Abstract
Substantial progress has been made in the past several years in establishing the stoichiometries of STIM and Orai proteins and understanding their influence on store-operated calcium entry. Depletion of ER Ca2+ triggers STIM1 to accumulate at ER-plasma membrane junctions where it binds and opens Ca2+ release-activated Ca2+ (CRAC) channels. STIM1 is a dimer, and release of Ca2+ from its two luminal domains is reported to promote their association as well as drive formation of higher-order STIM1 oligomers. The CRAC channel, originally thought to be tetrameric, is now considered to be a hexamer of Orai1 subunits based on crystallographic and electrophysiological studies. STIM1 binding activates CRAC channels in a highly nonlinear way, such that all six Orai1 binding sites must be occupied to account for the activation and signature properties of native channels. The structural basis of STIM1 engagement with the channel is currently unclear, with evidence suggesting that STIM1 dimers bind to individual or pairs of Orai1 subunits. This review examines evidence that has led to points of consensus and debate about STIM1 and Orai1 stoichiometries, and explains the importance of STIM-Orai complex stoichiometry for the regulation of store-operated calcium entry.
Keywords: Store-operated calcium entry, CRAC channel, STIM, Orai, stoichiometry, concatemer
Graphical abstract
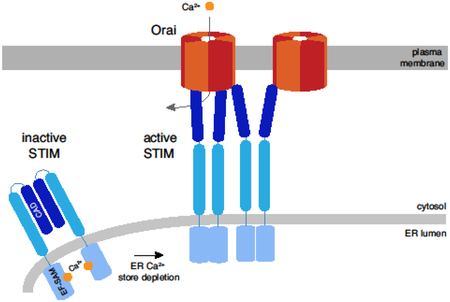
1. Introduction
The biological machinery of cells comprises diverse sets of proteins that are often organized as multi-protein complexes. The stoichiometry, or number of constituent proteins in each complex is a critical parameter underlying their structure and functional interactions. The dynamic interactions of STIM and Orai proteins that control the process of store-operated calcium entry (SOCE) are a good example. SOCE is initiated by cell surface receptors coupled to phospholipase C, which generates inositol 1,4,5-trisphosphate to release Ca2+ from the ER Ca2+ store [1,2]. The ensuing loss of luminal Ca2+ is sensed by the ER protein STIM1, which then accumulates at ER-plasma membrane (PM) junctions where it binds and traps Ca2+ release-activated Ca2+ channels (CRAC channels, made from Orai1) in the PM [3]. STIM1 binding to Orai1 triggers channel opening and the ensuing entry of Ca2+ promotes ER store refilling and a variety of essential cell functions, including immune cell activation, muscle development and function, bone and tooth development, and many others [2,4,5].
The stoichiometries of native STIM and Orai proteins as well as the STIM1-Orai1 complex that forms the functional core of the CRAC channel have been debated extensively since the identification of the STIM and Orai protein families. Questions surround the stoichiometry of STIM1 in its inactive resting state and as it becomes activated following store depletion. Orai1 stoichiometries ranging from two to six have been reported under various experimental conditions for the CRAC channel, and variable STIM1-Orai1 binding stoichiometries have been proposed. Gating (activation and inactivation) as well as the ion selectivity of the CRAC channel strongly depend on the number of STIM1s bound to each channel. Thus, to understand how CRAC channels function at the molecular level, it is essential to know how many Orai1 binding sites are bound by STIM1 under different conditions, how many STIM1s are needed to fill these sites, and ultimately how these binding reactions are regulated.
Important progress has been made in the past five years in determining the functional stoichiometries of STIM1 and Orai1, using biochemical, cell biological, photophysical, electrophysiological, and structural approaches. While each offers its own strengths in addressing stoichiometry, several of these approaches are indirect and subject to experimental caveats that may complicate interpretation. This review will examine the experimental evidence for the stoichiometries of STIM1, Orai1, and the CRAC channel STIM1-Orai1 complex. We will identify cases of consensus, attempt to explain discrepancies, and highlight current uncertainties and potential avenues for resolving these in the future.
2. The stoichiometry of STIM1
STIM proteins are single-pass ER membrane proteins with essential functional domains on both the luminal and cytosolic sides (Figure 1 A). On the luminal side, an EF-hand and an adjacent SAM domain are involved in Ca2+ sensing and regulating STIM1 oligomerization. The cytosolic side includes a domain that binds to Orai1, known as CAD (CRAC Activation Domain, aa 342-448) [6], SOAR (STIM-Orai Activation Region, aa 344-442) [7], or Ccb9 (aa 339-444) [8]). A putative coiled-coil 1 (CC1) region proximal to the ER membrane engages the CAD to limit its accessibility to Orai in the inactive resting state [9–14], and a polybasic domain at the C-terminus interacts with the plasma membrane to promote STIM accumulation at ER-PM junctions. For more comprehensive reviews of STIM1 domains and their functions, see [2,15].
Figure 1. STIM1 stoichiometry in the inactive and active states.
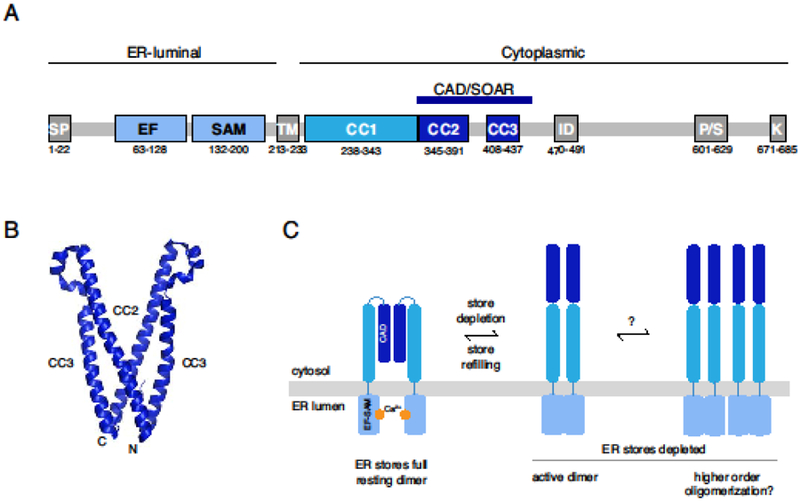
(A) Schematic of STIM1 domain architecture showing the signal peptide (SP) and the EF-SAM, ER transmembrane (TM), coiled-coil (CC1-CC3), CAD/SOAR, inactivation (ID), proline/serine-rich (P/S), and polybasic (K) domains. Numbered residues denote the domain boundaries for human STIM1. For further details see [2]. (B) Crystal structure of the dimeric CAD/SOAR (3TEQ.pdb) [13]. N- and C-terminal ends of the front subunit are indicated. (C) In the resting state, STIM1 is dimerized through interactions of the CAD domain, and the EF-SAM domains are separated in the ER lumen. After store depletion, the EF-SAM domains associate (left), extending the CAD towards the PM, and may also form higher-order oligomers through association of multiple STIM1 dimers (right). Regions C-terminal to the CAD have been omitted for clarity.
