Abstract
Introduction
Little is known about the association of urine metabolites with structural lesions in persons with diabetes.
Objectives
We examined the relationship between 12 urine metabolites and kidney structure in American Indians with type 2 diabetes.
Methods
Data were from a 6-year clinical trial that assessed renoprotective efficacy of losartan, and included a kidney biopsy at the end of the treatment period. Metabolites were measured in urine samples collected within a median of 6.5 months before the research biopsy. Associations of the creatinine-adjusted urine metabolites with kidney structural variables were examined by Pearson’s correlations and multivariable linear regression after adjustment for age, sex, diabetes duration, hemoglobin A1c, mean arterial pressure, glomerular filtration rate (iothalamate), and losartan treatment.
Results
Participants (n = 62, mean age 45 ± 10 years) had mean ± standard deviation glomerular filtration rate of 137 ± 50 ml/min and median (interquartile range) urine albumin:creatinine ratio of 34 (14–85) mg/g near the time of the biopsy. Urine aconitic and glycolic acids correlated positively with glomerular filtration surface density (partial r = 0.29, P = 0.030 and r = 0.50, P < 0.001) and total filtration surface per glomerulus (partial r = 0.32, P = 0.019 and r = 0.43, P = 0.001). 2-ethyl 3-OH propionate correlated positively with the percentage of fenestrated endothelium (partial r = 0.32, P = 0.019). Citric acid correlated negatively with mesangial fractional volume (partial r=−0.36, P = 0.007), and homovanillic acid correlated negatively with podocyte foot process width (partial r=−0.31, P = 0.022).
Conclusions
Alterations of urine metabolites may associate with early glomerular lesions in diabetic kidney disease.
Keywords: Metabolites, Biomarkers, Kidney structure, Type 2 Diabetes
1. Introduction
The mitochondrion is a major energy generating intracellular organelle responsible for optimizing the intracellular environment. Hyperglycemia is believed to damage mitochondrial DNA, leading to progressive impairment of mitochondrial metabolism and ultimately to cellular damage in various tissues (Czajka et al. 2015). We previously identified 13 metabolites with lower concentrations in the urine of persons with diabetic kidney disease (DKD) than in those without (Sharma et al. 2013). Bioinformatic analyses found that 12 of these metabolites were related directly to mitochondrial function. Lower urine concentrations of these metabolites were associated with reduced mitochondrial content and expression of PGC1α in kidney tissue—PGC1α is a master regulator of mitochondrial biogenesis. Lower urine concentrations were also associated with reduced mitochondrial DNA in urine exosomes from persons with DKD compared with diabetic controls.
In the present study, we examined the relationships of these metabolites with kidney structural lesions in American Indians with type 2 diabetes who were previously enrolled in a 6-year randomized clinical trial that evaluated the renoprotective efficacy of the angiotensin receptor blocker losartan in DKD (Weil et al. 2013). The morphometric data were obtained from a kidney biopsy performed at the end of the clinical trial. The metabolites were measured in urine samples obtained at a research examination that coincided with the biopsy and were adjusted for urine creatinine concentration by dividing urine metabolite concentration by urine creatinine concentration prior to analysis.
2. Materials and methods
2.1. Study participants and design
All patients from this study were enrolled in a randomized clinical trial (Weil et al. 2013) testing the renoprotective efficacy of losartan in early DKD (ClinicalTrials.gov number, NCT00340678). Glomerular filtration rate (GFR) was measured annually throughout the trial by the urinary clearance of iothalamate. At the end of the six-year clinical trial, 111 of the participants underwent percutaneous kidney biopsy to determine whether treatment was associated with preservation of kidney structure. Of the 111 biopsied participants, 62 had remaining urine samples available and were included in the present cross-sectional study. This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each subject signed an informed consent document.
2.2. Clinical and anthropometric measures
Blood pressure was measured while the subject was resting in the seated position. Mean arterial pressure (MAP) was calculated as (2 × diastolic blood pressure + systolic blood pressure)/3. Hemoglobin A1c, urine albumin and creatinine concentration, and GFR were measured as previously described (Saulnier et al. 2017).
2.3. Urine metabolites
Metabolites were measured in urine samples collected under standardized conditions and stored at −80 °C. Samples underwent one freeze–thaw cycle prior to assay. The samples were collected at the nearest annual research examination conducted prior to the date of the kidney biopsy. A urine sample collected at another annual research examination that occurred a median of 12.1 (interquartile range 11.7–12.5) months earlier (n = 60 [97%]) or later (n = 2 [3%]) was measured to assess biological stability of the metabolites over time.
Urine metabolites were measured in a blinded fashion in spot urine samples collected after an overnight fast at the onset of the renal clearance study. Samples were derivatized as described previously (Hallan et al. 2017) and metabolite concentrations were measured by gas chromatography coupled to triple quadrupole mass spectroscopy (GC-MS/MS), using a Bruker Scion-TQ GC-MS system. A control sample of pooled healthy urine was run after every 8 samples to control for batch variations and results were adjusted for batch effect. Concentrations of the metabolites were quantified using an 8-point calibration curve with R2 values above 0.98. Metabolite concentrations were normalized to urine creatinine concentrations measured in the same aliquot of urine by a biochemical assay using a creatinine assay kit from Cayman Chemical Company (Ann Arbor, MI). The results are reported in nmol of metabolite per µM of creatinine. Urine metabolite concentrations below the detection limit of the assay (i.e., 0.3 nmol for citric acid, 0.1 nmol for the other metabolites) were set to the detection limit. Of the 13 metabolites, previously related to mitochondrial dysfunction (Sharma et al. 2013) and identified in The Human Metabolome database (http://www.hmdb.ca), only 2-methyl-acetoacetic acid was not measured in the samples for technical reasons. The remaining 12 were measured in all study participants. Within-person reproducibility of the biomarker assays was assessed by intraclass correlation (ICC) with a second measurement from 20 duplicate samples blinded to the performance laboratory.
