Abstract
Obesity-related adverse health consequences occur predominately in individuals with upper body fat distribution commonly associated with increased central adiposity. Visceral adipose tissue accumulation is described to be the greatest driver of obesity-induced inflammation, however evidence also supports that the intestines fundamentally contribute to the development of obesity-induced metabolic disease. The visceral adipose depot shares the same vasculature and lymph drainage as the small intestine. We hypothesize that the visceral lymph node, which drains adipose tissue and the gastrointestinal tract, is central to the exacerbation of systemic pro-inflammation. Male C57BL/6 mice were fed CHOW or high fat diet (HFD) for 7 weeks. At termination the mesenteric depot, visceral lymph node and ileum, jejunum and Peyer’s patches were collected. Cytokine concentration was determined in adipose tissue whereas immune cell populations where investigated in the visceral lymph node and intestinal segments by flow cytometry. Visceral adipose tissue and the gastrointestinal tract mutually influence immune cells enclosed within the visceral lymph node. HFD increased visceral lymph node immune cell number. This likely resulted from 1.) an increase in immune cells migration from the small intestines likely from activated dendritic cells that travel to the lymph node and 2.) cytokine effluent from visceral adipose tissue that promoted expansion, survival and retention of pro-inflammatory immune cells. Overall, the visceral lymph node, the immune nexus of visceral adipose tissue and the small intestines, likely plays a fundamental role in exacerbation of systemic pro-inflammation by HFD-induced obesity.
The research of Tim Bartness greatly enhanced the understanding of adipose tissue regulation. Studies from his laboratory significantly contributed to our awareness of extrinsic factors that influence body fatness levels. Specifically, the work he produced eloquently demonstrated that adipose tissue was more complex than an insulating storage center; it was connected to our brains via the sympathetic and sensory nervous system. Mapping studies demonstrated that adipose tissue both receives and sends information to the brain. Further, his lab demonstrated that nervous system connections contributed to lipolysis, thermogenesis and adipocyte proliferation and growth. The work of Tim Bartness will continue to influence adipose tissue research. As such, Tim Bartness directly inspired the following research. Adipose tissue extrinsic factors are not limited to the peripheral nervous system. The lymphatic system is an additional extrinsic factor that cross talks with adipose tissue, however its role in this context is under emphasized. Here we begin to elucidate how the lymphatic system may contribute to the comorbidities associated with visceral adipose tissue accumulation.
Keywords: Lymphatics, Pro-Inflammation, Central Obesity, Metabolic Disease, Visceral Adiposity
INTRODUCTION
Obesity increases the risk of disease comorbidities including, but not limited to, cardiovascular disease [1], liver inflammation [2], insulin resistance and type-2-diabetes [3]. Systemic low-grade chronic inflammation is commonly described to drive tissue impairments that exacerbate disease risks [4–8]. Excessive adipose tissue accumulation in obesity is associated with regional inflammation, which subsequently drives systemic inflammation that contributes to deleterious health outcomes [9–12]. In particular, evidence supports that obesity-related adverse health consequences occur predominately in individuals with upper body fat distribution commonly associated with increased central adiposity [5, 13–15]. Here we question; why is visceral adiposity so detrimental?
Adipose tissue can be modified by both intrinsic and extrinsic factors. One extrinsic factor capable of adipose tissue cross talk is the lymphatic system. This association is particularly important in inflammatory processes. Specifically, lymph nodes are predominantly embedded in adipose tissue [16], and are the primary site for the development of regional protective immune responses. Here lymph nodes serve as conduits that facilitate interactions between immune cells (e.g. monocytes, macrophages, dendritic cells, neutrophils and lymphocytes) and antigens. The visceral lymph node located within the intra-abdominal cavity collects effluent from the visceral adipose depot. In adipose tissue, immune cells within the lymph nodes serve as the responders to tissue injury or pathogen invasion and are fundamental for the development of protective immune responses. These responses are orchestrated by the production and release of both pro- and anti-inflammatory cytokines. Hence, lymph nodes serve as posts that continuously survey and monitor exposure of adipose tissue to potentially harmful pathogens and metabolites [17, 18]. Immune cells within lymph nodes can be recruited and activated to defend adipose tissue against tissue damage, toxicity or impaired function [19]. In turn, research demonstrates adipose tissue serves as an energy reservoir to support immune functions, creating a reciprocal relation where enhanced pro-inflammation drives adipocyte proliferation [20]. The link between obesity and impaired immune function has been known for some time, yet there is very little understanding of how excessive adipose tissue deposition alters the lymphatic system. Thus far, excessive adiposity associated with obesity is a risk for immune detriments including, but not limited to, accelerated thymic aging, a reduction in vaccine efficacy and increased morbidity associated with pathogen infection [21–23]. We hypothesize that chronic adipose tissue-driven inflammation influences lymphatic tissue function, in part, by changes in resident immune cell populations. This, in turn, can affect systemic immunity and heighten disease risk. Obesity-mediated inflammation is therefore a disease state that involves adaptations in the extrinsic lymphatic system.
Human and rodent studies suggest the greatest driver of obesity-induced inflammation is visceral adipose tissue accumulation. As it accumulates and dysregulates during obesity, free fatty acids, adipokines and pro-inflammatory cytokines increase in circulation subsequently effecting insulin sensitive tissues such as the liver [24, 25]. However, the visceral adipose depot shares the same vasculature and lymph drainage as the small intestine. Hence, evidence also supports that the intestines fundamentally contribute to the development of obesity-induced metabolic disease [26] by way of inflammation.
The gastrointestinal (GI) tract is the first line of protection from antigens and immunomodulatory agents consumed from our diet or localized within our gut. The GI tract must continuously distinguish beneficial nutrients and commensal bacteria from harmful pathogens [27, 28]. Maintenance of immunologic defense and intestinal homeostasis engages ~70% of the body’s immune cells [29], hence the intestine has considerable influence on systemic immune function. Consequently, the gastrointestinal immune system within the small intestine must maintain physiological gut homeostasis despite continual exposure to varying lumen contents. Within the small intestine, this is accomplished by a semi-hierarchical organization of immune regions. Immune response can occur at the epithelial surface of the lumen and lamina propria, where regional immune cells attempt to eliminate noxious pathogens instantaneously (For review see:[30]). These same immune cells also release signaling molecules that can prompt activation of a greater immune response. Gut-associated lymphoid tissue (GALT), a network of lymphoid tissue containing immune cells (for review see: [31, 32]), also defends against pathogens. GALT associated immune cells play a critical role in the protection from and inhibition against the penetration of gut-derived pathogens. Overall, gut immune homeostasis is fundamental to the protection of systemic immunity.
