Abstract
Image cytometry enables quantitative cell characterization with preserved tissue architecture, thus it has been highlighted in the advancement of multiplex immunohistochemistry (IHC) and digital image analysis in the context of immune-based biomarker monitoring associated with cancer immunotherapy. However, one of the challenges in the current image cytometry methodology is a technical limitation in segmentation of nuclei and cellular components particularly in heterogeneously stained cancer tissue images. To improve detection and specificity of single cell segmentation in hematoxylin-stained images (which can be utilized for recently reported 12-biomarker chromogenic sequential multiplex IHC), we adapted a segmentation algorithm previously developed for hematoxlin and eosin-stained images, where morphological features are extracted based on Gabor-filtering, followed by stacking of image pixels into n-dimensional feature space and unsupervised clustering of individual pixels. Our proposed method showed improved sensitivity and specificity in comparison with standard segmentation methods. Replacing previously proposed methods with our method in multiplex IHC/image cytometry analysis, we observed higher detection of cell lineages including relatively rare TH17 cells, further enabling sub-population analysis into TH1-like and TH2-like phenotypes based on T-bet and GATA3 expression. Interestingly, predominance of TH2-like TH17 cells was associated with human papilloma virus (HPV)-negative status of oropharyngeal squamous cell carcinoma of head and neck, known as a poor-prognostic subtype in comparison with HPV-positive status. Furthermore, TH2-like TH17 cells in HPV-negative head and neck cancer tissues were spatiotemporally correlated with CD66b+ granulocytes, presumably associated with an immunosuppressive microenvironment. Our cell segmentation method for multiplex IHC/image cytometry potentially contributes to in-depth immune profiling and spatial association, leading to further tissue-based biomarker exploration.
Keywords: Cell segmentation, TH17 cell phenotypes, tumor immune microenvironment
INTRODUCTION
As emergence of immunotherapy has been revolutionizing cancer therapeutic strategies, the demands on biomarkers optimizing various treatment options have been constantly increasing (1–4). Profiling cellular complexities of tumor tissue provides a powerful platform to understand immune characteristics associated with clinical response to immunotherapy, thus in situ tumor profiling and imaging have been increasingly important toward tissue-based biomarker development.
Single cell segmentation is a key technology in digital image analysis, as it enables quantitative assessment of cell frequency, localization, and phenotypes (5–7). Previously, based on single cell segmentation, we built an image quantitation platform with multiplex immunohistochemistry (IHC) and image cytometry platform to evaluate in situ immune characteristics where 12 immune-related markers were quantitatively assessed by image cytometry (8). Image cytometry enables evaluation of cell morphology and signal intensity, analogous to flow cytometry, but where tumor architecture is maintained. However, a potential limitation of current image cytometry analysis is a technical challenge for single cell segmentation in hematoxylin images, where CellProfiler (CP) (9) has been adopted as a standard. Although CP performs well using image specific proper parameters, tweaking parameters is difficult, especially when dealing with clinical samples that show heterogeneity in shape, size and intensity. Thus, a fully automated and robust single cell segmentation method is required for not only providing a high-throughput cell segmentation but also enhancing image cytometry for multiplex IHC images.
In this study, we adapted our nuclei segmentation method (10) for hematoxylin and eosin (H&E) staining to provide stable cell segmentation and minimize the cost of frequent parameters tuning. In order to segment nuclei in hematoxylin-stained images, we extracted useful morphological features from the image using a set of Gabor filters (11). We mapped each image pixel to a point in an n-dimensional feature space and then clustered individual pixels with similar features in an unsupervised setting. By doing this, nuclei segmentation in hematoxylin-stained images can be effectively performed by partitioned groups. We validated our cell segmentation algorithm under the multiplex IHC setting and confirmed that the proposed method was able to detect higher cell numbers, although relative cellular ratios were maintained between CP-based segmentation in the previous study (8) and the proposed method. In addition, our method enabled detection of relatively rare cell populations, like TH17 cells, a subset of T lymphocytes associated with autoimmune diseases, but typically represent less than 1% of total immune cells (12). Use of this new segmentation method allowed for analysis of these cells. Analysis revealed that rare TH17 cell populations can be further divided into TH1-like and TH2-like subpopulations, which correlated with differential immune characteristics of tissue. Development of an image analytic platform based on robust and effective single cell segmentation can contribute to further tissue-based biomarker development.
BACKGROUND
In situ profiling cellular complexities of tissues and tumors provides a unique opportunity to quantitatively evaluate characteristic features of disease pathogenesis. With regards to inflammation associated with cancer development and the advent of therapies targeting immune-modulatory programs in tumors, evaluating the complexity and functional status of immune cells infiltrating tumor tissue provides information not only for patient stratification but also for monitoring metrics to discern response and/or resistance to anti-cancer therapy. As such, multiplex IHC platforms have emerged enabling robust detection of immune cell lineage and phenotype on single tissue sections. Rate limiting for these platforms is capabilities for automated high-throughput and quantitative segmentation methods. Thus to improve detection and specificity of single cell segmentation in hematoxylin-stained images and to provide capabilities for automated high-throughput and quantitative segmentation method, we developed a simple and effective methodology for a fully automated and robust single cell segmentation approach that reduces the cost of parameter tuning.