2.4. Morphometric methods
Digital images from the kidney biopsy tissue sections were collected and the renal structural parameters were quantified by morphometric methods as described previously (Weil et al. 2013). Parameters included were glomerular volume, glomerular basement membrane width, cortical interstitial fractional volume per glomerulus, mesangial fractional volume per glomerulus, glomerular filtration surface density, total filtration surface per glomerulus, number of podocytes per glomerulus, podocyte foot process width, percentage of podocyte detachment, percentage of normally fenestrated endothelium, and percentage of sclerotic glomeruli (Squarer et al. 1998). Morphometric variables for each individual were calculated as the mean of all measurements for that individual.
2.5. Statistical analysis
Patient characteristics were expressed as mean ± standard deviation, median (25th–75th percentile), or n (%). Pearson’s correlations were used to assess the relationships between metabolites, morphometric variables, and clinical characteristics. For regression analyses, we log2-transformed all variables with skewed distributions. Correlations between urine metabolites were partialled for urine creatinine concentration. Partial correlation analysis was used to study the relationships between biomarkers and structural measurements after adjustment for the effects of age, sex, diabetes duration, hemoglobin A1c, blood pressure, GFR, and treatment assignment during the clinical trial by multivariable linear regression. ACR was not included in these models because it is highly correlated with the underlying structural lesions. Treatment assignment was included as this was an important exposure in the 6 years leading up to the present observational study. Regression model fit was assessed for normality and leverage with ‘Studentized’ residuals. Relationships of urine metabolites with global glomerular sclerosis and percentage of podocyte detachment were not reported due to inadequate model fit. In addition, models for the relationships of 3-hydroxy isobutyrate and uracil with morphometric variables were reported after removing a highly influential point. Multicollinearity was assessed with eigenvalues and the condition index (Belsley et al. 1980). Each metabolite was also tested for interaction with treatment assignment and with angiotensin receptor blocker treatment. Associations between metabolites and morphometric measures were illustrated graphically by partial residual regression plots and were reported as partial Pearson’s correlation coefficients. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). P values < 0.05 were considered statistically significant.
3. Results
Clinical characteristics, creatinine-normalized urine metabolite concentrations, and morphometric variables from the 62 study participants are summarized in Table 1. Mean age of the participants was 45 ± 10 years, mean diabetes duration was 15.4 ± 6.1 years, mean hemoglobin A1c was 9.5 ± 1.9%, mean GFR was 137 ± 50 ml/min, and median ACR was 34 (14–85) mg/g at a research examination performed within a median of 6.5 (3.2–8.7) months before the research kidney biopsy. Thirty-one (50%) participants had normoalbuminuria (ACR < 30 mg/g), 18 (29%) had microalbuminuria (ACR 30 to < 300 mg/g), and 13 (21%) had macroalbuminuria (ACR ≥ 300 mg/g). Fifty-seven (92%) participants had measured GFR above 60 ml/min; the remaining five participants had values ranging from 40 to 57 ml/min.
Table 1.
Baseline characteristics of the study population. Data are presented as mean ± standard deviation, median (25th–75th percentiles), or number (%)
| Clinical characteristics | n = 62 |
|---|---|
| Age (years) | 44.6 ± 9.7 |
| Female:Male n (female %) | 49:13 (79) |
| Losartan treatment group n (%) | 33 (53) |
| Diabetes duration (years) | 15.4 ± 6.1 |
| Body mass index (kg/m2) | 35.4 ± 7.3 |
| Systolic blood pressure (mmHg) | 123 ± 14 |
| Diastolic blood pressure (mmHg) | 78 ± 10 |
| Mean arterial pressure (mmHg) | 93 ± 10 |
| HbA1c(%) | 9.5 ± 1.9 |
| Glomerular filtration rate (ml/min) | 137 ± 50 |
| Urine albumin/creatinine ratio (mg/g) | 34 (14–85) |
| Any oral antidiabetic drug treatment n (%) | 50 (81) |
| Metformin treatment n (%) | 36 (58) |
| Insulin treatment n (%) | 34 (55) |
| Urines metabolites (nmol/µmol creatinine) | |
| 2-ethyl 3-OH propionate | 3–3.64 1.77–7.19) |
| 3-hydroxy propionate | 5–3.66 (1.83–8.98) |
| 3-hydroxy isovalerate | 12.1 (8.1–21.5) |
| 3-methyl-crotonyl glycine | 0.49 (0.34–0.77) |
| 3-hydroxy isobutyrate | 14.8 (7.6–24.7) |
| Tiglyglycine | 1.69 (1.22–2.11) |
| Aconitic acid | 34.1 (22.7–42.6) |
| Citric acid | 201 (136–319) |
| Glycolic acid | 45.8 (26.7–76.7) |
| Homovanillic acid | 1.63 (1.24–2.02) |
| Uracil | 3.94 (2.34–6.21) |
| 3-methyl adipic acid | 1.06 (0.71–1.47) |
| Morphometric variables | |
| Glomerular volume (× 106 µm3) | 5.7 (4.9–7.4) |
| Cortical interstitial fractional volume (%) | 31 (26–35) |
| Mesangial fractional volume (%) | 19 (14–27) |
| Glomerular filtration surface density (µm2/ µm3) | 0.07 (0.05–0.08) |
| Total filtration surface per glomerulus (× 105 µm2) 3.8 (3.1–4.9) | |
| Glomerular basement membrane width (nm) | 524 (432–631) |
| Global glomerular sclerosis (%) | 7.1 (0–17.4) |
| Podocyte detachment (%) | 0.33 (0–1.49) |
| Podocyte cell number per glomerulus | 598 (458–725) |
| Foot process width (nm) | 449 (407–528) |
| Fenestrated endothelium (%) | 25.8 (21.8–31.7) |
Metabolite assay reproducibility, assessed by ICC with a second measurement obtained from 20 duplicate samples blinded to the performance laboratory, was excellent for 2-ethyl-3-hydroxy propionate (ICC = 0.814), fair to good for aconitic acid (ICC = 0.597), 3-hydroxy propionate (ICC = 0.596), 3-hydroxy isobutyrate (ICC = 0.586), homovanillic acid (ICC = 0.563), uracil (ICC = 0.562), tiglyglycine (ICC = 0.534), 3-methyl-crotonyl glycine (ICC = 0.465), 3-methyl adipic acid (ICC = 0.442), and citric acid (ICC = 0.423), and poor for 3-hydroxy isovalerate (ICC = 0.231), and glycolic acid (ICC = 0.128). Urine metabolite concentrations in samples taken at the research examination closest to the kidney biopsy correlated with samples taken from a research examination that occurred a median of 12.1 (11.7–12.5) months earlier or later, indicating stability of metabolite concentrations over this period (Table 2).