2.1. STIM1 stoichiometry in the resting state
Several indirect lines of evidence suggest STIM1 is a dimer in the resting state. Orthogonally labeled STIM1 proteins can be co-immunoprecipitated, and N-terminally labeled STIM1 molecules engage in FRET [16–18], indicating multimeric stoichiometry. Soluble protein fragments from the cytosolic domain of STIM1 generally form dimers in solution [7,10,19]. Chemical crosslinking studies in cells have provided the most direct evidence for resting dimers of full-length STIM1 [20–22]. While STIM1 was overexpressed in these studies, and its confinement to the two-dimensional ER membrane could artificially promote dimerization [23], STIM1 expressed at extremely low levels for single-particle tracking studies [24] diffuses at a rate similar to that of overexpressed protein as assessed by FRAP [17,18], supporting the notion that the endogenous protein is dimeric.
Which protein domains stabilize the dimeric form of STIM1? While the transmembrane (TM) domains in isolation have a tendency to dimerize [12], truncated STIM1 proteins containing the TM and luminal (EF-SAM) domains do not associate in resting cells as assessed by FRET [17], possibly due to steric constraints imposed by the Ca2+-bound EF-SAM domains. The CC1 domain by itself can support dimerization, but this is very weak [9,11,17]. Interactions between CAD domains provide the major forces for dimer stabilization [17] (Figure 1B, C), consistent with the tendency of cytosolic STIM1 fragments containing the CAD region to form dimers in solution (aa 233-685 [19]; aa 234-491 [10]; aa 336-485 [7]. A crystal structure of CAD shows that the dimer is held together through reciprocal hydrophobic and hydrogen bonding interactions near the N- and C-terminal ends of each subunit [13] (Figure 1B). Interestingly, the original study describing CAD showed the isolated fragment (aa 334-448) was a tetramer [6], and a later crystallization study of CAD reported a mixture of dimers and tetramers before the tetrameric fraction was eliminated by mutagenesis [13]. It is not clear why tetramers were observed in the isolated CAD studies; possible contributing factors include the deletion of N- and C-terminal flanking regions, as well as the attachment of histidine tags and the particular cell types and solutions that were employed for protein generation and purification.
2.2. STIM1 stoichiometry in the activated state
After store depletion, rearrangement of the luminal Ca2+-binding domains (Gudlur et al. 2018; Stathopulos et al. 2008) triggers dramatic conformational changes in the cytosolic region of the STIM1 dimer. The two CC1 helices are released from their interactions with CAD and pair with each other [12,22,26], releasing the C-terminal polybasic domain (PBD) of STIM1 to interact with PM phospholipids and drive STIM1 clustering at ER-PM junctions [6,18,28–30], while extending the CAD towards the PM to bind and activate Orai1. Dimeric stoichiometry of STIM proteins is essential to enable the polybasic domains to bind negatively-charged phospholipids in the PM, as assayed by the binding of isolated monomeric and dimeric cytosolic STIM domains to PIP2-containing liposomes [30,31],
Liou et al. [18] originally suggested that STIM1 oligomerization may be the triggering event leading to activation following store depletion (Figure 1C). FRET between STIM1 proteins labeled at their luminal N-termini increases shortly after Ca2+ release from stores [16–18], accompanied by a dramatic slowing of its diffusional mobility [17,18]. A role for oligomerization in STIM1 activation was supported by the finding that rapamycin-induced crosslinking of STIM1 proteins in which FK506 binding protein (FKBP) and FKBP-rapamycin binding (FRB) domains were substituted for the luminal EF-SAM domains triggered puncta formation and CRAC channel activation in cells with full Ca2+ stores [32]. More direct evidence for higher-order oligomerization of native STIM1 is that the Drosophila homolog dSTIM shifts from a dimeric to a higher-MW form after store depletion in cells exposed to crosslinkers [20], although this could also arise from binding to other proteins (e.g., STIMATE [33]).
In light of more recent findings, the interpretation of these earlier studies and the potential role of oligomerization in initiating STIM1 activation has been questioned [11,22,25]. Korzeniowski et al used rapamycin and multimers of FRB in the ER lumen to link together STIM proteins tagged with FKBP12 [22]. Interestingly, rapamycin-induced oligomerization failed to activate SOCE if the STIM1 N-terminus was intact, but did so when the entire luminal domain was replaced by FKBP12 (similar to the study by Luik et al [32]). These results imply that oligomerization per se is not sufficient to overcome the steric brake imposed by Ca2+ bound EF-SAM domains, and that a rearrangement of these domains is required. This rearrangement was subsequently probed directly by Gudlur et al. [25] in studies of a dimeric STIM1 construct lacking CAD/SOAR (a pair of CFP/YFP-labeled EFSAM-TM-CC1 fragments attached to a GrpE dimer). Without oligomerizing the dimers, store depletion triggered a FRET increase similar to that seen with full length STIM1. These results suggest that the FRET change of full length STIM1 could be largely accounted for by movements or rotations of the EF-SAM domains as they interact upon store depletion [34] and optimize the proximity or orientation of the FRET donor and acceptor within each dimer. Given this interpretation, the activating effect of crosslinking FRB to FKBP in the earlier studies [32] may have resulted from forced proximity of luminal domains within single STIM1 dimers, and the decreased mobility of STIM1 that was observed after store depletion might have resulted from interactions with cytoplasmic constituents by the extended cytosolic domain (see [24]) rather than formation of higher-order oligomers. However, while these recent studies are helping to clarify the initiating events underlying SOCE, they do not rule out store depletion-dependent oligomerization of STIM1 dimers. For example, following store depletion, STIM1 fragments containing only the EF-SAM and TM domains engage in FRET with full-length STIM1 and are weakly recruited to ER-PM junctions, suggesting that multiple STIM1 dimers may associate through interactions of their luminal or TM domains [17] (Figure 1C). There is still much to learn about how the stoichiometry and molecular composition of STIM1 complexes change over time after store depletion, and how this relates to the activation kinetics of SOCE.
3. The stoichiometry of the CRAC channel
Following the discovery of the Orai proteins [35–37], Orai1 was quickly established as the pore-forming subunit of the CRAC channel [38–40]. Soon after, a variety of approaches demonstrated that the CRAC channel is a multimer, including dominant negative effects of non-conducting Orai1 pore mutants [39,41], coimmunoprecipitation of orthogonally labeled subunits [42], and FRET between CFP- and YFP-tagged Orai1 [16,43]. However, its precise stoichiometry was not immediately clear.
Initially, most experimental evidence suggested the CRAC channel functions as a tetramer of Orai1 subunits. Tetrameric concatemers of Orai1, in which short linker sequences were used to connect the C terminus of each subunit with the N terminus of the next, displayed basic CRAC channel properties (Ca2+ conduction and an inwardly rectifying I/V relation with reversal potential >+50 mV) and were immune to inhibition by dominant negative mutant monomers, whereas Orai1 monomers and dimeric or trimeric concatemers were not [44]. The tetrameric view was further supported by single-particle fluorescence measurements, in which GFP attached to Orai1 monomers and concatemeric dimers, trimers and tetramers bleached in a stepwise manner consistent with a tetrameric channel stoichiometry [45]. Brightness analysis of labeled channels diffusing into a bleached region of the cell also supported a tetrameric arrangement [46]. Nevertheless, some studies hinted at higher-order assemblies. Crosslinking of cysteines introduced into the channel pore produced pentamers [47]; also, the molecular weight of purified Orai1 appeared much greater than expected for a tetramer, although the presence of detergent made it difficult to accurately estimate the protein size [6].