Single cell segmentation is vital for various fields of digital pathological evaluation despite technical obstacles such as morphological variation of tumor and stroma cells, and high cell density leading to back-to-back formations. As a consequence, many automatic segmentation, analysis and computer aided diagnosis methods have been proposed to alleviate pathologist’s burden (16–20). Various approaches have been proposed, ranging from relatively simple thresholding methods (Otsu or adaptive) to more sophisticated methods including active contour, level set, watershedding with multi-scaling seeds, unsupervised Bayesian, fuzzy c-means classification, supervised methods using machine/deep learning, etc. For nuclear segmentation purpose, these methods performance range from 75% to 96% accuracy (18), and are sensitive to the image quality and tissue type. Moreover, as the most recent literature (28) still shows new developments of a robust, practically usable segmentation algorithm, this indicates that developing novel segmentation methods is still an ongoing task.
In the H&E staining method, cell nuclei is stained blue by hematoxylin, followed by counter-staining with eosin, which colors other structures in various shades of red and pink (21). Although cell segmentation in H&E images is a highly challenging task, it is more difficult for multiplex IHC, because multiplex IHC requires hematoxylin-only images without eosin, which is resistant to signal stripping protocol. This results in a dramatic loss of contrast. In this study, we proposed an unsupervised method for cell segmentation in order to reduce an effort for parameter tuning for multiple experimental samples, enabling exploration of phenotypes and spatial distribution of relatively rare TH17 sub-populations. To do this, we utilize morphological features which are particularly appropriate for texture representation and discrimination,
MATERIALS AND METHODS
1. Nuclei and Cell Segmentation for Hematoxylin-only Image
Each pixel has intensity and represents a part of morphological features. In order to provide robust nuclei and cell segmentation and minimize the cost of frequent parameter tuning, we simply cluster individual pixels based on the similarity of their features by extracting useful morphological features from the image. To do so, Gabor filtering was adopted with different frequencies and orientations, which are particularly appropriate for texture representation and discrimination, i.e., edge detection in image processing (7). Also, various features such as intensities and Gabor filters’ impulse responses were stacked, where these features can be chosen by users. To avoid over-segmented noisy pixels, 2D Gaussian filtered images were added to the feature vector. Following mapping of each image pixel to a point in an n-dimensional feature space, each pixel is enhanced by chosen features. Then, k-means clustering was performed to differentiate foreground from background in different tissues, cells, or nuclei. Finally, after clustering and group selection, aggregated nuclei are discerned using mathematical morphology operations: first, an alternate sequential filter (consecutive openings and closings with structuring elements of increasing radii) reduced the noise and flattened the image by erasing small local gray level variation within the nuclei. Then, the resulting local minima (nuclei) were used into a seeded watershed on the gradient image.
2. Other Segmentation Methods for Comparison
2.1. Segmentation Using CellProfiler with Parameter Tuning
With manual parameters tuning procedure, segmentation was performed in CellProfiler version 2.2.0 using the “Identify Primary Objects” module with the “Threshold strategy” parameter set to “manual.” The primary parameters like the cell diameter (“Typical diameter of objects, in pixel units [Min, Max]”) and the object intensity minimum threshold (“Manual threshold”) were tuned for each image. After manual tuning of parameters, the pipeline was executed, and the results were inspected visually (using the “Convert Objects To Image” module). Parameters were re-tuned accordingly based on visual inspection of segmentation results. This process was repeated with as much different iteration of parameters necessary until convergence to the best possible segmentation (as determined by visual inspection) was achieved. Additional parameters were used to achieve satisfactory segmentation for certain images, including suppression of local maxima within a minimum allowed distance (using the “Automatically calculate minimum allowed distance between local maxima” parameter in the “Identify Primary Objects” module) and objects filtering based on maximum allowed eccentricity (using the “Filter Objects” module).
2.2. ImageJ/FIJI-based Segmentation
Segmentation in ImageJ/FIJI (16) was performed with a custom macro created to produce a binary nuclei mask from a hematoxylin-stained image. This macro takes the hematoxylin-stained image and performs color deconvolution to separate the hematoxylin stain from the background. Next, several preprocessing steps are performed to enhance the nuclei stain including Gaussian blur, median filter, contrast enhancement by 0.25% saturation, and rolling ball background subtraction (16). An automatic Otsu threshold is applied on the resulting image to select foreground (17). The “Find Maxima” function is run with the “Segmented Particles” option and noise tolerance as an argument provided from the user to create the binary mask. The noise parameter was visually examined at multiple values to determine the best noise value for each image. The output assumes each maximum belongs to a particle and segments the foreground area by watershed (18), producing a binary image of particles identifying cells by each maximum point and surrounding area under foreground selection. The binarized image is post processed to remove remaining background noise with erosion, removing outliers, watershed, median filter, “Fill holes,” dilation and watershed again to produce the final nuclei separation and mask. All functions can be found with full descriptions athttps://imagej.nih.gov/ij/docs/guide/https://imagej.nih.gov/ij/docs/guide/.
3. Multiplex IHC and Image Acquisition
Chromogenic sequential multiplex IHC and digital image acquisition were conducted as described before (8). Briefly, 5 μm of formalin fixed paraffin-embedded tissue sections were stained by hematoxylin, followed by whole-tissue scanning using Aperio ImageScope AT (Leica Biosystems). Following endogenous peroxidase blocking and heat-mediated antigen retrieval, sequential IHC consisting of iterative cycles of staining, scanning, and antibody and chromogen stripping was performed. Acquired digital images were co-registered so that cell features overlap down to a single-pixel level, using CellProfiler pipeline (8, 19). Then, pixel intensity and shape-size measurements were saved to a file format compatible with image cytometry data analysis software, FCS Express 5 Image Cytometry v.5.01.0029 (De Novo Software) (8).
