Abstract
Introduction:
Recruitment for Alzheimer’s disease (AD) prevention research studies is challenging due to lack of awareness among cognitively healthy adults coupled with the high screen fail rate due to participants not having a genetic risk factor or biomarker evidence of the disease. Participant recruitment registries offer one solution for efficiently and effectively identifying, characterizing, and connecting potential eligible volunteers to studies.
Methods:
Individuals age 55–75 who live in the United States and self-report not having a diagnosis of cognitive impairment such as MCI or dementia are eligible to join GeneMatch. Participants enroll online and are provided a cheek swab kit for DNA extraction and APOE genotyping. Participants are not told their APOE results, although the results may be used in part to help match participants to AD prevention studies.
Results:
As of August 2018, 75,351 participants had joined GeneMatch. Nearly 30% of participants have one APOE4 allele, approximately 3% have two APOE4 alleles. The percentages of APOE4 heterozygotes and homozygotes are inversely associated with age (p<0.001).
Discussion:
GeneMatch, the first trial-independent research enrollment program designed to recruit and refer cognitively healthy adults to AD prevention studies based in part on APOE test results, provides a novel mechanism to accelerate prescreening and enrollment for AD prevention trials.
1. Background
Alzheimer’s disease (AD) remains one of the greatest medical, economic, and societal burdens in the United States (US) and globally (1). An estimated 5.7 million people in the US currently have dementia due to AD—a number projected to more than double to nearly 14 million by 2050 (2). Interventions that delay the symptomatic onset of the disease by even by 1 or 2 years would have a major public health impact (3). As a result, the National Plan to Address Alzheimer’s Disease has set a goal of preventing AD by 2025. With a heightened sense of urgency, numerous AD prevention studies are underway, with many more planned. Unfortunately, the current approach to recruiting participants into AD prevention trials is lengthy, costly, and inefficient, leading some to conclude that the field has reached a crisis point (4).
The sharp growth in AD prevention trials requires an unprecedented screening and enrollment funnel (5). Specifically, researchers will need to screen tens of thousands of cognitively healthy older adults to identify the thousands of individuals eligible to enroll in prevention trials (6). The number needed to screen is further increased if AD prevention trials require specific enrichment strategies, such as biomarker evidence of AD or genetic risk for the disease. Overall, this recruitment benchmark confronts the AD field with a daunting challenge. In the US, regardless of disease area, the vast majority of studies (85–90%) experience significant delays in recruitment and enrollment (7). Nearly one-third of trials under-enroll, and only 7% meet their target enrollment number on deadline (8). Numerous factors contribute to these difficulties. Recruitment is time consuming, sometimes taking years to meet target sample sizes. This is in large part because screen failure rates can reach as high as 85%, chiefly due to inclusion criteria, such as requiring an AD biomarker or genetic risk factor to enroll in an AD prevention trial (9). Delayed or inefficient recruitment has scientific, financial, and ethical consequences (10). Improving recruitment methods has become a critical priority for the field (9;11–14).
As the number of AD prevention trials increase there is a growing need for mechanisms to quickly and efficiently reach out to, identify, characterize, and refer potential participants to trials, with the overarching goal of reducing the percentage of individuals who screen fail. Recruitment registries are innovative tools designed to fulfill this need. In the US, several AD-focused registries are currently being used on both the national and local levels, including the Alzheimer’s Prevention Registry (www.endALZnow.org) and its predecessor the Arizona Alzheimer’s Registry (15), the Alzheimer’s Association’s TrialMatch program (https://trialmatch.alz.org/find-clinical-trials), the Brain Health Registry (http://www.brainhealthregistry.org/) (16), Cleveland Clinic’s Healthy Brains program (https://healthybrains.org/), the University of California-Irvine Consent-to-Contact Registry (https://c2c.uci.edu/) (17), and the Wisconsin Registry for Alzheimer’s Prevention (18); this list does not include trial ready cohorts that often have a different focus and objective (19–21) or registries for autosomal dominant AD (22). Each of these recruitment registries approaches participant recruitment and engagement differently and the field is still gathering data on best practices for the design and conduct of recruitment registries (14). Some registries have begun to try to identify participants who are at elevated risk for symptomatic onset of AD, either based on change in cognition, biomarker evidence of the disease, or genetics, for eventual referral into AD prevention studies. For example, the Alzheimer’s Prevention Initiative (API) Generation Program is enrolling adults age 60–75 with one or two copies of the APOE4 gene (23;24), given that the APOE ε4 allele is associated with an increased risk of dementia due to AD in later life and younger age of onset of symptoms (25;26).
Here we describe the design and execution of, as well as enrollment metrics, participant demographics, and key lessons learned from GeneMatch, a program of the Alzheimer’s Prevention Registry and API, developed as a trial-independent recruitment registry to match individuals to AD prevention studies based in part on their APOE genotype. Although most studies perform APOE genotyping as part of screening to determine eligibility, GeneMatch was created as a trial-independent program that a) enables genetic information to be stored outside of a specific trial and b) allows for participant re-contact for a variety of studies for which they might be eligible, rather than just one trial.
2. Methods
In 2012, prior to the development of GeneMatch, Banner Alzheimer’s Institute launched the Alzheimer’s Prevention Registry (APR) (NCT02022943; www.endALZnow.org) as an online resource to connect individuals to AD-related studies taking place in their communities. APR members provide minimal contact and demographic information at signup and opt in to receiving monthly newsletters on the latest AD research as well as notifications when study opportunities are available in their community; approximately 320,000 have joined APR as of August 2018. In 2015, the APR platform was expanded to include GeneMatch (NCT02564692; https://www.endalznow.org/genematch). GeneMatch allows for online sign-up, consent, and submission of identifiable information while providing technical and physical safeguards of the data.