Table 2.
Median (25th–75th percentiles) urine metabolite concentrations measured at two time points about 1-year apart, and Pearsons correlations between the two measurements (n = 62)
| Urine metabolites | HMDB ID | Value #1* | Value #2* | r (95%CI) |
|---|---|---|---|---|
| 2-ethyl 3-OH propionate | HMDB00396 | 3.68 (1.98–6.05) | 3.64 (1.77–7.19) | 0.61 (0.43–0.75) |
| 3-hydroxy propionate | HMDB00700 | 3.66 (1.89–8.26) | 3.66 (1.83–8.98) | 0.48 (0.27–0.65) |
| 3-hydroxy isovalerate | HMDB00754 | 15.2 (9.2−-24.1) | 12.1 (8.1–21.5) | 0.64 (0.46–0.76) |
| 3-methyl-crotonyl glycine | HMDB00459 | 0.55 (0.39–0.88) | 0.49 (0.34–0.77) | 0.37 (0.14–0.57) |
| 3-hydroxy isobutyrate | HMDB00023 | 15.2 (8.0−-24.1) | 14.8 (7.6–24.7) | 0.57 (0.38–0.72) |
| Tiglyglycine | HMDB00959 | 1.45 (1.16–2.14) | 1.69 (1.22–2.11) | 0.28 (0.03–0.50) |
| Aconitic acid | HMDB00072 | 33.9 (21.2–45.7) | 34.1 (22.7–42.6) | 0.55 (0.35–0.70) |
| Citric acid | HMDB00094 | 200 (129–304) | 201 (136–319) | 0.61 (0.42–0.74) |
| Glycolic acid | HMDB00115 | 53.5 (23.6–81.2) | 45.8 (26.7–76.7) | 0.59 (0.39–0.73) |
| Homovanillic acid | HMDB00118 | 1.56 (1.24–2.21) | 1.63 (1.24–2.02) | 0.28 (0.03–0.49) |
| Uracil | HMDB00300 | 4.18 (2.15–5.57) | 3.94 (2.34–6.21) | 0.50 (0.29–0.67) |
| 3-methyl adipic acid | HMDB00555 | 0.97 (0.66–1.31) | 1.06 (0.71–1.47) | 0.34 (0.10–0.55) |
*Units are nmol/mol creatinine
Of the 12 metabolites measured in this study, seven correlated positively with GFR and three correlated negatively with ACR. Of the seven metabolites that correlated positively with GFR, six also correlated positively with hemoglobin A1c, five correlated negatively with systolic blood pressure, and one correlated negatively with BMI (Table 3). Concentrations of the metabolites were generally highly intercorrelated (Supplemental Table 1).
Table 3.
Pearson’s correlations (P-value) of urine metabolites with clinical variables in the study population
| Urine metabolites | Age | Diabetes duration | Systolic blood pressure | BMI | HbA1c | ACR | GFR |
|---|---|---|---|---|---|---|---|
| 2-ethyl 3-OH propionate | −0.20 (0.124) | 0.03 (0.822) | −0.27 (0.034) | −0.11 (0.389) | 0.56 (< 0.001) | −0.04 (0.745) | 0.45 (< 0.001) |
| 3-hydroxy propionate | −0.35 (0.006) | −0.11 (0.390) | − 0.27 (0.033) | −0.12 (0.337) | 0.43 (0.001) | − 0.17 (0.191) | 0.46 (< 0.001) |
| 3-hydroxy isovalerate | −0.37 (0.003) | −0.13 (0.324) | − 0.21 (0.106) | − 0.12 (0.349) | 0.47 (< 0.001) | − 0.11 (0.381) | 0.55 (< 0.001) |
| 3-methyl-crotonyl Glycine | −0.14 (0.262) | 0.05 (0.704) | − 0.09 (0.478) | −0.04 (0.762) | 0.30 (0.016) | 0.004 (0.977) | 0.24 (0.060) |
| 3-hydroxy isobu-tyrate | −0.16 (0.201) | 0.02 (0.899) | −0.14 (0.286) | − 0.01 (0.950) | 0.52 (< 0.001) | −0.08 (0.526) | 0.41 (0.001) |
| Tiglyglycine | −0.10 (0.443) | 0.01 (0.954) | −0.24 (0.061) | − 0.21 (0.095) | 0.25 (0.050) | −0.04 (0.729) | 0.12 (0.351) |
| Aconitic acid | −0.18 (0.160) | 0.01 (0.931) | − 0.34 (0.007) | − 0.26 (0.041) | 0.47 (< 0.001) | − 0.24 (0.062) | 0.42 (0.001) |
| Citric acid | − 0.05 (0.686) | − 0.03 (0.789) | −0.35 (0.006) | −0.11 (0.385) | 0.44 (< 0.001) | − 0.33 (0.009) | 0.32 (0.010) |
| Glycolic acid | − 0.34 (0.007) | −0.20 (0.114) | − 0.30 (0.016) | −0.09 (0.479) | 0.30 (0.017) | − 0.32 (0.010) | 0.42 (0.001) |
| Homovanillic acid | −0.15 (0.249) | − 0.03 (0.789) | −0.17 (0.193) | −0.06 (0.671) | 0.07 (0.580) | − 0.15 (0.244) | 0.02 (0.858) |
| Uracil | −0.07 (0.586) | 0.11 (0.401) | − 0.19 (0.131) | 0.25 (0.055) | 0.11 (0.389) | − 0.29 (0.024) | 0.17 (0.187) |
| 3-methyl adipic acid | − 0.12 (0.368) | −0.20 (0.126) | −0.29 (0.025) | −0.14 (0.288) | 0.22 (0.084) | − 0.14 (0.269) | 0.06 (0.636) |
P values < 0.05 are shown in boldface
ACR urine albumin/creatinine ratio, BMI body mass index, GFR glomerular filtration rate
3.1. Correlations between urine metabolites and kidney structure
