Abstract
Background and Aim:
Treatment options for patients with advanced biliary tract cancer are limited. Dysregulation of the immune system plays an important role in the pathogenesis of biliary tract cancer (BTC). This study aimed to investigate whether tremelimumab, an anti-CTLA4 inhibitor, could be combined safely with microwave ablation to enhance the effect of anti-CTLA4 treatment in patients with advanced BTC. Patients were enrolled to receive monthly tremelimumab (10mg/kg, intravenously) for 6 doses, followed by infusions every 3 months until off-treatment criteria were met. Thirty-six days after the first tremelimumab dose, patients underwent subtotal microwave ablation. Interval imaging studies were performed every 8 weeks. Adverse events were noted and managed. Tumor and peripheral blood samples were collected to perform immune monitoring and whole exome sequencing.
Results:
Twenty patients with refractory BTC were enrolled. Median age 56.5 years. No dose-limiting toxicities were encountered. The common treatment related adverse events included lymphopenia, diarrhea and elevated transaminases. Among sixteen patients evaluable for efficacy analysis, two (12.5%) patients achieved a confirmed partial response (lasting for 8.0 and 18.1 months, respectively), 5 patients (31.3%) achieved stable disease. Median progression free survival, and overall survival were 3.4 months (95% CI 2.5–5.2 months), and 6.0 months (95% CI 3.8–8.8 months), respectively. Peripheral blood immune cell subset profiling showed increased circulating activated (HLA-DR positive) CD8+ T cells. TCRβscreening showed tremelimumab expanded TCR repertoire but not reaching statistical significance (P=0.057).
Conclusions:
Tremelimumab in combination with tumor ablation is a potential new treatment strategy for patients with advanced BTC. Increased circulating activated CD8+ T cells and TCR repertoire expansion induced by tremelimumab may contribute to treatment benefit.
Keywords: anti-CTLA4, immune checkpoint inhibitor, liver cancer
Introduction
Biliary tract cancer (BTC) accounts for approximately 3% of all adult cancers, including cholangiocarcinoma (both intrahepatic and extrahepatic) and gallbladder cancer [1]. Surgical resection is the only curative approach for patients with local disease, however, the majority of patients present with advanced unresectable disease limiting the success of current treatment options [1–3]. The combination of gemcitabine and cisplatin chemotherapy has become the standard first-line treatment regimen for advanced BTC with a median survival of 11.7 months[4]. For patients who progress on first-line therapy, there is no standard second-line option [5]. Frequently, the chemotherapy regimens used are extrapolated from data in pancreatic and other gastrointestinal cancers with limited or uncertain benefit. Recently, biomarker based targeted therapies have shown meaningful clinical activity against small subset of patient chemotherapy-refractory cholangiocarcinoma baring specific gene mutations. A phase II study in patients with FGFR-altered advanced BTC found impressive antitumour activity of BGJ398 (a selective pan-FGFR kinase inhibitor), with a disease-control rate of 82%, and a manageable safety profile[6]. An interim analysis of data from BTC with IDH mutation showed a disease-control rate of 62% with IDH inhibitor [7].
Emerging data suggest encouraging clinical activity with immune checkpoint inhibitors in liver cancers [8–10]. However, the current response rates achievable with checkpoint inhibition alone in liver cancer is in the range of 17–20% [11–13]. Results from the biliary cohort of KEYNOTE-158 trial evaluating single agent pembrolizumab in 104 pateints with BTC showed overall response rate 5.8%[14]. Mounting evidence has shown that radiotherapy is able to enhance both local and systemic immunotherapy effects[15–17]. We have previously demonstrated positive antitumor activity of tremelimumab (an anti-CTLA-4 monoclonal antibody) in combination with radiofrequency ablation (RFA) in patients with advanced hepatocellular carcinoma (HCC) through the induced accumulation of intratumoral CD8+ T cells [18]. The study showed overall response rate was 26%. Based on the promsing results seen in HCC, we evaluated the safety and efficacy of tremelimumab in combination with microwave ablation in patients with refractory biliary tract cancer.
Patients and methods
Patients
Eligible patients were at least 18 years old and had pathologically confirmed BTC as defined by the Laboratory of Pathology at the National Cancer Institute (NCI) prior to enrollment. Additional eligibility criteria included: Eastern Cooperative Oncology Group (ECOG) performance status score 0–2; disease that was not amenable to potentially curative liver transplantation or resection; progression of disease on at least one line of chemotherapy for BTC; adequate organ and marrow function, and no history of chronic autoimmunity or inflammatory bowel disease. All enrolled patients had to have an intrahepatic lesion amenable to RFA (only intrahepatic lesions were ablated). All patients provided written informed consent and the study was approved by the NCI Institutional Review Board. All cases were discussed at a NCI multidisciplinary GI tumor board. All patients were evaluated by interventional radiologist for the suitability for the protocol-mandated interventional radiology procedure. The ClinicalTrials.gov identifier was: NCT01853618.
Study design
Patients who met the eligibility criteria were enrolled into the study. Tremelimumab at 10 mg/kg intravenously was administrated every 4 weeks for a total of 6 doses, followed by 3-monthly infusions until off-treatment criteria were met. On day 36 (± 4 days), patients underwent subtotal microwave ablation, as previously described [18]. The lesion subjected to ablation was chosen based on technical factors, including access and proximity to major vessels. Imaging studies were performed by contrast-enhanced CT or MRI scans every 8 weeks. Objective response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) in lesions not subjected to microwave ablation. Tumor biopsies were performed optionally before the first tremelimumab infusion (baseline) and at day 36 when patients underwent microwave ablation (post-treatment). The primary objective was to determine the feasibility of tremelimumab administration in combination with microwave ablation in patients with advanced BTCs who had received and progressed on at least one line of chemotherapy. Secondary objectives were to determine the immune response to treatment by measuring various immunologic parameters, as well as safety, toxicity and efficacy. Safety and toxicity was monitored and managed accordingly. Due to the delayed timing of the radiologic procedure, the assessment period for dose-limiting toxicities (DLT) was extended for the first 8 weeks of the study. The evaluable population was defined as all patients who had received at least one dose of both tremelimumab and ablation and had at least one post-baseline tumor response assessment using RECIST 1.1.