2.1. GeneMatch Enrollment
Individuals age 55–75, who live in the US (50 states and District of Columbia), and self report not having a diagnosis of a cognitive impairment such as MCI or dementia, are eligible to join GeneMatch. Individuals can enroll remotely or in person at one of 37 GeneMatch partner healthcare sites in 24 states.
All participants, whether they are enrolling remotely or at a partner site, complete the GeneMatch enrollment process via the program’s website www.endALZnow.org/genematch. Enrollment consists of five steps: learning about the program (education module), creating an account, providing consent and Health Insurance Portability and Accountability Act of 1996 (HIPAA) authorization, providing contact and demographic information, and confirming account information for enrollment. The education module provides information about AD, the APOE gene and associated risk of developing MCI or dementia due to AD, and an overview of the GeneMatch program. During this process, participants learn that GeneMatch does not disclose APOE test results to them directly, but those results may be used in part to help match them to research studies, which may in turn require participants to learn their test results as part of enrollment into a given study. Following the education module, individuals are prompted to answer five questions to reinforce the key learning concepts presented during the module (Table 3). Individuals are not required to answer the questions correctly to proceed with enrollment and all individuals are shown the correct response regardless of how they answered the question. Next, participants provide the following information: name, mailing and email addresses, phone number, date of birth, and biological sex at birth; information about family history of AD and race/ethnicity are optional. Participants can log in to their account at any time to update their information.
Table 3.
Education Module Quiz Response Rates
| Question | Answer Choices (correct answer in BOLD) | Percent Answered Correctly |
|---|---|---|
| 1. The common form of Alzheimer's disease does not have a single, definitive cause. | • True
• False • I don’t know |
89.1% |
| 2. How does having the APOE e4 gene affect the chances that someone will get Alzheimer’s disease? | • It increases the chance of getting Alzheimer’s disease • It decreases the chance of getting Alzheimer’s disease • It has no effect on the chance of getting Alzheimer’s disease • It guarantees Alzheimer’s disease • I don’t know |
80.5% |
| 3. Can the APOE genetic test predict with certainty whether or not someone will get Alzheimer’s disease? | • Yes • No • I don’t know |
89.1% |
| 4. I will learn my APOE test results through my participation in the Alzheimer’s Prevention Registry GeneMatch Program. |
• True • False • I don’t know |
70.7% |
| 5. GeneMatch will use my APOE test results to match me with studies which may or may not require me to learn my APOE test results. It is my choice to pursue these research opportunities. | • True
• False • I don’t know |
85.9% |
2.2. APOE Genotyping
After enrollment and consent, participants are either mailed a cheek swab kit to their homes for DNA collection, or handed a kit if enrollment is done at a partner site. The kit includes detailed instructions and pictures describing how to use the swab, with abbreviated instructions printed on the kit box. Participants are instructed to not eat or drink anything other than water for 1 hour prior to swabbing the inside of their cheek with the buccal swab. After the swab dries, participants close the swab tube and place the swab and laboratory requisition form in the addressed and postage-paid envelope for return to the lab for DNA extraction and APOE genotyping. The lab is accredited by the College of American Pathologists (CAP) and certified through Clinical Laboratory Improvement Amendments (CLIA). As an additional level of quality assurance, all samples include sex verification, which is cross-checked against the information provided at enrollment and on the laboratory requisition form. APOE results are stored on a HIPAA-compliant server, separate from other demographic information. All DNA samples are destroyed after APOE genotyping. In instances in which APOE genotyping and sex verification cannot be completed due to poor DNA yield, or sex verification results are discordant with self-reported genetic sex, participants are notified via email and are sent a new cheek swab kit to complete.
2.3. Timing of the education model
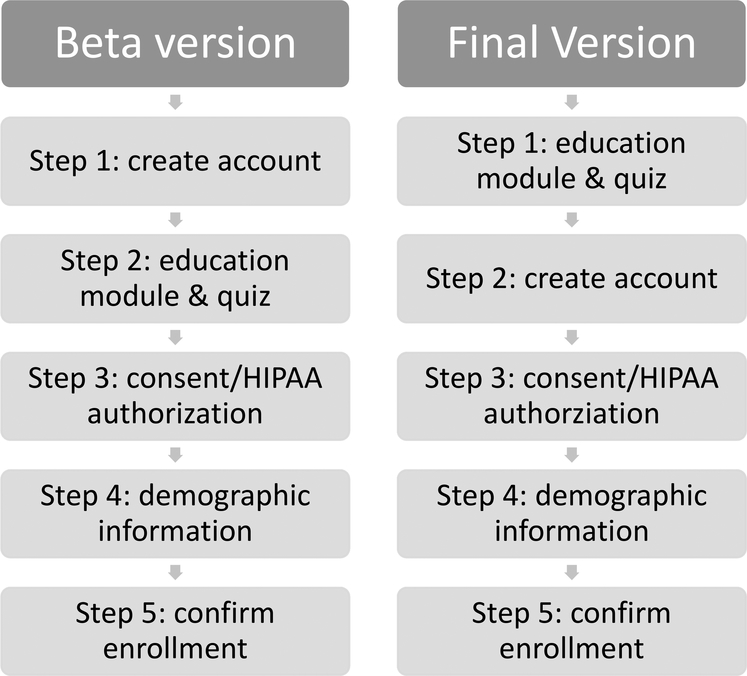
GeneMatch launched a beta version in November 2015 to fine tune the enrollment process. In the beta version, all interested individuals were required to first create an account enrolling in the program prior to completing the other enrollment steps (Figure 1). Based on unsolicited feedback submitted to the study team via email, phone, and social media channels from prospective volunteers as well as the need to ensure that prospective participants are fully briefed on the program before creating an account, the enrollment flow was modified, and the final version was launched in November 2016. In the final version, individuals are first presented with the education module in both video and bulleted text format and then answer the five-question assessment before they are prompted to create an account and complete the remaining enrollment steps (Figure 1).