4. Spatial Pattern Analysis
4.1. Extraction of Spatial Proximity and Distance Measurements
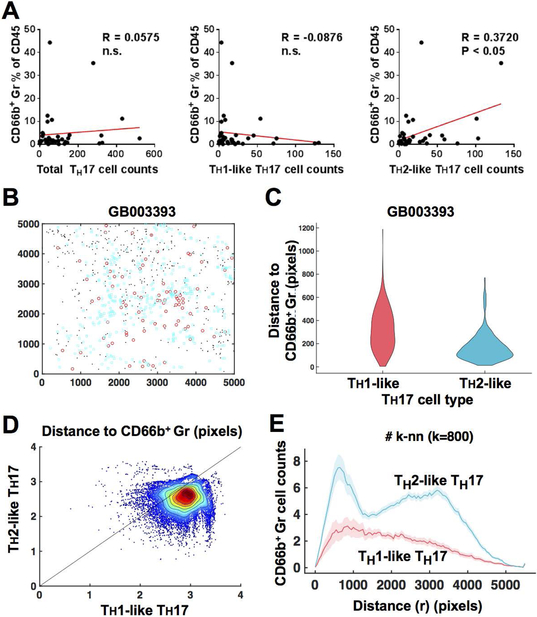
Positional data extracted from multiplex IHC images was used to measure distance from each individual neutrophil to the TH1-like TH17 cells and TH2-like TH17 cells. Using the Quickhull Algorithm (20), “dsearchn” function in MATLAB, the shortest distance between CD66b+ granulocyte centroids and the nearest TH17 cells was measured to determine spatial proximity between CD66b+ granulocyte distance to TH1-like TH17 cells and TH2-like TH17 cells, respectively. Scatter plot in Figure 4D represents the shortest distance from all neutrophil to the nearest TH1-like TH17 cells versus TH2-like TH17 cells.
Figure 4. Image cytometry with robust cell segmentation reveals TH17-associated tissue immunological characteristics.
(A) Spearman correlations were analyzed between TH17 cell density and cell percentages of CD66b+ granulocytes. (B) A representative case depicts close proximity between CD66b+ granulocytes (black dots) and TH2-like (cyan circles), but not TH1-like (red circles) TH17 cells. (C) Cell to cell distances from CD66b+ granulocyte to nearest TH1-like TH17 versus TH2-like TH17 cells were compared. Statistical significance was determined via Wilcoxon signed rank test. (D) A density plot presents overall distance distribution comparing distance from CD66b+ granulocytes to nearest TH1-like TH17 (x axis) and TH2-like TH17 (y axis) across 9 cases. (E) Microregional cell densities of TH1-like TH17 and TH2-like TH17 cells were shown depending on distance from nearest CD66b+ granulocytes (x axis).
4.2. Local Density Measurement by Adopting Radial Distribution Function
Since cell density can affect spatial proximity of cell-cell distances, the radial distance function was adopted as previously reported (21). Instead of using CD66b+ granulocytes as a reference point, each TH1-like TH17 or TH2-like TH17 cell was considered as a reference, enabling the calculation of the average density of CD66b+ granulocytes at a distance r. To do this, first, k-nearest neighbor CD66b+ granulocytes from individual TH1-like TH17 cells and TH2-like TH17 cells were counted across distance r. Then, only the k-nearest neighbor CD66b+ granulocyte was counted, instead of density to handle boundary conditions. Second, an ensemble distribution of CD66b+ granulocytes from TH1-like TH17 cells and TH2-like TH17 cells was individually assessed in differential k values as shown in Figure 4E and Supplementary Figure 5C.
5. Manual Cell Annotation
Hematoxylin images digitized at 20x magnification were utilized to manually count cell segments. By using the cell counter plugin in ImageJ, centroids of cell nucleus were manually marked by three physicians and recorded.
6. Patient Samples, IRB Description
All digital images from human cancer tissue were de-identically obtained under approval by institutional review board (IRB) (protocol 809, 3609 and 5886), and written informed consent was obtained.
7. Statistics
Mann-Whitney U and Wilcoxon signed rank tests were used to determine statistically significant differences in unpaired and paired data. Spearman rank correlation coefficient was used to assess correlations of cell percentages and densities among cell lineages. Mann-Whitney U and Wilcoxon signed rank tests were used for spatial relationship analysis, and a p-value of less than 0.05 was considered as statistically significant. All statistical calculations were performed by GraphPad Prism version 7.03. P < 0.05 was considered statistically significant.
RESULTS
We proposed a simple but effective methodology for fully automated and robust single cell segmentation with reduced cost of parameter tuning. To do this, we extracted useful morphological features from the image and group individual pixels using unsupervised clustering based on similar features to segment nuclei. Notably, our segmentation has been optimized with images stained only by hematoxylin, which lack the cytoplasmic staining usually provided by eosin staining (Figures 1A-C). This provides robust segmentation results across different cancer histological types with the same parameter setting. However, a limitation could be the dependence on image quality of hematoxylin staining, where low quality images lead to failure in cell detection or over/under-segmentation even with our segmentation approach. Our data indicates the proposed segmentation shows better detection of cells with higher sensitivity and specificity in three different cancer histological types. While we observed preserved cell ratio and composition (Supplementary Figure 4), our robust and effective cell detection enabled better detection of relatively rare TH17 cells (Figures 3A and 3B). Improved cell detection was particularly significant for sub-population analysis, which depends on a sufficiently large cell population.
