blood pressure (HR: 1.26, 95% CI: 1.03, 1.58) were associated with increased risk of breast cancer mortality among women with overweight/obesity. In sensitivity analysis considering non-breast cancer deaths as competing risk, the association between MetS (HR: 2.40, 95% CI: 1.74, 3.30), high WC (HR: 1.25, 95% CI: 1.05, 1.48), high cholesterol (HR: 1.12, 95% CI: 1.00, 1.25), high blood pressure (HR: 1.20, 95% CI: 1.00, 1.43) and high fasting glucose (HR: 1.61, 95% CI: 1.33, 1.94) with breast cancer mortality became stronger (data not shown).
In analyses stratified by menopausal status (Table 3), MetS was associated with a statistically significant 2-fold higher risk of breast cancer mortality among post-menopausal women in the fully adjusted model (HR: 2.07, 95% CI: 1.32, 3.25). Among this specific group, MetS was independent of breast cancer mortality in women with normal weight (HR: 0.91, 95% CI: 0.22, 3.66), but associated with higher but non-significantly increased risk of mortality among women with overweight/obesity (HR: 1.15, 95% CI: 0.81, 1.64). However, high cholesterol (HR: 1.39, 95% CI: 1.05, 1.84) and having one (HR: 1.72, 95% CI: 1.11, 2.67) or two (HR: 1.96, 95% CI: 1.14, 3.38) components of MetS in normal BMI post-menopausal women increased the risk of breast cancer mortality. The combination of high blood pressure, high fasting blood glucose and high WC was associated with 72% higher risk of breast cancer mortality (HR: 1.72, 95% CI: 1.05, 2.83), while other combinations did not reach statistical significance (Table 4). In models evaluating differences by hormone-receptor status (Table 5), MetS was associated with higher risk of breast cancer mortality for ER+/PR+ subtypes (HR: 1.28, 95% CI: 0.52, 3.16) and ER+/PR− (HR: 1.89, 95% CI: 0.36, 10.05), but lower risk of mortality for ER−/PR− subtypes (HR: 0.44, 95% CI: 0.11, 1.75) breast cancer. However, none of these associations by subtype reached statistical significance.
Table 3.
Hazard ratios (HRs)a and 95% confidence intervals for the association between MetS and breast cancer mortality by menopausal status
| Pre-menopausal | Post-menopausal | |||
|---|---|---|---|---|
| Overall | Overall | Normal BMI | Overweight/Obese | |
| N (Deaths)b | 3,693 (15) | 90,662 (592) | 41,652 (228) | 49,009 (364) |
| MetSδ | ||||
| Crude | NE | 2.24 (1.43, 3.51) | 1.45 (0.34, 6.15) | 1.66 (0.90, 3.08) |
| Adjusted § | NE | 2.07 (1.32, 3.25) | 0.91 (0.22, 3.66) | 1.15 (0.81, 1.64) |
| Components | ||||
| High WC | 1.34 (0.23, 7.80) | 1.45 (1.18, 1.78) | 1.19 (0.70, 2.01) | 1.32 (0.97, 1.79) |
| High cholesterol | 0.79 (0.27, 2.27) | 1.21 (1.03, 1.42) | 1.39 (1.05, 1.84) | 1.16 (0.94, 1.43) |
| High blood pressure | 2.44 (0.85, 7.03) | 1.28 (1.09, 1.51) | 1.18 (0.88, 1.58) | 1.21 (0.99, 1.49) |
| High fasting glucose | 1.42 (0.18, 11.09) | 1.40 (1.04, 1.89) | 1.38 (0.65, 2.94) | 1.19 (0.86, 1.66) |
| Number of MetS Components | ||||
| 0(Ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 0.75 (0.08, 7.48) | 1.49 (1.05, 2.12) | 1.72 (1.11, 2.67) | 1.02 (0.57, 1.83) |
| 2 | 1.21 (0.10, 13.93) | 2.05 (1.43, 2.94) | 1.96 (1.14, 3.38) | 1.45 (0.83, 2.53) |
| 3+ | NE | 2.09 (1.33, 3.28) | 1.39 (0.33, 5.89) | 1.42 (0.76, 2.64) |
Models are adjusted for age (crude model), plus BMI, region,race,physical activity,smoking, and marital status.
Death = number of breast cancer deaths; N=number of observations.
The reference category was women without any components of MetS.
Abbreviation: MetS, metabolic syndrome; NE, not estimable; Ref, reference; WC, waist circumference.