Safety and efficacy
All adverse events (AEs) and serious AEs occurring within 30 days of the last dose were reported according to the NCI Common Terminology Criteria for Adverse Events v4.0. The safety population was defined as all patients who received at least one dose of tremelimumab or ablation. Efficacy was assessed by response rate, as determined per RECIST 1.1 criteria by investigators, and reported along with an exact 95% confidence interval (CI). In addition, progression-free survival (PFS), time to progression (TTP) and overall survival (OS) were calculated by the Kaplan-Meier method, and reported along with 95% CI. PFS was the time from the first treatment included in the protocol until the first documented progression of disease or death. OS was defined as the time between the initiation of protocol therapy and date of death or last follow-up. Complete response (CR) was defined as disappearance of all target lesions. Any pathological lymph nodes (whether target or non-target) must have reduction in the short axis to <10 mm. Partial response (PR) was defined as at least a 30% decrease in the sum of the diameters of target lesions, taking as reference the baseline sum of diameters. Progressive disease (PD) was defined as at least a 20% increase in the sum of the diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. The appearance of one or more new lesions is also considered progressions. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum of diameters while on study.
Immune analysis of peripheral blood mononuclear cells
Peripheral blood was obtained by venipuncture and collected in Vacutainer tubes containing acid-citrate-dextrose anticoagulant. Peripheral blood mononuclear cells (PBMC) and plasma were separated by Ficoll density gradient centrifugation, and PBMCs were resuspended in a 90% FBS (Gemini) and 10% DMSO (Sigma-Aldrich) solution. PBMCs were viably cryopreserved and stored in vapor phase liquid nitrogen, whereas plasma was frozen and stored at −80°C for future use. Immune profiling of PBMCs was performed using an immune cell subsetting panel. Two million PBMCs were first incubated in a 12 × 75 staining tube, with Fc Block (BD Biosciences), followed by a surface stain with 4–1BB, ICOS, PD-1, PD-L1, TIM3, HLA-DR, viability dye, CD8 and CD4 for 25 minutes at 4°C. Following cell surface staining, cells were treated with the FOXP3 Fix/Perm solution (eBioscience) for 45 minutes at 4°C. Intranuclear staining was then performed for 30 minutes at 4º C using fluorescent antibodies against FOXP3 and CTLA-4. Matched isotype controls were used for ICOS, 4–1BB, HLA-DR, CTLA-4, PD-1, PD-L1, TIM3, and FOXP3. Cells were fixed with 1% paraformaldehyde (Sigma-Adrich) and acquired on a BD LSRII flow cytometer.
Tumor sample analysis
An optional tumor biopsy was performed at baseline and at the time of the ablative therapy for analysis of immune infiltration. Tumor tissue was processed by the Department of Pathology, NCI. After fixation with formalin and embed in paraffin, tumor slides were stained with H/E. The stained slides were scanned (Aperio ScanScope XT) and analyzed with an automated image analysis software (Aperio Positive Pixel Count v9). Immunohistochemistry was used to analyze MMR status and PD-L1 expression.
T cell receptor (TCR) sequencing
DNA was extracted from longitudinal pre- and post-treatment peripheral blood samples using the Qiagen DNA blood mini kit, respectively (Qiagen). TCR Vβ CDR3 sequencing was performed using the survey resolution Immunoseq platforms (Adaptive Biotechnologies). Bioinformatic analysis of productive clones was performed accordingly with ImmunoSeq software.
Exome and RNA sequencing of tumors
Patient’s FFPE tumor sample was proceeded for whole exome and RNA sequencing. One μg of DNA was sheared to approximately 200 bp by sonication (Covaris). Exome enrichment was performed using SureSelect Clinical Research Exome Kits according to manufacturer’s instructions (Aglient). Paired-end sequencing (2×75bp) was performed on an Illumina NextSeq500 instrument. A custom bioinformatic pipeline was used to detect somatic mutations including single-nucleotide variation, indels, and copy number alterations in each tumor sample comparing to its germline DNA as described. RNA-Seq libraries were constructed using Illumina TruSeq RNA Access Library Prep Kits and sequenced on an Illumina NextSeq500 instrument using 2×75bp sequencing (Illumina). RNA-seq data were processed and gene (immune) signature analysis in the tumor samples was performed using a similar method described elsewhere [19].
Statistical analysis
Paired t tests were used to assess differences in cell frequencies between visits, except that a Wilcoxon signed rank test was used when the paired difference was not normally distributed. Kaplan–Meier methods were used to estimate the PFS, TTP, and OS. Results are reported without adjustment for multiple testing. Flow cytometric analysis was performed using Flowjo software (Tree Star). Statistical analysis was performed using Prism software (GraphPad) or SAS Version 9.3 (SAS Institute, Cary NC).
