Abstract
On May 2017, the World Health Organization (WHO) recognized sepsis as a global health priority by adopting a resolution to improve the prevention, diagnosis and management of this deadly disease. While it has long been known that sepsis deeply perturbs immune homeostasis by inducing a tremendous systemic inflammatory response, pivotal observations based on clinical flow cytometry indicate that sepsis indeed initiates a more complex immune response that varies over time, with the concomitant occurrence of both pro- and anti-inflammatory mechanisms. As a resultant, some septic patients enter a stage of protracted immunosuppression. This paved the way for immunostimulation approaches in sepsis. Clinical flow cytometry permitted this evolution by drawing a new picture of pathophysiology and reshaping immune trajectories in patients. Additional information from cytometry by time of flight mass cytometry and other high-dimensional flow cytometry platform should rapidly enrich our understanding of this complex disease. This review reports on landmarks of clinical flow cytometry in sepsis and how this single-cell analysis technique permitted to breach the wall of decades of unfruitful anti-inflammatory-based clinical trials in sepsis.
Keywords: Sepsis, HLA-DR, monocyte, IL-7, PD-1, flow cytometry, Time of Flight mass spectrometry
1. Sepsis epidemiology and definition
On May 2017, the World Health Organization (WHO) recognized sepsis as a global health priority by adopting a resolution to improve the prevention, diagnosis and management of this deadly disease (1). Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (2). Septic shock is the most severe form of sepsis in which hypotension persists despite adequate volume resuscitation thus requiring the use of vasopressors. According to recent definitions, in response to an infectious trigger, the unbalanced host (immune) response is at the center of sepsis pathophysiology (2).
Sepsis represents a major healthcare problem worldwide. As an example, in the USA sepsis is more frequent than myocardial infarction, breast or colon cancers (3–6). Recent modeling of available data determined the number of cases of sepsis in the entire world to over 20–25 million corresponding to > 6 million deaths per annum (7,8). Over years, 28-day mortality has remained high, ranging from 20 % for sepsis to over 40 % for septic shock despite improvement in patients’ care (fluid resuscitation, source control, antimicrobial therapy). Whereas few therapeutic options evaluated in phase II clinical trials have demonstrated the potential efficacy of immunostimulation in sepsis, to date, no therapeutic intervention targeting host response has specifically been approved and sepsis remains the leading cause of mortality in intensive care units (ICU)(9,10). In addition, sepsis incidence has dramatically increased over the past decade and is expected to further augment. This rising incidence of documented cases of sepsis has been attributed not only to demographic changes, with a large population of patients with co-morbidities (cancer, diabetes, chronic inflammatory diseases) but also to the expansion of treatments with drugs or invasive medical devices that weaken the immune response. Improved coding of sepsis and its increased awareness has additionally been mentioned as a possible cause (4). More worrisome, the United Nations projects that the global human population over the age of 60 will increase by more than threefold in next decades and will exceed the size of the global population of young individuals by 2050. Thus, the burden of sepsis is expected to increase continuously in the forthcoming years as sepsis is a disease of the elderly (incidence increases exponentially with age). Importantly, according to the Centers for Disease Control and Prevention, over $ 22 billion is spent annually on hospitalizations for sepsis in the USA representing the most expensive hospitalization cause in this country (11). Moreover, during hospitalization, septic survivors are at high risk of developing nosocomial infections (due to induced immunosuppression – see below) that are associated not only with an increased morbidity, mortality and hospital length of stay but also with an important augmentation in patient’s hospitalization costs. Even after hospital / ICU discharge, infections remain the leading cause for patients’ readmission and death (12–14). Thus, in all respects, the cumulative burden of sepsis on public health is dramatically high and sepsis is likely to become “the quintessential medical disorder of the 21th century” (15).
2. Novel understanding of sepsis immunology
As early as 1975, MacLean, Meakins and colleagues (16–18) reported on the delayed hypersensitivity skin testing in response to recall antigens after injury. This lack of response was associated with enhanced mortality and risk of nosocomial infections which are evocative of immunosuppression. In 1991, the group of Cavaillon observed alterations in inflammatory cytokine response of septic patients’ blood upon ex vivo LPS challenge suggesting modifications of innate immunity (19). Meanwhile, the group of Richard Hotchkiss made several contributions to the field by reporting on the massive lymphocyte apoptosis in septic mice and patients supporting impairments of adaptive immunity. Simultaneously, the inability of various anti-inflammatory approaches aimed at blocking exuberant inflammation to show any clinical benefit (20) has led clinicians and researchers to reconsider their understanding of immune response in sepsis pathophysiology. From early 2000, it was progressively hypothesized that the host response to sepsis associates a pro-inflammatory phase and an anti-inflammatory compensatory response which could become immunosuppressive (21,22). The main mechanisms sustaining this process have been then progressively discovered and are still regularly reinvestigated at the light of recent technological and scientific advances (e.g., bioenergetic, epigenetic, transcriptomic). They have been extensively described in various reviews and they will not be reported here (23–25). In addition, the decades of inconclusive clinical trials with promising host targeted therapies are a reminder that septic patients comprise a heterogeneous group of individuals who are unlikely to respond uniformly to a treatment protocol (26). An important driver of this heterogeneity is patient’s individual immune status, which varies with age, sex, comorbidities (including infectious exposure through life), disease progression and underlying pathophysiology. This illustrates the importance of immune monitoring in sepsis to better describe patients’ immune status, improve the understanding of pathophysiology and identify novel therapeutic targets and associated biomarkers. In the present review, we will present seminal studies that used flow cytometry to describe sepsis induced immune alterations. We will focus on clinical studies that, by nature, take patients’ heterogeneity into account. These studies leveraged flow cytometry as a primary immuno-assay to precisely define immune trajectories in patients suffering from sepsis. Results from these flow cytometry-based clinical studies reshaped our understanding of sepsis immunology by demonstrating the occurrence of immunosuppression in patients. This is particularly important since features of immune failure in septic patients have striking similarities with those seen in cancer for which tailored targeted immunotherapies currently provide spectacular positive results (27,28). This constituted the rationale for translating immunostimulation strategies from cancer to sepsis in recent Randomized Controlled Trial (RCT) using IL-7 or anti-PD-1 antibodies (27). Other candidates that have proven to be efficient as adjunctive anti-cancer agents by acting on immunosuppressive mechanisms could thus be repurposed in sepsis.
3. Landmarks of flow cytometry in sepsis
Decreased monocyte HLA-DR (mHLA-DR) expression
Following several reports describing decreased expression of mHLA-DR after injury, the seminal work by Volk’s group in Berlin described the beneficial immunostimulant effect of IFN-γ in a small cohort of septic patients. This highlighted the major interest of this marker in identifying septic patients with altered immune response (29). Indeed, in this small clinical trial, patients’ stratification was based on low mHLA-DR expression and positive response to treatment was easily monitored by longitudinal mHLA-DR measurement. Loss of mHLA-DR clinically illustrates the phenomenon of endotoxin tolerance characterized by a reduced responsiveness to a secondary infectious challenge (i.e., refractory state to subsequent endotoxin challenge ex vivo) following a first inflammatory stress. Low mHLA-DR expression also reflects a decreased antigen presentation capacity since HLA-DR molecules belong to histocompatibility complex class II (MHC II) system (23). For now, a consensus exists for considering low monocyte HLA-DR (mHLA-DR) as a surrogate marker of sepsis-induced immunosuppression (30,31). mHLA-DR has been the most studied and validated biomarker in the field (> 200 publications). In clinics, the magnitude and the persistence overtime of mHLA-DR decrease is demonstrated to be associated with increased mortality and nosocomial infections. It is noteworthy that, after adjustment for usual clinical confounders in multivariate analyses, decreased mHLA-DR remains an independent predictor of mortality / occurrence of nosocomial infections after sepsis (32,33). Thus, immunosuppression illustrated by altered monocyte response contributes to increased risk of adverse events in sepsis and immune recovery is likely a key parameter contributing to patients’ favorable outcome. Since these initial studies, there has been a constant increase of citations matching “HLA-DR & sepsis” (figure 1) illustrating the rising interest and use of this biomarker in clinic. mHLA-DR is now used to stratify patients in RCT evaluating immunostimulation in sepsis (e.g., GRID study, NCT02361528) and to monitor drug efficacy (34,35).