Bold indicates statistically significant at α= 0.05.
Table 4.
Hazard ratios (HRs)a and 95% confidence intervals for the association of combinations of MetS components with breast cancer mortality
| Overall | Post-menopausal | |
|---|---|---|
| N (Deaths)b | 94,555 (607) | 90,662 (592) |
| High blood pressure, fasting glucose, and WC | 1.59 (0.84, 3.02) | 1.72 (1.05, 2.83) |
| High cholesterol, fasting glucose, and WC | 0.52 (0.07, 3.75) | 1.40 (0.66, 2.95) |
| High blood pressure, fasting glucose, and cholesterol | 1.87 (0.82, 4.27) | 1.72 (0.97, 3.05) |
| High blood pressure, cholesterol, and WC | 1.55 (0.94, 2.55) | 1.27 (0.86, 1.87) |
Models are adjusted for age, BMI, region,race,physical activity,smoking, and marital status.
Abbreviation: MetS, metabolic syndrome; WC, waist circumference.
Death = number of breast cancer deaths; N=sample size
Bold indicates a statistically significant value.
Table 5.
Hazard ratios (HRs)a and 95% confidence intervals for the association between MetS and breast cancer mortality by breast cancer subtype γ
| ER+/PR+ | ER+/PR− | ER−/PR− | |
|---|---|---|---|
| N (Deaths)b | 2,995 (169) | 587 (58) | 619 (89) |
| MetSδ | |||
| Crude | 1.87 (0.78, 4.49) | 3.10 (0.63, 15.43) | 0.62 (0.17, 2.30) |
| Adjusted§ | 1.28 (0.52, 3.16) | 1.89 (0.36,10.05) | 0.44 (0.11, 1.75) |
| Components | |||
| High WC | 1.15 (0.72, 1.84) | 1.11 (0.52, 2.33) | 1.21 (0.58, 2.55) |
| High Cholesterol | 1.21 (0.90, 1.66) | 1.53 (0.89, 2.63) | 1.02 (0.66, 1.56) |
| High blood pressure | 1.16 (0.85, 1.60) | 1.25 (0.73, 2.14) | 1.09 (0.70, 1.70) |
| High fasting glucose | 1.72 (1.00, 2.96) | 0.80 (0.24, 2.64) | 1.70 (0.84, 3.46) |
| Number of MetS Components | |||
| 0(Ref) | 1.00 | 1.00 | 1.00 |
| 1 | 1.18 (0.59, 2.35) | 1.86 (0.54, 6.40) | 0.66 (0.30, 1.45) |
| 2 | 1.91 (0.94, 3.88) | 2.39 (0.65, 8.72) | 0.97 (0.42, 2.25) |
| 3+ | 1.23 (0.47, 3.22) | 2.05 (0.38, 10.96) | 0.50 (0.13, 2.03) |
Crude model included MetS and age only.
Models were adjusted for age, race, BMI, education, region, physical activity, smoking, and marital status
Death, number of those who died of breast cancer; N=sample size
The reference category was women without any components of MetS.
ER and PR status only due to limited sample sizes on HER2 receptor status
Abbreviations: ER, estrogen receptor; MetS, metabolic syndrome; PR, progesterone receptor; Ref, reference; WC, waist circumference.
Bold indicates a statistically significant value.
DISCUSSION
In the large prospective NIH-AARP cohort study, MetS was significantly associated with increased risk of breast cancer mortality, especially among post-menopausal women in a dose-response manner. That is, the risk of breast cancer mortality increased significantly as the number of MetS components increased, with over a 3-fold increase in risk of breast cancer mortality observed among women with four components of MetS compared with women with none. MetS was also associated with increased risk of breast cancer mortality in women who had normal BMI and overweight/obesity; however, these increases were not statistically significant. Further, the combination of high WC, high cholesterol and high blood pressure was most strongly associated with increased risk of breast cancer mortality.