Results
Patient characteristics
Twenty patients were enrolled between March 2015 and December 2016. All patients had radiological evidence of progressive disease at enrollment. The baseline characteristics for the study population patients are shown in Table 1. Equal number of male and female patients were enrolled (50% vs 50%). The median age of the population was 56.5 years old (range 37–67). All enrolled patients had good performance status with ECOG Performance Status 0 (50%) or 1 (50%). Twelve patients (60%) had intrahepatic cholangiocarcinoma, six extrahepatic cholangiocarcinoma (30%, 2 common bile duct and 4 ampullar) and two gallbladder cancers (10%). None of the patients had hepatitis B/C infection or liver cirrhosis. The vast majority of patients (16/20, 80%) had metastatic disease. Seven patients had received primary tumor resection (7/20, 35%), including partial hepatectomy (2/7), cholecystectomy (1/7), or Whipple procedure (4/7). The majority of patients (16/20, 80%) progressed after at least two lines of chemotherapy and only 4 patients were enrolled after progression with first line gemcitabine and cisplatin (20%). Prior chemotherapy consisted of gemcitabine plus cisplatin (80%), FOLFOX (25%), gemcitabine plus paclitaxel (20%), gemcitabine monotherapy (20%), FOLFIRINOX (15%), FOLFIRI (10%), and gemcitabine plus oxaliplatin (5%). One patient received ramicurimab (5%) and one patient received pembrolizumab (5%) prior to enrollment. Two (10%) patients had received prior radiation therapy. The available molecular profiles of tumors from patients enrolled are listed in Supplemental Table S1. All tumors were mismatch repair proficient as shown by immunohistochemistry (7/20) or comprehensive genetic profiling (11/20). No tumor sample was available for further molecular evaluation from two patients. One patient with ampullary adenocarcinoma showed a germline MLH1 R487Q mutation (Tier 2). PD-L1 expression analysis in tumor only was tested in 11 available samples, 5 showed <1% to 2% positive staining, 6 samples were negative.
Table 1:
BTCs Patient Characteristics
| Number | 20 |
|---|---|
| Age, Median | 56.5 (37–67) |
| Sex | |
| Male | 10 |
| Female | 10 |
| Race | |
| Caucasion | 20 |
| Ethnicity | |
| Non-Hispanic | 19 |
| Hispanic | 1 |
| ECOG | |
| 0 | 10 |
| 1 | 10 |
| Viral hepatitis | 0 |
| Liver cirrohsis | 0 |
| M1 disease | |
| Yes | 16 |
| No | 4 |
| Primary disease | |
| Extrahepatic | 6 |
| Intrahepatic | 12 |
| Gallbladder | 2 |
| Prior stent placement | 4 |
| New stent placement during trial | 1 |
| Previous therapy | |
| Any Surgical resection | 7 |
| Any TACE | 1 |
| Any Chemotherapy | 20 |
| Any Radiation therapy | 2 |
| Reason for discontinuation | |
| PD | 17 |
| Toxicity | 3 |
| CA19.9 | |
| >200 U/mL | 8 |
| <200 U/mL | 12 |
Safety
Treatment-related toxicities are summarized in Table 2 and 3. This excludes toxicities which were directly attributable to the interventional radiology procedure (e.g. pain) as per the standard of care experience. All patients who withdrew received at least one dose of tremelimumab and all underwent ablation; therefore, the safety population consisted of 20 patients. All patients experienced at least one treatment related AE, with the majority being grade 1–2 (81.5%), where medical intervention was not required. The most commonly occurring treatment-related AEs were lymphopenia, elevated AST, elevated alkaline phosphatase, hyponatremia, thrombocytopenia, leukopenia, elevated ALT, diarrhea, skin rashes. Overall, five patients (25%) experienced at least one grade 4 AE of which 3 (15%) were determined to be related to the study treatment. This included lymphopenia, neutropenia and hypotension. Four patients were taken off treatment. Reasons for study termination included one patient who experienced aseptic meningitis 2 weeks after the first dose of tremelimumab (grade 3), one patient who rapidly progressed and developed bowel obstruction requiring surgery 9 days after he received second dose of tremelimumab, and one patient who suffered from grade 3 diarrhea secondary from biopsy confirmed immune colitis after the fifth dose of tremelimumab. The fourth patient had grade 3 insomnia not well controlled with medication after the third dose of tremelimumab. No DLT was encountered. No patient died of AEs. Eighteen patients (90%) died during follow up because of disease progression.
Table 2.
Treatment-Related Adverse Events
| AEs Among Patients, N=20 | Grade 2, No. | Grade 3, No. | Grade 4, No. |
|---|---|---|---|
| Diarrhea | 2 | 3 | 0 |
| Rash maculo-papular | 3 | 2 | 0 |
| Pruritus | 0 | 2 | 0 |
| Anorexia | 3 | 1 | 0 |
| Edema limbs | 2 | 0 | 0 |
| Vomiting | 1 | 0 | 0 |
| Cough | 1 | 0 | 0 |
| Nausea | 2 | 0 | 0 |
| Dehydration | 1 | 3 | 0 |
| Insomnia | 3 | 1 | 0 |
| Hypotension | 0 | 1 | 1 |
| Infusion related reaction | 2 | 0 | 0 |
| Colitis | 0 | 2 | 0 |
| Confusion | 1 | 0 | 0 |
| Flank pain | 1 | 0 | 0 |
| Generalized muscle weakness | 1 | 1 | 0 |
| Anaphylaxis | 0 | 1 | 0 |
| Meningitis | 0 | 1 | 0 |
| Restlessness | 1 | 0 | 0 |
| Stroke | 1 | 0 | 0 |
Table 3.