Much like adipose tissue, a diet high in fat can cause inflammation of the gastrointestinal tract. Acute or chronic treatment/intake of lipids can shift small intestine immunity toward pro-inflammation, this is best demonstrated in rodent models. For example, high fat diet (HFD) feeding increases tumor necrosis factor α (TNFα) expression and nuclear factor κβ (NF- κβ) activation, markers of pro-inflammation, in the small intestines of mice [33]. Consistent with this others demonstrate that HFD increases TNFα concentration within the lamina propria of the small intestines as well as other pro-inflammatory markers such as interleukin 6 and 17 (IL6 and 17) [34]. Epithelial cells also release IL6, as well as growth-related oncogene/cytokine-induced neutrophil chemoattractant-1 (GRO/CINC-1; stimulates locomotion and activation of neutrophils) in the presence of long chain fatty acids [35]. HFD-induced pro-inflammation in the small intestine likely results from epithelial cell injury, damaged morphology, that occurs as lipid transverses the enterocyte from the lumen [36]. Disruption of small intestine homeostasis by lipids causes epithelial and stroma cells of the small intestines to engage and activate lymphoid cells in attempt to sustain immunity, mediate inflammation and eventually incite resolution [37]. Overall, chronic inflammation, as occurs with intake of saturated fat diets, disrupts the mucosal barrier enhancing permeability of pathogens, which subsequently exacerbates pro-inflammation [38, 39]. Small intestines pro-inflammation, ensuing from acute or chronic exposure to HFD, influences systemic physiological and pathological processes.
We postulate that both visceral adipose tissue accumulation and the gastrointestinal tract influence systemic immunity during obesity. Both tissues are likely responsible for the comorbidities associated with obesity. We hypothesize that the visceral lymph node, which drains adipose tissue and the gastrointestinal tract, is central to the exacerbation of systemic pro-inflammation. A limitation of previous studies in this area include specific focus on one distinct gastrointestinal region. To examine comprehensively HFD-induced alterations of the small intestines requires distinction between different regions contained within. There are inherent anatomical and physiological differences between the duodenum, jejunum, and ileum (For review see:[30]). Primary differences that may influence immune regulation include differing populations of epithelial cells and absorption capacity. Therefore, characteristics of immunity may be specific to anatomical location among the gut. In addition, Peyers’s patches, organized lymphoid nodules located throughout the jejunum and ileum, play a fundamental role in the maintenance of intestinal immune homeostasis, yet the role of these nodules is often underemphasized. In this study, we used a Westernized diet to induce obesity in mice to investigate if diet-induced obesity differentially changed immune cell populations in the Peyer’s patches and distinct segments of the small intestines. Along with this we describe immune cell changes in the visceral lymph node, a structure proposed to control immigration of immune cells from the gut and enhance tolerance to gut derived pathogens [40]. Last, adipose tissue cytokine concentration was used to determine if visceral lymph node immune cell alterations are related to visceral adipose tissue signaling.
METHODS
Animals
Male C57BL/6 mice (n=10, aged 2–3 months, 27.7±0.22 g) from Jackson Laboratory, Bar Habor, Maine, were single housed under controlled conditions (12:12 light-dark cycle, 50–60% humidity, 25°C) and allowed one week of acclimation before experiment start. Following acclimation, mice were given free access to water and a standard CHOW or Westernized high fat and sugar diet (HFD) until termination at 7 weeks. Standard CHOW diet was (CHOW: Harlan Teklad LM485, Madison, WI.) 3.1 kcal/g with ~17% kcal from fat (~5.8% by weight of diet), ~25% protein, and ~58% carbohydrate. The fats in the diet consisted of mono- and poly-unsaturated and saturated fatty acids (1.3%, 2.9% and 0.8% by weight of diet respectively). Westernized high fat and sugar diet (HFD) (Harlan Teklad, TD.08811, Madison, WI) was 4.7 kcal/g with ~45% kcal from fat (~23% by weight of diet, main source milkfat), ~14.7% protein and ~40.7% carbohydrate (34% sucrose). The fats in the diet consisted of mono- and poly-unsaturated and saturated fatty acids (31%, 8% and 61% respectively). Body mass and food intake were recorded weekly. Procedures were reviewed and approved by the Colorado State University Institutional Animal Care and Use Committee.
Terminal Procedures (Blood Collection and Tissue Harvesting)
Termination occurred after mice were on respective diets for 7 weeks. Mice were anesthetized with isoflurane and terminated by decapitation. Adipose depots were collected and weighed and the mesenteric (visceral) depot was stored at −80°C. The visceral lymph node embedded inside the visceral depot was collected. Peyer’s patches (n = 5 mice, 4 collected Peyer’s Patches/mouse) were first collected from the small intestines (along the ileum and jejunum). Next 30mm portions of ileum and jejunum (n = 5 each), without visible Peyer’s patch portions, were collected. Intestine tissues and the visceral lymph node were placed into Hank’s balanced salt solution (HBSS) until further processed.
Adipose Tissue Cytokines
Adipose tissue accumulation, especially visceral (central) adiposity, is associated with increases in cytokines that contribute to pro-inflammation. Cytokine concentration was measured in the visceral depot to associate adipose depot changes with extrinsic lymph node immune cell signaling. Interleukin (IL) proteins were analyzed because of their role in regulating immune response. In particular, these ILs include proteins which generally contribute to pro-inflammation, IL-1β and IL-6, and those that are demonstrated to be higher in lean animals, IL-5 and IL-13. Visceral adipose tissue Interleukin -1β, -5, -6 and -13, as well as KC GRO (a.k.a. chemokine (C-X-C motif) ligand 1 (CXCL1) and tumor necrosis factor α (TNFα) protein concentration was measured by Mesoscale (Rockville, Maryland). Two kits were utilized, a V-PLEX Pro-inflammatory Panel 1 Mouse Kit (Mesoscale; Cat No. K15048D-1, Rockville, Maryland) and a U-PLEX customized IL-13 Mouse Kit (Mesoscale; Rockville, Maryland). Briefly, adipose tissues was placed in 2 ml tubes in HBSS+5% FBS (1mL), minced using scissors and sonicated on ice. Tissue homogenates were then treated according to kit protocols for antibody-based detection of cytokines.
Separation of Immune Cells from Stromal Tissues
Ileum, jejunum and Peyer’s patches samples were removed from HBSS, placed in collagenase solution (2 mL/sample, Sigma Aldrich, Cat#C9891) and minced. Tissues were placed in an incubator at 37°C for 25–30 minutes then removed and samples were triturated with 18 gauge needles on 3mL syringes. Tissues and collagenase solution were passed through a 40μm cell strainer, and rinsed with 10mL HBSS to deactivate collagenase. Visceral lymph nodes were dissociated and filtered through a 40 μm cell strainer without the use of collagenase. All cell suspensions were centrifuged at 2000 rpm at 4°C for 5 minutes. Supernatant was poured off and samples were re-suspended in 400μL of FACS buffer (1% BSA, 0.1% sodium azide, and PBS) for immediate cell count (see below: Cell Counting) then temporarily stored.
Cell Counting – Total and Viable
Tissue cell suspensions (see separation of immune cells from stromal tissues) were pipetted onto 96-well plates and mixed with trypan blue exclusion dye marker (ThermoFisher Scientific, Waltham, MA) at 1:1. Total and viable cell counts were evaluated on a Cellometer (Nexcelom, Lawrence, MA) using a pre-defined small immune cell program developed by Nexcelom. Immune cells were identified by diameter with a range from 4–20 microns.