These findings are consistent with two previous reports in the US showing that women with MetS had 26% [11] to 2-fold [18] higher risk of breast cancer mortality, and with a European study [15] documenting that women with MetS had 23% higher risk of breast cancer mortality. Other studies also support an association between MetS and poor breast cancer prognosis with results indicating an association of MetS with advanced cancer stage, metastasis, recurrence, and mortality [12,14]. Our findings on the associations between individual MetS components and risk of breast cancer mortality is also consistent with the result of a Norwegian study that found women in the highest tertiles of cholesterol and blood pressure had a 29% and 41% higher risk of breast cancer mortality, respectively [16]. A previous study observed that high blood glucose was associated with poor breast cancer prognosis [30]. Studies evaluating the differences in the association between MetS and breast cancer mortality by menopausal status have been limited and results are inconsistent [31]. Bjørge et al [15] evaluated the associations by age group (<50, 50–59 and ≥60 years) and found a positive association between MetS and breast cancer mortality in those ≥60 years old but no association in younger age groups. Some studies show that high WC is associated with poor breast cancer prognosis among post-menopausal women [32,33], while the association in pre-menopausal women is less clear [31,32]. To our knowledge, no study has simultaneously evaluated whether the association between MetS and breast cancer mortality varies by both BMI and menopausal status. Among post-menopausal women, there was consistently higher risk of breast cancer mortality associated with MetS, and a 45% increased risk of breast cancer mortality associated with high waist circumference. However, the association between MetS and breast cancer mortality among post-menopausal women were attenuated in BMI-stratified analysis. Among post-menopausal women with normal BMI, high cholesterol was associated with 39% higher risk of breast cancer mortality and the risk almost doubled in those with at least 2 components of MetS. Recent studies that evaluated the association of MetS with breast cancer mortality by subtype [17,20] found no significant association between MetS and breast cancer survival in models of subtype analysis; however, low HDL cholesterol was associated with poor survival among women with triple-negative breast cancer [20]. Although not statistically significant, we observed a trend towards increased breast cancer mortality among women with ER+ breast cancer, and an inverse association among women with ER- breast cancer, highlighting the need for more research in this area.
Several concerted pathways may predispose patients with MetS or metabolic dysregulation in general to poor breast cancer prognosis. Hyperinsulinemia, a common feature of MetS, promotes tumor growth, angiogenesis, and metastasis, and has anti-apoptotic properties [34,35]. Central obesity and the related increased adiposity may intensify aromatase activity, which converts androgens to estrogen, a hormone that promotes breast tumor growth and tumor cell survival [36–38]. The hormonal changes associated with menopause may also lead to central adiposity [39], and is seen as a driving factor for MetS after menopause [40]. Central adiposity is also related to a risk of insulin resistance, glucose intolerance, high blood pressure, and dyslipidemia [41]. Carriers of a single nucleotide polymorphism (SNP) APOA1 rs670 A/A associated with dyslipidemia, a component in MetS, were found to show about a 3-fold higher risk of breast cancer recurrence and nearly a 4-fold higher risk of breast cancer mortality [42], suggesting a potential role for gene-environment interaction driving MetS-associated breast cancer mortality risk. Abdominal obesity is also associated with chronic inflammation [43], which in turn is related to poor breast cancer prognosis [44,45], and studies suggest that cholesterol, another component of MetS, may be involved in cell signaling pathways in breast cancer [46] and may promote cancer metastasis [47,48]. The mechanisms through which MetS results in poor breast cancer outcomes are likely complex, multifaceted, and not yet fully elucidated.
There are several strengths and limitations relevant to the interpretation of these results. First, we were able to investigate the prospective association of MetS and components with breast cancer mortality in a large US cohort, and add important information to this literature regarding the association among pre- and post-menopausal women, those with normal weight and overweight/obesity, and by hormone-receptor subtype. Second, exposure and covariate data were obtained at baseline in a cancer-free cohort, reducing the risk of recall bias and/or reverse causality since measurement of these variables preceded diagnosis of breast cancer. Third, data on breast cancer mortality in the NIH-AARP cohort were obtained from cancer registries with high levels of ascertainment accuracy (>95% ascertainment rate) [49], thereby minimizing the risk of outcome misclassification [50]. The major limitations of this study were the limited sample size in sub-group analysis that may have limited our statistical power in detecting the existence of statistically significant associations, especially in analyses stratified by BMI and menopausal status. In addition, lack of detailed treatment data in the NIH-AARP cohort precluded our ability to adjust for these important variables, so future studies with treatment information will be needed to fill this critical gap. While we were able to evaluate associations by hormone-receptor subtype focusing on estrogen and progesterone receptors (ER and PR), we were limited by lack of data on HER2 status in the cohort to comprehensively evaluate the major breast cancer subtypes. Another limitation of this study is regarding the data on MetS exposure; since there were no clinical assessments of women in NIH-AARP cohort at baseline, we relied on self-reports of MetS components and could only assess 4 out of the 5 components. This may have resulted in underestimation of MetS prevalence. However, given the prospective cohort design, we expect that any misclassification would likely be non-differential in relation to the outcome and bias the estimates towards the null. Future studies with larger sample sizes and objective baseline measures of MetS are needed to fully validate our findings.