GFP-Orai1 photobleaching experiments also suggested other stoichiometries. Cahalan and colleagues proposed that Orai1 is dimeric in the absence of co-expressed STIM1 but tetrameric in its presence, prompting the idea that STIM1 may act like a chaperone to assemble Orai1 dimers into tetramers in the PM [20,48]. Using a similar photobleaching approach, Vig’s group later reported a mixture of dimers, tetramers, and hexamers with α-SNAP promoting roughly equivalent proportions of each, and loss of α-SNAP resulting in an increased fraction of hexameric bleaching events [49]. It is still not clear how these different apparent stoichiometries may be reconciled (see [50] for a discussion), but they raise intriguing possibilities for mechanisms of channel assembly that should be explored.
Against this backdrop it was quite a surprise when the crystal structure of Drosophila Orai (dOrai) was shown to be hexameric [51] (Figure 2A). A number of potential artifacts were considered, including possible differences among species as well as effects of detergent solubilization and mutations and truncations that were introduced to optimize crystallization. However, crosslinking dOrai in native membranes yielded six bands [51], and atomic force microscopy of purified wildtype human Orai1 decorated with antibodies also supported a hexameric stoichiometry [52], arguing against artifacts arising from solubilization, species, and mutations used in the crystallography study. The hexameric model of dOrai revealed a wealth of structural details that have informed many subsequent studies of Orai channel function.
Figure 2. The CRAC channel is a hexamer of Orai1.
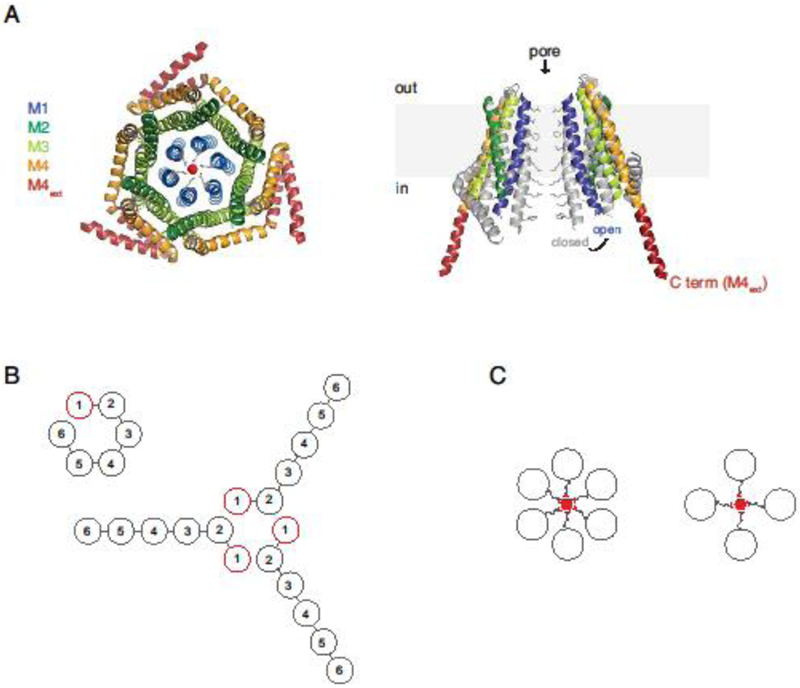
A. Top view of the closed dOrai structure with crossed M4 extensions (left; 4HKR.pdb) [51], and a side view of two opposing dOrai subunits, showing the closed structure in gray superimposed on the open H206A mutant structure (right; 6BBF.pdb), adapted from [67]. B. Two ways in which a hexameric Orai1 concatemer could in principle assemble into a hexameric channel: from a single polypeptide (left) or by combining the first two subunits of three concatemers (right). C. Schematic top views of the TM1 helices of hypothetical hexameric and tetrameric channels, showing glutamate side chains projecting into the pore to create the Ca2+ selectivity filter. The different geometries and valence of tetrameric and hexameric selectivity filters would be expected to produce significantly different affinities for Ca2+.
To assess the functional stoichiometry of CRAC channels in their native state, the physiological properties of hexameric concatemers of Orai1 were compared to those of endogenous CRAC channels and channels made from heterologous expression of Orai1 monomeric cDNA. Initially, hexameric Orai1 concatemers made with short (6-residue) linkers failed to reproduce the signature features of CRAC channels, including Ca2+ selectivity and Ca2+-dependent inactivation (CDI) [53]. Subsequent concatemer studies using longer linkers (24 or 36 residues) [54,55] were carried out with particular attention to verifying the subunit composition of concatemeric channels and applying more stringent biophysical tests to distinguish concatemeric from native CRAC channels. It is well known that channel protein concatemers do not always assemble as single polypeptides [56]; in some cases, only part of a concatemer is used, or subunits from multiple concatemers combine to form functional channels [57–59] (Figure 2B). Orai1 concatemeric hexamers were shown to produce a full-length protein in the plasma membrane fraction without generating significant amounts of smaller proteins that might assemble into channels [54,55]. To test whether all six subunits were used to make the channel, Yen et al. [55] introduced an L273D mutation to inhibit STIM1 binding to subunits 1, 3, or 6 within the hexamer. The mutation reduced the current level and CDI by similar amounts regardless of position, providing strong evidence that the hexamer assembled from single hexameric polypeptides. In contrast, Cai et al. [54] found that a non-conducting E106A mutation did not suppress current equally at all positions, possibly due to interference with channel assembly (Figure 2B). In this case, the unequal contributions of the six subunits within a single concatemer raised the possibility that channels were constructed from multiple concatemers and made uncertain the pore stoichiometry of the wildtype concatemer. These results illustrate some of the challenges involved in determining the stoichiometry of mutagenized concatemers, and why this is necessary in order to infer the stoichiometry of wildtype concatemers.
Pore properties of the hexameric Orai1 concatemers were compared to those of native CRAC channels. Here, it is pertinent to recognize the limitations of the commonly used but limited set of characteristics (Ca2+ conduction, rectifying I/V relation, and positive reversal potential) for detecting differences in pore structure. For example, Ca2+ selectivity over Na+ can appear normal for mutant Orai1 channels exposed to millimolar extracellular Ca2+ despite moderate decreases in the micromolar affinity of the selectivity filter [60,61], and inward rectification alone does not indicate Ca2+ selectivity because the Na+ current rectifies due to the transmembrane Na+ gradient. Importantly, the hexameric Orai1 concatemer displayed all of the essential pore properties of the native CRAC channel, including the affinity for Ca2+ block, the Cs+/Na+ permeability ratio under divalent-free conditions, and the unitary conductance [55]. Tetrameric and hexameric pore structures would likely exhibit differences in these characteristics given that they are expected to be highly sensitive to pore geometry (Figure 2C). Because the detailed pore properties of the concatemeric channels were indistinguishable from those of native CRAC channels, the native CRAC channel is considered to function as a hexamer of Orai1 subunits [55].
