Abstract
The chemical synthesis and incorporation of the phosphoramidite derivatives of 2′-O-photocaged ribonucleosides (A, C, G and U) with o-nitrobenzyl, α-methyl-o-nitrobenzyl or 4,5-dimethoxy-2-nitrobenzyl group into oligoribonucleotides are described. The efficiency of UV irradiated uncaging of these 2′-O-photocaged oligoribonucleotides was found in the order of α-methyl-o-nitrobenzyl > 4,5-dimethoxy-2-nitrobenzyl > 2′-O-o-nitrobenzyl.
Keywords: 2′-O-photocaged ribonucleosides; 2′-O-photocaged phosphoramdites; 2′-O-photocaged oligomers; 2′-O-o-nitrobenzyl; 2′-O-α-methyl-o-nitrobenzyl; 2′-O-4,5-dimethoxy-2-nitrobenzyl
INTRODUCTION
Photolabile protecting groups have been extensively investigated over the years to protect hydroxyl or amino groups in organic synthesis.[1,2] Photocaging provides a well-established strategy to cage functions of biologically important molecules until released by UV irradiation.[3–5] 2′-O-Photocaged oligonucleotides including the 3′-S-oligonucleotide and 5′-S-oligonucleotide phosphorothiolates as useful RNA analogues have been successfully utilized to investigate the mechanism of RNA catalyzed reactions[6–10] and pre-mRNA splicing.[11,12] Although a number of photolabile groups have been introduced into photocaged molecules for biological studies, to the best of our knowledge, only o-nitrobenzyl group has been introduced to 2′-O-caged RNAs.[6,10,13,14] For example, the 2′-O-o-nitrobenzyl derivatives of adenosine,[15] cytidine,[15] guanosine,[16] and uridine[17] were synthesized by Ohtsuka et al. and applied to the solution synthesis of 2′-O-caged dinucleotides and trinucleotides.[15,17,18] The first solid-phase synthesis of 2′-O-photocaged oligoribonucleotides was achieved by using 2′-O-o-nitrobenzyl-3′-H-phosphonate chemistry.[13] Later, the phosphoramidite derivatives of 2′-O-o-nitrobenzyl-N6-benzoyladenosine[6] and 2′-O-o-nitrobenzyl-N4-benzoylcytidine[10] were prepared and applied to synthesize the 2′-O-caged RNAs via phosphoramidite chemistry. These 2′-O-o-nitrobenzyl RNAs have proven useful as tools to probe RNA reactivity and dynamics via a photochemically-controlled manner.[6–12] The 2′-O-photolabile group and ultramild N-phenoxyacetyl group protected phosphoramidites are useful for the synthesis of oligoribonucleotides containing a 5′-S-phosphorothiolate linkage since these modified oligoribonucleotides are more susceptible to alkaline cleavage compared to the wild type RNAs.[7,19] Only three 2′-O-photolabile phosphoramidite derivatives (with 2′-O-o-nitrobenzyl and N-benzoyl or N-isobutyryl protection) have been synthesized and reported in the literature.[10,14,20] 2′-O-o-Nitrobenzyl caged RNAs synthesized by either solid phase synthesis or a two-step ligation method have been successfully applied to investigate the mechanisms of ribozyme catalyzed reactions.[7–9,19] However, the relatively long UV irradiation time (4 to 6 minutes)[9,19] for the full removal of o-nitrobenzyl group from 2′-O-photocaged RNAs may limit its applications in the case of unstable RNAs (or ribozymes) with UV exposure for a long time. To overcome this limitation, here we report the general synthetic methods to obtain 2′-O-photocaged phosphoramidites containing a number of more efficient 2′-O-photolabile and N-phenoxyacetyl protected ribonucleoside phosphoramidites.
Results and Discussion
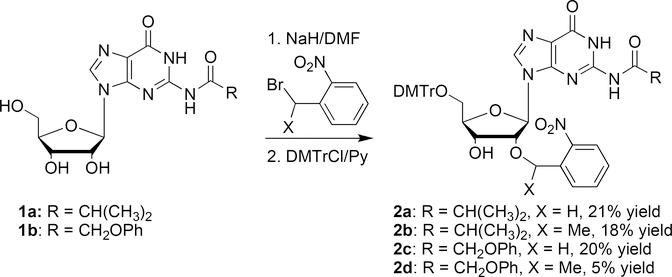
The 2′-O-photolabile 5′-O-DMTr-N2-butyrylguanosine derivatives (2a and 2b) were prepared in 21% and 18% yields, respectively, according to our previously improved procedures by the reactions of N2-butyrylguanosine (1a) with o-nitrobenzyl bromide or α-methyl-o-nitrobenzyl bromide, followed by the 5′-O-DMTr protection.[16,19] The corresponding 2′-O-photolabile 5′-O-DMTr-N2-phenoxyacetylguanosine derivatives (2c and 2d) were prepared from N2-phenoxyacetylguanosine (1b) in 20% and 5% yields, respectively (Scheme 1). It is worthy to note the free guanosine could not be selectively 2′-O-benzylated by the reaction with o-nitrobenzyl bromide due to the side reaction at N1 of the guanine ring.
Scheme 1.
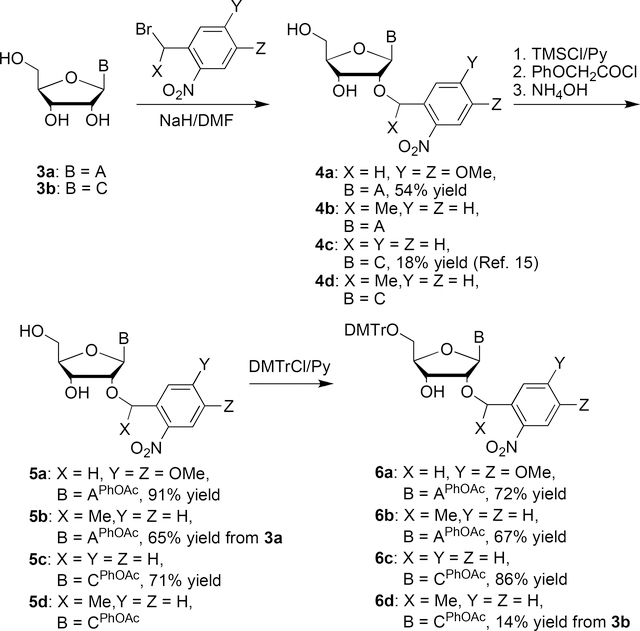
The 2′-O-photolabile 5′-O-DMTr-N-phenoxyacetyl adenosine and cytidine derivatives (6a-6d) could be prepared from their respective free ribonucleoside precursors (i.e. adenosine and cytidine) in three steps (Scheme 2). Without any protection both adenosine and cytidine could be selectively 2′-O-benzylated with 4,5-dimethoxy-2-nitrobenzyl bromide or α-methyl-o-nitrobenzyl bromide to give 4a-4d. The amino group of nucleobases of 4a-4d was then selectively protected by a phenoxyacetyl group to give compounds 5a-5d. 5′-O-DMTr protection of 5a-5d gave the corresponding phophoramidite precursors 6a-6d in three steps from free nucleosides 3a and 3b with 11–44% overall yields.