In conclusion, MetS was observed to be a strong risk factor for breast cancer mortality in a large US prospective cohort, especially among post-menopausal women. Future studies are needed to better elucidate the mechanisms linking metabolic dysregulation with tumor subtypes and mortality outcomes, especially among younger patients. Nevertheless, given the link between poor metabolic health and poor health outcomes in general, clinical strategies and lifestyle modification addressing MetS may be beneficial if incorporated into routine cancer care.
Funding:
TA was funded by grant K01TW010271 by the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Data Availability: The NIH-AARP dataset is publicly available for research investigations upon submission and approval of a research proposal. Details can be found at: https://www.nihaarpstars.com
Conflict of interest: The authors declare no conflict of interest.
Ethical approval: The Special Studies Institutional Review Board (IRB) of the U.S. National Cancer Institute approved the NIH-AARP Diet and Health Study (protocol number: OH95CN025)
REFERENCES
- 1.Nilsson PM,Engstrom G,Hedblad B (2007) The metabolic syndrome and incidence of cardiovascular disease in non-diabetic subjects--a population-based study comparing three different definitions. Diabet Med 24(5): 464–472. [DOI] [PubMed] [Google Scholar]
- 2.Athyros VG,Ganotakis ES,Elisaf MS,Liberopoulos EN,Goudevenos IA,Karagiannis A,Group, G-MC (2007) Prevalence of vascular disease in metabolic syndrome using three proposed definitions. Int J Cardiol 117(2): 204–210. [DOI] [PubMed] [Google Scholar]
- 3.Koren-Morag N,Goldbourt U,Tanne D (2005) Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: a prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke 36(7): 1366–1371. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM (2008) Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 28(4): 629–636. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamson PE,Gammon MD,Lund MJ,Flagg EW,Porter PL,Stevens J,Swanson CA,Brinton LA,Eley JW,Coates RJ (2006) General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev 15(10): 1871–1877. [DOI] [PubMed] [Google Scholar]
- 6.Chen H-l,Ding A,Wang M-l (2016) Impact of central obesity on prognostic outcome of triple negative breast cancer in Chinese women. SpringerPlus 5(594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogundiran TO,Huo D,Adenipekun A,Campbell O,Oyesegun R,Akang E,Adebamowo C,Olopade OI (2012) Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes Control 23(4): 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolucci A (2010) Epidemiological aspects of neoplasms in diabetes. Acta Diabetol 47(2): 87–95. [DOI] [PubMed] [Google Scholar]
- 9.Pereira A,Garmendia ML,Alvarado ME,Albala C (2012) Hypertension and the risk of breast cancer in Chilean women: a case-control study. Asian Pac J Cancer Prev 13(11): 5829–5834. [DOI] [PubMed] [Google Scholar]
- 10.Soler M,Chatenoud L,Negri E,Parazzini F,Franceschi S,la Vecchia C (1999) Hypertension and hormone-related neoplasms in women. Hypertension 34(2): 320–325. [DOI] [PubMed] [Google Scholar]
- 11.Calip GS,Malone KE,Gralow JR,Stergachis A,Hubbard RA,Boudreau DM (2014) Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res Treat 148(2): 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berrino F,Villarini A,Traina A,Bonanni B,Panico S,Mano MP,Mercandino A,Galasso R,Barbero M,Simeoni M, et al. (2014) Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat 147(1): 159–165. [DOI] [PubMed] [Google Scholar]
- 13.Maiti B,Kundranda MN,Spiro TP,Daw HA (2010) The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat 121(2): 479–483. [DOI] [PubMed] [Google Scholar]
- 14.Healy LA,Ryan AM,Carroll P,Ennis D,Crowley V,Boyle T,Kennedy MJ,Connolly E,Reynolds JV (2010) Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol (R Coll Radiol) 22(4): 281–288. [DOI] [PubMed] [Google Scholar]
- 15.Bjorge T,Lukanova A,Jonsson H,Tretli S,Ulmer H,Manjer J,Stocks T,Selmer R,Nagel G,Almquist M, et al. (2010) Metabolic syndrome and breast cancer in the mecan (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev 19(7): 1737–1745. [DOI] [PubMed] [Google Scholar]