Treatment-Related Laboratory Adverse Events
| AEs Among Patients, N=20 | Grade 2, No. | Grade 3, No. | Grade 4, No. |
|---|---|---|---|
| Lymphocyte count decreased | 13 | 3 | 2 |
| AST increased | 5 | 2 | 0 |
| ALP increased | 5 | 2 | 0 |
| Hyponatremia | 0 | 2 | 0 |
| Platelet count decreased | 2 | 1 | 0 |
| White blood cell decreased | 4 | 0 | 0 |
| ALT increased | 3 | 1 | 0 |
| Hypophosphatemia | 7 | 3 | 0 |
| Anemia | 0 | 6 | 0 |
| Neutrophil count decreased | 2 | 1 | 1 |
| Total bilirubin increased | 0 | 2 | 0 |
Efficacy
Sixteen patients had lesions that were evaluable for response excluding the areas treated with microwave ablation. Efficacy data is listed on Table 4. An overall response rate of 12.5% and a disease control rate of 50% was observed. Figure 1 shows efficacy data for the study population including overall change of disease burden in patients on treatment (Fig 1A), radiological response (Fig 1B), quality and duration of objective responses (Fig 1C) and relative change of tumor marker CA 19.9 on the course of treatment and follow up (Fig 1D). Of these patients, two patients (12.5%) achieved a confirmed partial response (one lasting for 8.0 months and the other ongoing after 18.1 months), and 5 patients (31.3%) achieved stable disease with the longest lasting for 6.2 months (range from 1.8 to 6.2 months). Median PFS, TTP, and OS were 3.4 months (95% CI 2.5–5.2 months) (Fig 2A), 3.3 months (95% CI: 2.5–4.6 months) and 6.0 months (95% CI 3.8–8.8 months) respectively in this small pilot cohort (Fig 2B). No disease-specific subset analyses were performed given the small number of patients.
Table 4.
Response to treatment
| Response | Investigator Assessment (%) |
|---|---|
| Best response | |
| CR | 0 |
| PR | 2 (12.5) |
| SD | 6 (37.5) |
| PD | 8 (50.0) |
| Overall response rate (CR or PR) | 2 (12.5) |
| Disease control rate (Cr, PR, or SD) | 8 (50.0) |
| Median PFS, months (95% CI) | 3.4 (2.5–5.2) |
| Median TTP, months (95% CI) | 3.3 (2.5–4.6) |
| Median OS, months (95% CI) | 6.0 (3.8–8.8) |
| Median duration of exposure (range) | 1.9 (1 day −6.1 months) |
Figure 1: Efficacy data from study population.
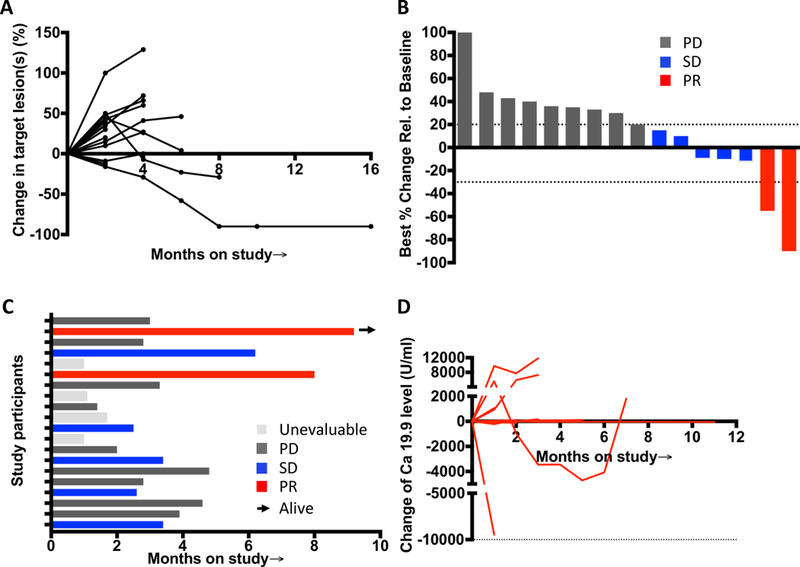
(A) Spider plot from left to right tracks the change in the overall disease burden of patients on treatment, and the rise or fall in the line represents a relative increase or decrease, respectively, in the overall size of measurable “target” lesions on imaging for each patient. These lines demonstrate the variability of the patterns seen, as well as the potential for prolonged response that may be of delayed onset. (B) Waterfall plot of all radiographic responses. Tumor responses were measured at regular intervals and values show the best fractional change of the sum of longest diameters from the baseline measurements of each measurable tumor. (C) Swimmer plot showed time on study and response status. The arrow indicates patients still alive. Gray represents as unevaluable case, black as progressive disease case, blue as stable disease case, red as partial response case. (D) Tumor marker CA 19.9 level change of patients during the treatment and follow-up.
Figure 2: Patient survival to CTLA-4 blockade and microwave ablation.