Scheme 2.
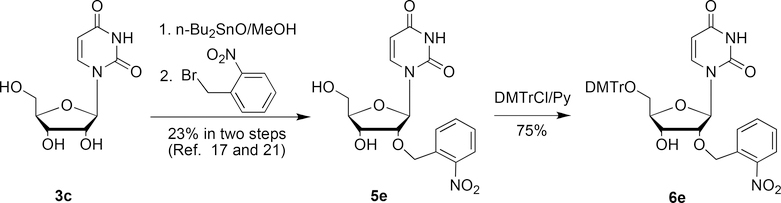
Ohtsuka and Ikehara reported the synthesis of 2′-O-o-nitrobenzyluridine (5e) in 24% yield by the reaction of 2′,3′-O-(dibutylstannyl)uridine[21] with o-nitrobenzyl bromide in DMF at 110 ºC for 4 hours (Scheme 3).[17] DMTr protection of 5e then yielded the corresponding 2′-O-photocaged uridine phosphoramidite precursor 6e in 75% yield. Unfortunately, we found this method cannot be extended for the preparation of 2′-O-α-methyl-o-nitrobenzyluridine by reacting of 2′,3′-O-(dibutylstannyl)uridine with α-methyl-o-nitrobenzyl bromide. No desired product was formed after this reaction, even at 110 ºC for 24 hours. According to the procedure for the synthesis of 2a, direct deprotonation of uridine with NaH in DMF, followed by reaction with o-nitrobenzyl bromide generated only O4-o-nitrobenzyluridine.
Scheme 3.
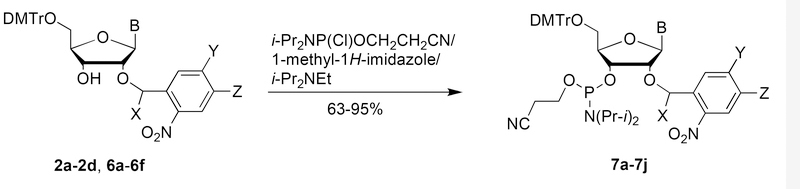
Phosphitylation of 2a-3d and 6a-6e with 2-cyanoethyl N,N-diisopropylchlorophosphoramidite yielded the corresponding 2′-O-photocaged nucleoside phosphoramidites 7a-7i including the 2′-O-photolabile N-phenoxyacetylribonucleoside phosphoramidites 7c-7h in good yields (Scheme 4 & Table 1).
Scheme 4.
Table 1.
Phosphoramidites 7a-7j prepared by the phosphitylation of 2a-2d and 6a-6f.
| Product | Precursor | X | Y | Z | B | Yield (%) |
|---|---|---|---|---|---|---|
| 7a | 2a | H | H | H | Gi-Bu | 95 |
| 7b | 2b | Me | H | H | Gi-Bu | 86 |
| 7c | 2c | H | H | H | GPhOAc | 77 |
| 7d | 2d | Me | H | H | GPhOAc | 63 |
| 7e | 6a | H | OMe | OMe | APhOAc | 88 |
| 7f | 6b | Me | H | H | APhOAc | 92 |
| 7g | 6c | H | H | H | CPhOAc | 92 |
| 7h | 6d | Me | H | H | CPhOAc | 91 |
| 7i | 6e | H | H | H | U | 86 |
| 7j | 6f | H | H | H | ABz | 82 |
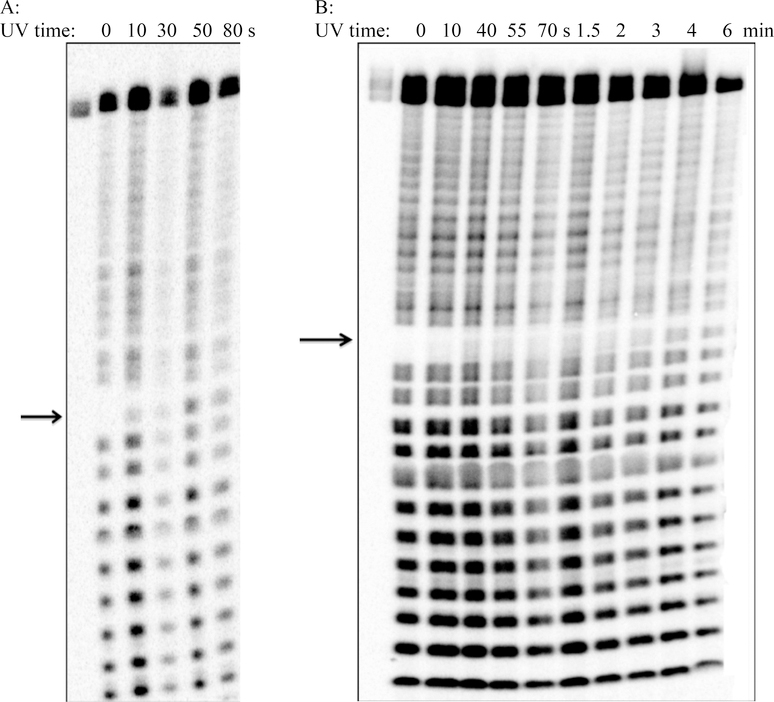
To compare the efficiency of the UV irradiated decaging of RNAs containing various photolabile groups, the known phosphoramidite derivative of 2′-O-o-nitrobenzyl-N6-benzoyladenosine 7j was also prepared (Scheme 4).[6,14] The adenosine phosphoramidites 7e (with 3,4-dimethoxy-2-nitrobenzyl group), 7f (with α-methyl-o-nitrobenzyl group), and 7j (with o-nitrobenzyl group) were then incorporated into a 30-mer RNA sequence (an oligomer fragment for the construction of glms ribozyme):[22] 5′-GGU AAA UUA UAG AXG CGC CAG AAC UAC ACC-3′, where X represented the coupling of 2′-O-photocaged phosphoramidites (7e, 7f or 7h) at the ribozyme cleavage site. The solid-phase synthesis was carried out on Expedite 8900 Nucleic Acid Synthesizer with a modified RNA 1 μmole protocol. The standard RNA 1 μmole protocol for X was modified to double coupling before capping and oxidation. The trityl yields for the coupling of 7e, 7f or 7h were comparable to the commercially available phosphoramidites. After deprotection with ammonium hydroxide, and desilylation with 1-methyl-2-pyrrolidinone (NMP)/Et3N/Et3N-3HF,[23] these RNAs were 5′-radiolabeled and purified by dPAGE gel as usual.[19] The progress of UV irradiated decaging of these RNAs was analyzed after alkaline hydrolysis and quantified by phosphorimager according to our reported method.[9,19] As expected, no alkaline hydrolysis was observed at the caged nucleotide site until UV irradiation (Figure 1). These data were then fitted into a first-order kinetics equation (y = y0 – A*e-kt); and the resulting kinetics rates are shown in Table 2. From Table 2, we conclude that 2′-O-caged RNA containing an α-methyl-o-nitrobenzyl group has the most prominent photolabile properties. An RNA oligomer with α-methyl-o-nitrobenzyl group is uncaged by UV at 365 nm about 10 times faster compared to an RNA oligomer with o-nitrobenzyl group, whereas an RNA strand with 3,4-dimethoxy-2-nitrobenzyl group is uncaged by UV about 5 times faster than an RNA oligomer with o-nitrobenzyl group at 365 nm. The electronic donating groups (α-methyl and 3,4-dimethoxy) may have stabilized the resonance isomer (intermediate) formation thus facilitating the UV irradiated deprotection.[24]
Figure 1. UV initiated deprotection and alkaline hydrolysis of 2′-O-caged RNAs containing 2′-O-photolabile groups.