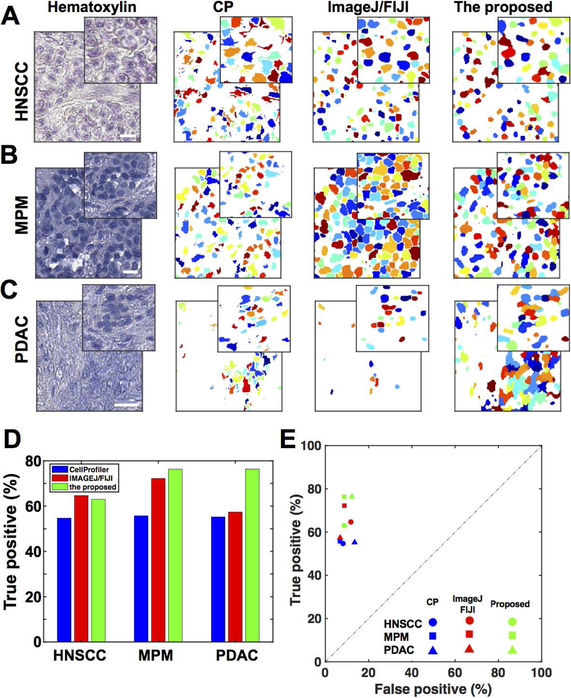
Figure 1. Improved cell segmentation for hematoxylin images.
(A-C) Cell segmentation by CellProfiler (CP), ImageJ/FIJI and our proposed method were compared in three different histological cancer types of head and neck squamous cell carcinoma (HNSCC) (A), malignant pleural mesothelioma (MPM) (B), and pancreatic ductal adenocarnoma (PDAC) (C). Hematoxylin image and nuclei segmentation label masks based on the three methods are shown. Scale bars = 100 μm. (D-E) Segmentation methods were compared in terms of true positive ratio and specificity/sensitivity in representative cancer histological types of HNSCC, MPM, and PDAC. For a better visual identification, the selected magnified images are shown on the top right corner for each image where low magnified images are shown in Figure S1.
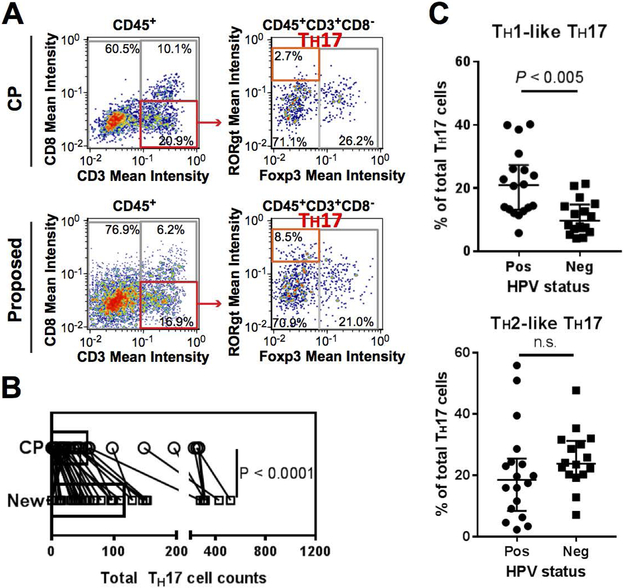
Figure 3. Robust cell segmentation in image cytometry enables subpopulation analysis for TH17 cells.
(A) Image cytometry plots are shown to compare frequency of TH17 cells detected by CellProfiler (top) and the proposed method (New, bottom). (B) Total cell counts of TH17 cells were compared between the two methods. Statistical significance was determined by a Wilcoxon signed rank test. (C) Percentages of TH1-like and TH2-like subpopulations in total TH17 cells were compared in HPV-positive (n = 21) and HPV-negative (n = 17) tumors of head and neck squamous cell carcinoma. Statistical significance was determined via Mann-Whitney U test.
1. A Cell Segmentation Method Based on Gabor Filtering and Pixel-Based Clustering Outperforms Standard Cell Segmentation Methods
To improve the detection and specificity of single cell segmentation, we adopted our previous method to provide better nuclei segmentation for multiplex IHC images [see Method section]. Next, we compared the new segmentation results with our previous CP-based method, which is composed of 4 steps: seeding, calculation of intensity gradient, watershed segmentation, and merging objects with weak borders. To perform a fair comparison, an expert user determined the optimal parameter by visual examination in order to achieve the best cell segmentation in CP. Our method effectively detected nuclei objects with fine edge identification, whereas the CP-based method occasionally failed to detect objects and showed over-segmentation (Figures 1A-C and Supplementary Figure 1).
To statistically verify those observations, we utilized nuclei objects manually identified by three independent physicians as a comparative baseline using moderate agreement between three examiners’ annotations, so called ground truth (Supplementary Figure 2 and Table 1). Three examiners marked the centroids of all visually recognizable cells in assigned images derived from different histological cancer types of head and neck squamous cell carcinoma, malignant mesothelioma and pancreatic ductal adenocarcinoma, where malignant mesothelioma had relatively homogenous cell size in contrast to moderate and high heterogeneity in head and neck cancer and pancreatic cancer, respectively (Figures 1A-C). Based on dilatation of annotated marks with various radii between 1 and 6 pixels, we evaluated overlap of dilated annotations to see matching and discrepancy of cell identification across three examiners. The manual cell annotations by three observers were moderately consistent, where matched annotations (overlapping or no further than 3 pixels) by all three observers ranged between 70% and 76.7%, reflecting technical challenges in heterogeneous cancer tissues (Supplementary Figure 2 and Supplementary Table 1).