Figure 1. “HLA-DR and sepsis” citations over years.
Yearly number of citations of articles matching terms HLA-DR and sepsis from 1997 to present time (publication year of landmark paper of Docke et al. is marked by a red arrow). Data from Web of Science.
It is also important to mention that, at admission, an extremely increased inaugural mHLA-DR value recently helped to unequivocally exclude a diagnosis of septic shock in a patient presenting with organ dysfunctions due to hemophagocytic lymphohistiocytosis (HLH) characterized by tremendous inflammatory responses(36). This preliminary report needs further assessment in various types of HLH. Upon confirmation, as septic shock and HLH would require opposing treatments (i.e., no steroids vs high dose of steroids), mHLA-DR may be of crucial help for clinicians regarding patients’ care and management. Thus flow cytometry may be useful clinical tool for the management of sepsis emergency medicine. In addition, it reopens the door for potential use of various immunomodulatory agents that have been initially found ineffective due to lack of stratification whereas they retrospectively demonstrated significant protective effect in the most inflammatory patients (37–39).
The more and more popular use of mHLA-DR is based on a standardized flow cytometry protocol that guaranties results reproducibility between laboratories thus permitting multicentric evaluation of this marker and comparable patients’ stratification between sites. In 2005, a group of experts (40) proposed a consensual protocol based on the use of calibrated beads (with known amounts of fluorochrome) to convert means of fluorescence intensities to numbers of antibodies bound per cell (AB/C). As this protocol requires only 2 monoclonal antibodies (anti-HLA-DR and anti-CD14 Abs), it is accessible to most inclusion centers with Flow facilities. Inter-laboratory assessment between centers equipped with cytometers from different manufacturers provided excellent results (41). However strict pre-analytical conditions are mandatory (staining within 1.5 h after blood collection or within 4 h if storage at + 4°C) and limit a wider use of mHLA-DR. To circumvent this drawback, a fully automated table top cytometer with simple operating procedures (injection of patient’s sample into a cartridge) was developed for use at the bedside or in emergency labs. Beta site evaluation for mHLA-DR evaluation showed convincing preliminary results (42).
Noteworthy, beyond sepsis, mHLA-DR is progressively becoming a popular immunomonitoring tool in other ICU contexts (traumas, burns), and other clinical conditions (gastroenterology, cancer, hematology, transplantation). Indeed, as monocytes are plastic cells fitted with capacity to both detect danger (and trigger inflammation) and resolve inflammation once danger is eliminated, the rapidly changing HLA-DR level on their surface likely reflects, at a given time point, the resultant of all immune forces applied on monocytes. Consequently, mHLA-DR regulation is not specific of any disease but rather appears as a relevant global inflammatory indicator of milieu intérieur. As such, it constitutes a useful biomarker in many clinical situations.
Immature neutrophils and myeloid-derived suppressor cells
For long, the markedly increased number of circulating immature neutrophils termed “band cells” is an established immunologic feature found in peripheral blood of septic patients. More recently, two independent groups reported on increased proportion of immature neutrophil with low CD10/CD16 expression and immunosuppressive properties in peripheral blood from septic patients. Increased percentage of these immature neutrophils was associated with increased mortality after sepsis (43,44). These recent results on immature/immunosuppressive neutrophils aggregate with the expanding description of myeloid-derived suppressor cells (MDSC) in infectious contexts (45). MDSC constitute a heterogeneous population of immature myeloid cells including progenitors / precursors of monocytes, neutrophils and dendritic cells (46,47). They are mainly characterized by their suppressive properties (both on innate and adaptive immunity) and are released upon various inflammatory / infectious signals. Recently, Uhel et al., described an association between increased MDSC number in blood and forthcoming occurrence of nosocomial infections after sepsis (48). Unfortunately, to date, Human MDSC definition lacks consensual phenotypic characterization (usable in whole blood for routine basis) and published results were obtained according to various phenotypes, either in whole blood or in Ficoll enriched fraction (49). Thus, further clinical investigations will require better standardization. Upon development of appropriate staining protocol, it is likely that analysis of MDSC populations will provide crucial information in clinical studies.
Lymphocyte alterations
In contrast to the initial innate inflammatory phase, until recently the adaptive immune response has received less attention in the description of sepsis-induced immune alterations. This is striking considering that more than 40 years ago, Meakins et al. described in septic patients the altered delayed–type hypersensitivity (16) which is mostly a reflection of lymphocyte dysfunction. Characterization of lymphocyte response in sepsis has nevertheless improved over the last 10 years.
Absolute lymphocyte count
By using hematological analyzers or flow cytometry, studies have described the major fall in absolute lymphocyte count (ALC) in septic patients. Seminal studies showed that apoptosis was playing a central role in that process and identified an association between the persistence of lymphopenia and increased mortality (50,51). It has been shown that sepsis represents the first cause of lymphopenia among patients in university hospital (52) and that severe lymphopenia is already present on patients’ admission and affects every lymphocyte subpopulations (53). However, the extend of this lymphopenia is variable depending on cell subpopulations, with some subpopulations such as regulatory T cells (Tregs) being less affected (see below). Because ALC is part of the complete blood cell count on hematology analyzers and is thus available 24/7 in every hospital, the association of this cellular alteration and poor outcomes in septic patients is now easily evaluated in large prospective clinical studies and can be retrospectively assessed in various sepsis cohorts (54–57). Overall, results homogenously show that decreased ALC is associated with initial severity upon ICU admission while persistent lymphopenia is associated with increased mortality and occurrence of nosocomial infections after sepsis. Consequently, lymphopenia depth has recently been selected as a stratification marker in the first clinical trial evaluating IL-7 in septic shock patients. Only patients with ALC < 900 cell/μl were included in the study (58).
Increased regulatory lymphocytes
While the presence of a subpopulation of CD4+ T cells with regulatory properties had been known for long in mice, the exact phenotype usable to identify these cells by flow cytometry in human was only described in 2001 (59,60). Based on this phenotype (i.e., CD4+CD25high) and the subsequent identification of Foxp3 as a specific intracellular marker for these cells (61,62), it was repeatedly observed that the percentage of Tregs is increased in septic shock patients with a maximum at day 3 after shock (63). It was proposed that this relative increase in percentage was due to Treg relative resistance to apoptotic mechanisms induced after sepsis (64). Later on, the role of these cells in sepsis-induced innate and adaptive alterations was showed (65,66). Thanks to improvement in flow cytometry phenotyping such as the addition of CD127 staining and standardization of intracellular Foxp3 staining for its use in clinic (67), large prospective multicenter clinical studies have evaluated this parameter in ICU patients (68). It was showed that increased percentage of Treg in critically ill patients is associated with increased risk of subsequent nosocomial infections and that this parameter could be part of an immunopanel to stratify patients suitable for immuno-intervention in a precision medicine approach (68).
Few data are available regarding B cell response in septic patients. The decrease in circulating absolute B cell number has been shown (53) while their relative percentage among total lymphocytes was increased in patients as observed for Tregs (69). Interestingly, remaining circulating B cells present with an altered phenotype and altered functions (69). While the presence of B cells with regulatory functions has been shown in mice models of infections and in some rare clinical contexts, preliminary data suggest that these cells might be induced after septic shock (70). This deserves to be further explored in sepsis.
Co-inhibitory molecule expressions
Increased co-inhibitory molecule expressions and role of co-inhibitory pathways in T cells exhaustion have been evaluated multiple times in cancer and chronic viral infections (71). In sepsis, a seminal work reported on the increased expressions of PD1 and PDL1 on circulating immune cells on a limited number of septic shock patients and on the role of these molecules in sepsis pathophysiology in mice model of sepsis (72). The associations between increased PD1 and PDL1 expressions and decreased T cell proliferation, increased risk of death and nosocomial infections were subsequently described in a larger cohort of septic shock and trauma patients (73). This observation was confirmed by other groups and was completed by the description of the increased expressions of other co-inhibitory molecules such as BTLA, Tim3, LAG3 (25). Based on these observations and on ex vivo studies showing the efficacy of anti-PD1 and anti-PD-L1 blocking antibodies in improving sepsis-induced T cell alterations (74–76), the first clinical trials evaluating immune checkpoint inhibitors that are currently revolutionizing cancer treatment have been completed in septic patients (NCT02960854, NCT02576457). Results from these clinical trials are strongly awaited.