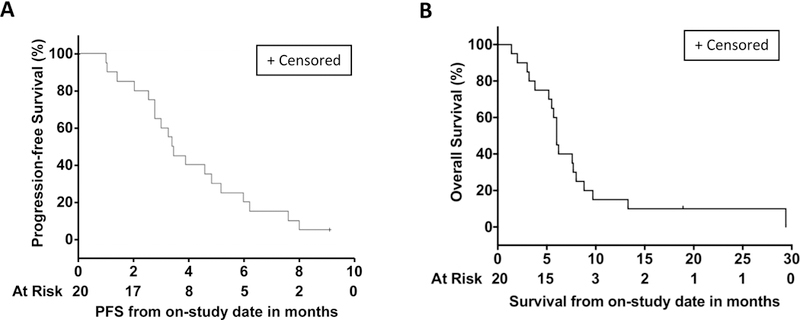
(A) PFS and (B) OS was assessed by Kaplan-Meier
Immune correlatives
Flow cytometry analyses were performed on PBMCs collected before treatment (baseline, D0), before the second dose of tremelimumab (D28), and before the third dose of tremelimumab (status post ablation, D56) to evaluate the effect of tremelimumab alone and in combination with microwave ablation on the immune parameters of patients with refractory BTC. Markers including 4–1BB, ICOS, HLA-DR, CTLA-4, and PD-1 are associated with T-cell activation. Tremelimumab caused a significant increase effect on CD8 activation, as evidenced by the increased HLA-DR marker expression at D28 compared with baseline. The median (25th-75th percentile) counts of HLA-DR–positive subsets of CD8 T cells at D0 versus D28 were 4.20 (1.67–9.23) vs. 12.48 (7.44–20.68) (P=0.0002, Figure 3A). There were no differences for individual CTLA-4, TIM3, PD-1, and PD-L1, ICOS positive CD8 T cells (Supplemental Figure S1). However, the median counts of 4–1BB–positive CD8 T cells at baseline versus D28 for 4–1BB–positive were 18.26 (8.14–41.20) vs. 4.02 (1.69–5.33) (P=0.012) and baseline versus D56 were 18.26 (8.14–41.2) vs. 8.72 (2.30–9.02) (P=0.038, Figure 3B), which were significantly decreased after the treatments. For CD4 T cells, the counts of individual HLA-DR, 4–1BB, CTLA-4, TIM3, PD-1, ICOS, and PD-L1 positive CD4 T cells remained unchanged from baseline to D28 and baseline to D56 (data not shown). The pattern of change of immune cell subset was different from the profiling of HCC patients who received the same treatment with tremelimumab with microwave ablation (Figure 3C) [13]. Stratification of CD8 or CD4 T cells activation by overall response did not reveal differences over time between the responders and non-responders, which might be attributed to the relatively small number of cases in each subgroup (data not shown).
Figure 3: Immune cell profiling determined by flow cytometry.
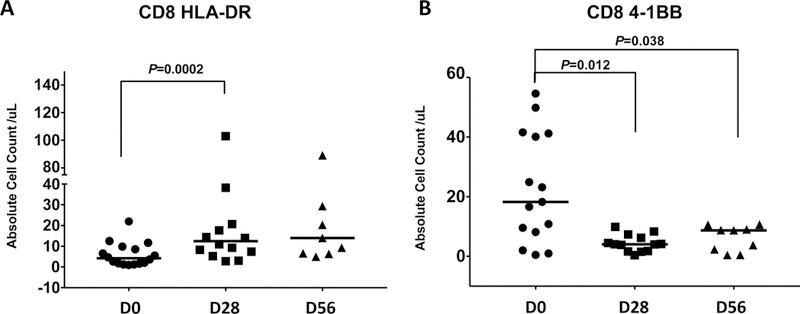
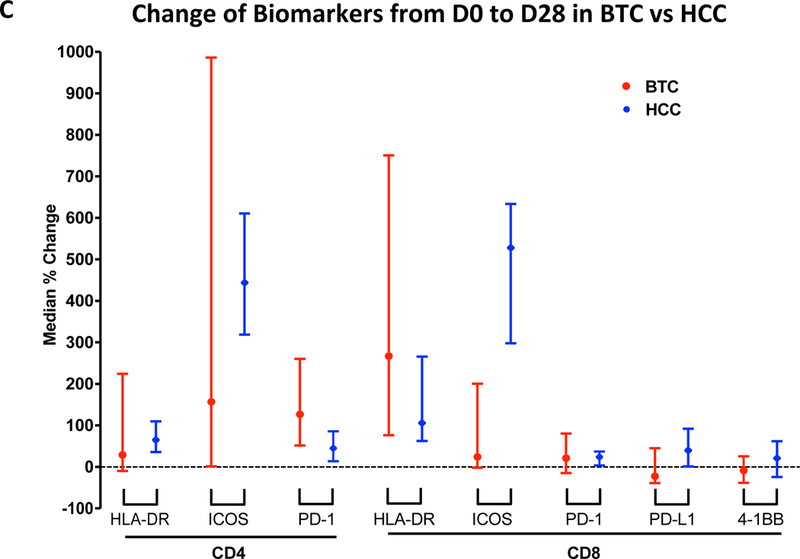
(A) Significantly increased HLA-DR positive CD8+ T cells at D28 compared with D0 (P=0.0002) as well as D56 compared with D0 (P=0.046). Horizon line represented median. (B) Suppressed 4–1BB–positive CD8 T cell count after the treatment (P=0.012 and P=0.038). Horizon line represented median. (C) Comparison of the change of immune cell profiling between BTC and HCC cohort treated with CTLA-4 blockade and microawave ablation. Bar represented upper and lower limit of cell count of individual subset and dot symbol as mean.
Next, we directly tested the hypothesis that clinical benefit from CTLA-4 blockade in advanced BTC patients is due to the induction of peripheral expansion of clonal T cells (Supplemental Figure S2). We used deep sequencing of TCR CDR3 regions (TCRseq) to evaluate T cell clonal representation in peripheral blood. We performed TCRseq on peripheral blood from two patients with a partial response, 4 with stable disease, and 2 with progressive disease. We demonstrated tremelimumab tends to expand T cell clones evidenced by the decreased TCR clonality and expanded diversity, although there was no statistically significant difference noted (P=0.057). No stratification by response were analyzed further given small number of cases for evaluation.