Figure 1:
GeneMatch Enrollment Process
2.4. GeneMatch partner healthcare sites
As noted above, GeneMatch currently has 37 partner healthcare sites in 24 states; new partner sites are added on an ongoing basis. GeneMatch launched its partner site program in July 2016 after receiving anecdotal feedback during the beta version that researchers and physicians who regularly hold community events as part of their recruitment and outreach strategy wanted a way to enroll interested individuals into GeneMatch without requiring participants to wait one to two weeks to receive their cheek swab kits in the mail. Partner healthcare sites can enroll participants on site (though all enrollment steps, including consent, are still completed via the GeneMatch website) and have site staff distribute the cheek swab kits rather than having the kit mailed to participants’ homes. Partner sites must cede IRB review to the GeneMatch IRB since consent is done online and there is only one informed consent document.
2.5. Participant Recruitment
Several recruitment strategies and tactics are used to enroll participants into GeneMatch, including community talks, flyers, regionally tailored postcards mailed to prospective volunteers, billboards, social media advertisements, and earned media coverage. In October 2017 a social media advertising campaign was launched to raise awareness about the GeneMatch program to men since men are underrepresented in the program.
2.6. Participant Retention and Engagement
Following enrollment, participants receive periodic updates via email from GeneMatch (e.g., when their kit orders are received, when the kits are mailed to their homes, when their completed kits are received by the lab, and for general GeneMatch program information). In addition, GeneMatch participants opt in to receive monthly email newsletters from the APR to keep them informed about the latest research on AD. Participants are notified by email and in some cases, by postal mail, when they have been matched to studies and are provided instructions should they be interested in learning more about a study or enrolling.
2.7. Ethical Considerations
GeneMatch enrollment criteria requires participants to self-identify as not having a diagnosis of cognitive impairment such as MCI or dementia due to AD, since one of the main objectives of the GeneMatch program is to match participants to AD prevention trials based in part on their APOE genotype. Moreover, enrollment and consent are done online, thus limiting our ability to assess capacity for understanding in individuals with a diagnosis of cognitive impairment. We acknowledge that it is possible that some individuals with cognitive impairment join GeneMatch since we do not assess participants’ cognitive function beyond what is required to navigate the GeneMatch website and complete the multi-step enrollment process. Nonetheless, online enrollment and consent is deemed appropriate given the minimal risks to participants.
The education module serves to reinforce key aspects of the GeneMatch program, something particularly important since consent is done online. The module includes information about AD, the APOE gene and associated risk of developing MCI or dementia due to AD. These concepts are reinforced in the 5-question quiz immediately after the education module and again in the consent document. These components help ensure participants are well-informed before they provide a sample of DNA.
GeneMatch does not disclose APOE test results to participants, though studies to which participants are match may disclose results as part of the study’s enrollment and screening process. Disclosure of genetic information is the practice of medicine and requires specific guidelines to be followed, including working with a genetic counselor or other licensed healthcare professional (regulations vary from state to state) (27).
GeneMatch is a trial-independent recruitment registry and works directly with study sponsors and researchers to develop the selection criteria or algorithm based on the unique needs of each study. For example, a study sponsor may wish to invite a ratio of APOE4 carriers to noncarriers age 65–75 who live within a 100-mile radius of study sites. Its standard operating procedures (SOPs) require GeneMatch to make participants aware of all study opportunities to which they have been matched. If a participant has been matched to more than one study, participants have the choice to pursue the study that is of interest to them (or decline both studies). GeneMatch does not reserve participants with specific APOE genotypes for specific studies, nor does GeneMatch choose which studies take priority in access to its participants.
2.8. Data Analyses
Z-test for two proportions was used to examine swab return rate data by year as well as swab return rate by enrollment source. In addition, since enrollment into GeneMatch requires participants to self-identify as not having a diagnosis of cognitive impairment and the ɛ4 variant of APOE is associated with a younger age of onset of symptoms, a Z-test for two proportions was used to examine APOE genotype results by age group. All analyses were conducted using MedCalc 17.9.7.
3. Results
3.1. Participant recruitment, enrollment, and characteristics
As of August 2018, 75,351 participants had enrolled in GeneMatch. Participant demographic characteristics and recruitment sources are shown in Table 1. Participants have a mean age of 65.0 (SD 5.4) and are predominately female (69%). Over half of participants (60%) joined GeneMatch via social media advertisements. A sizeable proportion of participants (79%) opted to receive email newsletters from the Alzheimer’s Prevention Registry. From November 2015 until October 2017, 21% of GeneMatch enrollees were men. From October 2017, when a social media campaign to raise awareness of the GeneMatch program to men was launched, until August 2018, 44% of GeneMatch enrollees were men which represented a statistically significant increase from the November 2015-October 2017 recruitment period (p<0.001).