Functional testing
Importantly, altered immune cell phenotypes are associated with functional alterations, such as altered cytokine production (23,30). Nevertheless, the potential added value of functional testing in clinical monitoring remains to be demonstrated. This is in particular because, as opposed to immunophenotyping, those tests lack of automatization and standardization: e.g., long incubation time, lengthy cell purification procedures, cell permeabilization, and complex protocols with numerous staining / wash cycles (77,78).
One specific aspect in which functional tests may appear informative is the control of treatments’ efficacy (see below). Indeed, functional testing may appear as a gold standard in this context because it directly measures ex vivo the capacity of a cell population to respond to an immune challenge. Such test may indicate whether a given therapy was truly effective in restoring immune functions in patients. Among immune functional assays that have been evaluated in clinic, measurement of intracellular cytokine content by flow cytometry appears as one of the most promising technique. Indeed, novel whole blood flow cytometry protocols provided significant information in septic patients regarding TNF-α production in monocytes (79) or TNF-α, IL-2, IFN-γ production by CD4+ and CD8+ lymphocyte (80). However, technical optimization remains to be done to specifically fit with sepsis monitoring (e.g., nature and concentration of stimulants, incubation time).
Is this immunosuppression?
Immunosuppression lacks specific clinical manifestation. As such, the definition of immunosuppression is based on the combination of immunological alteration (i.e., the quantitative or functional alteration of a given immune cell population) and the documented increased occurrence of infections. This definition describes all patients after sepsis (24,25). Indeed, on the one hand, we described above important immune alterations in patients. On the other hand, increasing numbers of clinical evidences highlight septic patients’ diminished capacity to fight secondary infections by weakly virulent germs (including fungi) or reactivation of dormant viruses (TTV, CMV, HSV). In this context, altered immune functions are reported as independent predictors of forthcoming infections in multivariate analyses including classic risk confounders (i.e., prior exposition to antibiotics, exposure to invasive devices such as central venous lines or intubation, comorbidities). In addition, the leading cause of readmission and long-term mortality within first years after surviving sepsis is infection (12–14). In line, an increased risk for cancer has also been recently reported after septic shock (81) that may constitute another clinical event related to chronic immunosuppression.
Taken together, according to definition, these data indicate that septic patients present with immunosuppression that is more or less pronounced and durable depending on individual. Some patients may retain persistent immune defects on a long term basis which may participate in their increased susceptibility to subsequent infections and aggravate occurrence of deleterious outcomes.
Thus, immunostimulation has appeared as a sound therapeutic option (24,25,82). However, appropriate therapeutic strategy relies on our capacity to identify, in a complex situation mixing both pro- and anti-inflammatory responses rapidly varying over time, the most immunosuppressed patients (figure 2). Moreover, it has been shown that variations in the human immune response are largely driven by non-heritable influences (83). Among various components, co-morbidities (including infectious exposure through life) and age are of major importance. As septic patients are older (median around 65 years) and present with various comorbidities, this reinforce the idea that an individual monitoring of immune parameters is of crucial importance as, depending on their own personal history, no patient will immunologically respond like another. International experts agree that, among other reasons, the heterogeneity of septic patients may have participated in the previous failure of clinical trials evaluating host targeted therapies in sepsis (84,85). The use of biomarker for patients’ stratification appears thus as a mandatory step in the design of next clinical trials evaluating immunoadjuvant treatments in sepsis (24,30,31). So far, as previously seen in this review, absolute lymphocyte count (including CD4+ and regulatory lymphocytes) and decreased expression of mHLA-DR appear as the most robust markers usable in multicenter studies (figure 3). Both measurements are standardized and their variations are associated with altered functions and deleterious outcomes (either nosocomial infection occurrence or mortality). In addition, monitoring of MDSC and / or immature neutrophils (as markers of bone marrow activity and of chronic low grade inflammation), lymphocyte PD-1 and monocyte PD-L1 expressions (as potential stratification markers for anti-PD-1 / anti-PD-L1 treatments – see below) are good candidates upon further validation in larger cohorts of patients.
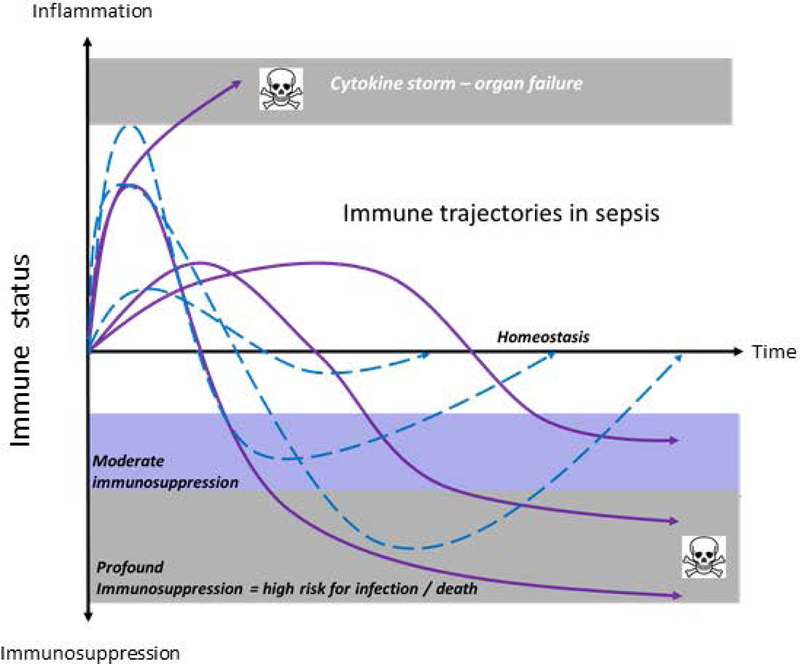
Figure 2. Immune trajectories in sepsis.
Theoretical evolutions over time of immune status of different septic patients are presented. Net inflammatory/suppressive immune responses are very heterogeneous in kinetics, duration and depth. Patients with dashed line return spontaneously to immune homeostasis overtime. Patients with continuous lines present with dysregulated immune responses. These patients should be identified to benefit from host directed immunointerventions.
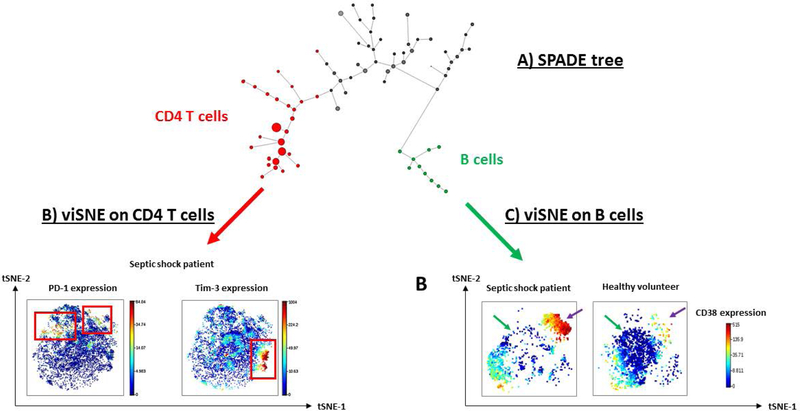
Figure 3. Illustrative example of unsupervised analysis of CyTOF results in healthy volunteers and septic patients.