Clinical Vignette
Two patients showed objective responses. Both responders were diagnosed with ampullary carcinomas. The first patient was a 63-year-old male (patient #1). He was initially diagnosed with poorly differentatiated ampllary adenocarcinoma and underwent Whipple procedure with negative surgical margin but positive lymphovascular invasion. He recieved adjuvant gemcitabine followed by chemoradiation then followed by gemcitabine alone for 5 months. He had recurrent disease and was treated with FOLFIRINOX. However, his disease continued progression. Before he was enrolled into this trial, he was treated with gemcitabine/abraxane. At study initiation, he had multiple enlarging liver lesions and retroperitoneal/mesenteric lymphadenopathy with all FDG avidity. He received 6 doses of tremelimumab and scheduled ablation procedure between his second and third treatment dose. He tolerated the treatment well except mild pain and skin itching. The treatment course was complicated by C. diff colitis and a urinary tract infection that was adequately treated. His best disease response was a partial response with a 55% reduction in target lesion size. However, his disease progressed after 7 months evidenced by multiple new liver lesions, and he died several weeks later.
The second patient was a 50-year-old female (patient #2). She was diagnosed as stage IV ampullary adenocarcinoma with metastases to liver. She received FOLFIRINOX but the disease was not controlled. She was on treatment with gemcitabine/abraxane before she was enrolled onto the trial (Figure 4A). Her radiological change during the clinical course in NIH is showed in Figure 4A. She received 3 doses of tremelimumab before she developed a liver abscess at the site of microwave ablation. She was successfully treated with metronidazole and levofloxacin and eventually switched to Moxifloxicin. She has remained on chronic parenteral antibiotic suppression while continuing treatment (Figure 4A). However, she developed grade 3 colitis after the fifth dose of tremelimumab and was taken off study. She was treated with parenteral prednisone with resolution of her symptoms. Despite being off treatment, her cancer has not recurred for over one year (Figure 4A). Repeat biopsy confirmed no evidence of recurrent disease (Figure 4B–4C). She does have residual enlarged mediastinal lymph nodes developed after the treatment. The lymph nodes were biopsied and showed non-necrotizing granulomatous inflammation (data not shown). RNA-seq of a pre-treatment tumor from this patient reaveled very low level of immune cell infiltration based on immune cell gene signature (sample AC indicated with arrow in Figure 4D). Interestingly, the whole exome sequecning of her peripheral blood sample detected a total of 7 germline mutations with the category of Tier 1 and 2, including 1 frameshift deletion (FANCD2), 1 frameshift substitution (ATF1), 2 non-frameshift substitutions (APOB and FANCD2), 2 stopgain (BRCA2 and MYD88), and 1 non-synonymous SNV mutations (MLH1) (Figure 4E, Supplemental Table S2). Her tumor sample exhibited a total of 122 somatic mutations, including 7 frameshift deletions, 4 non-frameshift deletions, 103 non-synonymous mutations and 7 stopgain mutations (Supplementary Table S3), representing a mutation burden of 4.05 per MB. There were 335 predicted potential neoantigen epitopes from 97 different genes to multiple HLA types (Supplementary Table S4). Among these, 64 epitope peptides from 18 genes that showed a peptide-HLA affinity of 500 nM or lower were expressed in her tumor sample (Supplementary Table S5).
Figure 4. Data of patient #2.
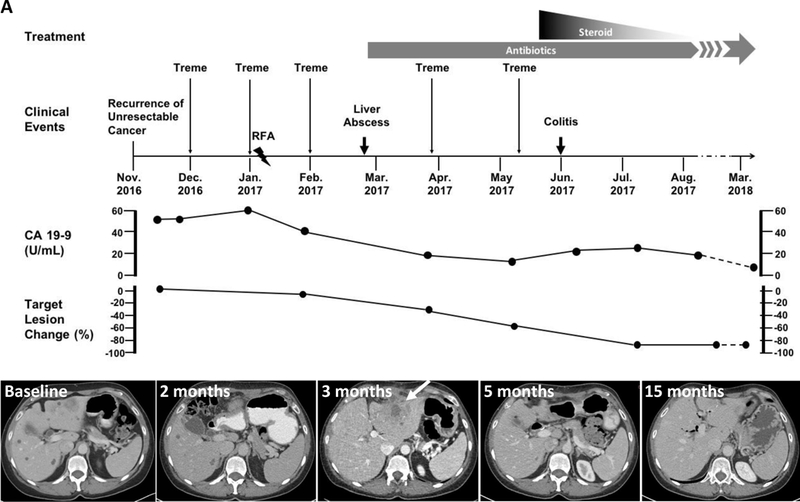
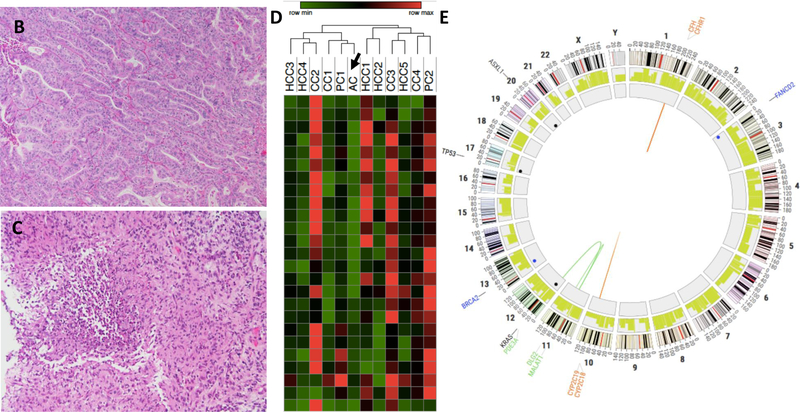
(A) Clinical events. Upper panel showed the timeline of clinical events, including therapy and disease status. Lower panel shows representative axial CT images at baseline, 2 months, 3 months, 5 months and 15 months after the tremelimumab infusion initiated. Arrow indicates liver abscess, confirmed with biopsy. (B–C): Representative H/E staining of biopsied tumor samples determined by immunohistochemistry (200x) (B: liver metastasis of putative ampullary carcinoma showed a moderately differentiated adenocarcinoma with minimal inflammatory infiltrate. C: follow-up liver biopsy of tissue near cyst. Only necrotizing granulomas were seen. There was no normal hepatic parenchyma and no tumor). (D): Heatmap based on immune cell signatures from RNA-seq. AC represents Patient #2 (arrow). (E): A Circos plot showed germline (Blue) and somatic (Black) mutation landscape for whole genome sequenced sample from one cholangiocarcinoma patient.