Univariate correlations of metabolites with morphometric variables are shown in Table 4. 3-hydroxy isovalerate correlated positively with podocyte number per glomerulus (r = 0.25, P = 0.050). 3-hydroxy isobutyrate and aconitic acid correlated positively with glomerular filtration surface density (r = 0.28, P = 0.030 and r = 0.30, P = 0.017). Citric acid correlated positively with glomerular filtration surface density and total filtration surface per glomerulus (r = 0.47, P < 0.001 and r = 0.31, P = 0.014) and negatively with mesangial fractional volume (r=−0.26, P = 0.020). Glycolic acid correlated negatively with mesangial fractional volume and cortical interstitial fractional volume (r=−0.26, P = 0.041 and r=−0.27, P = 0.034). Homovanillic acid correlated negatively with podocyte foot process width (r=−0.33, P = 0.008).
Table 4.
Pearson’s correlations (P-values) of urine metabolites with glomerular structural parameters in 62 participants with type 2 diabetes mellitus
| VG | Sv | %FE | Podo | TFS | GBM | VvMes | VvInt | FPW | |
|---|---|---|---|---|---|---|---|---|---|
| 2-ethyl 3-OH pro-pionate | −0.04 (0.765) | 0.21 (0.095) | 0.21 (0.105) | 0.17 (0.196) | 0.16 (0.202) | 0.02 (0.863) | −0.11 (0.384) | −0.10 (0.430) | −0.06 (0.667) |
| 3-hydroxy propionate | −0.04 (0.745) | 0.21 (0.101) | 0.15 (0.249) | 0.17 (0.188) | 0.19 (0.143) | 0.06 (0.641) | −0.14(0.267) | −0.24 (0.061) | −0.02 (0.865) |
| 3-hydroxy isovalerate | 0.03 (0.831) | 0.19 (0.135) | 0.06 (0.640) | 0.25 (0.050) | 0.21 (0.105) | −0.04 (0.764) | −0.19 (0.149) | −0.23 (0.069) | −0.06 (0.635) |
| 3-methyl-crotonyl glycine | 0.15 (0.238) | 0.04 (0.770) | −0.16 (0.202) | 0.02 (0.886) | 0.16 (0.204) | 0.17 (0.193) | 0.17 (0.177) | −0.03 (0.797) | 0.07 (0.596) |
| 3-hydroxy isobutyrate | 0.08 (0.538) | 0.22 (0.092) | 0.14 (0.295) | 0.19 (0.151) | 0.24 (0.066) | 0.04 (0.777) | −0.10 (0.462) | −0.15 (0.248) | −0.01 (0.929) |
| Tiglyglycine | −0.01 (0.935) | 0.05 (0.685) | 0.02 (0.875) | −0.03 (0.840) | 0.08 (0.546) | 0.12 (0.346) | 0.15 (0.254) | 0.11 (0.396) | −0.02 (0.879) |
| Aconitic acid | −0.11 (0.386) | 0.30 (0.017) | 0.18 (0.162) | 0.06 (0.628) | 0.24 (0.059) | 0.01 (0.942) | −0.17 (0.193) | −0.20 (0.111) | −0.11 (0.385) |
| Citric acid | −0.18 (0.165) | 0.47 (< 0.001) | 0.25 (0.053) | 0.16 (0.219) | 0.31 (0.014) | −0.14 (0.290) | −0.30 (0.020) | −0.18 (0.168) | −0.03 (0.811) |
| Glycolic acid | −0.10 (0.453) | 0.25 (0.053) | 0.14 (0.285) | 0.17 (0.181) | 0.20 (0.129) | −0.03 (0.806) | −0.24 (0.056) | −0.27 (0.036) | −0.13 (0.310) |
| Homovanillic acid | −0.10 (0.454) | 0.18 (0.162) | 0.08 (0.540) | −0.03 (0.794) | 0.11 (0.382) | −0.05 (0.693) | −0.07 (0.614) | −0.10 (0.455) | −0.33 (0.008) |
| Uracil | −0.15 (0.242) | 0.15 (0.264) | 0.11 (0.410) | −0.09 (0.469) | 0.06 (0.653) | −0.03 (0.798) | −0.21 (0.100) | −0.10 (0.426) | −0.10 (0.424) |
| 3-methyl adipic acid | 0.03 (0.819) | −0.04 (0.778) | −0.14 (0.283) | −0.08 (0.531) | 0.09 (0.494) | 0.21 (0.105) | 0.12 (0.336) | −0.08 (0.535) | −0.08 (0.560) |
P values < 0.05 are shown in boldface
VG glomerular volume, Sv glomerular filtration surface density, %FE percentage of fenestrated endothelium, Podo podocyte cell number per glomerulus, TFS total filtration surface per glomerulus, GBM glomerular basement membrane width, VvMes mesangial fractional volume, VvInt cortical interstitial fractional volume, FPW foot process width
Partial correlations between urine metabolites and kidney structural parameters, after adjustment for age, sex, diabetes duration, hemoglobin A1c, MAP, GFR, and losartan treatment assignment, are shown in Table 5. In general, lower concentrations of the urine metabolites correlated with greater structural lesions, and four of the 12 metabolites had statistically significant correlations with morphometric variables. 2-ethyl 3-OH propionate correlated positively with the percentage of fenestrated endothelium. Aconitic and citric acids correlated positively with glomerular filtration surface density and total filtration surface per glomerulus. Citric acid correlated negatively with mesangial fractional volume, and homovanillic acid correlated negatively with podocyte foot process width. Further adjustment for BMI did not modify these findings. For illustration, partial residual regression plots of the relationships of citric acid with glomerular filtration surface density, total filtration surface per glomerulus, and mesangial fractional volume are shown in Fig. 1.