A: Gel image corresponds to the RNA containing α-methyl-o-nitrobenzyl protecting group. B: Gel image corresponds to the RNA containing o-nitrobenzyl protecting group. Method: A 5′-radiolabeled 30 mer RNA fragment in water (20 μL) was irradiated at 365 nm and 0.5-μL aliquots were taken and diluted in water (3 μL). Alkaline hydrolysis ladders were performed by adding sodium bicarbonate, pH 9, and incubating at 90 °C for 15 min. The resulting ladder of RNA fragments was separated by denaturing PAGE, and deprotection was quantitated by measuring the intensity of the band corresponding to cleavage by the deprotected nucleophile. The first lane in the depicted gel corresponds to deprotected substrate prior to alkaline hydrolysis.
Table 2.
Efficiency of the UV (365 nm) irradiated uncaging of 2′-O-caged RNAs containing various 2′-O-photolabile groups.
| 2′-O-Caged RNAs | k (min−1)a | t1/2 (min)b | Time (min)c |
|---|---|---|---|
| 2′-O-α-methyl-o-nitrobenzyl-RNA | 3.0 | 0.23 | 0.83 |
| 2′-O-2-nitro-3,4-(MeO)2C6H3CH2-RNA | 1.6 | 0.43 | 1.5–3 |
| 2′-o-nitrobenzyl-RNA | 0.27 | 2.6 | 4–6 |
k is obtained from alkaline hydrolysis data of UV initiated uncaging of RNAs by fitting to the equation: y = y0 – A*e−kt.
t1/2 is the time when 50% RNAs are uncaged.
Time estimated for full uncaging.
In summary, we have described the synthesis of the phosphoramidite derivatives of adenosine, cytidine and guanosine with more 2′-O-photolabile and N-phenoxyacetyl protections. We also prepared the phosphoramidite derivative of 2′-O-o-nitrobenzyluridine. Solid phase synthesis using these phosphoramidites together with commercially available 2′-O-TBS-N-phenoxyacetylribonucleoside phosphoramidites would provide a general method to synthesize 2′-O-caged RNA oligonucleotides featured with ultramild deprotection conditions (NH4OH/rt, 2 hours). Application of this new class of ribonucleoside phosphoramidites in the synthesis of RNA oligomers containing a 2′-O-photocaged-5′-S-phosphorothiolate linkage are currently being further evaluated and the syntheses are expected to be reported elsewhere later. Overall, 2′-O-photocaged RNAs containing an α-methyl-o-nitrobenzyl group seems to be the most photolabile amongst the three photocaged groups studied.
Experimental Section
5′-O-(Dimethoxytrityl)-N2-isobutyryl-2′-O-(o-nitrobenzyl)guanosine (2a).[19]
Compound 2a was prepared from N2-isobutyrylguanosine (1a) in two steps (21% yield). Under argon N2-Isobutyrylguanosine (1a) (1.717 g, 4.86 mmol) was treated with NaH (292 mg, 12.2 mmol) in dry DMF (40 mL) at 0 ºC. After hydrogen gas generation ceased (about 40 min), o-nitrobenzyl bromide (1.575 g, 7.29 mmol) was added and the mixture was stirred for 5 h at 0 ºC. The reaction was quenched with EtOH and neutralized with 1 N HCl. The mixture was evaporated and the crude product was purified by silica gel chromatography, eluting with 4–6% methanol in dichloromethane, to give N2-isobutyryl-2′-O-(o-nitrobenzyl)guanosine[16,19] as a yellow foam: 0.76 g (32% yield). To a solution of N2-isobutyryl-2′-O-(o-nitrobenzyl)guanosine (192 mg, 0.393 mmol) in dry pyridine (4.0 mL) under dry argon, DMTrCl (400 mg, 1.18 mmol) was added. After the mixture was stirred at rt overnight, methanol (2.0 mL) was added to quench the reaction. The solvent was removed by rotary evaporation, and the crude product was purified by silica gel chromatography, eluting with 2% MeOH in CH2Cl2 containing 0.5% Et3N to give compound 2a[19] as a yellow foam (206 mg, 66% yield). 1H NMR (CDCl3) δ 12.02 (br s, 1H), 8.61 (br s, 1H), 8.01 (d, J = 8 Hz, 1H), 7.87 (s, 1H), 7.67–7.18 (m, 12H), 6.8 (m, 4H), 6.01 (d, J = 3.5 Hz, 1H), 5.37 (d, J = 15 Hz, 1H), 5.22 (d, J = 15 Hz, 1H), 4.62 (t, J = 4.5 Hz, 1H), 4.55 (m, 1H), 4.27 (m, 1H), 3.77 (s, 3H), 3.767 (s, 3H), 3.55 (d, J = 10.5 Hz, 3H), 3.35 (m, 1H), 3.21 (br s, 1H), 2.29 (m, 1H), 1.12 (d, J = 7 Hz, 1H), 1.01 (d, J = 7 Hz, 1H); 13C NMR (DMSO-d6) δ 231.0, 178.8, 158.6, 155.5, 147.5, 147.4, 146.9, 144.6, 137.5, 135.8, 135.6, 134.1, 133.8, 130.0, 129.9, 128.6, 128.5, 128.0, 127.9, 127.0, 124.7, 121.9, 113.2, 113.1, 87.0, 86.4, 83.6, 82.3, 69.4, 69.3, 63.1, 55.2, 36.2, 18.7, 18.6; HRMS calcd for C42H43N6O10 [MH+] 791.3035, found 791.3041.