Table 1:
Demographic Characteristics of GeneMatch Participants (n = 75,351)
| Age, mean (SD) | 65.0 (5.4) |
| Sex, female | 69% |
| Family history of AD or other dementia | |
| Yes | 40% |
| No | 18% |
| Unsure | 10% |
| Prefer not to answer | 32% |
| Race / ethnicity* | |
| Non-Hispanic, white | 63.9% |
| Hispanic or Latino | 1.2% |
| African American | 1.1% |
| Asian | 0.5% |
| Other | 4.5% |
| Prefer not to answer | 28.8% |
| Recruitment / enrollment source | |
| Alzheimer’s Prevention Registry | 22.5% |
| Visited GeneMatch website directly | 7.0% |
| Online or social media | 60.0% |
| Partner healthcare site | 6.4% |
| Other | 4.1% |
participants are able select multiple options, only those reported by 0.3% or more of participants are listed
3.2. GeneMatch Enrollment Based on Timing of Education Module
GeneMatch enrollment rates were compared before and after moving the education module relative to account creation (Table 2). Nearly all participants (90%) who watched the education video or reviewed the bulleted text completed the post-education quiz. Response rates to the education questions are displayed in Table 3. In the beta version, 40% of people who reviewed and confirmed they met GeneMatch eligibility criteria continued through the entire process to complete their enrollment and registration. In the final version, 49% of people completed their enrollment and registration. Continuation rates after creating the account were also improved from the beta version (79% vs 85%). Finally, the cumulative percentage of participants who completed enrollment and registration after creating an account was 62% in the beta version compared to 91% in the final version.
Table 2.
GeneMatch Enrollment Funnel Comparison: Before and After Moving Education Module Relative to Account Creation*
| Enrollment Step | % Continued | % Drop-Off | Cumulative % Continued from “Review Eligibility Criteria” | Cumulative % Continued from after “Create Account” Completed |
|---|---|---|---|---|
| Beta Enrollment Process | ||||
| 1. GeneMatch landing page | 61 | 39 | -- | -- |
| 2. Review GeneMatch eligibility criteria | 82 | 18 | 100 | -- |
| 3. Create account | 79 | 21 | 82 | -- |
| 4. View education module and complete 5-question quiz | 75 | 25 | 65 | 100 |
| 5. Review consent | 88 | 12 | 49 | 75 |
| 6. Provide contact information | 97 | 3 | 43 | 66 |
| 7. Review information | 96 | 4 | 42 | 64 |
| 8. Registration complete | -- | -- | 40 | 62 |
| Final Enrollment Process | ||||
| 1. GeneMatch landing page | 37 | 63 | -- | -- |
| 2. View education module | 69 | 31 | 100 | -- |
| 3. Complete 5-question quiz | 90 | 10 | 69 | -- |
| 4. Create account | 85 | 15 | 63 | -- |
| 5. Review consent | 92 | 8 | 53 | 100 |
| 6. Provide contact information | 97 | 3 | 49 | 92 |
| 7. Review information | 102‡ | −2 | 48 | 89 |
| 8. Registration complete | -- | 49 | 91 | |
Step 1 unique pageviews are not comparable across before vs. after time periods due to changes in measurement. Therefore, the before versus after comparison focuses on % continuation and % drop-off.
The number exceeds 100% because individuals can complete the enrollment process over multiple visits to the GeneMatch website.
3.3. Cheek Swab Return
The majority of participants returned their completed cheek swabs within 90 days, although the return rate varied across the 3 years, with 76% of participants who joined in 2015 returning their swabs within 90 days compared with 68% of those who enrolled in 2016 and 74% in 2017 (2015 vs 2016, p<0.001; 2016 vs 2017, p<0.001; 2015 vs 2017, p = 0.12). Examining swab return by GeneMatch recruitment/enrollment source, the highest swab return percentage was among individuals enrolling through a partner healthcare site (93.6%). A high swab return was demonstrated among people registering directly through the GeneMatch website, such as via organic traffic to the website or through email outreach directing people to the website (83.1%). 78.9% of those who registered for GeneMatch by first enrolling in the APR returned their completed swab. The swab return rate for individuals directed to GeneMatch through social media advertisements was lower than other registration sources (64.0%). The partner website return rate (93.6%) was significantly higher than all other enrollment sources (p<0.001 for all between-source comparisons).
3.4. APOE Genotype Results
Participants’ APOE genotypes are presented in Table 4. Consistent with genotype prevalence reported in the literature (28), over half of participants (56%) have the APOE ɛ3/ɛ3 genotype while approximately 3% have the APOE ɛ4/ɛ4 genotype. The APOE ɛ3/ɛ4 and ɛ4/ɛ4 genotypes are more prevalent among the younger age groups (ɛ3/ɛ4 55–59 = 29.54%, ɛ3/ɛ4 70–75 = 23.64%, p<0.001; ɛ4/ɛ4 55–59 = 4.08%, ɛ4/ɛ4 70–75 = 2.20%, p<0.001), likely the result of the enrollment criteria since the ε4 allele is associated with an increased risk of dementia due to AD and younger age of onset (25;26).
Table 4.
APOE Genotype Results
| APOE Genotype | % of All GeneMatch Participants | % By Age at Enrollment | |||
|---|---|---|---|---|---|
| 55–59 Years | 60–64 Years | 65–69 Years | 70–75 Years | ||
| ɛ2/ɛ2 | 0.5 | 0.4 | 0.4 | 0.5 | 0.5 |
| ɛ2/ɛ3 | 10.2 | 9.6 | 10.1 | 10.6 | 11.2 |
| ɛ2/ɛ4 | 2.3 | 2.9 | 2.6 | 2.7 | 2.8 |
| ɛ3/ɛ3 | 56.4 | 53.4 | 55.6 | 57.7 | 59.6 |
| ɛ3/ɛ4 | 26.9 | 29.5 | 27.8 | 25.5 | 23.6 |
| ɛ4/ɛ4 | 3.3 | 4.1 | 3.5 | 2.9 | 2.2 |
3.5. Barriers to Enrollment
Anecdotal feedback submitted to the study team by email, phone, and social media from prospective participants suggest common themes of concerns for participating in GeneMatch. One frequently cited theme relates to enrollment in an internet-based program because of risks regarding loss of privacy and confidentiality, particularly in the era of data and security breaches. To help address these concerns and provide credibility to the program, the GeneMatch program received a Certificate of Confidentiality from the National Institutes of Health and updated the program website in 2018 to more prominently feature the academic, non-profit organization leading the program (Banner Alzheimer’s Institute) as well as the program funders. Another theme centered around implications of the genetic results: for instance, if the results could be used as evidence of a pre-existing condition or as a rationale for denial of health care insurance. To try to address these concerns, the GeneMatch program website was updated in 2018 to feature a “Frequently Asked Questions” (FAQ) section, including information about the Genetic Information Nondiscrimination Act (GINA). Another theme centered around participants’ requests to receive genetic test results directly from GeneMatch. To address this concern, the FAQ section was updated to include information to educate prospective participants that APOE disclosure is not a standard practice of medicine in the US, nor are the results medically actionable.