A lingering issue is how to reconcile the earlier conclusion based on Orai1 concatemers that functional CRAC channels are tetrameric. Do tetrameric concatemers produce tetrameric channels, or could more than one tetrameric concatemer combine to form a hexamer? The answer is not known, although there is indirect evidence that a subset of subunits from multiple tetramers could be recruited into functional channels. Cai et al. reported that E106A mutations in the first two subunits of tetrameric concatemers completely abrogated SOCE, while mutations in subunits 3 and 4 had no effect [54]. To explain these and other results, the authors proposed a model in which the first two subunits of separate tetramers combine to form hexameric channels. Determining the number of subunits lining the pore and a more detailed analysis of channel properties will ultimately be needed to explain how tetrameric concatemers are processed to yield functional channels.
A related question is how to explain the GFP photobleaching data indicating dimers [20,48], tetramers [45], or a mix of dimers, tetramers and hexamers [49]. While again the answer is not known, it is relevant to note that the single-particle photobleaching technique is subject to a number of potential complications that can affect the number of observed bleaching steps [50]. Unlabeled endogenous Orai subunits may combine with labeled ones, pre-bleaching can occur during microscope focusing, and chemical fixation commonly used to immobilize channels for single-particle photobleaching also depletes Ca2+ stores [48] and may therefore promote channel clustering at ER-PM junctions. In addition, flickery GFP fluorescence makes it difficult to resolve distinct bleaching steps for complexes with more than 3-4 subunits, such that the majority of particles are excluded from analysis [45], potentially skewing the results towards channels with smaller numbers of actively fluorescent GFP attached.
In sum, the electrophysiological studies of hexameric concatemers provide strong functional support for the structural model depicting the CRAC channel as a hexamer of Orai1 subunits. By offering the ability to place mutations in a defined subset of subunits, hexameric concatemers also provide useful tools for future studies of permeation and gating mechanisms, as well as of functional interactions and cooperativity among subunits. However, as noted above, to allow a clear interpretation it is important to confirm that each subunit of mutant concatemers still contributes equally to the channel and that the channels are assembled from a single polypeptide.
4. The stoichiometry of the STIM1-Orai1 complex
Of the three cytosolic regions of Orai1, binding of STIM1 to the C terminus is the most thoroughly documented and widely accepted [2]. The stoichiometry of the STIM1-Orai1 complex is critical for trapping mobile CRAC channels at ER-PM junctions, for regulating their activation and inactivation gating, and for specifying their ion selectivity and conductance. In this way, stoichiometry of the assembled STIM1-Orai1 complex is central to the control of Ca2+ entry in response to store depletion.
The stoichiometric requirements differ for Orai1 trapping and gating at ER-PM junctions. Only a single STIM1 dimer appears necessary to trap Orai1 at junctions, but this is not sufficient for channel opening [62]. The requirements for opening are assessed from two viewpoints below. From the standpoint of the channel, how many binding sites need be occupied to open the channel and normalize its properties? From the STIM1 standpoint, how does STIM1 physically occupy those sites, and what is the stoichiometry of the STIM1-Orai1 complex?
4.1. How many Orai1 binding sites must be occupied to activate the CRAC channel?
The relation between the STIM1:Orai1 protein ratio at ER-PM junctions and CRAC current is highly nonlinear. In cells expressing similar amounts of STIM1, CRAC current increased with the level of exogenous Orai1 expression until the STIM1:Orai1 protein ratio fell below ~2 [62]. At this point the current abruptly decreased to a low value, and CDI declined in parallel (see also [63]). These results echoed earlier paradoxical observations that expression of Orai1 alone in cells tends to reduce rather than increase SOCE [2]. This paradox can be explained by an excess of Orai1 competing for a limited supply of STIM1, such that once the number of occupied sites on the channel falls below a threshold, channel activity and CDI are sharply reduced. The progressive loss of CDI at STIM1:Orai1 ratios <2 showed that channels with suboptimal STIM1 binding may still activate, but with very low efficiency. These studies could not address how many sites on the CRAC channel were bound to STIM1, because only the ratio of total STIM1 and Orai1 protein at junctions was measured, and binding may be nonlinear with STIM1 concentration, as suggested by the necessity for positive and negative cooperativity in fitting a model to the data [62].
The first attempt to titrate the number of binding sites on the CRAC channel utilized a tetrameric concatemer of Orai1 with L273D mutations in selected subunits [64]. Current declined by about half for each mutation, but a quantitative interpretation is complicated by the subsequent finding that the channel is hexameric, and as discussed above it is not clear how subunits of the tetrameric concatemer might assemble. A similar approach was later applied using a hexameric Orai1 concatemer [61]. In this case, each L273D mutation reduced CRAC current by 65%; surprisingly, however, noise analysis revealed that a single L273D mutation also increased the single-channel current (i) by ~3-fold. The open probability Po is given by Po = I/Ni, where I is the whole-cell current, and N is the number of channels at the cell surface. Given that N (as measured by antibody-labeling of Orai1 in the PM) was unchanged by the L273D mutation, the reduced current level (I) apparently underestimated the effect on Po by a factor of 3, indicating that inactivation of a single STIM1 binding site reduced the open probability to only ~10% of the WT channel. Thus, CRAC channel activation appears to be an extremely nonlinear function of STIM1 occupancy: binding to five sites barely opens the channel, while binding to the final site leads to a ~10-fold increase in opening [61].
Loss of a single binding site affected other CRAC channel pore properties in addition to the channel conductance [61]. Ca2+ affinity for the selectivity filter was reduced from a K1/2 of 16 μM to 47 μM. While this was not enough to prevent Ca2+ from blocking Na+ permeation in the presence of millimolar Ca2+o, selectivity for Na+ over Cs+ in the absence of divalent cations was significantly reduced. These results were consistent with a previous report linking the STIM1:Orai1 ratio to Cs+/Na+ permeability ratio (PCs/PNa) [65] (but see [63]). Interestingly, in that study cells transfected with a very low STIM1:Orai1 ratio produced small currents with a significant loss of Ca2+ selectivity over Na+ [65], suggesting that fewer than five sites occupied may also open with a further reduction of Ca2+ block affinity, although this has not been measured.
The strong dependence of gating and permeation on the number of Orai1 sites bound by STIM1 raises important questions about how STIM1 binding drives conformational changes in Orai1. Cysteine crosslinking experiments and molecular dynamics simulations support a hydrophobic gating model in which the six F99 residues lining the pore rotate by ~20o to allow water to enter and lower the energy barrier to Ca2+ conduction [66]. Reduction of Ca2+ affinity in the L273D mutant might then arise as a result of incomplete rotation of E106 residues located two turns above F99. It is less clear how incomplete STIM1 binding increases i and the PCs/PNa ratio. One possibility is that STIM1-mediated opening may also change pore structure in below F99, as suggested by structural models of constitutively active mutants (H206A dOrai or the equivalent H134A human Orai1) showing dilation or rotation of the inner pore helices [67,68] (Figure 2A, right).