Flow Cytometry
Between 5 × 105 and 1 × 106 cells from intestinal suspensions were plated per well. Cells were washed, spun and supernatant was removed and discarded. To prevent non-specific binding of antibodies cells were re-suspended in normal mouse block and briefly incubated. Primary antibodies were added at a 1:200 dilution in two different panels. Panel 1 included: anti-CD8 (clone 53-6.7, cat. no. 13-0081-85, eBioscience), anti-CD4 (clone RM4-5, cat. no. 48-0042-82, eBioscience), anti-CD3 (clone 145-2c11, cat. no. 11-0031-82 eBioscience), and intracellular Foxp3 (clone FJK-16a, cat. no. 12-5773-82, eBioscience) for T cell differentiation; anti-B220 (clone RA3-6B2, cat. no. 17-0452-82, eBioscience) for B cell identification; anti-NKG2D (clone CX5, cat. no. 13-5882-82, eBioscience) for natural killer cells. Panel 2) included: anti-CD11b (clone M1/70, cat. no. 48-0112-82, eBioscience), anti-CD11c (clone N418, cat. no. 417-0114-82, eBioscience), anti-F4/80 (clone BM8, cat. no. 25-5931-82, eBioscience), and anti-MHCII (clone M5/114.15.2, cat. no. 17-5321-81, eBioscience) for antigen presenting cell differentiation. After incubation and washes with FACS buffer secondary antibody streptavidin-pacific orange (cat. no. 532365, Invitrogen) was added at a dilution of 1:500. In addition, a permeabilization kit (cat. no. 00-5521, eBioscience) was used in accordance with kit instructions for FoxP3 intracellular staining. Last, stained cells were fixed in 4% PFA, washed and stored in FACS buffer for analysis the following day. Flow cytometry of immune cell populations were analyzed using MoFlo Legacy Cell Analyzer and Sorter (Beckman Coulter, Indianapolis, IN). Data were analyzed using Summit software version 4.2 (Beckman Coulter, Indianapolis, IN).
Statistics
Data are expressed as mean ± standard error of the mean (SEM). Food intake, body weight, intestine tissue cell percent and viable counts were analyzed with a One-way ANOVA for significance values among individual data points. Post-hoc tests of individual groups were made using LSD tests. Lymph node immune cell percent and viable number as well as adipose tissue cytokines were analyzed by T-test. For all experiments, differences among groups were considered statistically significant if p ≤ 0.05.
RESULTS
Food intake and body weight
Cumulative kcal intake was significantly higher in HFD mice compared with Chow (Chow 387.54 kcals ± 9.19, HFD 555.9 kcals ± 8.75; p = 7.3E-5) following 7 weeks of intake. As a result 7 weeks of HFD intake significantly increased body mass (Chow 28.9 ± 0.67, WD 39.39 ± 0.77; p = 1.67E-9) compared with Chow controls. Consistent with this individual adipose tissue, epididymal, visceral, perirenal and inguinal white adipose tissue, and total adiposity (previous adipose tissue depots added together) were significantly increased in the HFD group (Table 1; p ≤ 0.05).
Table 1. Total and individual adipose tissue weight depots in grams.
WD feeding magnifies total adipose, and expansion is.
| 7 week | p value | ||||||
|---|---|---|---|---|---|---|---|
| Chow | HFD | ||||||
| Total | 2.74 | ± | 0.48 | 5.34 | ± | 0.63 | 0.011 |
| Epididymal | 1.10 | ± | 0.16 | 1.87 | ± | 0.09 | 0.003 |
| Visceral | 0.32 | ± | 0.02 | 1.25 | ± | 0.08 | 0.001 |
| Perirenal | 0.59 | ± | 0.11 | 1.08 | ± | 0.15 | 0.030 |
| Inguinal | 0.61 | ± | 0.14 | 1.41 | ± | 0.20 | 0.010 |
Total adiposity is significantly greater in HFD fed mice compared with CHOW (p= .011). All individual depot weights are significantly greater in HFD fed mice compared with CHOW (≤.05).
Adipose Depot Cytokines
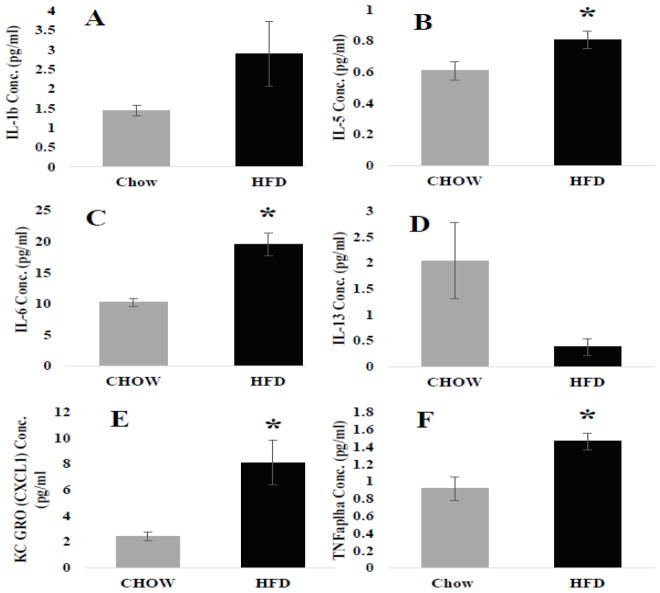
IL-1β, IL-5 and IL-6 (Figure 1 A–C) concentrations were increased in the visceral adipose depots of HFD mice, both IL-5 (Figure 1B; p = 0.05) and -6 (Figure 1C; p = 0.003) were significantly increased compared with controls. IL-13, however, decreased in the visceral depot of HFD animals (Figure 1 D). In addition, KC GRO (a.k.a. chemokine (C-X-C motif) ligand 1 (CXCL1) and tumor necrosis factor α (TNFα) protein concentration was measured. KC GRO is thus far characterized to be increased during pro-inflammation and obesity and aids as a chemoattractant for neutrophils [41]. TNFα is a protein involved in systemic inflammation. Both KC GRO (Figure 1E; p = 0.018) and TNFα (Figure 1F; p = 0.019) were significantly increased in HFD mice compared with control.
Figure 1. Visceral adipose tissue cytokine concentrations (pg/ml).
Compared with CHOW, HFD increased the concentration of pro-inflammatory interleukins IL-1b A.) and IL-6 (* = 0.003) C.). The anti-inflammatory IL-5 B.) was also significantly increased (* = 0.05) whereas the other IL-13 was decreased D.). Additionally pro-inflammatory cytokines that were significantly increased include KC GRO (* = 0.018) E.) and TNFα (* = 0.019) F.).