Based on ground truth defined by manual cell identification, our proposed segmentation method was statistically compared with CP and additional ImageJ/FIJI-based segmentation methods (https://imagej.nih.gov/ij/) with manually tuned optimal parameters [see Method section]. When ground truth was set to annotations selected by two or all physicians in consideration of inter-observer variability of cell identification, our proposed segmentation showed 65% to 78% sensitivity in the three cancer types, whereas CP-based method remained 55% (Figures 1D and 1E, and Supplementary Table 2). While our method and ImageJ/FIJI-based methods showed equivalent sensitivity in malignant mesothelioma and head and neck squamous cell carcinoma tissues, our method outperforms ImageJ/FIJI-based method in heterogeneous pancreatic ductal adenocarcinoma tissues (Figure 1D). Specificity of our method was approximately 90% when ground truth was set to annotations identified by one or more physicians (Figure 1E and Supplementary Table 2). Notably, our proposed segmentation method was applied without parameter tuning, whereas the other two methods were applied with the optimal or image specific proper parameters by an expert user across different cancer tissue samples. Given technical challenges in heterogeneous cancer tissues and moderate consistency across three examiners (70% to 76.7%), these observations suggest that our method achieves reasonable performance among different segmentation methods regardless of heterogeneity of cancer tissues.
2. New Cell Segmentation Method Was Validated for Image Cytometry-Based Quantification
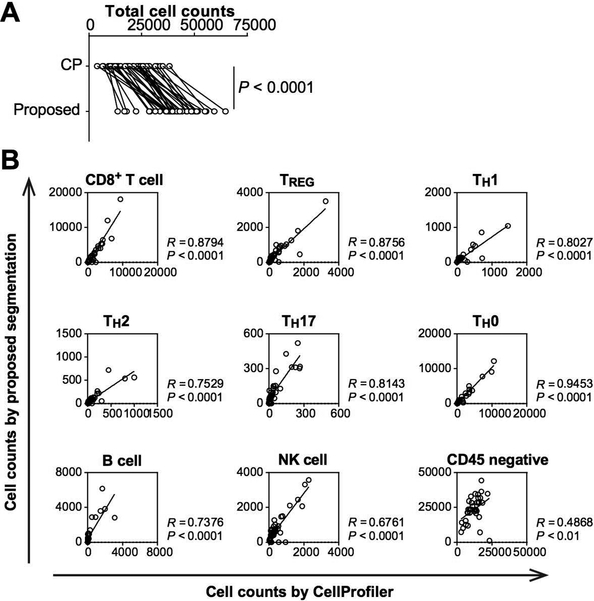
The new cell segmentation method was, then, tested for image cytometry-based quantification and was applied to a previously published tissue microarray of head and neck squamous cell carcinoma (N = 38), where immune cell lineages were quantitatively evaluated by CP-based cell segmentation and image cytometry (8). Image cytometry analysis with our new segmentation method was performed, according to matched gating strategies and lineage markers as CP-based segmentation was applied (Supplementary Figure 3). Cell percentages of total immune cells quantified by the two different segmentation methods were highly correlated (Supplementary Figure 4A), and cell compositions were also preserved regardless of cell segmentation methods (Supplementary Figure 4B). Importantly, our new segmentation method showed higher cell numbers in total cells (Figure 2A) and most cell lineages (Figure 2B) than CP-based segmentation, which was compatible with higher cell detection as observed in Figure 1. These results validate that the new method preserved cell composition together with better cell detection compared to the previous approach.
Figure 2. The proposed method improves detection of cell lineages in image cytometry analysis.
(A) Total cell counts were compared between CellProfiler and the proposed method. Statistical significance was determined via a Wilcoxon signed rank test. (B) The numbers of detected cells across various cell lineages were compared between the proposed method (vertical axes) and CellProfiler (horizontal axes). The proposed approach showed better cell detection in most of cell lineages than CellProfiler-based segmentation. Spearman correlation coefficient was used to assess correlations of cell counts among cell lineages.
3. Image Cytometry Based on the New Cell Segmentation Method Enables Analysis for Subpopulations of TH17 Cells
Since the improved cell detection could be advantageous for detection of rare cell populations, we next focused on relatively rare TH17 cells, whose role in the tumor microenvironment is still controversial (14). As was shown in Figure 3A, the new segmentation method detected a higher frequency of TH17 cells. The new segmentation method showed a statistically significant increase in TH17 cell detection across 38 samples in tissue microarray (Figure 3B), enabling subsequent analyses, including phenotyping and sub-classification. As the presence of subpopulations in TH17 cells has been shown in a previous report (15), we sought to dissect TH17 populations based on T-bet and GATA3 expression, which represent TH1 and TH2 phenotypes in helper T cell subsets, respectively. We observed that T-bet and GATA3 were expressed on TH17 cells, supporting the notion that TH17 cells can be further classified into TH1 and TH2-like subpopulations as was previously shown (15).
To assess the potential biological contexts of subpopulations of TH17, we next evaluated the correlation between TH1 versus TH2-like phenotypes and the presence of oncogenic human papilloma virus (HPV), which is associated with immunogenic characteristics (16). We found that HPV-positive tumors (N = 21) showed a significantly higher frequency of TH1-like TH17 cells than HPV-negative tumors (N = 17), potentially related to differential immune profiles between HPV-positive and negative tumors (Figure 3C).