5′-O-(Dimethoxytrityl)-N2-isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine (2b).[19]
According to the procedure described for the synthesis of 2a, N2-isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine[19] (636 mg, 26% yield) as a yellow foam was prepared from 1a (1.717 g, 4.86 mmol), NaH (292 mg, 12.2 mmol) and α-methyl-o-nitrobenzyl bromide (1.677 g, 7.29 mmol). Compound 2b was then prepared by the reaction of N2-isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine (307 mg, 0.61 mmol) with DMTrCl (621 mg, 1.83 mmol) in dry pyridine at rt overnight. Silica gel chromatography (1–2% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 2b[19] (345 mg, 70% yield) as a yellow foam. 1H NMR (CDCl3) δ 12.04 (s, 1H), 8.57 (s, 1H), 7.92–7.70 (m, 3H), 7.47–7.19 (m, 10H), 6.82–6.79 (m, 4H), 6.01 (d, J = 1.2 Hz, 1H), 5.77 (m, 1H), 4.33–4.26 (m, 2H), 3.91 (m, 1H), 3.770 (s, 3H), 3.767 (s, 3H), 3.57–3.45 (m, 2H), 2.72 (d, J = 8.3 Hz, 1H), 2.60 (m, 1H), 1.66 (d, J = 6.3 Hz, 3H), 1.27 (d, J = 6.9 Hz, 3H), 1.21 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3) δ 178.8, 158.5, 155.3, 147.8, 147.6, 147.0, 144.4, 139.0, 136.6, 135.6, 135.5, 134.7, 130.0, 128.8, 128.0, 127.9, 127.0, 124.6, 121.9, 113.2, 87.6, 86.6, 82.7, 80.4, 72.4, 69.1, 62.5, 55.2, 36.5, 24.1, 19,2, 18.4; HRMS calcd for C43H45N6O10 [MH+] 805.3192, found 805.3209.
5′-O-(Dimethoxytrityl)-2′-O-(o-nitrobenzyl)-N2-phenoxyacetylguanosine (2c).
According to the procedure described for the synthesis of 2a, 2′-O-(o-nitrobenzyl)-N2-phenoxyacetylguanosine (0.81 g, 30% yield) as a yellow foam was prepared from N2-phenoxyacetylguanosine (1b)[25] (2.027 g, 4.86 mmol), NaH (292 mg, 12.2 mmol) and o-nitrobenzyl bromide (1.575 g, 7.29 mmol). Compound 2c was then prepared by the reaction of 2′-O-(o-nitrobenzyl)-N2-phenoxyacetylguanosine (308 mg, 0.56 mmol) with DMTrCl (565 mg, 1.68 mmol) in dry pyridine at rt overnight. Silica gel chromatography (1–2% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 2c (317 mg, 67% yield) as a yellow foam. 1H NMR (DMSO-d6) δ 8.11 (s, 1H), 8.01 (d, J = 8.1 Hz, 1H), 7.73–7.69 (m, 2H), 7.55 (t, J = 6.6 Hz, 1H), 7.35–7.20 (m, 11H), 7.00–6.96 (m, 3H), 6.85–6.81 (m, 4H), 6.06 (d, J = 5.2 Hz, 1H), 5.47 (d, J = 2.6 Hz, 1H, disappeared with D2O), 5.06 (d, J = 14.7 Hz, 1H), 4.97 (d, J = 14.6 Hz, 1H), 4.85 (d, J = 2.5 Hz, 2H), 4.59 (t, J = 5.1 Hz, 1H), 4.42 (m, 1H), 4.12 (m, 1H), 3.72 (s, 6H), 3.28–3.18 (m, 2H); 13C NMR (CDCl3) δ 169.7, 158.6, 156.6, 147.7, 147.4, 144.3, 136.8, 135.5, 135.4, 133.8, 133.1, 130.0, 129.8, 128.9, 128.7, 128.1, 127.9, 127.0, 124.7, 122.6, 122.0, 114.8, 113.2, 86.7, 86.3, 83.6, 82.7, 69.8, 69.3, 67.0, 63.0, 55.2; HRMS calcd for C46H43N6O11 [MH+] 855.2984, found 855.2996.
5′-O-(Dimethoxytrityl)-2′-O-(α-methyl-o-nitrobenzyl)-N2-phenoxyacetylguanosine (2d).
According to the procedure described for the synthesis of 2a, a crude 2′-O-(α-methyl-o-nitrobenzyl)-N2-phenoxyacetylguanosine (530 mg, 19% yield) with some impurity as a yellow foam was prepared from 1b (2.09 g, 5.01 mmol), NaH (301 mg, 12.54 mmol) and α-methyl-o-nitrobenzyl bromide (1.729 g, 7.52 mmol). Compound 2d was then prepared by the reaction of 2′-O-(α-methyl-o-nitrobenzyl)-N2-phenoxyacetylguanosine (107 mg, 0.19 mmol) with DMTrCl (192 mg, 0.57 mmol) in dry pyridine at rt overnight. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 2d (47 mg, 28% yield) as a yellow foam. 1H NMR (DMSO-d6) δ 7.76 (d, J = 7.8 Hz, 1H), 7.70 (s, 1H), 7.45–7.17 (m, 15H), 7.07–6.74 (m, 7H), 5.80 (d, J = 4.7 Hz, 1H), 5.43 (m, 1H), 4.63 (s, 2H), 4.53 (t, J = 4.8 Hz, 1H), 4.40 (t, J = 4.8 Hz, 1H), 4.21 (m, 1H), 3.75 (s, 6H), 3.42 (dd, J = 2.4, 10.7 Hz, 1H), 3.35 (dd, J = 2.4, 10.7 Hz, 1H), 1.59 (d, J = 6.3 Hz, 3H); 13C NMR (CDCl3) δ 169.8, 158.5, 156.7, 147.4, 144.3, 138.7, 137.4, 135.5, 133.8, 129.9, 129.9, 129.7, 128.6, 128.0, 127.8, 127.4, 126.9, 123.8, 122.5, 114.7, 113.1, 86.5, 85.9, 84.1, 81.1, 73.9, 69.4, 67.0, 63.3, 55.1, 23.7; HRMS calcd for C47H45N6O11 [MH+] 869.3141, found 869.3158.
2′-O-(4,5-Dimethoxy-2-nitrobenzyl)adenosine (4a).
According to the procedure described for the synthesis of 2a, compound 4a was prepared from adenosine (1.51 g, 5.43 mmol), NaH (196 mg, 8.17 mmol) and 4,5-dimethoxy-2-nitrobenzyl bromide (2.248 g, 8.14 mmol). Silica gel chromatography (0–8% methanol in dichloromethane) of the crude product yielded 4a (1.41 g, 54% yield) as a yellow foam. 1H NMR (DMSO-d6) δ 8.35 (s, 1H), 8.06 (s, 1H), 7.56 (s, 1H), 7.35 (brs, 2H), 7.20 (s, 1H), 6.13 (d, J = 5.0 Hz, 1H), 5.50 (d, J = 5.0 Hz, 1H), 5.42 (m, 1H), 5.07 (d, J = 15.0 Hz, 1H), 4.87 (d, J = 15.0 Hz, 1H), 4.56 (t, J = 5.0 Hz, 1H), 4.40 (m, 1H), 4.05 (m, 1H), 3.82 (s, 3H), 3.71 (s, 3H), 3.70 (m, 1H), 3.59 (m, 1H); 13C NMR (DMSO-d6) δ 156.1, 153.2, 152.4, 148.9, 147.3, 139.5, 139.0, 129.4, 119.3, 110.1, 107.8, 86.3, 86.1, 81.3, 68.9, 67.9, 61.3, 56.0, 55.8; HRMS calcd for C19H23N6O8 [MH+] 463.1572, found 463.1560.