4. Discussion
Globally, several national and local AD recruitment registries exist (16–18;20;22;29;30), but, to our knowledge, GeneMatch is the first trial-independent program designed to recruit and connect community-dwelling adults to AD prevention studies based in part on APOE test results, providing a novel mechanism to accelerate prescreening and enrollment for AD prevention trials. Although still early in its development, GeneMatch has produced initial evidence that individuals are willing and able to participate in a primarily internet-based recruitment registry that requires participants to complete a cheek swab for APOE genotyping. Although participants are not told their APOE test results, this information is used in part to match them to AD prevention studies. Invitations to studies are done in a manner that does not inadvertently disclose genetic results to participants. For example, study invitations may be sent to genetically eligible individuals and a pragmatic ratio of genetically ineligible individuals. Since GeneMatch is independent from trial programs, participants are able to be re-contacted for a variety of AD prevention-related studies ranging from clinical trials to observational studies, a practice that was modeled after the API Colombia Registry (22). Moreover, GeneMatch is able to refer participants to studies enriching based on AD risk factors other than APOE4, such as elevated brain amyloid, since APOE4 is associated with a greater risk for elevated brain amyloid and younger age at onset (31).
Based on the anecdotal feedback received during the beta phase, the order of the enrollment steps was changed, moving the education module step to before account creation. This modification resulted in an improvement in the enrollment and drop-off rates. The rationale for establishing partner healthcare sites was also resulted from anecdotal feedback during the beta phase. Over time, the partner healthcare site model has evolved, e.g., modifying email communications and study invitations sent to participants who enrolled at a partner healthcare site to explicitly remind them where they joined GeneMatch. It is important to note that in both cases the anecdotal feedback was not collected systematically and as a result, reflects only a subset of attitudes towards GeneMatch. The goal of the personalized emails is to reinforce the connection and relationship established between the GeneMatch participant and the partner healthcare site.
GeneMatch has used a variety of recruitment strategies and tactics to enroll participants, such as community talks, re-contacting databases of prospective volunteers by mail or email, and social media advertisements. Social media advertisements have resulted in the greatest number of enrollees, though these individuals have a slightly lower swab return rate compared to those recruited from other sources. Future work will examine whether source of initial enrollment into GeneMatch is a factor in participants’ acceptance rates of their study invitations as well as the return on investment for the different recruitment strategies and tactics.
The overall percentages of APOE4 heterozygotes (APOE ɛ3/ɛ4, ɛ2/ɛ4) and homozygotes (APOE ɛ4/ɛ4) are consistent with previously reported prevalence estimates(28), although the percentages are higher in the younger age groups. This difference is likely the result of the GeneMatch inclusion criteria, since participants must self-report not having a diagnosis of cognitive impairment and the ɛ4 variant of APOE is a risk factor for MCI and dementia due to AD, and a younger age of onset of symptoms (25;26).
Despite using a variety of recruitment strategies and tactics, GeneMatch participants are predominantly female and self-report being of non-Hispanic white race / ethnicity, similar to reports from other internet-based recruitment registries (16). To address this gender disparity, social media advertisements targeting men were launched in October 2017 and although the percentage of men enrolled has increased, more needs to be done to better understand the barriers and facilitators to enrollment for men. Similarly, a concerted effort is needed to address the lack of racial and ethnic diversity among GeneMatch participants, including understanding why a sizeable percentage prefer not to provide their race/ethnicity during initial enrollment, perhaps adapting strategies found to be effective at a local level to online registries (32–34). However, it is important to note that, although other groups have been successful in increasing enrollment of individuals from traditionally underrepresented racial and ethnic groups into their registries, these efforts did not translate to a high rate of enrollment into an AD prevention study (30). Identification and removal of these potential barriers, as well as implementation of new recruitment solutions is critically important to meet the goal of enrolling diverse populations into AD prevention trials (35).
We acknowledge several limitations of GeneMatch. For instance, participants are not representative of the general population. All participants, including those who join at a partner healthcare site, must have an email address to enroll in GeneMatch. This requirement is a potential barrier for individuals who do not have access to or use email on a routine basis. GeneMatch participants are not representative of the general population with regard to gender, race, or socioeconomic status, although such bias is similar to what is observed in healthy controls / cohorts enrolled in AD observational studies and is reflective of reported demographic characteristics of participants enrolled in AD prevention trials. As discussed previously, it is important for GeneMatch to try to increase the enrollment of men and individuals from underrepresented racial/ethnic populations in order to help meet the goal of increasing diversity among participants enrolled in AD trials (35;36). GeneMatch does not assess participants’ cognitive functioning and, as a result, some participants with a diagnosis of cognitive impairment may have joined GeneMatch, and some participants who did not have a diagnosis at the time of enrollment may indeed be impaired when they are matched to a study. For these and other reasons, GeneMatch encourages participants to review study inclusion criteria when they have been matched to a study and emphasizes to study sites the importance of prescreening GeneMatch referrals. Regarding the GeneMatch program itself, we did not conduct focus groups during the beta version. Feedback from individuals was unsolicited and not collected in a systematic manner, nor was a qualitative assessment conducted. As a result, the barriers to enrollment described previously may only reflect a subset of attitudes towards GeneMatch. Lastly, it remains unknown whether GeneMatch accelerates enrolment into AD prevention trials and reduces the screen fail rate. Currently, two AD prevention trials and two observational studies are using GeneMatch as a recruitment tool. We will report the findings in a future publication when these studies complete their enrollment.