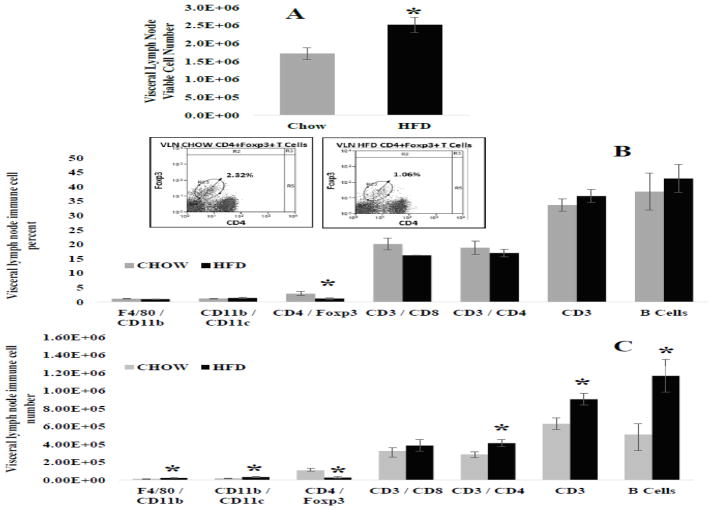
Visceral Lymph Node Cell Number and Flow Cytometry
7 weeks of HFD significantly increased the number of viable cells within the visceral lymph node (Figure 2A; p = 0.02). In general, HFD did not shift immune cell percent within the visceral lymph node, with the exception of CD4+Foxp3+ regulatory T cells (Tregs) immune cells that play a role in suppression of immune response. The percent of Tregs were significantly decreased in HFD mice compared with CHOW control (Figure 2B; p = 0.039). Absolute numbers of distinct immune cell subsets were determined by multiplying percent of representative cell populations by the total viable cell count. This data is represented in Figure 2C. HFD intake did not cause vast shifts in immune cell frequencies, but did cause multiple significant changes in viable visceral lymph node immune cell numbers. Specifically, mice fed HFD had significantly higher numbers of F4/80+CD11b+ macrophages (p = 0.022), CD11b+CD11c+ dendritic cells (p = 0.017), CD3+CD4+ helper T cells (p = 0.024), CD3+ general T cells (p = 0.02) and B220+ B cells (p = 0.002) within the visceral lymph node compared with CHOW control (Figure 2C). In opposition, CD4+Foxp3+ regulatory T cells viable number was decreased in HFD mice (Figure 2C; p = 0.011).
Figure 2. Visceral lymph node immune cell number and frequency.
A.) Total visceral lymph node viable cells number - HFD significantly increased total number of immunes cells within the visceral lymph node compared with CHOW (* = 0.02). B.) Percent frequencies (%) of individual immune cell populations – CD4+FoxP3+ cells (regulatory T) were the only immune cells to be significantly decreased in HFD fed mice compared with CHOW (* = 0.039). The inset represents scatter plots of the significant population enclosed within the circle. C.) Viable immune cells for distinct populations - F4/80+CD11b+macrophages (* = 0.022), CD11b+CD11c+ dendritic cells (* = 0.017), CD3+CD4+helper T cells (* = 0.024), CD3+ pan T cell (* = 0.02), and B220+ B cells (* = 0.002) were increased in the visceral lymph node of HFD fed mice compared with CHOW. CD4+FoxP3+ regulatory T cells, however, were decreased in HFD mice (** = 0.011)
Intestine Cell number and Flow Cytometry
7 weeks of HFD did not alter total viable cell number in ileum, jejunum or Peyer’s patch samples (Supplemental Figure 1). A limitation of this study was the processing of whole intestinal sections. This method of collection interferes with the determination of viable immune cell populations because of the numerous different cells types located within the intestines that are a similar size to immune cells of interest. Hence, viable cell number for the intestines would not be reflective of the actual immune cell number in the intestine. Therefore, only percent frequency will be reported for the ileum, jejunum and Peyer’s patches.
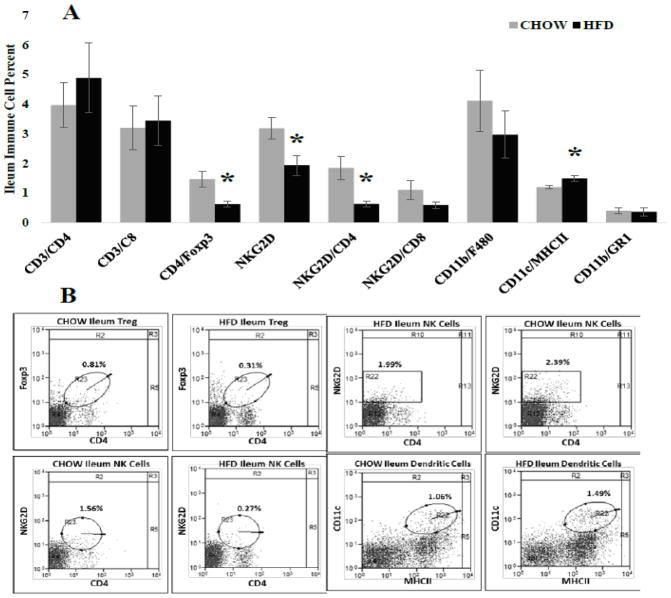
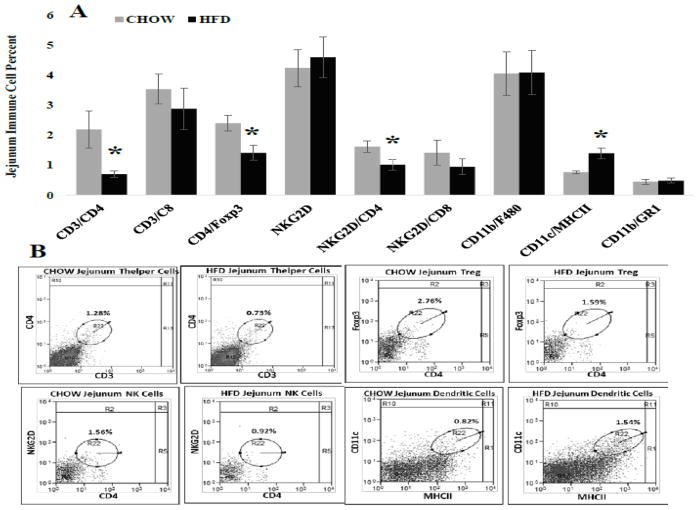
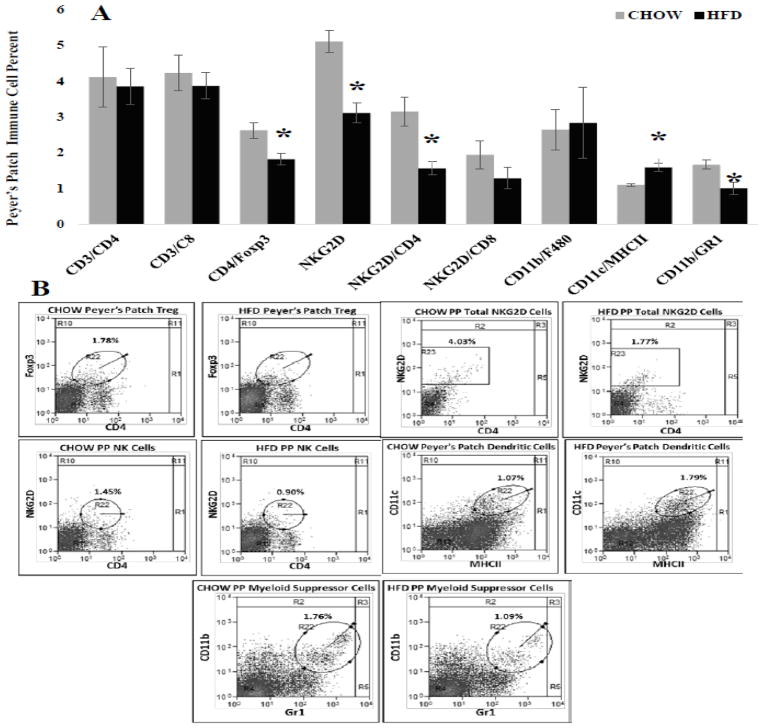
Immune cell frequency was evaluated in several distinct intestine regions because of the inherent anatomical and physiological differences between intestine segments (For review see:[30]). Differences in epithelial cell number, absorption capacity and numerous other factors can influence immune regulation. In general, changes in immune cell frequency where similar between intestine regions. First, in the ileum HFD caused significant decreases (Figure 3A) in percent of CD4+Foxp3+ regulatory T (p = 0.011), NKG2D+ (p = 0.002) and NKG2D+CD4+ (P = 0.014) immune cells while significantly increasing percent of CD11c+MCHII+ dendritic cells (p = 0.016) compared with CHOW. Similarly, in the jejunum the percent of CD4+Foxp3+ regulatory T (p = 0.015) and NKG2D+CD4+ (P = 0.034) immune cells were significantly decreased and CD11c+MCHII+ dendritic cells (p = 0.008) frequency was significantly increased in HDF mice. Additionally, HFD significantly decreased the percent of jejunum CD3+CD4+ helper T cells (Figure 4A; p = 0.011) compared with CHOW. Last, Peyer’s patches located along the ileum and jejunum (Figure 5) of HFD mice had significant decreases in percent of CD4+Foxp3+ regulatory T (p = 0.011), NKG2D+ (p = 0.002), NKG2D+CD4+ (P = 0.014) and CD11b+GR1+ myeloid suppressor (p = 0.042) immune cells. Similar to the ileum and jejunum CD11c+MCHII+ dendritic cells (p = 0.005) in Peyer’s patches were significantly increased in HFD mice compared with CHOW (Figure 5).