2′-O-(4,5-Dimethoxy-2-nitrobenzyl)-N6-phenoxyacetyladenosine (5a).
Compound 4a (0.68 g, 1.47 mmol) was dried by co-evaporation with dry pyridine (3 × 50 mL) under vacuum, and resuspended in dry pyridine (30 mL) under Ar. Chlorotrimethylsilane (1.5 mL) was added to the suspension at 0 ºC. After the reaction mixture was stirred at rt for 45 min, a solution of phenoxyacetyl chloride (310 μL, 2.21 mmol) and 1,2,4-triazole (155 mg, 2.26 mmol) in dry pyridine/acetonitrile (20 mL, 1:1) was slowly added. After the mixture was stirred at 55 ºC overnight, it was cooled to rt, and the reaction was quenched by addition of H2O (1.5 mL). After stirring for 5 min, concentrated aqueous NH4OH (1 mL) was added at 0 ºC, and the mixture was stirred for 30 min. The solvent was removed, the crude product was purified by silica gel chromatography, eluting with 3–5 % MeOH in CH2Cl2 to give 5a (797 mg, 91% yield) as a yellow foam. 1H NMR (CDCl3) δ 9.67 (br s, 1H), 8.71 (s, 1H), 8.17 (s, 1H), 7.44 (s, 1H), 7.37–7.33 (m, 2H), 7.07–7.04 (m, 3H), 6.76 (s, 1H), 6.07 (d, J = 7.3 Hz, 1H), 5.03 (d, J = 12.8 Hz, 1H), 4.96–4.93 (m, 3H), 4.71–4.65 (m, 2H), 4.40 (s, 1H), 3.99 (dd, J = 1.5, 13.0 Hz, 1H), 3.87 (s, 3H), 3.82 (s, 3H), 3.81 (dd, J = 1.5, 13.0 Hz, 1H); 13C NMR (CDCl3) δ 167.3, 157.1, 153.1, 152.0, 150.4, 149.1, 148.5, 143.7, 140.2, 129.9, 129.7, 126.7, 123.8, 115.0, 114.6, 111.4, 108.1, 89.1, 88.1, 81.2, 70.7, 70.1, 68.3, 65.1, 63.1, 56.5, 56.4; HRMS calcd for C27H29N6O10 [MH+] 597.1940, found 597.1937.
2′-O-(o-Nitrobenzyl)-N4-phenoxyacetylcytidine (5c).[19]
2′-O-(o-Nitrobenzyl)cytidine (4c)[15] (3.58 g, 9.46 mmol) was dried by co-evaporation with dry pyridine (3 × 100 mL) under vacuum and resuspended in dry pyridine (180 mL) under argon. Chlorotrimethylsilane (9.0 mL) was added to the suspension at 0 ºC. After the mixture was stirred at rt for 45 min, a solution of phenoxyacetyl chloride (2.00 mL, 14.4 mmol) and 1,2,4-triazole (0.980 g, 14.3 mmol) in pyridine-acetonitrile (120 mL, 1:1) was slowly added. After the mixture was stirred at 55 ºC overnight, it was cooled to rt, and the reaction was quenched by addition of H2O (10 mL). After stirring for 5 min, concentrated aqueous NH4OH (6.5 mL) was added at 0 ºC, and the mixture was stirred for 30 min. The solvent was removed, and the crude product was purified by silica gel chromatography, eluting with 3–5 % MeOH in CH2Cl2 to give 5c[19] (3.44 g, 71% yield) as a yellow foam. 1H NMR (DMSO-d6) δ 11.04 (s, 1H), 8.51 (d, 1H, J = 7.5 Hz), 8.08 (dd, 1H, J = 1.3, 8.2 Hz), 7.94 (d, 1H, J = 7.7 Hz), 7.77 (m, 1H), 7.57 (m, 1H), 7.35–7.25 (m, 2H), 7.11 (d, 1H, J = 7.5 Hz), 6.96 (m, 1H), 6.92 (m, 2H), 5.94 (d, 1H, J = 2.4 Hz), 5.34 (d, 1H, J = 6.7 Hz), 5.25 (t, 1H, J = 5.0 Hz), 5.15 (s, 2H), 4.83 (s, 2H), 4.12 (m, 1H), 4.15 (s, 1H), 4.03–3.95 (m, 3H), 3.79 (m, 1H), 3.64 (m, 1H); HRMS calcd for C24H25N4O9 [MH+] 513.1622, found 513.1621.
5′-O-(Dimethoxytrityl)-2′-O-(4,5-dimethoxy-2-nitrobenzyl)-N6-phenoxyacetyladenosine (6a).
According to the procedure described for the synthesis of 2a, compound 6a was prepared by the reaction of 5a (797 mg, 1.34 mmol) with DMTrCl (1.25 g, 4.02 mmol) in dry pyridine at rt overnight. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 6a (864 mg, 72% yield) as a yellow foam. 1H NMR (CDCl3) δ 9.47 (brs, 1H), 8.66 (s, 1H), 8.26 (s, 1H), 7.59 (s, 1H), 7.44–6.81 (m, 19H), 6.30 (d, J = 4.1 Hz, 1H), 5.24 (d, J = 14.0 Hz, 1H), 5.08 (d, J = 14.0 Hz, 1H), 4.86 (s, 2H), 4.74 (t, J = 4.6 Hz, 1H), 4.57 (t, J = 4.9 Hz, 1H), 4.32 (m, 1H), 3.96 (s, 3H), 3.91 (s, 3H), 3.78 (s, 6H), 3.57 (dd, J = 3.2, 10.8 Hz, 1H), 3.46 (dd, J = 4.1, 10.7 Hz, 1H), 2.92 (b, 1H); 13C NMR (CDCl3) δ 158.7, 157.1, 153.6, 152.6, 151.4, 148.4, 148.3, 144.5, 141.9, 140.1, 135.62, 135.60, 130.2, 130.0, 128.2, 128.0, 127.8, 127.1, 123.2, 122.5, 115.0, 113.3, 110.6, 108.2, 87.1, 86.9, 84.3, 81.9, 70.1, 69.8, 68.2, 62.9, 56.5, 56.4, 55.3; HRMS calcd for C48H47N6O12 [MH+] 899.3246, found 899.3244.
5′-O-(Dimethoxytrityl)-2′-O-(α-methyl-o-nitrobenzyl)-N6-phenoxyacetyladenosine (6b).