Discussion
In this pilot study, we explored the feasibility, safety and efficacy of the combination of tremelimumab and microwave ablation in patients with advanced BTC. This study was based on the hypothesis that the blockade of CTLA-4 checkpoint in combination with microwave ablation would transiently and selectively enhance antitumor immunity to improve PFS and OS. To our knowledge, this is the first study to examine the efficacy of combining tremelimumab with microwave ablation in advanced BTC.
In our study, treatment was well tolerated with less than 10% of patients experiencing grade 3–4 toxicity, which was mainly hematologic. Only one patient developed grade 3 immune related colitis (which resolved with systemic steroid therapy), that led to treatment discontinuation. No toxicity-related deaths were observed. The overall toxicity profile for tremelimumab in combination with microwave ablation in our study was mild to moderate. Thus, these results suggest this combination strategy does not lead to excess toxicity in advanced BTC.
Historically, second-line chemotherapy demonstrated a median PFS and OS of 2.8 and 7.5 months, respectively, based on a large retrospective study [20]. In this study, 80% participants in our current study had progressive disease on at least two lines of chemotherapy. Among 16 patients evaluable for efficacy analysis, two (12.5%) patients achieved a confirmed durable partial response and 5 patients (31.3%) achieved stable disease with the longest lasting for 6.2 months. Median PFS was 3.4 months and OS 6.0 months. Thereby, our data is comparable to previous reports. However, it is important to note that the primary objective of this study was to evaluate the feasibility and safety of tremelimumab in combination with microwave ablation in advanced BTC patients. Therefore, we acknowledge that the interpretation of our outcomes is limited by the small size of evaluable study population (n=16).
The success of immune checkpoint inhibit is dependent on the presence of an already existing endogenous antitumor immune response, which can then be amplified. Because a small proportion of tumor tissue was obtained during microwave ablation, immune cell profiling of the tumor was not performed. Instead, we evaluated the systemic immune response by analyzing the immune profile of PBMCs with flow cytometry and TCR before and during treatment. It showed limited activation of CD8+ and CD4+ T cells in refractory BTC samples, with the evidence of only increased HLA-DR–positive subsets of CD8. The pattern of immune profile change between the BTC and HCC cohort after the treatment is different as shown in Figure 3C. The underlying mechanism leading to this difference is unclear although the etiologies of these two cancers are distinctive. For instance, 75% of the HCC patients had hepatitis B or C infections and over 70% had liver cirrohsis in contrast to all BTC patients [18]. It is not clear whether this disparity may contribute to the different outcome between these two entities. Other obvious resaons may be a difference in the genetic signatures or the tumor stroma of these different tumor types.
Surprisingly, the amount of the 4–1BB positive CD8 subset, indicating activation of CD8+ cells, was suppressed after treatment. It is well known that 4–1BB-positive CD8+ T cells are related with immunostimulation. Therefore, it is likely that the synergistic combination treatment with different immune checkpoint blockade could improve the efficacy for refractory BTC patients in the future. Recently, a study reported that suppression of tumor growth was achieved in B16 melanoma-bearing mice with the combination of anti-PD-1 with the agonistic antibody anti- 4–1BB in vivo, although no obvious anti-tumor activity was observed using the anti-4–1BB mAb alone [21].
Previous studies have shown increasing TCR diversity and decreasing TCR clonality on the PBMC after tremelimumab administration. Our data showed the trend of decreasing TCR clonality, that is consistent with previous reports that CTLA-4 blockade promotes global T cell expansion and/or gain of T cell clonotype diversity [22, 23]. However, the change is not significantly different, likely due to small sample sizes and the suboptimal timing of sample collection. In the study of TCR clonality in HCC patients treated by tremelimumab and ablation (data no shown), we were not able to detect significant changes in response to ablation after tremelimumab treatment, though several patients responded to this combination[18]. It is hypothesed that prior tremelimumab treatment will mask any ablation induced changes in TCR clonality. There is evidence that post-chemotherapy delivery of immunotherapy was more effective than pretreatment [24]. The timing of such immunotherapy is probably important. Our ongoing clinical study with the combination of checkpoint inhibitor and traditional chemotherapy on BTCs will partially answer this question (NCT03111732).
Interestingly, 2 patients with ampullary cholangiocarcinoma had durable responses. One of the responders bears a germline mutation (e.g BRCA2) and somatic mutations (e.g TP53 and KRAS). It is not clear if a correlation exists between these mutations and immune checkpoint. Recently, the analysis of TCGA database [25] and clinical samples[26, 27] have shown that the BRCA mutation presents a potent immunogenic phenotype, independent of tumor mutation burden. Further studies are necessary to expand on this potential link. It has recently reported that patients with MMR deficient BTC may benefit from treatment with pembrolizumab [28, 29]. The patient, who showed an exceptional responde in our study, carried a germline MLH1 R487Q mutant (Tier 2, Supplemental Table S2). However, this did not lead to a high tumor mutation burden, Thus, it remains unclear if this germline mutation of MLH1 contributes to this patient’s partial response.
Despite these limitations, our study combining CTLA-4 blockade with microwave ablation in patients with advanced BTC demonstrated intriguing clinical activity. The correlation between global immune response elicited by tremelimumab and locally antitumor immunity within tumor microenvironments needs further exploration.