Figure 3. Immune cell frequency (%) for specific populations within the ileum.
A.) CD4+FoxP3+ regulatory T (* = 0.011), NKG2D+ (* = 0.002) and NKG2D+CD4+ (* = 0.014) immune cells were significantly decreased in HFD fed mice compared with CHOW, whereas CD11c+MHCII+dendritic cells (* = 0.014) were significantly increased. B.) Representative scatter plots of immune cell frequency for populations significantly altered by HFD. Circles indicate double positive populations while the square represents all cells positive for a single marker, NKG2D.
Figure 4. Immune cell frequency (%) for specific populations within the jejunum.
A.) CD4+FoxP3+ regulatory T (* = 0.015), NKG2D+CD4+ (* = 0.034), and CD3+CD4+ helper T cells (* = 0.011) were significantly decreased in HFD fed mice compared with CHOW, whereas CD11c+MHCII+dendritic cells (* = 0.008) were significantly increased. B.) Representative scatter plots of immune cell frequency for populations significantly altered by HFD. All populations are double positive and indicated by circles.
Figure 5. Immune cell frequency (%) for specific populations within the Peyer’s patches collected along the Ileum and jejunum.
A.) Significant decreases occurred in CD4+FoxP3+ regulatory T (* = 0.011), NKG2D+ (*= 0.002), NKG2D+CD4+ (p= 0.014) and CD11b+Gr1+ Myeloid suppressor (* = 0.042) immune cells of HFD mice compared with CHOW. The frequency of CD11b+MHCII+ dendritic cells, however, were significantly increased in HFD mice (* = 0.005). B.) Representative scatter plots of immune cell frequency for populations significantly altered by HFD. The circles indicate double positive populations while the square indicates all cells positive for a single marker NKG2D.
DISCUSSION
Obesity induces chronic low-grade inflammation. It is suggested that obesity associated inflammation is incited and exacerbated by excessive dysregulated adipose tissue deposition. Dysregulation of adipose tissue endocrine function, pro-inflammatory cytokines and deleterious cytokines are implicated in pathophysiology of numerous diseases. Visceral adipose tissue accumulation, in particular, is highly associated with adverse metabolic profiles [42]. Emerging evidence additionally supports a relation between dysregulated visceral adipose tissue and gut dysfunction [43]. Specifically, visceral adipose tissue pro-inflammation causes gastrointestinal leakiness [44, 45] and gut inflammation drives adipose tissue accumulation [46], both factors together exacerbate systemic pro-inflammation. Because the gastrointestinal tract and visceral adipose tissue commonly drain to visceral lymph node, we proposed to investigate the associated changes between the three intra-abdominal tissues during obesity. Here we demonstrated that both the visceral adipose depot and the gastrointestinal tract likely influence immune cells enclosed within the visceral lymph node. This occurred by HFD-induced increases in visceral adipose tissue pro-inflammatory cytokines and a reduction in the tolerant milieu of the intestines needed to suppress unnecessary immune reaction to commensal bacteria and beneficial nutrients.
Visceral adipose tissue accumulation is highly associated with metabolic disease [24, 25]. Deleterious effects of excessive visceral depot expansion, in part, are due to increased production and release of pro-inflammatory cytokines [47] that circulate directly to the liver and systemically. Cytokine release from adipose tissue originate from both adipocytes and immune cells contained within the depot in response to the changing milieu induced by HFD. Consistent with previous studies, we demonstrate that HFD-induces deleterious cytokine release from visceral adipose tissue. Il-1 plays a fundamental role in immune response. These molecules comprise a family of pro-inflammatory cytokines that induce a complex network of additional pro-inflammatory cytokines/mediators via interaction with leukocytes. Specifically, IL-1b in adipose tissue is known as mononuclear cell factor and a lymphocyte activating factor that originates from macrophages and is activated by adipocyte-specific caspase-1 [48]. Specifically, release of IL-1b, in a situation such as obesity, enhances antigen-mediated expansion of pro-inflammatory cell types such as Th1 and Th17 T cells [49]. IL-1b also promotes the recruitment and retention of macrophages [50]. Similar to IL-1b, IL-6 is a fundamental regulator of T cells. Of the numerous immune-modulating cytokines increased in obesity, IL-6 dysregulation significantly contributes to pro-inflammation and metabolic dysregulation [51, 52]. Release of Il-6 causes numerous events to occur that allow for T cell survival. This includes promoting T cell development [53] and activation and tissue invasion [54, 55]. In addition, Il-6 permits effector T cells to overcome suppression by regulatory T cells [55, 56] while correspondingly inhibiting immune suppression T cells [57]. In opposition are interleukins associated with anti-inflammation that are typically decreased in obesity including IL-5 and IL-13. IL-5 plays a role in maintaining immune cell populations that contribute to metabolic homeostasis including glucose tolerance, and protection against diet-induced obesity in adipose tissue [58, 59]. Similarly, IL-13 is also associated with conservation of glucose homeostasis [60]. In response to a pathogen TNFα is typically the first cytokine released [61]. Within tissues TNFα recruits cells towards pro-inflammatory stimuli by inducing vasodilation, which allows increased infiltration of immune cells such as lymphocytes, neutrophils and monocytes [62]. TNFα also induces the release of KC GRO (CXCL1) from immune cells such macrophages and neutrophils [63, 64], this further facilitates pro-inflammatory immune cell recruitment. Obesity increases both TNFα [65] and CXCL1 release [63]. Overall, our data demonstrates that 7 weeks of HFD increases pro-inflammatory cytokines while decreasing immune-suppressing cells types. This subsequently creates a signal in adipose tissue that permits increased infiltration of pro-inflammatory cells types. We propose effluent from visceral adipose tissue into the visceral lymph node influence immune cells incased within.