According to the procedure described for the synthesis of 2a, a crude 2′-O-(α-methyl-o-nitrobenzyl)adenosine (4b) (1.42 g) as a yellow foam was prepared from adenosine (2.00 g, 7.48 mmol), NaH (269 mg, 11.2 mmol) and α-methyl-o-nitrobenzyl bromide (2.51 g, 10.9 mmol). Compound 5b was then prepared from the crude 4b (1.42 g, 3.41 mmol), TMSCl (3.5 mL), and phenoxyacetyl chloride (0.71 mL, 5.12 mmol) as described for the synthesis of 5a. Silica gel chromatography (5% methanol in dichloromethane) of the crude product yielded 5b (1.20 g, 65% yield, two steps from adenosine) as a yellow foam. 1H NMR (CDCl3) δ 9.55 (s, 1H), 8.71 (s, 1H), 8.13 (s, 1H), 7.72–7.06 (m, 9H), 6.01 (d, J = 7.0 Hz, 1H), 5.42 (m, 1H), 5.11 (m, 1H), 4.93 (s, 2H), 4.85 (m, 1H), 4.40 (d, J = 4.6 Hz, 1H), 4.32 (d, J = 1.3 Hz, 1H), 3.90 (m, 1H), 3.76 (m, 1H), 2.69 (s, 1H), 1.33 (d, J = 6.4 Hz, 3H). Compound 6b was then prepared by the reaction of 5b (295 mg, 0.538 mmol) with DMTrCl (546 mg, 1.61 mmol) according to the procedure described for synthesis of 2a. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 6b (305 mg, 67% yield, or 44% from 3a) as a yellow foam. 1H NMR (CDCl3) δ 9.36 (brs, 1H), 8.68 (s, 1H), 8.63–8.61 (m, 3H), 8.25 (s, 1H), 7.94–6.81 (m, 18H), 6.25 (d, J = 2.1 Hz, 1H), 5.68 (m, 1H), 4.86 (s, 2H), 4.34 (m, 1H), 4.29–4.25 (m, 2H), 3.79 (s, 6H), 3.55 (dd, J = 2.7, 10.8 Hz, 1H), 3.44 (dd, J = 4.0, 10.8 Hz, 1H), 2.56 (d, J = 8.0 Hz, 1H), 1.65 (d, J = 6.3 Hz, 3H); HRMS calcd for: C47H45N6O10, [MH+]: 853.3197, found 853.3200.
5′-O-(Dimethoxytrityl)-2′-O-(o-nitrobenzyl)-N-phenoxyacetylcytidine (6c).[19]
According to the procedure described for the synthesis of 2a, compound 6c was prepared by the reaction of 5c (1.24 g, 2.42 mmol) with DMTrCl (2.46 g, 7.26 mmol) in dry pyridine at rt overnight. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 6c[19] (1.67 g, 86% yield) as a yellow foam. 1H NMR (DMSO-d6) δ 11.03 (s, 1H), 8.34 (d, J = 7.5 Hz, 1H), 8.08 (d, J = 8.2 Hz, 1H), 7.98 (d, J = 7.7 Hz, 1H), 7.76 (m, 1H), 7.57 (m, 1H), 7.40–6.85 (m, 19H), 5.94 (s, 1H), 5.4 (d, J = 7.3 Hz, 1H), 5.21 (dd, J = 15.2, 25.4 Hz, 2H), 4.82 (s, 2H), 4.35 (m, 1H), 4.15 (s, 1H), 4.01 (d, J = 4.8 Hz, 1H), 3.70 (s, 3H), 3.69 (s, 3H), 3.42–3.36 (m, 2H); HRMS calcd for C45H43N4O11 [MH+] 815.2928, found 815.2930.
5′-O-(Dimethoxytrityl)-2′-O-(α-methyl-o-nitrobenzyl)-N-phenoxyacetylcytidine (6d).
According to the procedure described for the synthesis of 2a, a crude 2′-O-(α-methyl-o-nitrobenzyl)cytidine (4d) (~1.42 g) containing impurities as a yellow foam was prepared from cytidine (0.97 g, 4.0 mmol), NaH (192 mg, 8.00 mmol) and α-methyl-o-nitrobenzyl bromide (1.43 g, 6.22 mmol). The molecular weight of 4d was confirmed by MS (ES-API), calcd for C17H21N4O7 [MH+] 393.1, found 393.2. According to the procedure described for the synthesis of 5c and 6c, intermediate 4d (~1.42 g, 3.60 mmol) was first protected with phenoxyacetyl group to give a crude 2′-O-(α-methyl-o-nitrobenzyl)-N4-phenoxyacetylcytidine (5d) (1.35 g). The molecular weight of 5d was also confirmed by MS (ES-API), calcd for C25H27N4O9 [MH+] 527.2, found 527.2. Compound 6d was then prepared by the reaction of crude 5d (1.35 g) with DMTrCl (2.57 g, 7.58 mmol) in dry pyridine at rt overnight. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 6d (459 mg, three steps 14% yield from cytidine) as a yellow foam. 1H NMR (CDCl3) δ 9.78 (brs, 1H), 8.36 (d, J = 7.5 Hz, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.59 (m, 1H), 7.40–7.20 (m, 14H), 7.09 (d, J = 7.5 Hz, 1H), 7.01 (t, J = 7.5 Hz, 1H), 6.86 (d, J = 8.5 Hz, 4H), 5.61 (q, J = 6.5 Hz, 1H), 5.56 (s, 1H), 4.65 (s, 2H), 4.35 (m, 1H), 4.11 (m, 1H), 3.92 (m, 1H), 3.81 (s, 6H), 3.62–3.52 (m, 2H), 1.64 (d, J = 6.5 Hz, 3H); 13C NMR (CDCl3) δ 168.5, 161.8, 158.8, 156.9, 154.8, 148.0, 145.0, 144.2, 139.4, 135.6, 135.3, 133.3, 130.18, 130.15, 129.8, 128.3, 128.1, 127.2, 124.4, 122.4, 114.6, 113.4, 96.4, 88.5, 87.2, 83.4, 82.2, 75.0, 68.2, 67.6, 61.2, 55.3, 23.4; HRMS calcd for C46H44N4O11Na [MNa+] 851.2904, found 851.2874.
5′-O-(Dimethoxytrityl)-2′-O-(o-nitrobenzyl)uridine (6e).
According to the procedure described for the synthesis of 2a, compound 6e was prepared by the reaction of 2′-O-(o-nitrobenzyl)uridine (5e)[17] (540 mg, 1.42 mmol) with DMTrCl (1.083 g, 3.20 mmol) in dry pyridine at rt overnight. Silica gel chromatography (1.5–2% methanol in dichloromethane) of the crude product yielded 6e (727 mg, 75% yield) as a yellow foam. 1H NMR (CDCl3) δ 10.05 (brs, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.95 (d, J = 8.5 Hz, 1H), 7.68 (d, J = 8.0 Hz, 1H), 7.56 (m, 1H), 7.44–7.35 (m, 3H), 7.33–7.25 (m, 7H), 6.83 (d, 4H, J = 8.5 Hz), 6.05 (d, J = 1.8 Hz, 1H), 5.31 (d, J = 8.5 Hz, 1H), 5.28 (d, J = 13.5 Hz, 1H), 5.15 (d, J = 13.5 Hz, 1H), 4.53 (dd, J = 7.5, 5.5 Hz, 1H, 1H), 4.13–4.08 (m, 2H), 3.77 (s, 6H), 3.58 (m, 2H); 13C NMR (CDCl3) δ 163.8, 158.7, 150.5, 147.9, 144.4, 139.9, 135.3, 135.1, 133.6, 133.0, 130.2, 130.1, 129.8, 128.8, 128.2, 128.0, 127.2, 124.8, 113.3, 102.2, 87.4, 87.1, 83.2, 82.6, 69.4, 68.7, 61.3, 55.3; HRMS calcd for C37H35N3O10Na [MNa+] 704.2220, found 704.2200.