These results have been obtained from reference (70) (A) SPADE clustering analysis was performed in order to group peripheral blood mononuclear cell sub-populations into “nodes” according to phenotype similarities. These nodes and their relationships are represented in a minimum spanning tree format (i.e. SPADE tree). Size of the nodes illustrates the number of cells within the clusters. In this analysis, CD4 expressing cells are highlighted as red nodes while CD19 expressing cells are represented as green nodes. (B) viSNE visualization tool was then performed on selected CD4+ cells from SPADE nodes. Based on the t-Distributed Stochastic Neighbor Embedding (t-SNE) algorithm, viSNE determines the two dimensional representation of single-cell data sets that best matches their respective local and global geometry. Cells get unique x- and y-coordinates (tSNE1 and tSNE2) according to their expression of parameters from CyTOF panel. Their relative positions on the two-dimensional plot indicate similarities in terms of expression pattern. In the current analysis, we used color as a third dimension to visualize specific marker expression levels (PD-1 and Tim-3) in one representative example of a septic patient. Red and blue color intensities respectively represent high and low expression levels of the indicated marker. In this illustrative septic shock patient, PD-1 and Tim-3 are clearly not co-expressed by same CD4 T cell subpopulations (red squares). (C) Alternatively, viSNE algorithm was performed on selected CD19+ B cells to compare sepsis vs healthy volunteers. Expression level of CD38, a marker of B cell maturation was visualized on the viSNE plots in one representative septic shock patient and one healthy donor. A depletion of cells from the center of the map (green arrows), but an enrichment within the upper right cell cluster (purple arrows) are noticed in the patient. This latter cluster was constituted of CD38hi cells, suggesting a shift towards differentiated B cells in septic shock patients.
4. Current multicenter studies and clinical trials
Early Flow on patients’ admission
Most flow cytometric studies of sepsis have focused on delayed immune consequences (i.e., after the first 48 hours). However recent studies have examined the role of flow cytometry in the early management of septic patients. Regarding sepsis diagnosis, increased neutrophil CD64 expression has been shown to be a highly sensitive (>95%) and specific marker for systemic infection and sepsis in adults, neonates and children (86). Low HLA-DR expression associated with PCT determination (i.e., usual biochemical marker of sepsis) tended to reinforce sepsis diagnosis (87) as well as increased CD8/CD19 ratio in another study (88). In contrast, very high values of mHLA-DR may be indicative of HLH (see above and (36)). These studies pave the way for the use of flow cytometry in the diagnosis of sepsis. However, flow cytometry approaches will need to be assessed in comparison with reference markers (i.e., PCT and CRP) since the measurements of these proteins are inexpensive, fully automated (24/7 available) and readily available.
Multicenter studies and improved standardization
Identifying the most severe patients is another opportunity for the clinical application of Flow in sepsis. In a pilot study, percentage of immature granulocytes (CD10low/CD16low) was demonstrated to predict early clinical deterioration at the acute phase of sepsis (44). Those preliminary results were recently confirmed in a multicentric multicolor flow cytometric study (SEPTIFLUX-2) including 781 patients in 11 centers (56). An increased circulating immature neutrophil percentage at the acute phase of sepsis was linked to clinical worsening, especially when combined with T-cell lymphopenia. The authors concluded that early flow cytometry could help clinicians to target patients at high risk of clinical deterioration. Beyond these interesting results, this study was the first to highlight the feasibility of performing large multicentric multicolor flow cytometry studies in ICU patients. Both Beckman Coulter and BD Biosciences flow cytometers were harmonized as previously established for lymphoma classification (89). In line, the ExPRES-Sepsis study recently reinforced this report. In this study evaluating standardized multi-site flow cytometry in about 400 acutely ill patients with suspected infections from 4 different centers, 47 leukocyte biomarkers were simultaneously assessed (90). Markers of immune suppression (increased neutrophil CD24 and PD-1 and decreased mHLA-DR expressions) showed the strongest associations with clinical outcomes. Moreover, a multicenter, prospective observational cohort study of critically ill patients in four UK ICUs has been published (INFECT study)(68). Three cell surface markers associated with immune cell dysfunctions (CD88 expression on neutrophil, mHLA-DR, percentage of regulatory T cells) were assayed. This study reported that these markers could predict subsequent risk of secondary infections. This study (90) along with SEPTIFLUX-2 (56) and ExPRES-Sepsis (68), all published in 2018, demonstrated that harmonizing Flow protocols is now an achievable objective. Before starting studies, ensuring intercenter reproducibility in setting up thresholds and gates should be performed (e.g., listmodes blindly analyzed). Then, standardization would imply the use of stabilized blood samples as controls (when possible), common standard operating procedures based on antibodies from same batch from a single manufacturer, commercially available standards for results comparison across labs (calibrated beads, e.g., setup and tacking beads) as well as internal (provided by manufacturers) and external controls (e.g., UK NEQAS). In addition, the development of lyophilized pre-formulated antibody panels also represents a major improvement for FCM standardization in RCTs. As an example, FOXP3-lyophilized tubes are currently used for regulatory T cells determination (67) in an RCT evaluating low-dose IL-2 treatment for type 1 diabetes (DIABIL-2 study, NCT02411253). Furthermore, new technical developments (low cost compact portable flow cytometer, bedside flow cytometry or chip-based flow cytometry) are now being proposed that will facilitate the use of Flow at the bedside in ICU (see above in mHLA-DR paragraph (42)). For now, such techniques remain devoted to very simple applications but we may expect major developments in the forthcoming years, including multicolor flow cytometry.
As septic patients form a heterogeneous population, biomarkers characterizing patients’ immune trajectories are useful to identify subgroups of patients that may benefit the most from an immune modulatory intervention. These biomarkers should be viewed as markers for patients’ enrichment more than tailored stratification stricto sensu. So far, patients’ stratification was mostly based on Flow which remains the most appropriate tool to individualize immunotherapy (Table 1). Recent phase II trials in the field used either mHLA-DR or lymphocyte count to stratify patients (34,91). In addition, one may expect further developments in PD-1 stratification as anti-PD-1 and anti-PD-L1 are believed to be good candidates in sepsis (92).
Table 1.
Biomarker-guided clinical cases and trials with immunostimulatory drugs in sepsis.
| Interventions | Targeted cells (for stratification) | Biomarkers (at inclusion) | Number of patients | Randomization | Bibliographic Reference or NCT |
|---|---|---|---|---|---|
| IFN-γ | Monocytes | HLA-DR | 35 | No | (29) |
| IFN-γ | Monocytes | HLA-DR | 9 | No | (123) |
| IFN-γ | Monocytes | HLA-DR* | 21 | yes | (124) |
| IFN-γ | Monocytes | HLA-DR | 1 | No | (125) |
| IFN-γ | Monocytes | HLA-DR | 2 | No | (126) |
| IFN-γ | Monocytes | HLA-DR | 1 | No | (127) |
| IFN-γ | Monocytes | HLA-DR | Not published yet | yes | NCT03332225 |
| GM-CSF | Monocytes | HLA-DR | 38 | Yes | (34) |
| GM-CSF | Monocytes | HLA-DR | Not published yet | Yes | NCT02361528 |
| GM-CSF | Monocytes | HLA-DR | 1 | No | (128) |
| GM-CSF | Monocytes | HLA-DR | 1 | No | (129) |
| GM-CSF | Monocytes | Whole blood TNF release | 14 | Yes | (130) |
| GM-CSF | Monocytes | Whole blood TNF release | Not published yet | Yes | NCT03769844** |
| GM-CSF | Neutrophils | Phagocytic capacity | 38 | Yes | (35) |
| Anti-PD-1 | Lymphocytes | PD-1 expression | 1 | No | (125) |
| IL-7 | Lymphocytes | Absolute count | 27 | Yes | (58) |
: mHLA-DR was assessed in broncho-alveolar lavages,
: inclusion criteria is organ failure (including sepsis).
Another important application of Flow-based biomarkers is to monitor drug efficacy. The restoration of mHLA-DR expression has been successfully used to control IFN-γ or GM-CSF treatment effects (29,34). Recently, in critically ill patients, a sharp peak of mHLA-DR expression was observed in patients receiving GM-CSF (35). In HLH patient, mHLA-DR was successfully used to increase dosage of corticosteroids to control inflammation process (36). In the recent IRIS study, efficacy of IL-7 was measured by decreased expression of CD127 (i.e., IL-7 receptor) and by a peak of increased Ki67 as surrogate marker of lymphocyte proliferation (58). Of note, in controlling drug efficacy, functional testing might be an appropriate tool as it really reflects cell restoration in response to an immune challenge (i.e, more appropriate than cell counting or phenotyping).