The visceral lymph node, however, is not limited to visceral adipose depot effluent. Intestine health can also influence the visceral lymph node. The gastrointestinal (GI) tract is the first line of protection from antigens and immunomodulatory agents consumed from our diet or localized within our gut. Hence, it is likely that immune dysregulation within the gut can directly influence immune cells encased within the visceral lymph node. In the present study, we comprehensively investigated how HFD altered immune cell populations within the small intestines by comparing distinct intestinal regions with inherent anatomical and physiological differences. The jejunum and ileum as well as Peyer’s patches contained throughout these two regions were evaluated for differential response to HFD. Distinct intestine sections were evaluated to determine if regional differences in specialized epithelial cells, receptor types, antigen uptake or absorption capacity could disparately influence HFD outcomes. Although there were some minor differences between gut regions, most changes among the three sections evaluated were similar.
First this includes HFD-induced decrease in regulatory T cells (Tregs: CD4+foxp3+) in all three intestine tissues. These regulatory cells are fundamental in maintaining homeostasis within the gut as demonstrated in numerous inflammatory disease models (For review see: [66]). Generally, Tregs are upregulated during homeostatic conditions and consequently downregulated in order to elicit an immune response to pathogens or other inflammatory stimuli [67]. This is the first indication that HFD induced obesity is potentially driving a shift towards the pro-inflammatory environment associated with obesity. As previously discussed, this shift in Treg cells is well characterized within the ileum and jejunum in obesity as well as other inflammatory bowel disorders [66] but to the best of our knowledge, this is the first time this has been documented within Peyer’s patches in response to diet induced obesity. Second are the dendritic cells (DCs: CD11c+MHCII+) which are antigen presenting cells. Within the intestines, dendritic cells function to extend cellular extensions between epithelial cells to sample pathogens or commensal factors of the intestinal lumen. Dendritic cell percent was increased in all three HFD intestinal samples. Research demonstrates that harmful pathogens can trigger dendritic cells to elicit a pro-inflammatory cascade that includes 1.) both attracting and stimulating maturation and differentiation of B and T lymphocytes to mature into effector cells [68, 69], 2.) sustainability of recirculating T lymphocytes [70], and 3.) stimulation of antibody production from B cells by IL-12 secretion [71]. We would speculate these changes in immune system milieu would lead to a shift in percent increase of effector immune cells that play a role in pro-inflammation, yet this did not occur within the small intestine. Hence, the third common change to occur among our three tissue regions was decreased NKG2D+ immune cells, in particular those that where NKG2D+CD4+. The role of CD4 T cells expressing the NKG2D receptor is best described in Crohn’s disease [72–74]. NKG2D+CD4+ cells in the lamina propria of patients presenting with Crohn’s disease were associated with a pro-inflammatory cytokine profile [72] with a high expression of both IFN-y, TNF-α and increased cytotoxic capability [72]. Taken together, this demonstrates that NKG2D+CD4+ cells may play an important role in shaping the pro-inflammatory profile of the small intestine during HFD induced obesity and inflammation. However, we observed a decrease in NKG2D+CD4+ cells. Similarly, but specific to the Jejunum, there was also a decrease in small intestine CD3+CD4+ effector T cells, this again was contradictory to our prediction. Last, studies demonstrate that myeloid derived suppressor cells (MDSCs), characterized as CD11B+GR1+, induce intestinal tolerance by suppressing T-cell [75]. In Peyer’s patches only, HFD decreased MDSCs compared with CHOW. Taken together, in the intestines HFD induces release of suppressive immune cell types typically needed for toleration of commensal bacteria and variable lumen components. Release of this suppression caused a pro-inflammatory environment with an increase in antigen presenting dendritic cells that present to and activate effector cells. Hence, an increase in dendritic cell migration to the intestine increases antigen presentation and consequently T and B cell response [76]. However, there was not a corresponding increase in the percent of effector cells within the intestine instead there was a decrease. We propose this decrease in immune cell frequency may be due to immune cell migration from the gut.
Data supports that gastrointestinal immunity and systemic tolerance is predominately mediated by the visceral lymph node [40]. Gastrointestinal tolerance is the ability of immune cells to resist an unnecessary pro-inflammatory response to harmless food antigens and commensal bacteria and remain in a homeostatic state (for review see: [77]). The visceral lymph node serves as a conduit that continuously surveys and monitors exposure of the small intestine to potentially harmful pathogens and metabolites [17, 18]. Within this lymph node immune cells can be recruited and activated to defend against damage, pathogens or impaired function [19]. A fundamental role of the visceral lymph node is to create tolerance to beneficial nutrients and commensal bacteria. This process is prompted by anti-inflammatory antigen presenting dendritic cells that constitutively traffic from the intestinal epithelium and Peyer’s patches to the visceral lymph node where T cell tolerization can occur [78–80]. Consequently, this creates a protective barrier within the lymph node, via enrichment in tolerant T cells, which prevents unnecessary systemic pro-inflammatory priming reactions [81–83]. HFD, however, releases immune tolerance leading to enhanced pro-inflammation. As we have demonstrated, HFD-induced obesity is associated with an increase in dendritic cells within the intestines; we propose these cells are primed for immune response and not tolerance (pro-inflammatory dendritic cells). As such, we would expect an associated increase in dendritic activated immune cells that defend against antigen presented to them (effector cells). This effector immune response did not occur within the intestine. The visceral lymph node, however, swelled in size as a response to HFD intake. The increase in size was due to an influx/proliferation of immune cells. In general, there was not a change in percent distribution of immune cell type within the visceral lymph node, besides decreased percent of T regs, but there was an increase in dendritic cells, macrophages and effector T and B cell types. Previous studies demonstrate that immune cells migrate from PPs [84, 85] to lymphatics in the mesenteric lymph nodes [85]. The movement of lymphocyte populations is described to occur in reaction to mobile antigen presenting cells, such as dendritic cells (DCs) [86, 87]. Upon antigen/pathogen presentation dendritic cells mature and release cytokines while migrating from PPs to the mesentery. The release of cytokines is described to attract recently activated T lymphocytes [86]. We propose the diet-induced increase in the percent of dendritic cells and release of immune tolerance in the intestine causes immune cell expansion in the visceral lymph node. Here the visceral lymph node functions as a primary barrier that, in the case of HFD-induced inflammation, attempts to prevent the spread of regional pathogens and inflammatory stimuli.