5′-O-(Dimethoxytrityl)-N2-isobutyryl-2′-O-(o-nitrobenzyl)guanosine 3′-N,N-Diisopropyl(cyanoethyl)phosphoramidite (7a).[20]
To a solution of compound 2a[19] (47 mg, 0.059 mmol) in dry CH2Cl2 (5.0 mL) under Ar, N,N-diisopropylethylamine (52 μL, 0.30 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (42 mg, 0.18 mmol) and 1-methylimidazole (5.0 μL, 0.059 mmol) were added. The mixture was stirred at rt until all starting material was consumed (1 h). The reaction was quenched with MeOH (1 mL) and stirred for 5 min. After the solvent was removed, the crude product was purified by silica gel chromatography, eluting with 1 % MeOH in CH2Cl2 containing 0.5% Et3N to give phosphoramidite 7a[20] (56 mg, 95% yield) as a yellow foam. 31P NMR (CD3CN) δ 152.8, 152.6; HRMS calcd for C51H60N8O11P [MH+]: 991.4119, found: 991.4110.
5′-O-(Dimethoxytrityl)-N2-isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine 3′-N,N-Diisopropyl(cyanoethyl)phosphoramidite (7b).
Phosphoramidite 7b was prepared from 5′-O-(dimethoxytrityl)-N2-isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine (2b)[19] (255 mg, 0.317 mmol), N,N-diisopropylethylamine (277 μL, 1.59 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (224 mg, 0.95 mmol), and 1-methylimidazole (27 μL, 0.32 mmol) as described for 7a. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7b (275 mg, 86% yield) as a yellow foam. 31P NMR (CD3CN) δ 152.7, 152.4; HRMS calcd for C52H62N8O11P [MH+]: 1005.4276, found: 1005.4276.
5′-O-(Dimethoxytrityl)-2′-O-(o-nitrobenzyl)-N2-phenoxyacetylguanosine 3′-N,N-Diisopropyl(cyanoethyl)phosphoramidite (7c).
hosphoramidite 7c was prepared from 2c (284 mg, 0.332 mmol), N,N-diisopropylethylamine (290 μL, 1.66 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (234 mg, 0.996 mmol), and 1-methylimidazole (28 μL, 0.33 mmol) as described for 7a. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7c (270 mg, 77% yield) as a yellow foam. 31P NMR (CD3CN) δ 153.0, 152.7; HRMS calcd for C55H60N8O12P [MH+]: 1055.4069, found: 1055.4075.
5′-O-(Dimethoxytrityl)-2′-O-(α-methyl-o-nitrobenzyl)-N2-phenoxyacetylguanosine 3′-N,N-Diisopropyl(cyanoethyl)phosphoramidite (7d).
Phosphoramidite 7d was prepared from 2d (156 mg, 0.18 mmol), N,N-diisopropylethylamine (157 μL, 0.90 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (127 mg, 0.54 mmol), and 1-methylimidazole (15 μL, 0.18 mmol) as described for 7a. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7d (121 mg, 63% yield) as a yellow foam. 31P NMR (CD3CN) δ 152.6, 152.5; HRMS calcd for C56H62N8O12P [MH+]: 1069.4225, found: 1069.4235.
5′-O-(Dimethoxytrityl)-2′-O-(4,5-dimethoxy-2-nitrobenzyl)-N6-phenoxyacetyladenosine 3′-N,N-diisopropyl (cyanoethyl)phosphoramidite (7e).
Phosphoramidite 7e was prepared from 6a (210 mg, 0.234 mmol), N,N-diisopropylethylamine (194 μL, 1.11 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (156 mg, 0.66 mmol), and 1-methylimidazole (18 μL, 0.21 mmol) as described for 7a. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7e (225 mg, 88% yield) as a yellow foam. 31P NMR (CD3CN) δ 152.8, 152.7; HRMS calcd for C57H64N8O13P [MH+] 1099.4325, found 1099.4309.
5′-O-(Dimethoxytrityl)-2′-O-(α-methyl-o-nitrobenzyl)-N6-phenoxyacetyladenosine 3′-N,N-Diisopropyl(cyanoethyl)phosphoramidite (7f).
Phosphoramidite 7f was prepared from 6b (284 mg, 0.33 mmol), N,N-diisopropylethylamine (276 μL, 1.58 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (221 mg, 0.94 mmol), and 1-methylimidazole (26 μL, 0.30 mmol) as described for 7a. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7f (322 mg, 92% yield) as a yellow foam. 31P NMR (CD3CN) δ 152.6, 152.0; HRMS calcd for C56H62N8O11P [MH+]: 1053.4276, found: 1053.4277.
5′-O-(Dimethoxytrityl)-2′-O-(o-nitrobenzyl)-N-phenoxyacetylcytidine 3′-N,N-Diisopropyl-(cyanoethyl)phosphoramidite (7g).
Phosphoramidite 7g was prepared from 5′-O-(dimethoxytrityl)-2′-O-(o-nitrobenzyl)-N-phenoxyacetylcytidine (6c)[19] (185 mg, 0.23 mmol), N,N-diisopropylethylamine (192 μL, 1.10 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (154 mg, 0.66 mmol), and 1-methylimidazole (18 μL, 0.21 mmol) as described for 7a. Silica gel chromatography (0–4% acetone in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7g (215 mg, 92% yield) as a yellow foam. 31P NMR (CD3CN) δ 152.4, 151.6; HRMS calcd for C54H60N6O12P, [MH+]: 1015.4007, found: 1015.4011.
5′-O-(Dimethoxytrityl)-2′-O-(α-methyl-o-nitrobenzyl)-N-phenoxyacetylcytidine 3′-N,N-Diisopropyl(cyanoethyl)phosphoramidite (7h).
Phosphoramidite 7h was prepared from 6d (150 mg, 0.18 mmol), N,N-diisopropylethylamine (158 μL, 0.91 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (129 mg, 0.54 mmol), and 1-methylimidazole (15 μL, 0.18 mmol) as described for 7a. Silica gel chromatography (0–1% acetone in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7h (169 mg, 91% yield) as a white foam. 31P NMR (CD3CN) δ 151.2, 149.4; HRMS calcd for C55H62N6O12P, [MH+]: 1029.4163, found: 1029.4143.
5′-O-(Dimethoxytrityl)-2′-O-(o-nitrobenzyl)uridine 3′-N,N-Diisopropyl(cyanoethyl)-phosphoramidite (7i).
Phosphoramidite 7i was prepared from 6e (121 mg, 0.18 mmol), N,N-diisopropylethylamine (158 μL, 0.91 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (129 mg, 0.54 mmol), and 1-methylimidazole (15 μL, 0.18 mmol) as described for 7a. Silica gel chromatography (0–2% acetone in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7i (135 mg, 86% yield) as a white foam. 31P NMR (CD3CN) δ 150.9, 150.1; HRMS calcd for C46H53N5O11P, [MH+]: 882.3479, found: 882.3469.