Table 5.
Partial Pearson’s correlation matrix (P-values) between urine metabolite concentrations and morphometric variables
| VG | Sv | %FE | Podo | TFS | GBM | VvMes | VvInt | FPW | |
|---|---|---|---|---|---|---|---|---|---|
| 2-ethyl 3-OH propionate | 0.02 (0.884) | 0.22 (0.109) | 0.32 (0.019) | 0.22 (0.102) | 0.19 (0.174) | −0.15 (0.259) | −0.25 (0.067) | −0.08 (0.581) | −0.17 (0.202) |
| 3-hydroxy propionate | 0.04 (0.776) | 0.14 (0.299) | 0.16 (0.249) | 0.15 (0.260) | 0.18 (0.190) | −0.03 (0.837) | −0.15 (0.288) | −0.18 (0.187) | −0.06 (0.656) |
| 3-hydroxy isovalerate | 0.04 (0.768) | 0.15 (0.282) | 0.07 (0.626) | 0.24 (0.072) | 0.15 (0.271) | −0.20 (0.149) | −0.24 (0.084) | −0.17 (0.204) | −0.14 (0.317) |
| 3-methyl-crotonyl glycine | 0.15 (0.262) | 0.05 (0.735) | −0.16 (0.254) | 0.01 (0.916) | 0.16 (0.242) | 0.10 (0.457) | 0.15 (0.286) | 0.01 (0.931) | 0.05 (0.697) |
| 3− hydroxy isobutyrate | 0.13 (0.354) | 0.21 (0.119) | 0.21 (0.126) | 0.22 (0.107) | 0.25 (0.069) | −0.09 (0.505) | −0.19 (0.169) | −0.18 (0.201) | −0.12 (0.391) |
| Tiglyglycine | 0.04 (0.761) | 0.01 (0.949) | 0.02 (0.878) | −0.02 (0.876) | 0.08 (0.546) | 0.08 (0.541) | 0.15 (0.278) | 0.22 (0.105) | 0.01 (0.940) |
| Aconitic acid | −0.02 (0.909) | 0.29 (0.030) | 0.19 (0.155) | 0.05 (0.713) | 0.32 (0.019) | −0.09 (0.528) | −0.22 (0.113) | −0.21 (0.125) | −0.23 (0.095) |
| Citric acid | −0.07 (0.627) | 0.50 (< 0.001) | 0.25 (0.062) | 0.18 (0.179) | 0.43 (0.001) | −0.25 (0.066) | − 0.36 (0.007) | −0.21 (0.117) | −0.12 (0.394) |
| Glycolic acid | −0.05 (0.708) | 0.15 (0.290) | 0.10 (0.458) | 0.12 (0.386) | 0.13 (0.340) | −0.09 (0.509) | −0.22 (0.104) | −0.17 (0.206) | −0.14 (0.300) |
| Homovanillic acid | −0.004 (0.980) | 0.15 (0.288) | 0.05 (0.693) | −0.03 (0.846) | 0.17 (0.216) | − 0.07 (0.628) | −0.05 (0.706) | 0.03 (0.826) | − 0.31 (0.022) |
| Uracil | − 0.09 (0.500) | 0.07 (0.597) | 0.06 (0.648) | −0.12 (0.369) | 0.04 (0.754) | − 0.04 (0.794) | − 0.24 (0.075) | −0.07 (0.640) | −0.11 (0.440) |
| 3-methyl adipic acid | 0.16 (0.249) | −0.18 (0.176) | − 0.23 (0.092) | −0.13 (0.345) | 0.06 (0.658) | 0.22 (0.113) | 0.19 (0.172) | −0.01 (0.928) | −0.03 (0.805) |
P values < 0.05 are shown in boldface
Correlations are adjusted for age, sex, diabetes duration, hemoglobin A1c, mean arterial pressure, GFR, and treatment assignment
VG glomerular volume, SV glomerular filtration surface density, %FE percentage of fenestrated endothelium, Podo podocyte cell number per glomerulus, TFS total filtration surface per glomerulus, GBM glomerular basement membrane width, VvMes mesangial fractional volume, VvInt cortical interstitial fractional volume, FPW foot process width
Fig. 1.