4.1. Future Directions
We aim to have numerous studies use GeneMatch as a recruitment tool, including studies enriching for risk factors other than (or in addition to) APOE4, such as elevated brain amyloid. The eligibility age range for GeneMatch was selected to maximize resources and ensure as many participants as possible are matched with a study opportunity. If, in the future, new AD preventions studies become available for people outside of the current age range, then the program may be adapted accordingly. Due to consent requirements and to minimize costs, GeneMatch only tests for APOE and all DNA samples are destroyed after genotyping. If, in the future, there is another genetic marker of interest that would be used for accelerating enrollment into AD prevention studies, GeneMatch may attempt to re-contact all participants, obtain consent, and collect new DNA samples for genetic testing. A concerted effort was made when designing GeneMatch to ensure that it was compliant with State law for collection of DNA and laboratory analysis. If laws change, GeneMatch may need to adapt accordingly. Similarly, GeneMatch may, in the future, need to adapt and offer disclosure of APOE results; research studies in which genetic risk disclosure protocols have been developed may provide guidance (37). Separate efforts are underway via several ancillary studies to GeneMatch and the API Generation Program to understand 1) the shorter- and longer-term psychological and emotional impact of APOE disclosure as part of screening for the API Generation Program (23), 2) whether disclosure of APOE results is associated with worsening of subjective and objective cognitive functioning, and 3) how to design efficient, scalable models for delivery of APOE results (38).
4.2. Conclusion
With the growing number of current and planned AD prevention studies, it is increasingly important to have efficient mechanisms to accelerate participant enrollment into trials and reduce the screen fail rate. Current processes are generally inefficient, contributing to the expense and duration of trials. In the US, recent reviews show that 85–90% of all studies, not just those focused on AD, have delays in recruitment and enrollment (7), with 30% under-enrolling and only 7% of sites enrolling the projected number of participants in their originally stated timelines(8). Despite its limitations, GeneMatch has demonstrated that it is feasible to enroll tens of thousands of adults across the US into a predominantly online, trial-independent genetic recruitment registry. The majority of enrollees complete their cheek swabs at home and return them to the laboratory for genetic testing. Importantly, GeneMatch does not return APOE test results to participants. Although we are optimistic that GeneMatch will be an effective resource for efficiently referring potential participants to AD prevention studies and reducing screen fail rates, we do not yet have the data necessary to confirm this. Future publications will report on the effectiveness of GeneMatch for accelerating recruitment and enrollment into AD prevention trials.
Research in Context.
Systematic review: The authors reviewed the literature by traditional sources (PubMed), meeting abstracts and presentations, and had personal communication with researchers. The relevant research on Alzheimer’s participant recruitment registries is appropriately cited.
Interpretation: GeneMatch has enrolled over 75,000 participants since its inception. Approximately 3% of enrollees are APOE4 homozygotes and the percentage of APOE4 carriers is inversely associated with age. The program has demonstrated the feasibility of an online recruitment registry incorporating APOE genotyping to accelerate prescreening and enrollment for Alzheimer’s prevention studies.
Future Directions: Continue to enroll new participants to provide an even larger pool of prospective volunteers for Alzheimer’s prevention studies, bring on new study opportunities to offer GeneMatch participants, report on the effectiveness of GeneMatch for accelerating recruitment and enrollment into Alzheimer’s prevention studies, and study the barriers to and motivators of joining a recruitment registry such as GeneMatch to help address the lack of diversity among participants.
Acknowledgements
The GeneMatch program is supported by the NIA (1UF1AG046150), the Alzheimer’s Association, Banner Alzheimer’s Foundation, the Flinn Foundation, GHR Foundation, and the state of Arizona (Arizona Alzheimer’s Consortium). The authors would like to acknowledge Drs. Mike Malek-Ahmadi, Doris Zallen, Linda Patrick-Miller, Gary Marchant; Jodie Nichols, Hayley Graf, Laura Jakimovich, John Nicholson, Mile Curcic, and the research teams at our GeneMatch partner healthcare sites for their contributions to the GeneMatch program. The authors thank Emily Kuhl, PhD for her early contributions to the manuscript and the Alzheimer’s Prevention Registry executive committee members for their early support of GeneMatch.
Jessica Langbaum: research support from National Institute on Aging (UF1 AG046150, R01AG055444), Genentech/Roche, Novartis, Amgen, Alzheimer’s Association (API-16-388841), Arizona Alzheimer’s Consortium (state of Arizona), Banner Alzheimer’s Foundation, FBRI, Flinn Foundation, GHR, Nomis Foundation; and consulting fees from Biogen and Lilly.
Jason Karlawish: research support from the National Institute on Aging (1UF1AG046150); site investigator for API Generation Program (Novartis) and A4 Trial (Lilly) and a consultant for Squintmetrics.