- 16.Emaus A,Veierod MB,Tretli S,Finstad SE,Selmer R,Furberg AS,Bernstein L,Schlichting E,Thune I (2010) Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat 121(3): 651–660. [DOI] [PubMed] [Google Scholar]
- 17.Simon MS,Beebe-Dimmer JL,Hastert TA,Manson JE,Cespedes Feliciano EM,Neuhouser ML,Ho GYF,Freudenheim JL,Strickler H,Ruterbusch J, et al. (2018) Cardiometabolic risk factors and survival after breast cancer in the Women’s Health Initiative. Cancer 124(8): 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gathirua-Mwangi WG,Song Y,Monahan PO,Champion VL,Zollinger TW (2018) Associations of metabolic syndrome and C-reactive protein with mortality from total cancer, obesity-linked cancers and breast cancer among women in NHANES III. Int J Cancer 143(3): 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phipps AI,Malone KE,Porter PL,Daling JR,Li CI (2008) Reproductive and hormonal risk factors for postmenopausal luminal, HER2-overexpressing, and triple-negative breast cancer. Cancer 113(7): 1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y,Ding X,Wang J,Ma F,Yuan P,Li Q,Zhang P,Xu B (2015) Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers 30(2): e200–207. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki R,Orsini N,Saji S,Key TJ,Wolk A (2009) Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer 124(3): 698–712. [DOI] [PubMed] [Google Scholar]
- 22.Chan DS,Vieira AR,Aune D,Bandera EV,Greenwood DC,McTiernan A,Navarro Rosenblatt D,Thune I,Vieira R,Norat T (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25(10): 1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan DS,Norat T (2015) Obesity and breast cancer: not only a risk factor of the disease. Curr Treat Options Oncol 16(5): 22. [DOI] [PubMed] [Google Scholar]
- 24.Schatzkin A,Subar AF,Thompson FE,Harlan LC,Tangrea J,Hollenbeck AR,Hurwitz PE,Coyle L,Schussler N,Michaud DS, et al. (2001) Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 154(12): 1119–1125. [DOI] [PubMed] [Google Scholar]
- 25.Etemadi A,Sinha R,Ward MH,Graubard BI,Inoue-Choi M,Dawsey SM,Abnet CC (2017) Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. The BMJ 357(j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti KG,Eckel RH,Grundy SM,Zimmet PZ,Cleeman JI,Donato KA,Fruchart JC,James WP,Loria CM,Smith SC Jr., et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16): 1640–1645. [DOI] [PubMed] [Google Scholar]
- 27.Park Y,Leitzmann MF,Subar AF,Hollenbeck A,Schatzkin A (2009) Dairy food, Calcium, and Risk of Cancer in the NIH-AARP Diet and Health Study. Archives of internal medicine 169(4): 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiller L,Marshall A,Dunn J (2015) Assessing violations of the proportional hazards assumption in Cox regression: does the chosen method matter? Trials 16(Suppl 2): P134–P134. [Google Scholar]
- 29.Rong SS,Chen LJ,Leung CKS,Matsushita K,Jia L,Miki A,Chiang SWY,Tam POS,Hashida N,Young AL, et al. (2016) Ethnic specific association of the CAV1/CAV2 locus with primary open-angle glaucoma. Scientific Reports 6(27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monzavi-Karbassi B,Gentry R,Kaur V,Siegel ER,Jousheghany F,Medarametla S,Fuhrman BJ,Safar AM,Hutchins LF,Kieber-Emmons T (2016) Pre-diagnosis blood glucose and prognosis in women with breast cancer. Cancer & Metabolism 4(7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandera EV,Maskarinec G,Romieu I,John EM (2015) Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Advances in Nutrition 6(6): 803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borugian MJ,Sheps SB,Kim-Sing C,Olivotto IA,Van Patten C,Dunn BP,Coldman AJ,Potter JD,Gallagher RP,Hislop TG (2003) Waist-to-Hip Ratio and Breast Cancer Mortality. American Journal of Epidemiology 158(10): 963–968. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M,Cai H,Bao P,Xu W,Qin G,Shu XO,Zheng Y (2017) Body mass index, waist-to-hip ratio and late outcomes: a report from the Shanghai Breast Cancer Survival Study. Scientific Reports 7(6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang S,Song J,Kang H,Kim S,Lee Y,Park D (2003) Insulin can block apoptosis by decreasing oxidative stress via phosphatidylinositol 3-kinase- and extracellular signal-regulated protein kinase-dependent signaling pathways in HepG2 cells. Eur J Endocrinol 148(1): 147–155. [DOI] [PubMed] [Google Scholar]