Last, considerations to take into account include data supporting a reciprocal relation among adipose tissue and the immune system regulation. Though this link remains to be elucidate, research supports that adipocytes also respond to signals emanating from embedded lymph nodes and/or leaky lymphatic vessel. Indeed, visceral adipose tissue is most sensitive to lymph node alterations and best demonstrates the functional basis for the anatomical association between the two tissues [88]. Pond et al. characterized adipose tissue surrounding lymph nodes to play a fundamental role in lymph node enlargement and immune response [89] by providing fatty acids via lipolysis from triacylglycerol stores [90–92]. In addition, lymph node stimulation is proposed to facilitate adipogenesis, where mild or chronic inflammation of lymphatic tissue is associated with adipose depot enlargement [93]. In support of this lymphatic vessel fluid is also demonstrated to cause adipocyte hypertrophy [94]. Our studies support and extend that adipose tissue is an active endocrine organ involved in immunological processes. In our model, adipose tissue accumulation occurs by excessive food intake. However, adipose tissue accumulation and associated pro-inflammatory cytokine release could also be due to intestinal alterations that cause increases in pro-inflammatory immune milieu in the visceral lymph node. Further studies are needed to elucidate relative contributions of lymph node and adipose tissue response in the progression of obesity-induced systemic inflammation. It is likely they both contribute and exacerbate obesity-induced inflammation.
Taken together this data demonstrates that visceral adipose tissue and the gastrointestinal tract mutually influence immune cells enclosed within the visceral lymph node. During HFD-induced obesity, excessive adipose tissue deposition leads to a shift in deleterious immune cell types subsequently leading to increased release of several cytokines. This effluent is taken up by the visceral lymph node. Within the visceral lymph node adipose derived pro-inflammatory cytokines aid in the expansion, survival and retention of pro-inflammatory T cells (For example Th1 and Th17) and macrophages. Gastrointestinal dysfunction also contributes to this diet-induce metabolic dysregulation. The gastrointestinal tract and visceral adipose tissue likely cause an additive pro-inflammatory response to HFD-induced obesity in the visceral lymph node. Gastrointestinal tolerance is the ability of immune cells to resist an unnecessary pro-inflammatory response to harmless food antigens and commensal bacteria. The visceral lymph node contributes to gastrointestinal tolerance while also protecting systemic immunity by providing a barrier to prevent spread of gastrointestinal pathogens. However, diet-induced obesity leads to an inhibition of gastrointestinal tolerance, which consequently promotes migration of immune reactive cells to the visceral lymph node in an attempt to stimulate an immune barrier to protect systemic immunity. We propose, however, that chronic effluent of pro-inflammatory cytokines from excessive adipose tissue expansion will eventually disrupt this process and gratuitously exacerbate lymph node immune cell expansion. We predict in obesity, this can result in immune cell dysfunction, exhaustion and immunosuppression within the intra-abdominal cavity.
Supplementary Material
Highlights.
Visceral adipose tissue and the gastrointestinal tract mutually influence immune cells of the visceral lymph node.
Within the visceral lymph node adipose cytokine effluent aids in the expansion, survival and retention of pro-inflammatory T cells.
HFD inhibits gastrointestinal tolerance, which promotes migration of immune reactive cells to the visceral lymph node.
Acknowledgments
This work was supported by the National Institutes of Health [Grant Numbers P30 DK048520 and R03 DK099425]
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 1996;16(12):1509–15. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 2.Miyake T, et al. Body mass index is the most useful predictive factor for the onset of nonalcoholic fatty liver disease: a community-based retrospective longitudinal cohort study. J Gastroenterol. 2013;48(3):413–22. doi: 10.1007/s00535-012-0650-8. [DOI] [PubMed] [Google Scholar]
- 3.Sanada H, et al. High body mass index is an important risk factor for the development of type 2 diabetes. Intern Med. 2012;51(14):1821–6. doi: 10.2169/internalmedicine.51.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 5.Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14(12):1132–43. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 6.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilecik NA, et al. Prevalence of metabolic syndrome in women with rheumatoid arthritis and effective factors. Int J Clin Exp Med. 2014;7(8):2258–65. [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CG, et al. Visceral abdominal obesity is associated with an increased risk of irritable bowel syndrome. Am J Gastroenterol. 2015;110(2):310–9. doi: 10.1038/ajg.2014.422. [DOI] [PubMed] [Google Scholar]
- 9.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2014 doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 10.Guarner V, Rubio-Ruiz ME. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol. 2015;40:99–106. doi: 10.1159/000364934. [DOI] [PubMed] [Google Scholar]
- 11.Esser N, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222(3):R113–27. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 13.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74(4):761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 14.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4(1):20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 15.Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10(4):493–6. [PubMed] [Google Scholar]
- 16.Pond CM, Mattacks CA. Interactions between adipose tissue around lymph nodes and lymphoid cells in vitro. J Lipid Res. 1995;36(10):2219–31. [PubMed] [Google Scholar]
- 17.Arngrim N, et al. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes (Lond) 2013;37(5):748–50. doi: 10.1038/ijo.2012.98. [DOI] [PubMed] [Google Scholar]
- 18.Weitman ES, et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 2013;8(8):e70703. doi: 10.1371/journal.pone.0070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von der Weid PY, Rainey KJ. Review article: lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32(6):697–711. doi: 10.1111/j.1365-2036.2010.04407.x. [DOI] [PubMed] [Google Scholar]
- 20.Pond CM, Mattacks CA. THE ACTIVATION OF THE ADIPOSE TISSUE ASSOCIATED WITH LYMPH NODES DURING THE EARLY STAGES OF AN IMMUNE RESPONSE. Cytokine. 2002;17(3):131–139. doi: 10.1006/cyto.2001.0999. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, et al. Obesity accelerates thymic aging. Blood. 2009;114(18):3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y-H, et al. Diet-induced obesity dramatically reduces the efficacy of a 2009 pandemic H1N1 vaccine in a mouse model. Journal of Infectious Diseases. 2011;205(2):244–251. doi: 10.1093/infdis/jir731. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Experimental biology and medicine. 2010;235(12):1412–1424. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen S, et al. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113(11):1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Poorten D, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48(2):449–57. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 26.de Wit NJ, et al. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics. 2008;1:14. doi: 10.1186/1755-8794-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3(4):331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 28.Helander HF, Fandriks L. Surface area of the digestive tract - revisited. Scand J Gastroenterol. 2014;49(6):681–9. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 29.Vighi G, et al. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153(Suppl 1):3–6. doi: 10.1111/j.1365-2249.2008.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–85. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 31.Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Advanced Drug Delivery Reviews. 2012;64(6):523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Wershil BK, Furuta GT. 4. Gastrointestinal mucosal immunity. J Allergy Clin Immunol. 2008;121(2 Suppl):S380–3. doi: 10.1016/j.jaci.2007.10.023. quiz S415. [DOI] [PubMed] [Google Scholar]
- 33.Ding S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5(8):e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novotny Nunez I, et al. Lactobacillus casei CRL 431 administration decreases inflammatory cytokines in a diet-induced obese mouse model. Nutrition. 2015;31(7–8):1000–7. doi: 10.1016/j.nut.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, et al. Fatty acids enhance GRO/CINC-1 and interleukin-6 production in rat intestinal epithelial cells. J Nutr. 2001;131(11):2943–50. doi: 10.1093/jn/131.11.2943. [DOI] [PubMed] [Google Scholar]