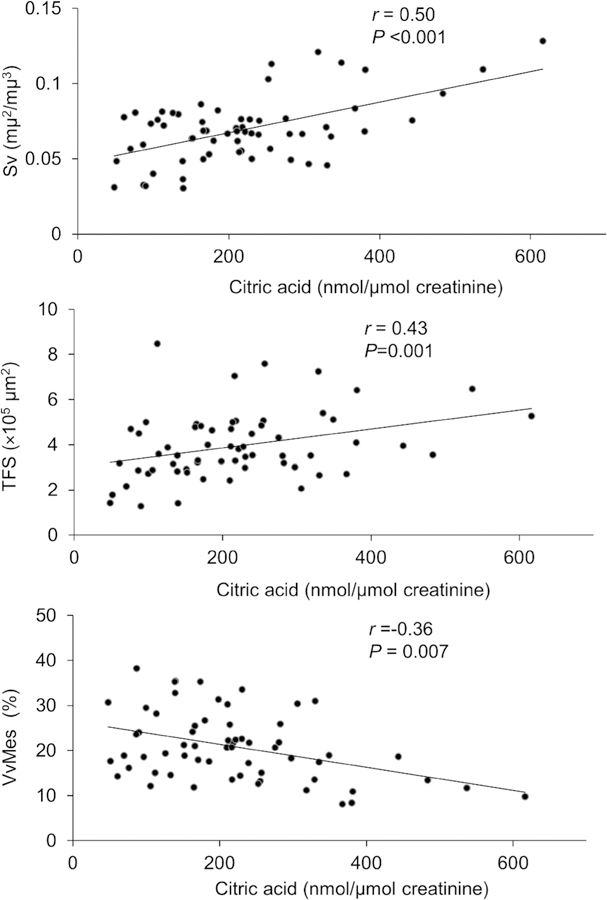
Partial regression residual plot of the associations between urine citric acid and morphometric variables. The residuals were computed from regressing each of these variables on age, sex, diabetes duration, hemoglobin A1c, mean arterial pressure, GFR, and treatment assignment. Pearson’s partial r and the corresponding P value are shown. Abbreviations: Sv glomerular filtration surface density, VvMes mesangial fractional volume, TFS total filtration surface per glomerulus
4. Discussion
In this study we show that lower concentrations of urine metabolites previously identified as potential biomarkers of DKD are associated with the glomerular structural lesions of early DKD in American Indians with type 2 diabetes. Lower urine metabolite concentrations showed the strongest association with loss of filtration surface and increased mesangial fractional volume after controlling for the effects of relevant clinical covariates. These findings provide evidence that lower urine concentrations of these metabolites coincide with some early lesions in specialized filtration structures in type 2 diabetes. The metabolites included in this study are closely connected to mitochondrial metabolism in that they are either generated within the tricarboxylic acid (TCA) cycle or regulated by mitochondrial enzymes and are involved in major metabolic pathways, including branched-chain amino acid (BCAA) catabolism, the TCA cycle, dopamine metabolism, and glycolic acid metabolism. Further research is required to determine whether the lower concentrations of these urine metabolites that we observed in those with kidney structural abnormalities reflect mitochondrial dysfunction at early stages of DKD.
2-ethyl 3-OH propionate is a short-chain fatty acid (SCFA) derived from the metabolism of BCAAs (Macfarlane and Macfarlane 2003). BCAAs—i.e. valine, leucine, and isoleucine—are essential amino acids. Despite their beneficial contribution to normal human protein and lipid synthesis, increased fasting concentrations of circulating BCAAs are associated with an increased risk of insulin resistance and obesity (Lynch and Adams 2014). SCFAs are produced by the microbiota in the gut where they are absorbed and enter the circulation and are filtered into the urine. They act via G protein coupled receptors (GPCR) and through non-GPCR mediated mechanisms to modulate various physiological functions (Natarajan and Pluznick 2014). In mice, SCFAs modulate renin release and blood pressure (Pluznick et al. 2013) and ameliorate the effects of hypoxia in kidney epithelial cells by improving mitochondrial biogenesis (Andrade-Oliveira et al. 2015). In rats with CKD, a high fermentable fiber diet increases SCFA production and is associated with improvement in kidney function and reduction in kidney inflammation and fibrosis (Vaziri et al. 2014). Persons with end-stage renal disease (ESRD) exhibit a decreased capacity to produce SCFAs compared with healthy individuals (Wong et al. 2014), and plasma concentrations of 4 BCAA metabolites were negatively associated with the risk of progression to ESRD in persons with type 2 diabetes (Niewczas et al. 2014). In addition, BCAAs activate the mammalian target of rapamycin complex 1 pathway, which favors diabetic glomerulopathy (Inoki 2008). Our findings highlight the association of the lower urine SCFA 2-ethyl 3-OH propionate with the loss of endothelial fenestrae in the glomerular capillary—one of the earliest structural changes of DKD that occurs prior to the onset of reduced GFR and, in some cases, elevated ACR, and is known to predict the decline in GFR (Ellis at al. 1986; Fufaa et al. 2016; Moriya et al. 2014; Nosadini et al. 2000).
Citric acid and aconitic acid are TCA cycle intermediates, with citric acid produced by citrate synthase, which is then converted to aconitic acid by aconitase. Fractional excretion of citrate is highly regulated (Simpson 1983). Citrate is actively reabsorbed in the proximal tubules via a sodium-citrate co-transporter and is metabolized by renal cortical citrate lyase. Metabolic acidosis and hypokalemia both increase renal cortical ATP citrate lyase activity and the activity of the sodium-citrate cotransporter in the apical membrane, leading to lower urine citrate concentrations (Demigné et al. 2004). According to preclinical data, angiotensin converting enzyme (ACE) inhibitors could reduce urine citrate concentrations via increased cytosolic citrate lyase activity (Melnick et al. 1998) even though we found no difference in urinary citrate concentration according to ACE inhibitor use in our study. Aconitic acid excretion is also influenced by pH and hypokalemia due to changes in the activity of cortical aconitase (Demigné et al. 2004). Contradictory findings are reported regarding urine concentrations of TCA intermediates in murine models of DKD (Li et al. 2013; Stec et al. 2015). Like the SCFAs, lower TCA cycle intermediates were associated with loss of filtration surface and with mesangial expansion in the present study. Importantly, nearly all participants in this study had normal or elevated GFR, so the reduction in these metabolites cannot be explained by a reduction of GFR.