- 35.Djiogue S,Nwabo Kamdje AH,Vecchio L,Kipanyula MJ,Farahna M,Aldebasi Y,Seke Etet PF (2013) Insulin resistance and cancer: the role of insulin and IGFs. Endocrine-Related Cancer 20(1): R1–R17. [DOI] [PubMed] [Google Scholar]
- 36.Chumsri S,Howes T,Bao T,Sabnis G,Brodie A (2011) Aromatase, aromatase inhibitors, and breast cancer. The Journal of steroid biochemistry and molecular biology 125(1–2): 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subbaramaiah K,Howe LR,Bhardwaj P,Du B,Gravaghi C,Yantiss RK,Zhou XK,Blaho VA,Hla T,Yang P, et al. (2011) Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prevention Research 4(3): 329–346. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Lorincz AM,Sukumar S (2006) Molecular links between obesity and breast cancer. Endocrine-Related Cancer 13(2): 279–292. [DOI] [PubMed] [Google Scholar]
- 39.Lobo RA (2008) Metabolic syndrome after menopause and the role of hormones. Maturitas 60(1): 10–18. [DOI] [PubMed] [Google Scholar]
- 40.Carr MC (2003) The Emergence of the Metabolic Syndrome with Menopause. The Journal of Clinical Endocrinology & Metabolism 88(6): 2404–2411. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro R,Azevedo I (2010) Chronic inflammation in obesity and the metabolic syndrome. Mediators of Inflammation 2010(289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu M-C,Lee K-T,Hsiao W-C,Wu C-H,Sun H-Y,Lin I-L,Young K-C (2013) The dyslipidemia-associated SNP on the APOA1/C3/A5 gene cluster predicts post-surgery poor outcome in Taiwanese breast cancer patients: a 10-year follow-up study. BMC Cancer 13(1): 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen SB,Lee YC,Ser KH,Chen JC,Chen SC,Hsieh HF,Lee WJ (2009) Serum C-reactive protein and white blood cell count in morbidly obese surgical patients. Obes Surg 19(4): 461–466. [DOI] [PubMed] [Google Scholar]
- 44.Allin KH,Nordestgaard BG,Flyger H,Bojesen SE (2011) Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res 13(3): R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villaseñor A,Flatt SW,Marinac C,Natarajan L,Pierce JP,Patterson RE (2013) Postdiagnosis C-reactive protein and breast cancer survivorship: findings from the WHEL study. Cancer Epidemiology Biomarkers & Prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou P,Li B,Liu B,Chen T,Xiao J (2018) Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clinica Chimica Acta 477(94–104. [DOI] [PubMed] [Google Scholar]
- 47.Jeon JH,Kim SK,Kim HJ,Chang J,Ahn CM,Chang YS (2010) Lipid raft modulation inhibits NSCLC cell migration through delocalization of the focal adhesion complex. Lung Cancer 69(2): 165–171. [DOI] [PubMed] [Google Scholar]
- 48.Reverter M,Rentero C,Garcia-Melero A,Hoque M,Vilà de Muga S,Álvarez-Guaita A,Conway JRW,Wood P,Cairns R,Lykopoulou L, et al. (2014) Cholesterol regulates syntaxin 6 trafficking at trans-golgi network endosomal boundaries. Cell Reports 7(3): 883–897. [DOI] [PubMed] [Google Scholar]
- 49.Hermansen SW,Leitzmann MF,Schatzkin A (2009) The impact on National Death Index ascertainment of limiting submissions to Social Security Administration Death Master File Matches in epidemiologic studies of mortality. American Journal of Epidemiology 169(7): 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schatzkin A,Subar AF,Thompson FE,Harlan LC,Tangrea J,Hollenbeck AR,Hurwitz PE,Coyle L,Schussler N,Michaud DS, et al. (2001) Design and Serendipity in Establishing a Large Cohort with Wide Dietary Intake Distributions The National Institutes of Health–American Association of Retired Persons Diet and Health Study. American Journal of Epidemiology 154(12): 1119–1125. [DOI] [PubMed] [Google Scholar]