The electrophysiological analysis of hexameric Orai1 concatemers suggests that native CRAC channels normally operate with STIM1 bound to all six C-terminal sites. This conclusion has interesting implications for events leading up to opening (Figure 3). CRAC current generally shows constant ion selectivity and CDI beginning with the earliest signs of current induction following store depletion [69]. This implies that as single channels diffuse into the ER-PM junction and bind progressive amounts of STIM1, they spend very little time with only five sites occupied. How this is achieved is not understood. Compounding the mystery is that endogenous STIM1 expression is more than ten-fold lower than the levels achieved by heterologous expression in most electrophysiological studies. Factors that may contribute to complete binding under such conditions include pre-clustering of STIM1 prior to contact with Orai1, accessory proteins that enhance STIM1 binding to Orai1 (e.g., CRACR2A [21]), binding cooperativity, and channel clustering (see below). There is much left to explore and understand about the process of CRAC channel activation under physiological conditions.
Figure 3. Nonlinear effects of STIM1-Orai1 binding on CRAC channel gating and permeation.
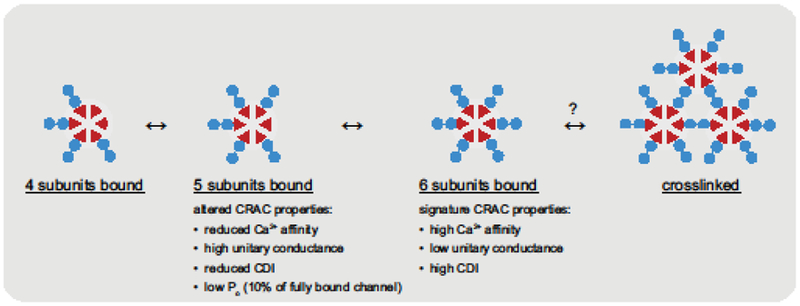
Hexameric CRAC channels (red) bind STIM1 dimers (blue) upon entering ER-PM junctions, but show little activity until five sites become occupied. The quintuplybound channels have altered properties, including reduced Po, Ca2+ blocking affinity, and CDI, and elevated unitary conductance and PCs/PNa compared to the fully bound channel or native CRAC channels. Binding to all six subunits is needed to produce the effective activation and permeation characteristics of native CRAC channels.
4.2. How does STIM1 engage Orai1 and what is the stoichiometry of the interaction?
A persistent question is how STIM1 binds all six Orai1 binding sites to open the channel and confer its signature properties. Unfortunately, attempts to derive a structure of the full STIM1-Orai1 complex have been unsuccessful so far, probably due to the flexibility of STIM1 and the low affinity of its interaction with Orai1. In the absence of direct structural evidence, two alternative models for STIM1-Orai1 binding have emerged.
A dimer binding model, in which a dimer of STIM1s bind to a pair of Orai1 C termini (1:1 stoichiometry) is based on an NMR structure of a fragment of STIM1, comprising part of CC1 and the CC2 helix of CAD/SOAR (aa 312-387), in complex with a short peptide derived from the cytoplasmic extension of the Orai1 M4 helix (aa 272-292) [70] (Figure 4A). The STIM1 fragments assemble spontaneously in vitro to form an interlocking structure with two identical hydrophobic grooves that bind a pair of Orai1 C-terminal fragments. An attractive feature of this model is that the angled orientation of bound Orai1 fragments resembles that of the paired M4 extensions depicted in the original dOrai structure, which associate in a coiled-coil configuration [51]. Thus, binding by this mechanism would require only modest conformational changes in Orai1. Mutagenesis of many of the predicted interaction residues in the structure inhibited function as expected [70]; however, it is still uncertain whether this structure accurately describes STIM1-Orai1 interactions in vivo. Expression of the STIM1 fragments themselves is not sufficient to activate Orai1, probably due to the absence of the CAD CC3 domain. The model has also been challenged recently by the structure of a constitutively open dOrai H206A mutant suggesting that the crossed M4 extensions would sterically prevent the opening movement of the pore and associated TM2/TM3 helices [67] (Figure 2A, right). While it remains to be shown that the open mutant channel precisely replicates the open structure of the STIM1-Orai1 complex, these results raise questions about whether the complex of STIM1 and Orai1 fragments represents a physiological conformational state, and if so, whether it is an intermediate or a final open state.
Figure 4. Dimeric and monomeric models of STIM1-Orai1 binding.
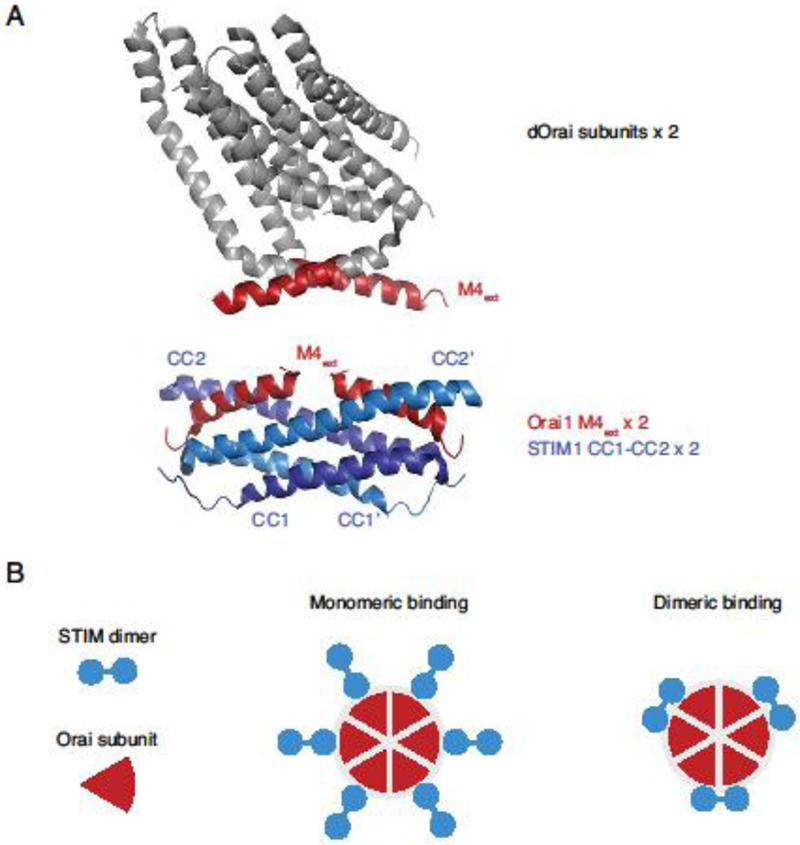
A. An example of a dimeric STIM-Orai binding model. Two subunits from the crystal structure of dOrai [51] are shown at top, with the cytoplasmic M4 regions marked in red (4HKR.pdb). The NMR structure below [70] shows two human Orai1 M4 fragments (aa 272-292) bound to a dimer of CC1-CC2 STIM1 fragments (aa 312-383) (2MAK.pdb). B. Schematic views of monomeric and dimeric binding models in which six or three STIM1 dimers are bound to the six Orai1 C termini, respectively.