Supplementary Material
(A) TCR sequencing was performed on peripheral T cell samples obtained before and after CTLA-4 blockade. Representative frequency change of TCR clones were shown from patient #2 with Pair-Wise Scatter Plot. Red dot represents contracted TCR clones after treatment, blue dots expanded TCR clones after treatment. (B) Productive clonality change after CTLA-4 blockade (N=8, P=0.057)
Acknowledgments
Financial support
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and a Cooperative Research and Development Agreement between NCI and AstraZeneca. TFG is supported by the Intramural Research Program of the NIH, NCI (ZIA BC 011343).
Abbreviations
- BTC
biliary tract cancer
- RFA
radiofrequency ablation
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- RECIST
Response Evaluation Criteria in Solid Tumors
- DLT
dose-limiting toxicities
- AEs
Adverse events
- CI
confidence interval
- PFS
progression-free survival
- TTP
time to progression
- OS
overall survival
- CR
Complete response
- PR
Partial response
- PD
Progressive disease
- SD
Stable disease
- PBMC
Peripheral blood mononuclear cells
- TCR
T cell receptor
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References:
- 1.Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov 2017; 7: 943–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang SJ, Lemieux A, Kalpathy-Cramer J, Ord CB, Walker GV, Fuller CD, Kim JS, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011; 29: 4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995; 270: 985–988. [DOI] [PubMed] [Google Scholar]
- 4.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 5.Ulahannan SV, Rahma OE, Duffy AG, Makarova-Rusher OV, Kurtoglu M, Liewehr DJ, Steinberg SM, et al. Identification of active chemotherapy regimens in advanced biliary tract carcinoma: a review of chemotherapy trials in the past two decades. Hepat Oncol 2015; 2: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol 2018; 36: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowery MA, Abou-Alfa GK, Burris HA, Janku F, Shroff RT, Cleary JM, Azad NS, et al. Phase I Study of AG-120, an IDH1 Mutant Enzyme Inhibitor: Results From the Cholangiocarcinoma Dose Escalation and Expansion Cohorts [Abstract]. J Clin Oncol 2017; 35 (Suppl): 1.28034063 [Google Scholar]
- 8.Bang YJ, Doi T, De Braud F, Piha-Paul S, Hollebecque A, Abdul Razak AR, Lin CC, et al. Safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: Interim results of KEYNOTE-028 [Abstract]. Eur J Cancer 2015; 51 (Suppl): S112. [Google Scholar]
- 9.Shimomura A, Fujiwara Y, Kondo S, Kodaira M, Iwasa S, Kitano S, Tanabe Y, et al. Tremelimumab-associated tumor regression following after initial progression: two case reports. Immunotherapy 2016; 8: 9–15. [DOI] [PubMed] [Google Scholar]
- 10.Duffy AG, Makarova-Rusher OV, Greten TF. The case for immune-based approaches in biliary tract carcinoma. Hepatology 2016; 64: 1785–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59: 81–88. [DOI] [PubMed] [Google Scholar]
- 13.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018. [DOI] [PubMed]
- 14.Ueno M, Chung HC, Nagrial A, Marabelle A, Kelley RK, Xu L, Mahoney J, et al. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase 2 KEYNOTE-158 study. Annals of Oncology 2018; 29: 1. [Google Scholar]
- 15.Hansler J, Wissniowski TT, Schuppan D, Witte A, Bernatik T, Hahn EG, Strobel D. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol 2006; 12: 3716–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, Nakachi K, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol 2012; 40: 63–70. [DOI] [PubMed] [Google Scholar]
- 17.Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, Cerioni S, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology 2010; 138: 1931–1942. [DOI] [PubMed] [Google Scholar]
- 18.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017; 66: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei JS, Kuznetsov IB, Zhang S, Song YK, Asgharzadeh S, Sindiri S, Wen X, et al. Clinically Relevant Cytotoxic Immune Cell Signatures and Clonal Expansion of T Cell Receptors in High-risk MYCN-not-amplified Human Neuroblastoma. Clin Cancer Res 2018. [DOI] [PMC free article] [PubMed]
- 20.Walter T, Horgan AM, McNamara M, McKeever L, Min T, Hedley D, Serra S, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 2013; 49: 329–335. [DOI] [PubMed] [Google Scholar]
- 21.Hosoi A, Takeda K, Nagaoka K, Iino T, Matsushita H, Ueha S, Aoki S, et al. Increased diversity with reduced “diversity evenness” of tumor infiltrating T-cells for the successful cancer immunotherapy. Sci Rep 2018; 8: 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, Mok S, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res 2014; 20: 2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, Fong L. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med 2014; 6: 238ra270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer 2005; 5: 397–405. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Sun C, Feng Y, Jia Q, Zhu B. Potent immunogenicity in BRCA1-mutated patients with high-grade serous ovarian carcinoma. J Cell Mol Med 2018. [DOI] [PMC free article] [PubMed]
- 26.Wieser V, Gaugg I, Fleischer M, Shivalingaiah G, Wenzel S, Sprung S, Lax SF, et al. BRCA1/2 and TP53 mutation status associates with PD-1 and PD-L1 expression in ovarian cancer. Oncotarget 2018; 9: 17501–17511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, Garber JE, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 2016; 7: 13587–13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) TCR sequencing was performed on peripheral T cell samples obtained before and after CTLA-4 blockade. Representative frequency change of TCR clones were shown from patient #2 with Pair-Wise Scatter Plot. Red dot represents contracted TCR clones after treatment, blue dots expanded TCR clones after treatment. (B) Productive clonality change after CTLA-4 blockade (N=8, P=0.057)