4. Robust Cell Segmentation Elucidates Imaging-Based TH17 Cell Phenotypes in Tumor Immune Microenvironment
To further explore the significance of TH17-associated immune characteristics, subpopulations of TH17 cells were analyzed in tissue immunological contexts. We analyzed correlations between TH17 subpopulation profiles and immune cell frequency of 14-different lineages quantified in a previous publication (Table 1). Without stratification into TH1 versus TH2 phenotypes, total TH17 cells showed a positive correlation with CD8+ T cellsas well as a negative correlation with CD163− macrophages, suggested by a previous murine study (8). TH2 cells correlated with TH2-like TH17 cells, suggesting an association with TH2-driven cytokine profiles. Although TH17 has been known to recruit granulocytes via cascades of cytokine production (17), total TH17 cells did not correlate with CD66b+ granulocyte infiltration. Interestingly, CD66b+ granulocyte infiltration negatively correlated with TH1-like TH17 cells, but positively correlated with TH2-like TH17 cells, indicating that granulocyte recruitment might be associated with TH2-polarized phenotypes in TH17 cells, rather than total TH17 cells in the cancer microenvironment (Figure 4A).
Table 1.
Spearman correlations of TH17 cell densities versus immune cell percentages of total CD45+ cells.
| Total Thl7 | Thl-like Thl7 | Th2-like Thl7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spearman r | 95% confidence interval | P value | Spcrman r | 95% confidence interval | P value | Spcrman r | 95% confidence interval | P value | ||||
| vs.CD8 | 0.6137 | 0.3573 to 0.7841 | <0.0001 | **** | 0.6878 | 0.4642 to 0.829 | <0.0001 | **** | 0.3805 | 0.0595 to 0.6302 | 0.0184 | * |
| vs.Treg | 0.2953 | −0.03669 to 0.5686 | 0.0719 | ns | 0.308 | −0.02273 to 0.578 | 0.0599 | ns | 0.08274 | −0.2526 to 0.4003 | 0.6214 | ns |
| vs.Thl | 0.04298 | −0.2896 to 0.3663 | 0.7978 | ns | 0.1935 | −0.1441 to 0.4908 | 0.2444 | ns | −0.2531 | −0.5369 to 0.08216 | 0.1252 | ns |
| vs.Th2 | 0.4043 | 0.08743 to 0.6468 | 0.0118 | * | 0.3093 | −0.02127 to 0.579 | 0.0588 | ns | 0.5279 | 0.2413 to 0.7298 | 0.0007 | *** |
| vs.Th17 | 0.592 | 0.3272 to 0.7706 | <0.0001 | **** | 0.5369 | 0.2531 to 0.7356 | 0.0005 | *** | 0.3754 | 0.0536 to 0.6266 | 0.0202 | * |
| vs.Th0 | 0.2447 | −0.09104 to 0.5305 | 0.1386 | ns | 0.2471 | −0.08858 to 0.5323 | 0.1348 | ns | −0.01238 | −0.3395 to 0.3174 | 0.9412 | ns |
| vs.B cell | 0.2825 | −0.05066 to 0.5591 | 0.0857 | ns | 0.2786 | −0.05486 to 0.5562 | 0.0903 | ns | 0.06616 | −0.2681 to 0.3862 | 0.6931 | ns |
| vs.NK | −0.3093 | −0.579 to 0.02128 | 0.0588 | ns | −0.4006 | −0.6443 to −0.08305 | 0.0127 | * | 0.03123 | −0.3003 to 0.356 | 0.8523 | ns |
| VS.CD163- TAM | −0.5171 | −0.7228 to −0.2272 | 0.0009 | *** | −0.4746 | −0.6947 to-0.1731 | 0.0026 | ** | −0.4429 | −0.6734 to −0.1339 | 0.0054 | ** |
| VS.CD163+ TAM | −0.3116 | −0.5807 to 0.01873 | 0.0568 | ns | −0.2432 | −0.5294 to 0.09263 | 0.1412 | ns | −0.1745 | −0.4757 to 0.1633 | 0.2948 | ns |
| vs.DC-SIGN+ | −0.2149 | −0.5076 to 0.1221 | 0.195 | ns | −0.1889 | −0.4872 to 0.1487 | 0.2559 | ns | −0.07157 | −0.3908 to 0.2631 | 0.6694 | ns |
| vs.CD83+ DC | −0.02497 | −0.3505 to 0.306 | 0.8817 | ns | −0.05691 | −0.3783 to 0.2767 | 0.7343 | ns | 0.1584 | −0.1794 to 0.4627 | 0.3423 | ns |
| vs.CD66b+ | 0.05749 | −0.2762 to 0.3788 | 0.7317 | ns | −0.08761 | −0.4044 to 0.248 | 0.6009 | ns | 0.372 | 0.04957 to 0.6242 | 0.0215 | * |
| vs.Mast cell | −0.1116 | −0.4245 to 0.2251 | 0.5048 | ns | −0.1408 | −0.4485 to 0.1967 | 0.3991 | ns | −0.1253 | −0.4358 to 0.2119 | 0.4536 | ns |
| vs.Other CD45+ | −0.5602 | −0.7505 to −0.284 | 0.0003 | *** | −0.4546 | −0.6813 to-0.1483 | 0.0041 | ** | −0.6055 | −0.779 to −0.3458 | <0.0001 | **** |
The potential relationship between TH2-like TH17 cells and CD66b+ granulocytes was further investigated in terms of spatial distribution of the two cell populations in cancer tissues. For validation, we utilized another cohort of HPV-negative head and neck squamous cell carcinoma (N = 9), as we observed a high density of TH2-like TH17 cells in HPV-negative tumors (Figure 3C). Interestingly, TH2-like TH17 cells were located close to CD66b+ granulocytes (Figures 4B and 4C, and Supplementary Figure 5A and 5B). Compared with TH1-like TH17 cells, TH2-like TH17 cells were significantly closer to CD66b+ granulocytes (Figures 4D). In order to investigate potential bias derived from cell frequency, we also evaluated local cell densities surrounding CD66b+ granulocytes (Figure 4E, and Supplementary Figure 5C) with varying distance radius (r) and the k-nearest neighbors (k). Notably, two peaks were observed only in TH2-like TH17 cell density, but not in TH1-like TH17 cells, suggesting the presence of certain biological interactions. The population density of CD66b+ granulocytes is higher in TH2-like TH17 cell regions compared with TH1-like TH17 cell regions (first peak in Figure 4E). As distance radius (r) increases, we can see the second peak of CD66b+ granulocyte density in TH2-like TH17 cell regions, although high population densities of CD66b+ granulocytes only exist at the small distance region (r=1000) in TH1-like TH17 cell regions. Supplementary Figure 5C shows the population density of CD66b+ granulocyte with different numbers of nearest neighbor cells (k). For small numbers of k (<100), the high density peak location of TH2-like TH17 cells is shorter than the TH1-like TH17 cells peak. For larger numbers of k (>100), within the same radius from each cell type, the overall CD66b+ granulocyte density from TH2-like TH17 cells is higher than TH1-like TH17 cells density. These all suggest the presence of a spatial relationship between TH2-like TH17 cells and CD66b+ granulocytes. Together, increased detection of rare TH17 cell populations based on this new segmentation method potentially contributes to in-depth immune profiling and spatial association, leading to further tissue-based biomarker exploration.