5. The promise of Cytometry by Time of Flight mass spectrometry
The advent of high content immune profiling technologies provides new opportunities for the in-depth characterization of septic patients’ immune states. Cytometry by Time of Flight mass spectrometry (CyTOF), or mass cytometry, is a particularly promising single-cell technology, as it allows for the comprehensive monitoring of the distribution and activities of all immune cell subsets present in a blood sample (93,94). Rather than fluorescent reporters used in traditional flow cytometry, mass cytometry utilizes antibodies conjugated to stable isotopes of rare earth metals. By exploiting the resolution, dynamic range and near absence of background noise offered by mass spectrometry, mass cytometry allows for the simultaneous detection of over 50 parameters on a cell-by-cell basis (95,96). The high parametrization afforded by mass cytometry allows for the precise phenotyping and distribution of virtually every immune cell subset, as well as a number of functional attributes which are assessed simultaneously at the single-cell level. Functional attributes routinely measured with a mass cytometry assay include cell proliferation, apoptosis, intracellular signaling responses, and intracellular cytokine production (97). In addition, recent developments in mass cytometry reagents allow the single-cell assessment of epigenetic modifications (98) and mRNA expression (99,100).
The general concept of utilizing mass cytometry to characterize immune states associated with disease progression or response to therapy has been demonstrated in multiple clinical contexts (101), including malignancies (102–104), rheumatological diseases (105), aging (106), traumatic injury (107–109), and pregnancy (110,111). While the use of mass cytometry in sepsis is still in its infancy (112), observational studies in the context of traumatic injury provide the groundwork for the deep immune profiling of complex inflammatory states such as sepsis. Not unlike sepsis, traumatic injury produces a conserved inflammatory response that engages both the innate and adaptive branches of the immune system (113). However, recent mass cytometry analyses of patients undergoing major surgery showed that underlying the canonical immune response to traumatic injury were distinct immune phenotypes associated with surgical recovery outcomes (108,109). In sepsis, the use of mass cytometry in a preliminary study led to the description of novel immune alterations (70). For instance, it was observed that increased checkpoints inhibitor expressions were not uniformly co-expressed on T lymphocyte population (e.g, TIM-3 and PD-1, Figure 3) but that each immune checkpoint was overexpressed on different T lymphocyte subsets. Putative consequences of such disparate expression remain to be investigated. Another novel result was the identification in septic patients of a very mature subset of B lymphocytes (Figure 3) absent from controls. These cells might be circulating regulatory B cells never reported before in septic patients (70). Thus, the field of sepsis research is primed for similar translational studies that bring mass cytometry “to the bedside”. Exciting applications of this technology will include creating a high-resolution cellular atlas of the human immune response to sepsis, identifying immune signatures predictive of clinical outcomes, and tailoring promising immune therapies such as anti-PD-L1 and IL-7 to individualized immune phenotypes.
The mass cytometry analysis of clinical samples has certain limitations. The technology requires complete ionization of immune cells to their elemental composition, which precludes further cell-based assay once the sample is analyzed. Although the number of antibodies measured per single cell is unparalleled, developing a new mass cytometry assay requires a priori selection of markers of interest. Combining mass cytometry with traditional fluorescence-based cell sorting (114) and untargeted RNA sequencing will become increasingly useful for informing the design of future mass cytometry antibody panels. Most importantly, the increasing dimensionality of mass cytometry datasets – often comprised of thousands of immune features – comes at a price, referred to as the “curse of dimensionality” (115). Extraction of features using traditional analysis by human experts is time consuming a potential source of error (116,117) but a broad range of algorithms have been developed for objective extraction of immunophenotypes from mass cytometry data (118,119). The structure of mass cytometry data is also highly intercorrelated, reflecting the interconnected nature of the human immune system. Novel analytical methods – such as the recently developed cell-signaling Elastic Net algorithm (110) – that account for the dimensionality and correlated nature of large mass cytometry datasets are needed to ensure the statistical validity and generalizability of identified immune predictors of clinical outcomes.
These high dimensional analysis methods will allow not only the integration of multiple classes of single-cell mass cytometry measurements but also the integration of mass cytometry data with other high-throughput biological assays (such as metabolomics, transcriptomics, an proteomics assays) (120). Such multi-omic modeling of inflammation in humans may reveal novel biological connections between functional readouts from discrete cell types and circulating molecular factors, which hold promise for the development of integrative bioassays with high predictive performance (121,122).
Taken together, mass cytometry has now reached the level of technological and computational maturity needed for deep clinical profiling of sepsis to lay the foundation for the design of precision-interventions based on the status of a patient’s immune system.
6. Conclusion
As described in this review, sepsis is entering the era of precision medicine. Clinical Flow has played a critical role in this evolution by drawing a new picture of pathophysiology and by cracking the wall of decades of unfruitful anti-inflammatory-based clinical trials. Considering the current revolution in cancer treatment and the parallels that can be drawn between the host immune responses to cancer and sepsis, immunotherapeutic interventions hold great promise for the targeted treatment of septic patients. That said, sepsis being a complex syndrome with deleterious consequences on all organs, it is plainly obvious that immunostimulation would not save all septic patients. It will preferentially work in the most immunosuppressed patients and this reasoning is all the more applicable since we can use flow cytometry to stage each patient’s immune status and depict immune trajectories. Hopefully, we may soon obtain complementary information from mass cytometry and other high-dimensional flow cytometry platform. So far, mHLA-DR, immature neutrophils and/or MDSC count, lymphocyte count along with PD-1 molecules expression – all measured by flow cytometry - offer relevant information for identifying individual trajectories. Recent IL-7 RCT results and recent observational multicenter studies indicate the feasibility of the approach of flow-based RCT. Taken together, Flow cytometry reshaped sepsis immunology and paved a new way of success for tackling this hitherto deadly disease.
Funding
GM, MG and FV are supported by Hospices Civils de Lyon and Univeristy Lyon-1. BG is funded by National Institute of Health K23GM111657.