Homovanillic acid is a stable dopamine metabolite produced by catechol O-methyltransferase or monoamine oxidase/aldehyde dehydrogenase. Intra-renal dopamine is synthesized by the proximal tubule epithelium (Hagege, and Richet 1985), and may act in an autocrine/paracrine fashion by modulating water/sodium homeostasis (Zhang and Harris 2015). Decreased renal synthesis of dopamine is associated with hypertension (Gill et al. 1988), and decreased urine homovanillic acid concentration is associated with impaired kidney function in adults with chronic glomerular disease (Pestana et al. 1998). In addition, urine tyrosine, an upstream metabolite of dopamine, is significantly lower in persons with type 2 diabetes who increase their urinary albumin excretion compared to those with type 2 diabetes and persistent normoalbuminuria (Pena et al. 2014). Nonetheless, higher urine homovanillic acid concentrations are also associated with a greater risk of acute kidney injury in children after cardiac surgery (Beger et al. 2008). In the present study, lower urine concentrations of homovanillic acid were associated with increased podocyte foot process width, another structural determinant of progressive DKD (Fufaa et al. 2016).
The strengths of this study include stability of the urine metabolites and the availability of research kidney biopsies. GFR was measured by a urinary clearance method rather than estimated by a creatinine equation, and urine samples for metabolite assays were collected under standardized conditions and stored at −80 °C, undergoing only one freeze–thaw cycle prior to assay. The study also has limitations. By having tissue from only a single kidney biopsy, we were not able to assess structural changes that may occur over time in relation to the urine metabolite concentrations. In addition, urine samples were obtained a median of 6.5 months prior to the kidney biopsies and metabolite concentrations in the urine could fluctuate due to a number of factors. Urine concentration of these metabolites may also be affected by secretion, reabsorption, or metabolism in the proximal tubule epithelium. Nevertheless, urine samples obtained a median of 12.1 months earlier or later demonstrated stability of the urine metabolite concentrations over time. We have no direct measure of mitochondrial function in study participants, nor do we have direct microscopic measures of mitochondrial structure to demonstrate whether mitochondrial structure is perturbed in those with lower urine mitochondrial metabolites. Nearly all our morphometric measures were glomerular, and none of the urine metabolites correlated with the single tubulointerstitial measurement (cortical interstitial fractional volume) after adjustment for potential confounders. Given that most study participants had early or no clinical DKD, we would expect their diabetic kidney lesions to predominantly involve the glomerulus, hence our focus primarily on glomerular measures. Losartan is thought to attenuate mitochondrial dysfunction through several protective mechanisms (de Cavanagh et al. 2011), but we observed no effect of treatment assignment to either losartan or placebo on the relationship between the urine mitochondrial metabolites and the morphometric variables. Two of the urine metabolites—glycolic acid and 3-hydroxy isovalerate—had poor reproducibility as assessed by the ICC, suggesting that their associations reported here may be less reliable than those for the other urine metabolites. Two other urine metabolites—3-hydroxy isobutyrate and uracil—were analyzed after removing a highly influential point. By excluding this point, we may have introduced some selection bias into the analysis. We did not adjust for multiple comparisons in our analyses because this was an exploratory study, and we did not want to mask potential relationships of interest. The consistency of the relationships of the urine metabolites with the morphometric variables of progressive DKD provides some assurance against false positive results.
5. Conclusion
Lower concentrations of a set of urine metabolites are associated with kidney structural lesions in American Indians with type 2 diabetes, and may serve as biomarkers for early glomerular lesions in DKD. These findings may implicate reduced mitochondrial function in the pathogenesis of diabetic kidney disease, although additional work is needed to establish this relationship.
Supplementary Material
Acknowledgements
The authors thank the participants and the doctors, nurses, and support staff for their role in collecting and processing the data. Portions of this work were presented in abstract form at the 2016 ADA scientific meeting in New Orleans, LA.
Funding This research was supported by the Intramural Research Program at the National Institute of Diabetes and Digestive and Kidney Diseases and by the American Diabetes Association (Clinical Science Award 1–08-CR-42).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11306–018-1380–6) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest Pierre-Jean Saulnier, Manjula Darshi, Kevin M. Wheelock, Helen C. Looker, Gudeta D. Fufaa, William C. Knowler, E. Jennifer Weil, Stephanie K. Tanamas, Kevin V. Lemley, Rintaro Saito, Loki Natarajan, Robert G. Nelson, and Kumar Sharma declare that they have no conflict of interest.