An alternative monomer binding model proposes that rather than engaging two adjacent Orai1 subunits, each dimer of STIM1 binds to a single Orai1 C terminus through one of its two subunits, giving a 2:1 STIM1:Orai1 stoichiometry (Figure 4B). This model was based on evidence that heteromeric mutant SOAR concatemers (in which one subunit was rendered binding incompetent by an F394H mutation) bound to and activated Orai1 to the same extent as homomeric WT SOAR concatemers, suggesting that only one of the subunits in each STIM1 dimer was required to bind [71].
Interestingly, monomeric binding would provide a potential explanation for how CAD or SOAR concatemers crosslink channels [72], as shown by clusters of Orai1 particles observable by negative stain electron microscopy as well as by fluorescent Orai1 puncta and a slowing of Orai1 diffusion in cells expressing CAD or SOAR with Orai1 [6,72]. In addition, a freeze-fracture EM study showed Orai1 particles at ER-PM junctions with a preferred spacing of ~15 nm, close to the expected distance of Orai1 channels bridged by the two CAD subunits of a STIM1 dimer [73]. The formation of interconnected clusters of channels might also explain the prevalence of immobile STIM1 and Orai1 particles at ER-PM junctions observed in living cells after store depletion [24].
STIM1-mediated crosslinking of Orai1 has been reported to enhance CRAC channel activity [72]. Such an effect might be explained by increased avidity of the STIM1-Orai1 interaction in crosslinked channels which could lengthen the time channels spend in the fully occupied state, or by possible changes in the force applied to the channel. One caveat is that these crosslinking phenomena have thus far only been observed with highly overexpressed proteins which may favor clustering, and it will be important to test whether crosslinking occurs at physiological levels of STIM1 and Orai1 expression.
As with the dimeric binding model, there are also uncertainties with the monomeric model. The monomeric model is based on the ability of the WT/F394H SOAR heterodimer to activate Orai1 as well as WT/WT homodimers, with the assumption that F394H completely prevents binding by one of the two SOAR subunits. However, the F394H mutation appears to weaken but not eliminate interactions with Orai1 [71,74], raising the possibility that the WT SOAR subunit anchored to the channel could increase the local concentration of the mutant subunit enough to bind an adjacent Orai1 subunit through a dimeric interaction. Also, a single SOAR domain, or a dimer with inhibitory LQ>AA mutations in one subunit, does not activate current maximally when attached to Orai1, even though each would be expected to produce a functional SOAR subunit for each Orai1 subunit [64]. These issues are difficult to address without more direct structural information showing how the SOAR concatemers fold and associate with Orai1.
In one attempt to reconcile the monomer and dimer binding models, a FRET assay was used to monitor binding of STIM1, CAD, or dimeric SOAR concatemers to CRAC channels that were made from dimeric Orai1 concatemers [61]. As expected from earlier work [64], L273D in all six subunits completely precluded FRET between STIM1 or CAD and Orai1. Surprisingly, however, a C terminus with the L273D mutation did contribute to FRET if it was located next to a WT subunit. This result suggests that the L273D C terminus can actually bind STIM1 to some degree if the local concentration of STIM1 is high enough, and that STIM1 or CAD dimers interact with pairs of Orai1 subunits. The simplest explanation is that each STIM1/CAD dimer is bound simultaneously to a pair of Orai1 C termini (as in the dimer binding model). Alternatively, a STIM1 dimer may simply bind to one WT subunit (as in the monomer binding model) and hop by diffusion to bind transiently to the neighboring L273D subunit. While the first explanation intuitively seems more probable, more precise information about the binding interface or evidence for simultaneous occupancy of two C termini will be needed to distinguish it from monomer binding combined with hopping. An unexpected finding of this study was that unlike STIM1 and CAD, SOAR concatemer binding was not inhibited by Orai1 mutations downstream of L273, implying that it binds differently than native STIM1 or CAD to Orai1 [61]. Thus, in future studies it may be advisable to use non-concatenated STIM1 proteins to obtain definitive structures of the STIM1-Orai1 complex.
Finally, it is possible that STIM1 could bind by both monomeric and dimeric modes to Orai1. Palty et al. [74] reported that partial activation of CRAC channels allows mutant SOAR domains that normally do not bind Orai1 to bind and activate the channel, and concluded that activation may proceed through a two-step process. One of the partially activating mutations (P245L) is likely to promote the transition of the crossed M4 extensions into a more extended form like that seen in the open H206A dOrai structure [67] (Figure 2A). Thus, an intriguing possibility is that initial binding may be dimeric and elicit partial activity, while full activation results from interactions with the extended M4 helices. There are clearly many questions to be addressed relating to such a mechanism. Does Orai1 spontaneously assume the crossed and extended M4 conformations in vivo, and if so, can STIM1 bind to either? Does STIM1 binding bias the time spent in either state, and which states correspond to the partially and fully active channel? Answers to these questions will require new approaches to determine the structures of the STIM1-Orai1 complex, including intermediates on the path to full activation.
5. Conclusions and future perspectives
The stoichiometry of STIM1 and Orai1 proteins is intimately connected with their functions. There is strong evidence that STIM1 is a dimer, and the initial event in STIM1 activation is likely to be association of paired EF-SAM domains [34], but the stoichiometry and regulation of its further oligomerization and clustering at ER-PM junctions remain to be determined.
There is now wide agreement that the CRAC channel is a hexamer of Orai1 subunits, and STIM1 appears to bind to all six channel subunits to effectively activate it and establish its characteristic low conductance and high selectivity. However, more needs to be learned about the timing of STIM1-Orai1 binding and unbinding at ER-PM junctions after store depletion and how the channel avoids spending significant time in incompletely bound states with altered characteristics as it becomes activated. It will be interesting to assess the potential contributions of STIM1 pre-clustering through interactions with the PM, monomeric vs. dimeric binding modes, channel crosslinking, and accessory proteins to the efficiency of assembling STIM1-Orai1 complexes following store depletion.
Finally, the precise way in which STIM1 engages Orai1 still needs clarification. Evidence for monomeric and dimeric binding modes is indirect and/or based on association of protein fragments that may interact differently than full-length proteins. A structure of the STIM1-Orai1 complex would carry us a long way towards defining these interactions and illuminating the sequence of conformational changes in STIM1 and Orai1 that underlie binding and its coupling to channel gating.