Literature cited
- 1.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med 2017;377:414–417. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016;193:259–72. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann C, Thomas-Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S, Dennler U, Reinhart K. Hospital Incidence and Mortality Rates of Sepsis. Dtsch Arztebl Int 2016;113:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754–61. [DOI] [PubMed] [Google Scholar]
- 6.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014;5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840–51. [DOI] [PubMed] [Google Scholar]
- 8.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010;376:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers 2016;2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 2017;17:407–420. [DOI] [PubMed] [Google Scholar]
- 11.Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. 2006. [PubMed]
- 12.Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest 2016;126:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar-Hari M, Rubenfeld GD. Understanding Long-Term Outcomes Following Sepsis: Implications and Challenges. Curr Infect Dis Rep 2016;18:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennelly PJ, Martin-Loeches I. Long term mortality following sepsis. Ann Transl Med 2016;4:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 2014;40:463–75. [DOI] [PubMed] [Google Scholar]
- 16.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg 1977;186:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLean LD, Meakins JL, Taguchi K, Duignan JP, Dhillon KS, Gordon J. Host resistance in sepsis and trauma. Ann Surg 1975;182:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christou NV, Meakins JL, Gordon J, Yee J, Hassan-Zahraee M, Nohr CW, Shizgal HM, MacLean LD. The delayed hypersensitivity response and host resistance in surgical patients. 20 years later. Ann Surg 1995;222:534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest 1991;88:1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 1997;25:1095–100. [DOI] [PubMed] [Google Scholar]
- 21.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med 2001;163:316–21. [DOI] [PubMed] [Google Scholar]
- 22.Pugin J Sepsis and the immune response. Intensive Care Med 1999;25:1027–8. [DOI] [PubMed] [Google Scholar]
- 23.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med 2008;14:64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 2013;13:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013;13:862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med 2014;20:195–203. [DOI] [PubMed] [Google Scholar]
- 27.Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol 2018;14:121–137. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med 2014;371:380–3. [DOI] [PubMed] [Google Scholar]
- 29.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 1997;3:678–81. [DOI] [PubMed] [Google Scholar]
- 30.Venet F, Lukaszewicz AC, Payen D, Hotchkiss R, Monneret G. Monitoring the immune response in sepsis: a rational approach to administration of immunoadjuvant therapies. Curr Opin Immunol 2013;25:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monneret G, Venet F. Sepsis-induced immune alterations monitoring by flow cytometry as a promising tool for individualized therapy. Cytometry B Clin Cytom 2015;90:376–86. [DOI] [PubMed] [Google Scholar]
- 32.Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 2006;32:1175–83. [DOI] [PubMed] [Google Scholar]
- 33.Landelle C, Lepape A, Voirin N, Tognet E, Venet F, Bohe J, Vanhems P, Monneret G. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med 2010;36:1859–66. [DOI] [PubMed] [Google Scholar]
- 34.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 2009;180:640–8. [DOI] [PubMed] [Google Scholar]
- 35.Pinder EM, Rostron AJ, Hellyer TP, Ruchaud-Sparagano MH, Scott J, Macfarlane JG, Wiscombe S, Widdrington JD, Roy AI, Linnett VC, Baudouin SV, Wright SE, Chadwick T, Fouweather T, Juss JK, Chilvers ER, Bowett SA, Parker J, McAuley DF, Conway Morris A, Simpson AJ. Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis. Thorax 2018;73:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remy S, Gossez M, Belot A, Hayman J, Portefaix A, Venet F, Javouhey E, Monneret G. Massive increase in monocyte HLA-DR expression can be used to discriminate between septic shock and hemophagocytic lymphohistiocytosis-induced shock. Crit Care 2018;22:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welte T, Dellinger RP, Ebelt H, Ferrer M, Opal SM, Singer M, Vincent JL, Werdan K, Martin-Loeches I, Almirall J, Artigas A, Ignacio Ayestaran J, Nuding S, Ferrer R, Sirgo Rodriguez G, Shankar-Hari M, Alvarez-Lerma F, Riessen R, Sirvent JM, Kluge S, Zacharowski K, Bonastre Mora J, Lapp H, Wobker G, Achtzehn U, Brealey D, Kempa A, Sanchez Garcia M, Brederlau J, Kochanek M, Reschreiter HP, Wise MP, Belohradsky BH, Bobenhausen I, Dalken B, Dubovy P, Langohr P, Mayer M, Schuttrumpf J, Wartenberg-Demand A, Wippermann U, Wolf D, Torres A. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensive Care Med 2018;44:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berlot G, Vassallo CM, Busetto N, Nieto Yabar M, Istrati T, Baronio S, Quarantotto G, Bixio M, Barbati G, Dattola R, Longo I, Chillemi A, Scamperle A, Iscra F, Tomasini A. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann Intensive Care 2018;8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med 2016;44:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Docke WD, Hoflich C, Davis KA, Rottgers K, Meisel C, Kiefer P, Weber SU, Hedwig-Geissing M, Kreuzfelder E, Tschentscher P, Nebe T, Engel A, Monneret G, Spittler A, Schmolke K, Reinke P, Volk HD, Kunz D. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem 2005;51:2341–7. [DOI] [PubMed] [Google Scholar]
- 41.Demaret J, Walencik A, Jacob MC, Timsit JF, Venet F, Lepape A, Monneret G. Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytometry B Clin Cytom 2013;84:59–62. [DOI] [PubMed] [Google Scholar]
- 42.Zouiouich M, Gossez M, Venet F, Rimmele T, Monneret G. Automated bedside flow cytometer for mHLA-DR expression measurement: a comparison study with reference protocol. Intensive Care Med Exp 2017;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demaret J, Venet F, Friggeri A, Cazalis MA, Plassais J, Jallades L, Malcus C, Poitevin-Later F, Textoris J, Lepape A, Monneret G. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J Leukoc Biol 2015;98:1081–90. [DOI] [PubMed] [Google Scholar]
- 44.Guerin E, Orabona M, Raquil MA, Giraudeau B, Bellier R, Gibot S, Bene MC, Lacombe F, Droin N, Solary E, Vignon P, Feuillard J, Francois B. Circulating immature granulocytes with T-cell killing functions predict sepsis deterioration*. Crit Care Med 2014;42:2007–18. [DOI] [PubMed] [Google Scholar]
- 45.Ost M, Singh A, Peschel A, Mehling R, Rieber N, Hartl D. Myeloid-Derived Suppressor Cells in Bacterial Infections. Front Cell Infect Microbiol 2016;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res 2017;5:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol 2018;19:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhel F, Azzaoui I, Gregoire M, Pangault C, Dulong J, Tadie JM, Gacouin A, Camus C, Cynober L, Fest T, Le Tulzo Y, Roussel M, Tarte K. Early Expansion of Circulating Granulocytic Myeloid-derived Suppressor Cells Predicts Development of Nosocomial Infections in Patients with Sepsis. Am J Respir Crit Care Med 2017;196:315–327. [DOI] [PubMed] [Google Scholar]
- 49.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 1999;27:1230–51. [DOI] [PubMed] [Google Scholar]
- 51.Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drenou B. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 2002;18:487–94. [DOI] [PubMed] [Google Scholar]
- 52.Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population--what does it signify? Aust N Z J Med 1997;27:170–4. [DOI] [PubMed] [Google Scholar]
- 53.Venet F, Davin F, Guignant C, Larue A, Cazalis MA, Darbon R, Allombert C, Mougin B, Malcus C, Poitevin-Later F, Lepape A, Monneret G. Early Assessment of Leukocyte Alterations at Diagnosis of Septic Shock. Shock 2010;34:358–363. [DOI] [PubMed] [Google Scholar]
- 54.Adrie C, Lugosi M, Sonneville R, Souweine B, Ruckly S, Cartier JC, Garrouste-Orgeas M, Schwebel C, Timsit JF, group Os. Persistent lymphopenia is a risk factor for ICU-acquired infections and for death in ICU patients with sustained hypotension at admission. Ann Intensive Care 2017;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014;42:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daix T, Guerin E, Tavernier E, Mercier E, Gissot V, Herault O, Mira JP, Dumas F, Chapuis N, Guitton C, Bene MC, Quenot JP, Tissier C, Guy J, Piton G, Roggy A, Muller G, Legac E, de Prost N, Khellaf M, Wagner-Ballon O, Coudroy R, Dindinaud E, Uhel F, Roussel M, Lafon T, Jeannet R, Vargas F, Fleureau C, Roux M, Allou K, Vignon P, Feuillard J, Francois B. Multicentric Standardized Flow Cytometry Routine Assessment of Patients With Sepsis to Predict Clinical Worsening. Chest 2018;154:617–627. [DOI] [PubMed] [Google Scholar]