- 36.Kvietys PR, et al. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol. 1991;261(3 Pt 1):G384–91. doi: 10.1152/ajpgi.1991.261.3.G384. [DOI] [PubMed] [Google Scholar]
- 37.Miura S, et al. Modulation of intestinal immune system by dietary fat intake: relevance to Crohn’s disease. J Gastroenterol Hepatol. 1998;13(12):1183–90. [PubMed] [Google Scholar]
- 38.Lam YY, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7(3):e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam YY, et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity (Silver Spring) 2015;23(7):1429–39. doi: 10.1002/oby.21122. [DOI] [PubMed] [Google Scholar]
- 40.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203(3):497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira MC, et al. Acute and sustained inflammation and metabolic dysfunction induced by high refined carbohydrate-containing diet in mice. Obesity (Silver Spring) 2013;21(9):E396–406. doi: 10.1002/oby.20230. [DOI] [PubMed] [Google Scholar]
- 42.Porter SA, et al. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32(6):1068–75. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam YY, et al. Role of the gut in visceral fat inflammation and metabolic disorders. Obesity (Silver Spring) 2011;19(11):2113–20. doi: 10.1038/oby.2011.68. [DOI] [PubMed] [Google Scholar]
- 44.Cani PD, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 45.Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gambero A, et al. Mesenteric adipose tissue alterations resulting from experimental reactivated colitis. Inflamm Bowel Dis. 2007;13(11):1357–64. doi: 10.1002/ibd.20222. [DOI] [PubMed] [Google Scholar]
- 47.Booth A, Magnuson A, Foster M. Detrimental and protective fat: body fat distribution and its relation to metabolic disease. Horm Mol Biol Clin Investig. 2014;17(1):13–27. doi: 10.1515/hmbci-2014-0009. [DOI] [PubMed] [Google Scholar]
- 48.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12(6):593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Sasson SZ, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106(17):7119–24. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rider P, et al. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187(9):4835–43. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 51.Pickup JC, et al. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40(11):1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 52.Kern PA, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745–51. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 53.Atreya R, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6(5):583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 54.McLoughlin RM, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102(27):9589–94. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nish SA, et al. T cell-intrinsic role of IL-6 signaling in primary and memory responses. Elife. 2014;3:e01949. doi: 10.7554/eLife.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida H, et al. Anti-IL-6 receptor antibody suppressed T cell activation by inhibiting IL-2 production and inducing regulatory T cells. Eur J Pharmacol. 2010;634(1–3):178–83. doi: 10.1016/j.ejphar.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 57.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 58.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210(3):535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang LQ, et al. Autocrine role of interleukin-13 on skeletal muscle glucose metabolism in type 2 diabetic patients involves microRNA let-7. Am J Physiol Endocrinol Metab. 2013;305(11):E1359–66. doi: 10.1152/ajpendo.00236.2013. [DOI] [PubMed] [Google Scholar]
- 61.Beutler BA. The role of tumor necrosis factor in health and disease. J Rheumatol Suppl. 1999;57:16–21. [PubMed] [Google Scholar]
- 62.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nunemaker CS, et al. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J Endocrinol. 2014;222(2):267–76. doi: 10.1530/JOE-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griffin GK, et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188(12):6287–99. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun M, et al. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015;8(5):969–978. doi: 10.1038/mi.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Josefowicz SZ, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482(7385):395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adema GJ, et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387(6634):713–7. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 69.Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 70.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186(8):1223–32. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dubois B, et al. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185(5):941–51. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allez M, et al. CD4+NKG2D+ T cells in Crohn’s disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology. 2007;132(7):2346–58. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 73.Pariente B, et al. Activation of the receptor NKG2D leads to production of Th17 cytokines in CD4+ T cells of patients with Crohn’s disease. Gastroenterology. 2011;141(1):217–26. 226 e1–2. doi: 10.1053/j.gastro.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 74.Camus M, et al. Oligoclonal expansions of mucosal T cells in Crohn’s disease predominate in NKG2D-expressing CD4 T cells. Mucosal Immunol. 2014;7(2):325–34. doi: 10.1038/mi.2013.51. [DOI] [PubMed] [Google Scholar]
- 75.Haile LA, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135(3):871–81. 881 e1–5. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 76.Kerjaschki D, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15(3):603–12. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 77.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 78.Huang FP, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191(3):435–44. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Worthington JJ, et al. Intestinal dendritic cells specialize to activate transforming growth factor-beta and induce Foxp3+ regulatory T cells via integrin alphavbeta8. Gastroenterology. 2011;141(5):1802–12. doi: 10.1053/j.gastro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun JB, Czerkinsky C, Holmgren J. Sublingual ‘oral tolerance’ induction with antigen conjugated to cholera toxin B subunit generates regulatory T cells that induce apoptosis and depletion of effector T cells. Scand J Immunol. 2007;66(2–3):278–86. doi: 10.1111/j.1365-3083.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- 81.Hadis U, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34(2):237–46. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 82.Worbs T, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203(3):519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schulz O, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206(13):3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 85.Koboziev I, Karlsson F, Grisham MB. Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation. Ann N Y Acad Sci. 2010;1207(Suppl 1):E86–93. doi: 10.1111/j.1749-6632.2010.05711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189(4):611–4. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9(1):10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 88.Pond CM, Mattacks CA. The activation of the adipose tissue associated with lymph nodes during the early stages of an immune response. Cytokine. 2002;17(3):131–9. doi: 10.1006/cyto.2001.0999. [DOI] [PubMed] [Google Scholar]
- 89.Pond CM, Mattacks CA. The source of fatty acids incorporated into proliferating lymphoid cells in immune-stimulated lymph nodes. Br J Nutr. 2003;89(3):375–83. doi: 10.1079/BJN2002784. [DOI] [PubMed] [Google Scholar]
- 90.Pond CM, Mattacks CA. In vivo evidence for the involvement of the adipose tissue surrounding lymph nodes in immune responses. Immunol Lett. 1998;63(3):159–67. doi: 10.1016/s0165-2478(98)00074-1. [DOI] [PubMed] [Google Scholar]
- 91.Mattacks CA, Pond CM. Interactions of noradrenalin and tumour necrosis factor alpha, interleukin 4 and interleukin 6 in the control of lipolysis from adipocytes around lymph nodes. Cytokine. 1999;11(5):334–46. doi: 10.1006/cyto.1998.0442. [DOI] [PubMed] [Google Scholar]
- 92.Mattacks CA, Sadler D, Pond CM. Site-specific differences in the action of NRTI drugs on adipose tissue incubated in vitro with lymphoid cells, and their interaction with dietary lipids. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135(1):11–29. doi: 10.1016/s1532-0456(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 93.Mattacks CA, Sadler D, Pond CM. The cellular structure and lipid/protein composition of adipose tissue surrounding chronically stimulated lymph nodes in rats. J Anat. 2003;202(6):551–61. doi: 10.1046/j.1469-7580.2003.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Medina M, Orgill DP. Discussion: regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg. 2012;129(4):835–7. doi: 10.1097/PRS.0b013e3182450b7a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.