Highlights:
The ER Ca2+ sensor STIM1 is a dimer, and Ca2+ release activates it via association of its luminal domains and possibly higher-order oligomerization
The CRAC channel is structurally and functionally a hexamer of Orai1 subunits
STIM1 binds to all six Orai1 subunits to effectively activate the CRAC channel and confer its signature pore properties
Current models propose STIM1 binding to individual or pairs of Orai1 C-termini
Acknowledgments
The authors gratefully acknowledge support from National Institutes of Health grant R37 GM45347 and the Mathers Charitable Foundation (to R.S.L.) and by the National Science Foundation Graduate Research Fellowship Program and National Institutes of Health training grant 5T32AI007290 to the Stanford Immunology Graduate Program (M.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Putney JW, A model for receptor-regulated calcium entry, Cell Calcium 7 (1986) 1–12. [DOI] [PubMed] [Google Scholar]
- [2].Prakriya M, Lewis RS, Store-operated calcium channels, Physiol. Rev 95 (2015) 1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lewis RS, The molecular choreography of a store-operated calcium channel, Nature 446 (2007) 284–287. [DOI] [PubMed] [Google Scholar]
- [4].Nguyen NT, Han W, Cao W-M, Wang Y, Wen S, Huang Y, Li M, Du L, Zhou Y, Store-operated calcium entry mediated by ORAI and STIM, Compr. Physiol 8 (2018) 981–1002. [DOI] [PubMed] [Google Scholar]
- [5].Lacruz RS, Feske S, Diseases caused by mutations in ORAI1 and STIM1, Ann. NY Acad. Sci 1356 (2015) 45–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS, STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1, Cell 136 (2009) 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yuan J, Zeng W, Dorwart M, Choi Y, Worley P, Muallem S, SOAR and the polybasic STIM1 domains gate and regulate Orai channels, Nat. Cell Biol 11 (2009) 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kawasaki T, Lange F, Feske S, A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels, Biochem. Biophys. Res. Commun 385 (2009) 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fahmer M, Muik M, Schindl R, Butorac C, Stathopulos P, Zheng L, Jardin I, Ikura M, Romanin C, A coiled-coil clamp controls both conformation and clustering of stromal interaction molecule 1 (STIM1), J. Biol. Chem 289 (2014) 33231–33244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler F, Plenk P, Lackner B, Groschner K, Ikura M, Romanin C, STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation, EMBO J. 30 (2011) 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos PB, Ikura M, Rao A, Hogan PG, Initial activation of STIM1, the regulator of store-operated calcium entry, Nat. Struct. Mol. Biol 20 (2013) 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ma G, Wei M, He L, Liu C, Wu B, Zhang SL, Jing J, Liang X, Senes A, Tan P, Li S, Sun A, Bi Y, Zhong L, Si H, Shen Y, Li M, Lee M-S, Zhou W, Wang J, Wang Y, Zhou Y, Inside-out Ca2+ signalling prompted by STIM1 conformational switch, Nat. Commun 6 (2015) 7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang X, Jin H, Cai X, Li S, Shen Y, Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1), Proc. Natl. Acad. Sci. USA 109 (2012) 5657–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Korzeniowski MK, Manjarrés IM, Varnai P, Balla T, Activation of STIM1-Orai1 involves an intramolecular switching mechanism, Sci. Signal 3 (2010) ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Novello MJ, Zhu J, Feng Q, Ikura M, Stathopulos PB, Structural elements of stromal interaction molecule function, Cell Calcium 73 (2018) 88–94. [DOI] [PubMed] [Google Scholar]
- [16].Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C, Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation, J. Biol. Chem 283 (2008) 8014–8022. [DOI] [PubMed] [Google Scholar]
- [17].Covington ED, Wu MM, Lewis RS, Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1, Mol. Biol. Cell 21 (2010) 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liou J, Fivaz M, Inoue T, Meyer T, Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion, Proc. Natl. Acad. Sci. USA 104 (2007) 9301–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou Y, Meraner P, Kwon HT, Machnes D, Masatsugu O, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG, STIM1 gates the store-operated calcium channel ORAI1 in vitro, Nature Structural & Molecular Biology 17 (2010) 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Penna A, Demuro A, V Yeromin A, Zhang SL, Safrina O, Parker I, Cahalan MD, The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers, Nature 456 (2008) 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Srikanth S, Jung H-J, Kim K-D, Souda P, Whitelegge J, Gwack Y, A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells, Nat. Cell Biol 12 (2010) 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Korzeniowski MK, Wisniewski E, Baird B, Holowka DA, Balla T, Molecular anatomy of the early events in STIM1 activation - oligomerization or conformational change?, J. Cell Sci 130 (2017) 2821–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carrasco S, Meyer T, STIM proteins and the endoplasmic reticulum-plasma membrane junctions, Annu. Rev. Biochem 80 (2011) 973–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu MM, Covington ED, Lewis RS, Single-molecule analysis of diffusion and trapping of STIM1 and Orai1 at endoplasmic reticulum-plasma membrane junctions, Mol. Biol. Cell 25 (2014) 3672–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gudlur A, Zeraik AE, Hirve N, Rajanikanth V, Bobkov AA, Ma G, Zheng S, Wang Y, Zhou Y, Komives EA, Hogan PG, Calcium sensing by the STIM1 ER-luminal domain, Nat. Commun 9 (2018) 4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hirve N, Rajanikanth V, Hogan PG, Gudlur A, Coiled-coil formation conveys a STIM1 signal from ER lumen to cytoplasm, Cell Rep. 22 (2018) 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stathopulos PB, Zheng L, Li G-Y, Plevin MJ, Ikura M, Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry, Cell 135 (2008) 110–122. [DOI] [PubMed] [Google Scholar]
- [28].Walsh CM, Chvanov M, Haynes LP, Petersen OH, V Tepikin A, Burgoyne RD, Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry, Biochem. J 425 (2010) 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Korzeniowski M, Popovic M, Szentpetery Z, Varnai P, Stojilkovic S, Balla T, Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides, J. Biol. Chem 284 (2009) 21027–21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ercan E, Momburg F, Engel U, Temmerman K, Nickel W, Seedorf M, A conserved, lipid-mediated sorting mechanism of yeast Ist2 and mammalian STIM proteins to the peripheral ER, Traffic 10 (2009) 1802–1818. [DOI] [PubMed] [Google Scholar]
- [31].Bhardwaj R, Müller H-M, Nickel W, Seedorf M, Oligomerization and Ca2+/calmodulin control binding of the ER Ca2+-sensors STIM1 and STIM2 to plasma membrane lipids, Biosci. Rep 33 (2013) 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS, Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation, Nature 454 (2008) 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jing J, He L, Sun A, Quintana A, Ding Y, Ma G, Tan P, Liang X, Zheng X, Chen L, Shi X, Zhang SL, Zhong L, Huang Y, Dong M-Q, Walker CL, Hogan PG, Wang Y, Zhou Y, Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca2+ influx, Nat. Cell Biol 17 (2015) 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stathopulos P, Li G, Plevin M, Ames J, Ikura M, Stored Ca2+ depletion-induced oligomerization of STIM1 via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry, J. Biol. Chem 281 (2006) 35855–35862. [DOI] [PubMed] [Google Scholar]
- [35].Zhang SL, V Yeromin A, Zhang XH-F, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD, Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity, Proc. Natl. Acad. Sci. USA 103 (2006) 9357–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S-H, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A, A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function, Nature 441 (2006) 179–185. [DOI] [PubMed] [Google Scholar]