- 57.Girardot T, Rimmele T, Venet F, Monneret G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis 2016;22:295–305. [DOI] [PubMed] [Google Scholar]
- 58.Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmele T, Blood T, Morre M, Gregoire A, Mayo GA, Blood J, Durum SK, Sherwood ER, Hotchkiss RS. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 2018;3:e98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 2001;193:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med 2001;193:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–61. [DOI] [PubMed] [Google Scholar]
- 62.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–6. [DOI] [PubMed] [Google Scholar]
- 63.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med 2003;31:2068–71. [DOI] [PubMed] [Google Scholar]
- 64.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Monneret G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25- lymphocytes. Crit Care Med 2004;32:2329–31. [DOI] [PubMed] [Google Scholar]
- 65.Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, Ayala A. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol 2008;83:523–35. [DOI] [PubMed] [Google Scholar]
- 66.Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F, Bohe J, Lepape A, Ayala A, Monneret G. Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (−)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med 2009;35:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitoiset F, Barbie M, Monneret G, Braudeau C, Pochard P, Pellegrin I, Trauet J, Labalette M, Klatzmann D, Rosenzwajg M. A standardized flow cytometry procedure for the monitoring of regulatory T cells in clinical trials. Cytometry B Clin Cytom 2018;94:621–626. [DOI] [PubMed] [Google Scholar]
- 68.Conway Morris A, Datta D, Shankar-Hari M, Stephen J, Weir CJ, Rennie J, Antonelli J, Bateman A, Warner N, Judge K, Keenan J, Wang A, Burpee T, Brown KA, Lewis SM, Mare T, Roy AI, Hulme G, Dimmick I, Rossi AG, Simpson AJ, Walsh TS. Cell-surface signatures of immune dysfunction risk-stratify critically ill patients: INFECT study. Intensive Care Med 2018;44:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gustave CA, Gossez M, Demaret J, Rimmele T, Lepape A, Malcus C, Poitevin-Later F, Jallades L, Textoris J, Monneret G, Venet F. Septic Shock Shapes B Cell Response toward an Exhausted-like/Immunoregulatory Profile in Patients. J Immunol 2018;200:2418–2425. [DOI] [PubMed] [Google Scholar]
- 70.Gossez M, Rimmele T, Andrieu T, Debord S, Bayle F, Malcus C, Poitevin-Later F, Monneret G, Venet F. Proof of concept study of mass cytometry in septic shock patients reveals novel immune alterations. Sci Rep 2018;8:17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol 2017;47:765–779. [DOI] [PubMed] [Google Scholar]
- 72.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 2009;106:6303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Cheron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, Venet F. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 2011;15:R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang K, Svabek C, Vazquez-Guillamet C, Sato B, Rasche D, Wilson S, Robbins P, Ulbrandt N, Suzich J, Green J, Patera AC, Blair W, Krishnan S, Hotchkiss R. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care 2014;18:R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol 2016;100:1239–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patil NK, Guo Y, Luan L, Sherwood ER. Targeting Immune Cell Checkpoints during Sepsis. Int J Mol Sci 2017;18:2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Segre E, Fullerton JN. Stimulated Whole Blood Cytokine Release as a Biomarker of Immunosuppression in the Critically Ill: The Need for a Standardized Methodology. Shock 2016;45:490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Vught LA, Wiewel MA, Hoogendijk AJ, Scicluna BP, Belkasim-Bohoudi H, Horn J, Schultz MJ, van der Poll T. Reduced Responsiveness of Blood Leukocytes to Lipopolysaccharide Does not Predict Nosocomial Infections in Critically Ill Patients. Shock 2015;44:110–4. [DOI] [PubMed] [Google Scholar]
- 79.Monneret G, Demaret J, Gossez M, Reverdiau E, Malergue F, Rimmele T, Venet F. Novel Approach in Monocyte Intracellular TNF Measurement: Application to Sepsis-Induced Immune Alterations. Shock 2016;47:318–322. [DOI] [PubMed] [Google Scholar]
- 80.Letessier W, Demaret J, Gossez M, Allam C, Venet F, Rimmele T, Monneret G. Decreased intra-lymphocyte cytokines measurement in septic shock patients: A proof of concept study in whole blood. Cytokine 2018;104:78–84. [DOI] [PubMed] [Google Scholar]
- 81.Hu WS, Lin CL. Acute critical illness and cancer risk: Implications from a nationwide population based study in Asia. Int J Cardiol 2018;270:319–323. [DOI] [PubMed] [Google Scholar]
- 82.Leentjens J, Kox M, van der Hoeven JG, Netea MG, Pickkers P. Immunotherapy for the adjunctive treatment of sepsis: from immunosuppression to immunostimulation. Time for a paradigm change? Am J Respir Crit Care Med 2013;187:1287–93. [DOI] [PubMed] [Google Scholar]
- 83.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, Maecker HT, Davis MM. Variation in the human immune system is largely driven by non-heritable influences. Cell 2015;160:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, Tracey K, van der Poll T, Pelfrene E. Sepsis: a roadmap for future research. Lancet Infect Dis 2015;15:581–614. [DOI] [PubMed] [Google Scholar]
- 85.Perner A, Gordon AC, Angus DC, Lamontagne F, Machado F, Russell JA, Timsit JF, Marshall JC, Myburgh J, Shankar-Hari M, Singer M. The intensive care medicine research agenda on septic shock. Intensive Care Med 2017;43:1294–1305. [DOI] [PubMed] [Google Scholar]
- 86.Hoffmann JJ. Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin Chem Lab Med 2009;47:903–16. [DOI] [PubMed] [Google Scholar]
- 87.Almansa R, Martin S, Martin-Fernandez M, Heredia-Rodriguez M, Gomez-Sanchez E, Aragon M, Andres C, Calvo D, Rico-Feijoo J, Esteban-Velasco MC, Vaquero-Roncero LM, Ortega A, Gomez-Pesquera E, Lorenzo-Lopez M, de Cenarruzabeitia IL, Benavides D, Lopez-Sanchez J, Doncel C, Gonzalez-Sanchez C, Zarca E, Rios-Llorente A, Diaz A, Sanchez-Barrado E, de Heredia JB, Calvo-Vecino JM, Munoz-Bellvis L, Gomez-Herreras JI, Aldecoa C, Tamayo E, Bermejo-Martin JF. Combined quantification of procalcitonin and HLA-DR improves sepsis detection in surgical patients. Sci Rep 2018;8:11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frattari A, Polilli E, Primiterra V, Savini V, Ursini T, Di Iorio G, Parruti G. Analysis of peripheral blood lymphocyte subsets in critical patients at ICU admission: A preliminary investigation of their role in the prediction of sepsis during ICU stay. Int J Immunopathol Pharmacol 2018;32:2058738418792310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solly F, Rigollet L, Baseggio L, Guy J, Borgeot J, Guerin E, Debliquis A, Drenou B, Campos L, Lacombe F, Bene MC. Comparable flow cytometry data can be obtained with two types of instruments, Canto II, and Navios. A GEIL study. Cytometry A 2013;83:1066–72. [DOI] [PubMed] [Google Scholar]
- 90.Shankar-Hari M, Datta D, Wilson J, Assi V, Stephen J, Weir CJ, Rennie J, Antonelli J, Bateman A, Felton JM, Warner N, Judge K, Keenan J, Wang A, Burpee T, Brown AK, Lewis SM, Mare T, Roy AI, Wright J, Hulme G, Dimmick I, Gray A, Rossi AG, Simpson AJ, Conway Morris A, Walsh TS. Early PREdiction of sepsis using leukocyte surface biomarkers: the ExPRES-sepsis cohort study. Intensive Care Med 2018;44:1836–1848. [DOI] [PubMed] [Google Scholar]
- 91.Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmele T, Blood T, Morre M, Gregoire A, Mayo GA, Blood J, Durum SK, Sherwood ER, Hotchkiss RS. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moldawer LL, Hotchkiss R. Immunotherapy: It is not just for cancer anymore. J Leukoc Biol 2018;103:9–11. [DOI] [PubMed] [Google Scholar]
- 93.Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe’er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011;332:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bjornson ZB, Nolan GP, Fantl WJ. Single-cell mass cytometry for analysis of immune system functional states. Curr Opin Immunol 2013;25:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ornatsky O, Bandura D, Baranov V, Nitz M, Winnik MA, Tanner S. Highly multiparametric analysis by mass cytometry. J Immunol Methods 2010;361:1–20. [DOI] [PubMed] [Google Scholar]