Ethical approval This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. All procedures involving human participants were in accordance with the ethical standards of the Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, et al. (2015). Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. Journal of the American Society of Nephrology, 26, 1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger RD, Holland RD, Sun J, Schnackenberg LK, Moore PC, Dent CL, et al. (2008). Metabonomics of acute kidney injury in children after cardiac surgery. Pediatric Nephrology, 23, 977–984. [DOI] [PubMed] [Google Scholar]
- Belsley DA, Kuh E, & Welsch RE (1980). Regression Diagnostics: Identifying Influential Observations and Sources of Collinearity Hoboken: John Wiley and Sons. [Google Scholar]
- Czajka A, Ajaz S, Gnudi L, Parsade CK, Jones P, Reid F, et al. (2015). Altered Mitochondrial Function, Mitochondrial DNA and Reduced Metabolic Flexibility in Patients With Diabetic Nephropathy. EBioMedicine, 2, 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cavanagh EM, Inserra F, & Ferder L (2011). Angiotensin II blockade: A strategy to slow ageing by protecting mitochondria? Cardiovascular Research, 89, 31–40. [DOI] [PubMed] [Google Scholar]
- Demigné C, Sabboh H, Puel C, Rémésy C, & Coxam V (2004). Organic anions and potassium salts in nutrition and metabolism. Nutrition Research Reviews, 17, 249–258. [DOI] [PubMed] [Google Scholar]
- Ellis EN, Steffes MW, Goetz FC, Sutherland DE, & Mauer SM (1986). Glomerular filtration surface in type I diabetes mellitus. Kidney International, 29, 889–894. [DOI] [PubMed] [Google Scholar]
- Fufaa GD, Weil EJ, Lemley KV, Knowler WC, Brosius FC, Yee B, et al. (2016). Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clinical Journal of the American Society of Nephrology, 11, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JR Jr., Gullner G, Lake CR, Lakatua DJ, & Lan G (1988). Plasma and urinary catecholamines in salt-sensitive idiopathic hypertension. Hypertension, 11, 312–319. [DOI] [PubMed] [Google Scholar]
- Hagege J, & Richet G (1985). Proximal tubule dopamine histofluorescence in renal slices incubated with L-dopa. Kidney International, 27, 3–8. [DOI] [PubMed] [Google Scholar]
- Hallan S, Afkarian M, Zelnick LR, Kestenbaum B, Sharma S, Saito R, et al. (2017). Metabolomics and gene expression analysis reveal down-regulation of the citric acid (TCA) cycle in non-diabetic CKD patients. EBioMedicine, 26, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K (2008). Role of TSC-mTOR pathway in diabetic nephropathy. Diabetes research and clinical practice, 82(Suppl 1), S59–S62. [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Aa J, Qin W, Zha W, Ge Y, et al. (2013). GC/TOFMS analysis of metabolites in serum and urine reveals metabolic perturbation of TCA cycle in db/db mice involved in diabetic nephropathy. American Journal of Physiology-Renal Physiology, 304, F1317–F1324. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, & Adams SH (2014). Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Reviews Endocrinology, 10, 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S, & Macfarlane GT (2003). Regulation of short-chain fatty acid production. Proceedings of the Nutrition Society, 62, 67–72. [DOI] [PubMed] [Google Scholar]
- Melnick JZ, Preisig PA, Haynes S, Pak CY, Sakhaee K, & Alpern RJ (1998). Converting enzyme inhibition causes hypocitraturia independent of acidosis or hypokalemia. Kidney International, 54, 1670–1674. [DOI] [PubMed] [Google Scholar]
- Moriya T, Suzuki Y, Inomata S, Iwano M, Kanauchi M, & Haneda M (2014). Renal histological heterogeneity and functional progress in normoalbuminuric and microalbuminuric Japanese patients with type 2 diabetes. BMJ Open Diabetes Research and Care, 2, e000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan N, & Pluznick JL (2014). From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. American Journal of Physiology-Cell Physiology, 307, C979–C985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, et al. (2014). Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney International, 85, 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosadini R, Velussi M, Brocco E, Bruseghin M, Abaterusso C, Saller A, et al. (2000). Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes, 49, 476–484. [DOI] [PubMed] [Google Scholar]
- Pena MJ, Lambers Heerspink HJ, Hellemons ME, Friedrich T, Dallmann G, Lajer M, et al. (2014). Urine and plasma metabolites predict the development of diabetic nephropathy in individuals with Type 2 diabetes mellitus. Diabetic Medicine, 31, 1138–1147. [DOI] [PubMed] [Google Scholar]
- Pestana M, Jardim H, Serrão P, Soares-da-Silva P, & Guerra L (1998). Reduced urinary excretion of dopamine and metabolites in chronic renal parenchymal disease. Kidney and Blood Pressure Research, 21, 59–65. [DOI] [PubMed] [Google Scholar]
- Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. (2013). Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proceedings of the National Academy of Sciences USA, 110, 4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier PJ, Wheelock KM, Howell S, Weil EJ, Tanamas SK, Knowler WC, et al. (2017). Advanced Glycation End-Products Predict Loss of Renal Function and Correlate with Lesions of Diabetic Kidney Disease in American Indians with Type 2 Diabetes. Diabetes, 65, 3744–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. (2013). Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. Journal of the American Society of Nephrology, 24, 1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DP (1983). Citrate excretion: A window on renal metabolism. American Journal of Physiology-Renal Physiology, 244, F223–F234. [DOI] [PubMed] [Google Scholar]
- Squarer A, Lemley KV, Ambalavanan S, Kristal B, Deen WM, Sibley R, et al. (1998). Mechanisms of progressive glomerular injury in membranous nephropathy. Journal of the American Society of Nephrology, 9, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Stec DF, Wang S, Stothers C, Avance J, Denson D, Harris R, et al. (2015). Alterations of urinary metabolite profile in model diabetic nephropathy. Biochemical and Biophysical Research Communications, 456, 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, et al. (2014). High amylose resistant starch diet ameliorates oxidative stress, inflammation and progression of chronic kidney disease. PLoS ONE, 9, e114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil EJ, Fufaa G, Jones LI, Lovato T, Lemley KV, Hanson RL, et al. (2013). Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes, 62, 3224–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, & Vaziri ND (2014). Expansion of urease-and uricase-containing, indole-and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. American Journal of Nephrology, 39, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MZ, & Harris RC (2015). Antihypertensive mechanisms of intra-renal dopamine. Current Opinion in Nephrology and Hypertensions, 24, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