DISCUSSION
We proposed a simple but effective methodology for fully automated and robust single cell segmentation with reduced cost of parameter tuning. To do this, we extract useful morphological features from the image and group individual pixels using unsupervised clustering based on similar features to segment nuclei. Notably, our segmentation has been optimized with images stained only by hematoxylin, which lack the cytoplasmic staining usually provided by eosin staining (Figures 1A-C). This provides robust segmentation results across different cancer histological types with the same parameter setting. However, a limitation could be the dependence on image quality of hematoxylin staining, where low quality images lead to failure in cell detection or over/under-segmentation even with our segmentation approach. Our data indicates the proposed segmentation shows better detection of cells with higher sensitivity and specificity in three different cancer histological types. While we observed preserved cell ratio and composition (Supplementary Figure 4), our robust and effective cell detection enabled better detection of relatively rare TH17 cells (Figures 3A and 3B). Improved cell detection was particularly significant for sub-population analysis, which depends on a sufficiently large cell population.
Given that the immunological properties of TH17 cells have not been fully elucidated, we sought to dissect the characteristics of TH17 cells based on multiplex IHC and image cytometry analysis. Then, sub-populations reflecting TH1 versus TH2 balance correlated with HPV status in head and neck cancer, where the immunogenic background of viral-related malignancy could be associated with TH1-based anti-tumor immunity (Figure 3C). Interestingly, our cell density analysis revealed that TH2-like TH17 cells correlated with granulocytes. This observation was further validated in another cohort of HPV-negative head and neck cancer tissues. A major advantage of image cytometry is that it retains tissue context with preserved tissue architectural information, enabling spatial relationship analysis. Thus, we demonstrated that TH2-like, but not TH1-like TH17 cells, showed closer proximities to granulocytes (Figure 4). In addition to previous experimental results, our data provides human tissue-based evidence of distinctive TH17 subpopulations and spatial association with other immune subsets. Taken together, stratification of TH2-like TH17 cells can contribute to further understanding of the roles of TH17 subpopulations in tumor immunity. Simultaneously, our data highlights the capability for improved cell segmentation and image cytometry, enabling better resolution of immune complexity analysis with spatial relationships.
In this study, we demonstrated a proof of concept where combinations of general image processing techniques such as Gabor/Gaussian filters and mathematical morphology operations could improve work flow for single cell segmentation without dependence on manual parameter tuning. As improvement of usability comes after the early stage of proving concepts, we did not release a single package at this time. However, we are currently implementing our pipeline as a user command line tool and integrating it into Galaxy (scientific workflow system) to improve usability. This requires software development so that a wide range of users can run our pipeline upon our release.
In summary, this study proposed a significantly improved cell segmentation approach for image cytometry analysis based on multiplex IHC, which increased detection of rare cell populations including TH17 subpopulations. The image cytometry analyisis with improved cell detection may provide in-depth immune profiling with maintained spatial association, thereby leading to further tissue-based biomarker exploration.
Supplementary Material
Figure S1. Hematoxylin and cell segmentation images in support of Figure 1. Representative histological cancer types of head and neck squamous cell carcinoma (HNSCC) (A), malignant pleural mesothelioma (MPM) (B), and pancreatic ductal adenocarcinoma (PDAC) (C) are shown with hematoxylin-stained images and their nuclei segmentation label mask based on CellProfiler, ImageJ/FIJI-based segmentation and the proposed method. The boxes depict magnified areas. Scale bars = 250 μm (low magnification) and 100 μm (high magnification).
Figure S2. Manual cell identification toward validation of cell segmentation. Manually identified nuclei objects by three independent physicians (Red: physician #1, green: physician #2, blue: physician #3) by dilation with different radii to evaluate matching and discrepancy of cell identification.