- 96.Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, Pavlov S, Vorobiev S, Dick JE, Tanner SD. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal Chem 2009;81:6813–22. [DOI] [PubMed] [Google Scholar]
- 97.Spitzer MH, Nolan GP. Mass Cytometry: Single Cells, Many Features. Cell 2016;165:780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheung P, Vallania F, Dvorak M, Chang SE, Schaffert S, Donato M, Rao AM, Mao R, Utz PJ, Khatri P, Kuo AJ. Single-cell epigenetics - Chromatin modification atlas unveiled by mass cytometry. Clin Immunol 2018;196:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frei AP, Bava FA, Zunder ER, Hsieh EW, Chen SY, Nolan GP, Gherardini PF. Highly multiplexed simultaneous detection of RNAs and proteins in single cells. Nat Methods 2016;13:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schulz D, Zanotelli VRT, Fischer JR, Schapiro D, Engler S, Lun XK, Jackson HW, Bodenmiller B. Simultaneous Multiplexed Imaging of mRNA and Proteins with Subcellular Resolution in Breast Cancer Tissue Samples by Mass Cytometry. Cell Syst 2018;6:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baca Q, Cosma A, Nolan G, Gaudilliere B. The road ahead: Implementing mass cytometry in clinical studies, one cell at a time. Cytometry B Clin Cytom 2017;92:10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Good Z, Sarno J, Jager A, Samusik N, Aghaeepour N, Simonds EF, White L, Lacayo NJ, Fantl WJ, Fazio G, Gaipa G, Biondi A, Tibshirani R, Bendall SC, Nolan GP, Davis KL. Single-cell developmental classification of B cell precursor acute lymphoblastic leukemia at diagnosis reveals predictors of relapse. Nat Med 2018;24:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, Fong L, Nolan GP, Engleman EG. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 2017;168:487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D’Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nair N, Mei HE, Chen SY, Hale M, Nolan GP, Maecker HT, Genovese M, Fathman CG, Whiting CC. Mass cytometry as a platform for the discovery of cellular biomarkers to guide effective rheumatic disease therapy. Arthritis Res Ther 2015;17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, Ganio EA, Fragiadakis GK, Spitzer MH, Douchet I, Daburon S, Moreau JF, Nolan GP, Blanco P, Dechanet-Merville J, Dekker CL, Jojic V, Kuo CJ, Davis MM, Faustin B. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 2017;23:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seshadri A, Brat GA, Yorkgitis BK, Keegan J, Dolan J, Salim A, Askari R, Lederer JA. Phenotyping the Immune Response to Trauma: A Multiparametric Systems Immunology Approach. Crit Care Med 2017;45:1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fragiadakis GK, Gaudilliere B, Ganio EA, Aghaeepour N, Tingle M, Nolan GP, Angst MS. Patient-specific Immune States before Surgery Are Strong Correlates of Surgical Recovery. Anesthesiology 2015;123:1241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, Huddleston JI, Goodman SB, Davis MM, Bendall SC, Fantl WJ, Angst MS, Nolan GP. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med 2014;6:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aghaeepour N, Ganio EA, McIlwain D, Tsai AS, Tingle M, Van Gassen S, Gaudilliere DK, Baca Q, McNeil L, Okada R, Ghaemi MS, Furman D, Wong RJ, Winn VD, Druzin ML, El-Sayed YY, Quaintance C, Gibbs R, Darmstadt GL, Shaw GM, Stevenson DK, Tibshirani R, Nolan GP, Lewis DB, Angst MS, Gaudilliere B. An immune clock of human pregnancy. Sci Immunol 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fragiadakis GK, Baca QJ, Gherardini PF, Ganio EA, Gaudilliere DK, Tingle M, Lancero HL, McNeil LS, Spitzer MH, Wong RJ, Shaw GM, Darmstadt GL, Sylvester KG, Winn VD, Carvalho B, Lewis DB, Stevenson DK, Nolan GP, Aghaeepour N, Angst MS, Gaudilliere BL. Mapping the Fetomaternal Peripheral Immune System at Term Pregnancy. J Immunol 2016;197:4482–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gaudilliere B, Angst MS, Hotchkiss RS. Deep Immune Profiling in Trauma and Sepsis: Flow Is the Way to Go! Crit Care Med 2017;45:1577–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med 2011;208:2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aghaeepour N, Simonds EF, Knapp D, Bruggner RV, Sachs K, Culos A, Gherardini PF, Samusik N, Fragiadakis GK, Bendall SC, Gaudilliere B, Angst MS, Eaves CJ, Weiss WA, Fantl WJ, Nolan GP. GateFinder: projection-based gating strategy optimization for flow and mass cytometry. Bioinformatics 2018;34:4131–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Newell EW, Cheng Y. Mass cytometry: blessed with the curse of dimensionality. Nat Immunol 2016;17:890–5. [DOI] [PubMed] [Google Scholar]
- 116.Finak G, Langweiler M, Jaimes M, Malek M, Taghiyar J, Korin Y, Raddassi K, Devine L, Obermoser G, Pekalski ML, Pontikos N, Diaz A, Heck S, Villanova F, Terrazzini N, Kern F, Qian Y, Stanton R, Wang K, Brandes A, Ramey J, Aghaeepour N, Mosmann T, Scheuermann RH, Reed E, Palucka K, Pascual V, Blomberg BB, Nestle F, Nussenblatt RB, Brinkman RR, Gottardo R, Maecker H, McCoy JP. Standardizing Flow Cytometry Immunophenotyping Analysis from the Human ImmunoPhenotyping Consortium. Sci Rep 2016;6:20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol 2012;12:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aghaeepour N, Chattopadhyay P, Chikina M, Dhaene T, Van Gassen S, Kursa M, Lambrecht BN, Malek M, McLachlan GJ, Qian Y, Qiu P, Saeys Y, Stanton R, Tong D, Vens C, Walkowiak S, Wang K, Finak G, Gottardo R, Mosmann T, Nolan GP, Scheuermann RH, Brinkman RR. A benchmark for evaluation of algorithms for identification of cellular correlates of clinical outcomes. Cytometry A 2015;89:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weber LM, Robinson MD. Comparison of clustering methods for high-dimensional single-cell flow and mass cytometry data. Cytometry A 2016;89:1084–1096. [DOI] [PubMed] [Google Scholar]
- 120.Ghaemi MS, DiGiulio DB, Contrepois K, Callahan B, Ngo TTM, Lee-McMullen B, Lehallier B, Robaczewska A, McIlwain D, Rosenberg-Hasson Y, Wong RJ, Quaintance C, Culos A, Stanley N, Tanada A, Tsai A, Gaudilliere D, Ganio E, Han X, Ando K, McNeil L, Tingle M, Wise P, Maric I, Sirota M, Wyss-Coray T, Winn VD, Druzin ML, Gibbs R, Darmstadt GL, Lewis DB, Partovi Nia V, Agard B, Tibshirani R, Nolan G, Snyder MP, Relman DA, Quake SR, Shaw GM, Stevenson DK, Angst MS, Gaudilliere B, Aghaeepour N. Multiomics modeling of the immunome, transcriptome, microbiome, proteome and metabolome adaptations during human pregnancy. Bioinformatics 2019;35:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ghaemi MS, DiGiulio DB, Contrepois K. Multiomics modeling of the immunome, transcriptome, microbiome, proteome and metabolome adaptations during human pregnancy. Bioinformatics 2018:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, Gustafsson A, Bernhardsson AK, Zhang C, Bohlin K, Brodin P. Stereotypic Immune System Development in Newborn Children. Cell 2018;174:1277–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nierhaus A, Montag B, Timmler N, Frings DP, Gutensohn K, Jung R, Schneider CG, Pothmann W, Brassel AK, Schulte Am Esch J. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med 2003;29:646–51. [DOI] [PubMed] [Google Scholar]
- 124.Nakos G, Malamou-Mitsi VD, Lachana A, Karassavoglou A, Kitsiouli E, Agnandi N, Lekka ME. Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med 2002;30:1488–94. [DOI] [PubMed] [Google Scholar]
- 125.Grimaldi D, Pradier O, Hotchkiss RS, Vincent JL. Nivolumab plus interferon-gamma in the treatment of intractable mucormycosis. Lancet Infect Dis 2017;17:18. [DOI] [PubMed] [Google Scholar]
- 126.Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, Faivre V, Boval B, Payen D. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med 2009;37:2746–52. [DOI] [PubMed] [Google Scholar]
- 127.Nalos M, Santner-Nanan B, Parnell G, Tang B, McLean AS, Nanan R. Immune effects of interferon gamma in persistent staphylococcal sepsis. Am J Respir Crit Care Med 2012;185:110–2. [DOI] [PubMed] [Google Scholar]
- 128.Protti A, L’Acqua C, Spinelli E, Lissoni A, Porretti L, Frugoni C, Maraschini A, Gattinoni L, Bonara P. Granulocyte-macrophage colony stimulating factor for non-resolving legionellosis. Anaesth Intensive Care 2014;42:804–6. [PubMed] [Google Scholar]
- 129.Skirecki T, Kawiak J, Hoser G, Tarnowski W, Zlotorowicz M, Zielinska-Borkowska U. Effect of granulocyte colony-stimulating factor on circulating immune and stem cells in septic shock: revisit the basics and consider giving it another chance. Am J Respir Crit Care Med 2013;187:217–9. [DOI] [PubMed] [Google Scholar]
- 130.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, Carcillo JA. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med 2011;37:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]