J. Scott Roberts: research support from the National Institute on Aging (1UF1AG046150)
Beth McCarty Wood: research support from the National Institute on Aging (1UF1AG046150)
Angela Bradbury: research support from the National Institute on Aging (1UF1AG046150)
Pierre Tariot: research support National Institute on Aging (UF1 AG046150, R01AG055444), grants from Novartis, Amgen, Banner Alzheimer’s Foundation, and Genentech/Roche; research support from Arizona Department of Health Services, Alzheimer’s Association, Banner Alzheimer’s Foundation, FBRI, GHR, Nomis Foundation, and the Flinn Foundation; consultant fees from Acadia, Abbott Laboratories, AbbVie, AC Immune, Auspex, Boehringer-Ingelheim, Brain Test Inc., California Pacific Medical Center, Chase Pharmaceuticals, CME Inc., GliaCure, Insys Therapeutics, Pfizer, and T3D; consulting fees and research support from AstraZeneca, Avanir, Lilly, Lundbeck, Merck & Co., Roche, and Takeda; research support only from Amgen, Avid, Biogen, Elan, Functional Neuromodulation [f(nm)], GE, Genentech, Novartis, and Targacept; and stock options in Adamas Pharmaceuticals.
Eric Reiman: National Institute on Aging (P30 AG19610, R01 AG055444, UF1 AG046150, R01 AG031581, NIH Office of the Director UG3OD023171, NINDS U01NS093334, grants from Novartis, Amgen, Banner Alzheimer’s Foundation, and Genentech/Roche; research support from, Alzheimer’s Association, Banner Alzheimer’s Foundation, FBRI, GHR, NOMIS Foundation, and the Flinn Foundation; Compensated consultation services to Aural Analytics, Alkahest, Alzheon, Denali, Green Valley, Roche (expenses only), and Zinfandel Pharma; research contracts from Avid, Eli Lilly, Genentech/Roche, Novartis, Amgen.
Footnotes
Conflicts of interest
Nellie High: no financial conflicts
Trisha Walsh: no financial conflicts
David Gordon: no financial conflicts
Carolyn Langlois: no financial conflicts
Raj Aggarwal: no financial conflicts
Peter Davis: no financial conflicts
Carter Stowell: no financial conflicts
Lane Trisko: no financial conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, Jonsson L, Liu Z, Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017. January;13(1):1–7. PMCID:PMC5232417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. 2018 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2018;14(3):367–429 [Google Scholar]
- 3.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am.J.Public Health 1998. September;88(9):1337–42. PMCID:PMC1509089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: An action plan for solutions. Alzheimers Dement. 2016. November;12(11):1113–5 [DOI] [PubMed] [Google Scholar]
- 5.Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N.Y.) 2018;4:195–214. PMCID:PMC6021548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alber J, Lee AKW, Menard W, Monast D, Salloway SP. Recruitment of At-Risk Participants for Clinical Trials: A Major Paradigm Shift for Alzheimer’s Disease Prevention. J Prev Alzheimers Dis 2017;4(4):213–4 [DOI] [PubMed] [Google Scholar]
- 7.Dowling NM, Olson N, Mish T, Kaprakattu P, Gleason C. A model for the design and implementation of a participant recruitment registry for clinical studies of older adults. Clin.Trials 2012. April;9(2):204–14. PMCID:PMC3325341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strasser JE, Cola PA, Rosenblum D. Evaluating various areas of process improvement in an effort to improve clinical research: discussions from the 2012 Clinical Translational Science Award (CTSA) Clinical Research Management workshop. Clin.Transl.Sci 2013. August;6(4):317–20. PMCID:PMC3740438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grill JD, Galvin JE. Facilitating Alzheimer disease research recruitment. Alzheimer Dis.Assoc.Disord 2014. January;28(1):1–8. PMCID:PMC3945167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin.Nurs 2010. January;19(1–2):227–33 [DOI] [PubMed] [Google Scholar]
- 11.Schneider LS. Recruitment methods for United States Alzheimer disease prevention trials. J Nutr.Health Aging 2012. April;16(4):331–5 [DOI] [PubMed] [Google Scholar]
- 12.Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer’s disease clinical trials. Alzheimers.Res.Ther 2010;2(6):34 PMCID:PMC3031880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vellas B, Hampel H, Rouge-Bugat ME, Grundman M, Andrieu S, Abu-Shakra S, Bateman R, Berman R, Black R, Carrillo M, et al. Alzheimer’s disease therapeutic trials: EU/US Task Force report on recruitment, retention, and methodology. J Nutr.Health Aging 2012. April;16(4):339–45 [DOI] [PubMed] [Google Scholar]
- 14.Aisen P, Touchon J, Andrieu S, Boada M, Doody RS, Nosheny RL, Langbaum JB, Schneider LS, Hendrix S, Wilcock G, et al. Registries and cohorts to accelerate early phase Alzheimer’s trials. A report from the E.U./U.S. Clinical Trials in Alzheimer’s Disease Task Force. J Prev Alz Dis 2016;3(2):68–74 [DOI] [PubMed] [Google Scholar]
- 15.Saunders KT, Langbaum JB, Holt CJ, Chen W, High N, Langlois C, Sabbagh M, Tariot PN. Arizona Alzheimer’s Registry: strategy and outcomes of a statewide research recruitment registry. J Prev Alz Dis 2014. February;1(2):74–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner MW, Nosheny R, Camacho M, Truran-Sacrey D, Mackin RS, Flenniken D, Ulbricht A, Insel P, Finley S, Fockler J, et al. The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 2018. May 8; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grill JD, Hoang D, Gillen DL, Cox CG, Gombosev A, Klein K, O’Leary S, Witbracht M, Pierce A. Constructing a Local Potential Participant Registry to Improve Alzheimer’s Disease Clinical Research Recruitment. J Alzheimers Dis. 2018;63(3):1055–63 [DOI] [PubMed] [Google Scholar]