5′-O-(Dimethoxytrityl)-2′-O-(o-nitrobenzyl)-N6-benzoyladenosine 3′-N,N-Diisopropyl-(cyanoethyl)phosphoramidite (7j).[6,14]
Phosphoramidite 7j was prepared from 5′-O-(dimethoxytrityl)-2′-O-(o-nitrobenzyl)-N6-benzoyladenosine (6f)[6,14,19] (99 mg, 0.12 mmol), N,N-diisopropylethylamine (106 μL, 0.61 mmol), 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (221 mg, 0.36 mmol), and 1-methylimidazole (10 μL, 0.12 mmol) as described for 7a. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the crude product yielded 7j[6,14] (101 mg, 82% yield) as a yellow foam. 31P NMR (CD3CN) δ 152.9, 152.7.
Acknowledgment:
We thank Dr. Qing Dai, Mr. Saurja Dasgupta, Dr. Armando Hernandez, Mr. Hao Huang and Dr. Sandip Shelke for helpful discussions and critical comments on the manuscript. This work was supported by an N.I.H. grant to J.A.P. (1R01-AI081987).
References:
- 1.Pillai VNR Photoremovable protecting groups in organic synthesis, Synthesis 1980, 1–26. [Google Scholar]
- 2.Greene TW; Wuts PGM Protective Groups in Organic Synthesis, 3rd ed., John Wiley & Sons Inc., New York, 1999. [Google Scholar]
- 3.Adams SR; Tsien RY Controlling cell chemistry with caged compounds, Annu. Rev. Physiol 1993, 55, 755–784. [DOI] [PubMed] [Google Scholar]
- 4.Tang X; Dmochowski IJ Regulating gene expression with light-activated oligonucleotides, Mol. Biosyst 2007, 3, 100–110. [DOI] [PubMed] [Google Scholar]
- 5.Casey JP; Blidner RA; Monroe WT Caged siRNAs for spatiotemporal control of gene silencing, Mol. Pharm 2009, 6, 669–685. [DOI] [PubMed] [Google Scholar]
- 6.Chaulk SG; MacMillan AM Caged RNA: photo-control of a ribozyme reaction, Nucleic Acids Res. 1998, 26, 3173–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das SR; Piccirilli JA General acid catalysis by the hepatitis delta virus ribozyme, Nat. Chem. Biol 2005, 1, 45–52. [DOI] [PubMed] [Google Scholar]
- 8.Kath-Schorr S; Wilson TJ; Li N-S; Lu J; Piccirilli JA; Lilley DM General acid-base catalysis mediated by nucleobases in the hairpin ribozyme, J. Am. Chem. Soc 2012, 134, 16717–16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson TJ; Li N-S; Lu J; Frederiksen JK; Piccirilli JA; Lilley DM Nucleobase-mediated general acid-base catalysis in the Varkud satellite ribozyme, Proc. Natl. Acad. Sci. USA 2010, 107, 11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtsuka E; Ohta T; Koizumi M Sequence-specific cleavage of RNA by designed ribozymes, Supramolecular Chemistry 1993, 2, 197–200. [Google Scholar]
- 11.Chaulk SG; MacMillan AM Separation of spliceosome assembly from catalysis with caged pre-mRNA Substrates, Angew. Chem. Int. Ed 2001, 40, 2149–2152. [DOI] [PubMed] [Google Scholar]
- 12.Fica SM; Tuttle N; Novak T; Li N-S; Lu J; Koodathingal P; Dai Q; Staley JP; Piccirilli JA RNA catalyzes nuclear pre-mRNA splicing, Nature 2013, 503, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka T; Tamatsukuri S; Ikehara M Solid phase synthesis of oligoribonucleotides using the o-nitrobenzyl group for 2’-hydroxyl protection and H-phosphonate chemistry, Nucleic Acids Res. 1987, 15, 7235–7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaulk SG; MacMillan AM Synthesis of oligo-RNAs with photocaged adenosine 2’-hydroxyls, Nat. Protoc 2007, 2, 1052–1058. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsuka E; Tanaka S; Ikehara M Studies on transfer ribonucleic acids and related compounds. XVI. Synthesis of ribooligonucleotides using a photoseneitive o-nitrobenzyl protection for the 2’-hydroxyl group, Chem. Pharm. Bull 1977, 25, 949–959. [Google Scholar]
- 16.Ohtsuka E; Tanaka S; Ikehara M Studies of transfer ribonucleic-acids and related compounds. XVIII. Photolabile 2’-ether of guanosine as an intermediate for oligonucleotide synthesis, Synthesis 1977, 453–454. [Google Scholar]
- 17.Ohtsuka E; Tanaka S; Ikehara M Studies on transfer ribonucleic acids and related compounds. IX. Ribooligonucleotide synthesis using a photosensitive o-nitrobenzyl protection at the 2’-hydroxyl group, Nucleic Acids Res. 1974, 1, 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtsuka E; Tanaka T; Tanaka S; Ikehara M Studies on transfer ribonucleic acids and related compounds. 20. A new versatile ribooligonucleotide block with 2’-(o-nitrobenzyl) and 3’-phosphorodianilidate groups suitable for elongation of chains in the 3’ and 5’ directions, J. Am. Chem. Soc 1978, 100, 4580–4584. [Google Scholar]
- 19.Li N-S; Frederiksen JK; Koo SC; Lu J; Wilson TJ; Lilley DM; Piccirilli JA A general and efficient approach for the construction of RNA oligonucleotides containing a 5’-phosphorothiolate linkage, Nucleic Acids Res. 2011, 39, e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N-S; Tuttle N; Staley JP; Piccirilli JA Synthesis and incorporation of the phosphoramidite derivative of 2’-o-photocaged 3’-s-thioguanosine into oligoribonucleotides: substrate for probing the mechanism of RNA catalysis, J. Org. Chem 2014, 79, 3647–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner D; H. VJP; Moffatt JG Preparation and synthetic utility of some organotin derivatives of nucleosides, J. Org. Chem 1974, 39, 24–30. [Google Scholar]
- 22.Viladoms J; Scott LG; Fedor MJ An active-site guanine participates in glmS ribozyme catalysis in its protonated state, J. Am. Chem. Soc 2011, 133, 18388–18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wincott F; DiRenzo A; Shaffer C; Grimm S; Tracz D; Workman C; Sweedler D; Gonzalez C; Scaringe S; Usman N Synthesis, deprotection, analysis and purification of RNA and ribozymes, Nucleic Acids Res. 1995, 23, 2677–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelliccioli AP; Wirz J Photoremovable protecting groups: reaction mechanisms and applications, Photochem. Photobiol. Sci 2002, 1, 441–458. [DOI] [PubMed] [Google Scholar]
- 25.Singh KK; Nahar P An improved method for the synthesis of N-phenoxyacetylribonucleosides, Synth. Commun 1995, 25, 1997–2003. [Google Scholar]