- [37].Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet J-P, CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry, Science 312 (2006) 1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yeromin A, Zhang S, Jiang W, Yu Y, Safrina O, Cahalan M, Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai, Nature 443 (2006) 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vig M, Beck A, Billingsley J, Lis A, Parvez S, Peinelt C, Koomoa D, Sobol off J, Gill D, Fleig A, Kinet J, Penner R, CRACM1 multimers form the ionselective pore of the CRAC channel, Curr Biol 16 (2006) 2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan P, Orai1 is an essential pore subunit of the CRAC channel, Nature 443 (2006) 230–233. [DOI] [PubMed] [Google Scholar]
- [41].Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems D, Hogan P, Rao A, Biochemical and functional characterization of Orai proteins, J. Biol. Chem 282 (2007) 16232–16243. [DOI] [PubMed] [Google Scholar]
- [42].Li Z, Lu J, Xu P, Xie X, Chen L, Xu T, Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation, J. Biol. Chem 282 (2007) 29448–29456. [DOI] [PubMed] [Google Scholar]
- [43].Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M, STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy, J. Physiol 586 (2008) 5383–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mignen O, Thompson JL, Shuttleworth TJ, Orai1 subunit stoichiometry of the mammalian CRAC channel pore, J. Physiol 586 (2008) 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ji W, Xu P, Li Z, Lu J, Liu L, Zhan Y, Chen Y, Hille B, Xu T, Chen L, Functional stoichiometry of the unitary calcium-release-activated calcium channel, Proc. Natl. Acad. Sci. USA 105 (2008) 13668–13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Madl J, Weghuber J, Fritsch R, Derler T, Fahrner M, Frischauf T, Lackner B, Romanin C, Schütz GJ, Resting-state Orai1 diffuses as homotetramer in the plasma membrane of live mammalian cells, J. Biol. Chem 285 (2010) 41135–41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhou Y, Ramachandran S, Oh-hora M, Rao A, Hogan PG, Pore architecture of the ORAI1 store-operated calcium channel, Proc. Natl. Acad. Sci. USA 107 (2010) 4896–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Demuro A, Penna A, Safrina O, V Yeromin A, Amcheslavsky A, Cahalan MD, Parker I, Subunit stoichiometry of human Orai1 and Orai3 channels in closed and open states, Proc. Natl. Acad. Sci. USA 108 (2011) 17832–17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li P, Miao Y, Dani A, Vig M, α-SNAP regulates dynamic, on-site assembly and calcium selectivity of Orai1 channels, Mol. Biol. Cell 27 (2016) 2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen L, Xu T, On the stoichiometry of resting and activated CRAC channels, Curr. Top. Membr 71 (2013) 95–108. [DOI] [PubMed] [Google Scholar]
- [51].Hou X, Pedi L, Diver MM, Long SB, Crystal structure of the calcium release-activated calcium channel Orai, Science 338 (2012) 1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Balasuriya D, Srivats S, Murrell-Lagnado RD, Edwardson JM, Atomic force microscopy (AFM) imaging suggests that stromal interaction molecule 1 (STIM1) binds to Orai1 with sixfold symmetry, FEBS Lett. 588 (2014) 2874–2880. [DOI] [PubMed] [Google Scholar]
- [53].Thompson JL, Shuttleworth TJ, How many Orai’s does it take to make a CRAC channel?, Sci. Rep 3 (2013) 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cai X, Zhou Y, Nwokonko RM, Loktionova NA, Wang X, Xin P, Trebak M, Wang Y, Gill DL, The Orai1 store-operated calcium channel functions as a hexamer, J. Biol. Chem 291 (2016) 25764–25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yen M, Lokteva LA, Lewis RS, Functional analysis of Orai1 concatemers supports a hexameric stoichiometry for the CRAC channel, Biophys. J 111 (2016) 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sack JT, Shamotienko O, Dolly JO, How to validate a heteromeric ion channel drug target: assessing proper expression of concatenated subunits, J. Gen. Physiol 131 (2008) 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, North RA, Contribution of individual subunits to the multimeric P2X2 receptor: estimates based on methanethiosulfonate block at T336C, Mol. Pharmacol 56 (1999) 973–981. [DOI] [PubMed] [Google Scholar]
- [58].McCormack K, Lin L, Iverson LE, Tanouye MA, Sigworth FJ, Tandem linkage of Shaker K+ channel subunits does not ensure the stoichiometry of expressed channels, Biophys. J 63 (1992) 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nicke A, Rettinger J, Schmalzing G, Monomeric and dimeric byproducts are the principal functional elements of higher order P2X1 concatamers, Mol. Pharmacol 63 (2003) 243–252. [DOI] [PubMed] [Google Scholar]
- [60].Mullins FM, Yen M, Lewis RS, Orai1 pore residues control CRAC channel inactivation independently of calmodulin, J. Gen. Physiol 147 (2016) 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yen M, Lewis RS, Physiological CRAC channel activation and pore properties require STIM1 binding to all six Orai1 subunits, J. Gen. Physiol 150 (2018) 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hoover PJ, Lewis RS, Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1), Proc. Natl. Acad. Sci. USA 108 (2011) 13299–13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Scrimgeour N, Litjens T, Ma L, Barritt GJ, Rychkov GY, Properties of Orai1 mediated store-operated current depend on the expression levels of STIM1 and Orai1 proteins, J. Physiol 587 (2009) 2903–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Li Z, Liu L, Deng Y, Ji W, Du W, Xu P, Chen L, Xu T, Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits, Cell Res 21 (2011) 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McNally BA, Somasundaram A, Yamashita M, Prakriya M, Gated regulation of CRAC channel ion selectivity by STIM1, Nature 482 (2012) 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yamashita M, Yeung PS-W, Ing CE, McNally BA, Pomès R, Prakriya M, STIM1 activates CRAC channels through rotation of the pore helix to open a hydrophobic gate, Nat. Commun 8 (2017) 14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hou X, Burstein SR, Long SB, Structures reveal opening of the store-operated calcium channel Orai, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Frischauf I, Litviňuková M, Schober R, Zayats V, Svobodová B, Bonhenry D, Lunz V, Cappello S, Tociu L, Reha D, Stallinger A, Hochreiter A, Pammer T, Butorac C, Muik M, Groschner K, Bogeski I, Ettrich RH, Romanin C, Schindl R, Transmembrane helix connectivity in Orai1 controls two gates for calcium-dependent transcription, Sci. Signal 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zweifach A, Lewis RS, Rapid inactivation of depletion-activated calcium current (Icrac) due to local calcium feedback, J. Gen. Physiol 105 (1995) 209–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi-Seabrook GM, Muik M, Romanin C, Ikura M, STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry, Nat. Commun 4 (2013) 2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhou Y, Wang X, Wang X, Loktionova NA, Cai X, Nwokonko RM, Vrana E, Wang Y, Rothberg BS, Gill DL, STIM1 dimers undergo unimolecular coupling to activate Orai1 channels, Nat. Commun 6 (2015) 8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhou Y, Nwokonko RM, Cai X, Loktionova NA, Abdulqadir R, Xin P, Niemeyer BA, Wang Y, Trebak M, Gill DL, Cross-linking of Orai1 channels by STIM proteins, Proc. Natl. Acad. Sci. USA 115 (2018) E3398–E3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Perni S, Dynes JL, V Yeromin A, Cahalan MD, Franzini-Armstrong C, Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry, Proc. Natl. Acad. Sci. USA 112 (2015) E5533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Palty R, Fu Z, Isacoff EY, Sequential steps of CRAC channel activation, Cell Rep. 19 (2017) 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]