- 18.Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, Bendlin BB, Engelman CD, Okonkwo OC, Hogan KJ, et al. The Wisconsin Registry for Alzheimer’s Prevention: A review of findings and current directions. Alzheimers Dement.(Amst.) 2018;10:130–42. PMCID:PMC5755749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings J, Aisen P, Barton R, Bork J, Doody R, Dwyer J, Egan JC, Feldman H, Lappin D, Truyen L, et al. Re-Engineering Alzheimer Clinical Trials: Global Alzheimer’s Platform Network. J Prev Alzheimers Dis. 2016. June;3(2):114–20. PMCID:PMC5408881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermunt L, Veal CD, Ter ML, Chrysostomou C, van der Flier W, Frisoni GB, Guessous I, Kivipelto M, Marizzoni M, Martinez-Lage P, et al. European Prevention of Alzheimer’s Dementia Registry: Recruitment and prescreening approach for a longitudinal cohort and prevention trials. Alzheimers Dement. 2018. June;14(6):837–42 [DOI] [PubMed] [Google Scholar]
- 21.Sperling R, Cummings J, Donohue M, Aisen P. Global Alzheimer’s Platform Trial Ready Cohorts for the Prevention of Alzheimer’s Dementia. J Prev Alzheimers Dis 2016;3(4):185–7 [DOI] [PubMed] [Google Scholar]
- 22.Rios-Romenets S, Lopez H, Lopez L, Hincapie L, Saldarriaga A, Madrigal L, Piedrahita F, Navarro A, Acosta-Uribe J, Giraldo M, et al. The Colombian Alzheimer’s Prevention Registry. Alzheimer’s & Dementia 2017. May;13(5):602–5 [Google Scholar]
- 23.Lopez LC, Caputo A, Liu F, Riviere ME, Rouzade-Dominguez ML, Thomas RG, Langbaum JB, Lenz R, Reiman EM, Graf A, et al. The Alzheimer’s Prevention Initiative Generation Program: Evaluating CNP520 Efficacy in the Prevention of Alzheimer’s Disease. J Prev Alzheimers Dis. 2017;4(4):242–6 [DOI] [PubMed] [Google Scholar]
- 24.Reiman EM, Langbaum JBS, Tariot PN. Alzheimer’s Prevention Initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomarkers in Medicine 2010. February 1;4(1):3–14. PMCID:PMC2850446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993. August;261(5123):921–3 [DOI] [PubMed] [Google Scholar]
- 26.Qian J, Wolters FJ, Beiser A, Haan M, Ikram MA, Karlawish J, Langbaum JB, Neuhaus JM, Reiman EM, Roberts JS, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts. PLoS.Med 2017. March;14(3):e1002254 PMCID:PMC5360223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman JS, Hahn SE, Catania JW, LaRusse-Eckert S, Butson MB, Rumbaugh M, Strecker MN, Roberts JS, Burke W, Mayeux R, et al. Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet.Med 2011. June;13(6):597–605. PMCID:PMC3326653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashford JW. APOE genotype effects on Alzheimer’s disease onset and epidemiology. J Mol.Neurosci 2004;23(3):157–65 [DOI] [PubMed] [Google Scholar]
- 29.Krysinska K, Sachdev PS, Breitner J, Kivipelto M, Kukull W, Brodaty H. Dementia registries around the globe and their applications: A systematic review. Alzheimers Dement. 2017. September;13(9):103147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero HR, Welsh-Bohmer KA, Gwyther LP, Edmonds HL, Plassman BL, Germain CM, McCart M, Hayden KM, Pieper C, Roses AD. Community engagement in diverse populations for Alzheimer disease prevention trials. Alzheimer Dis Assoc.Disord 2014. July;28(3):269–74. PMCID:PMC4139415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Aalten P, Aarsland D, Alcolea D, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015. May 19;313(19):1924–38. PMCID:PMC4486209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams MM, Scharff DP, Mathews KJ, Hoffsuemmer JS, Jackson P, Morris JC, Edwards DF. Barriers and facilitators of African American participation in Alzheimer disease biomarker research. Alzheimer Dis.Assoc.Disord 2010. July;24 Suppl:S24–S29. PMCID:PMC2939138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, Mungas D. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis.Assoc.Disord 2010. July;24(3):234–41. PMCID:PMC2946798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dilworth-Anderson P, Williams SW. Recruitment and retention strategies for longitudinal African American caregiving research: the Family Caregiving Project. J Aging.Health 2004. November;16(5 Suppl):137S–56S [DOI] [PubMed] [Google Scholar]
- 35.Watson JL, Ryan L, Silverberg N, Cahan V, Bernard MA. Obstacles and opportunities in Alzheimer’s clinical trial recruitment. Health Aff.(Millwood.) 2014. April;33(4):574–9. PMCID:PMC4167360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Together we make the difference: National strategy for recruitment and participation in Alzheimer’s and related dementias clinical research National Institutes of Health National Institute on Aging. 2018. Oct. https://www.nia.nih.gov/sites/default/files/2018-10/alzheimers-diseaserecruitment-strategy-final.pdf [DOI] [PMC free article] [PubMed]
- 37.Roberts JS, Christensen KD, Green RC. Using Alzheimer’s disease as a model for genetic risk disclosure: implications for personal genomics. Clin.Genet 2011. November;80(5):407–14. PMCID:PMC3191239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradbury AR, Patrick-Miller LJ, Egleston BL, Hall MJ, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, et al. Randomized Noninferiority Trial of Telephone vs In-Person Disclosure of Germline Cancer Genetic Test Results. J Natl.Cancer Inst 2018. February 27;110(9):985–93. PMCID:PMC6136932 [DOI] [PMC free article] [PubMed] [Google Scholar]