Figure S3. Image cytometry analysis for quantification of TH17 cell sub-populations. Gating strategies of image cytometry analysis to identify TH17 cell populations were shown. Gating thresholds for qualitative identification were determined based on data in negative controls. The x and y axes are shown on logarithmic scales.
Figure S4. Cell composition comparison using image cytometry analysis based on both of CP-based and our new segmentation method. (A) Spearman correlation coefficient was utilized for comparing various cell percentages of total CD45+ leukocytes quantified by the proposed method (vertical axes) and CellProfiler (horizontal axes). Overall immune cells composition was preserved and highly correlated with each other. (B) A heat map according to a color scale (right) was shown with immune cell compositions across individual patient samples (N=38).
Figure S5. TH17-associated tissue immunological characteristics are analyzed via image cytometry. (A) Dot plots represent distribution of CD66b+ granulocytes (black dots) and TH2-like (cyan circles), but not TH1-like (red circles) TH17 cells. (B) Cell to cell distances from CD66b+ granulocyte to nearest TH1-like TH17 versus TH2-like TH17 cells were compared. Statistical significance was determined via a Wilcoxon signed rank test. (C) Microregional cell densities of TH1-like TH17 and TH2-like TH17 cells were shown depending on distance from nearest CD66b+ granulocytes (x axis).
Table S1. Manually identified nuclei objects and inter-observer variability by three independent physicians. Three physicians marked the centroids of all visually recognizable cells in assigned images derived from different histological cancer types of squamous cell carcinoma, adenocarcinoma, and malignant mesothelioma where “P_i only” represents cells which only i-physician annotated. “P_ij only” represents cells which physician-i and physician-j annotated. “P_ik only” represents cells which physician-i and physician-k annotated. “P_ij or P_ik” represents union of “P_ij only” and “P_ik only.” “P_ijk only” represents cells which all physicians annotated. “N_total” represents the number of cells which physician annotated. “All agree” represents “P_ijk only” divided by “N_total” x 100. “R_ij” represents “P_ij only” divided by “N_total” x 100. “R_jk” represents “P_jk only” divided by “N_total” x 100. “Only 2 agree” represents “P_ij or P_ik” divided by “N_total” x 100. “Mismatch” represents “P_i” divided by “N_total” x 100. “R” represents dilated radius, and “P1”, “P2” and “P3” represent anonymize physicians.
Table S2. Comparison of cell segmentation methods with manual cell annotations. “P1 only” represents the number of segmented cells which only Pathologist 1 annotated. “P2 only” represents the number of segmented cells which only Pathologist 2 annotated. “P3 only” represents the number of segmented cells which only Pathologist 3 annotated. “P12” represents the number of segmented cells which Pathologist 1 and Pathologist 2 annotated. “P23” represents the number of segmented cells which Pathologist 2 and Pathologist 3 annotated. “P13” represents the number of segmented cells, which Pathologist 1 and Pathologist 3 annotated. “P123” represents the number of segmented cells, which all Pathologists annotated. “FALSE” represents the number of segmented cells, which do not have Pathologist’s annotation. “Discrepancy” represents sum of “P1 only,” “P2 only” and “P3 only.” “F2” represents sum of “FALSE” and “Discrepancy.” “TRUE (P12 + P23 + P13 + P123)” represents the number of segmented cells, which at least two pathologists annotated. “TRUE(ALL)” represents the number of segmented cells, which any pathologists annotated. “Total” represents sum of “P1 only,” “P2 only,” “P3 only,” “P12,” “P23,” “P13,” “P123,” and “FALSE.” We also compared with the recent method proposed by Wang et al. (28), because the authors propose a general framework able to segment cells acquired from different imaging modalities, and claim “a higher accuracy compared to past work.” We used the code provided by the authors, and the results presented are the best obtained after parameters tuning.
Acknowledgement:
This study was supported by the Knight Cancer Institute (P30 CA069533), and the Oregon Clinical and Translational Research Institute (OCTRI, #UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH) and Grant-in-Aid for Scientific Research (17H07016) from the Japan Society for the Promotion of Science (TT). LMC acknowledges support from the Brenden-Colson Center for Pancreatic Health, and a Stand Up To Cancer – Lustgarten Foundation Pancreatic Cancer Convergence Dream Team Translational Research Grant (SU2C-AACR-DT14–14). YHC acknowledges grant support from NCI U54CA209988, the Brenden-Colson Center for Pancreatic Care and the OHSU Center for Spatial Systems Biomedicine (OCSSB).
REFERENCE
- 1.Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO, Kvistborg P, Maccalli C, Maecker HT, Page DB, Robins H, Song W, Stack EC, Wang E, Whiteside TL, Zhao Y, Zwierzina H, Butterfield LH, Fox BA. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4:3 Epub 2016/01/21. doi: 10.1186/s40425-016-0107-3. PubMed PMID: 26788324; PMCID: PMC4717548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scutti JAB. Importance of immune monitoring approaches and the use of immune checkpoints for the treatment of diffuse intrinsic pontine glioma: From bench to clinic and vice versa (Review). Int J Oncol. 2018;52(4):1041–56. Epub 2018/02/28. doi: 10.3892/ijo.2018.4283. PubMed PMID: 29484440; PMCID: PMC5843403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assi HI, Kamphorst AO, Moukalled NM, Ramalingam SS. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer. 2018;124(2):248–61. Epub 2017/12/07. doi: 10.1002/cncr.31105. PubMed PMID: 29211297. [DOI] [PubMed] [Google Scholar]
- 4.Varn FS, Wang Y, Mullins DW, Fiering S, Cheng C. Systematic Pan-Cancer Analysis Reveals Immune Cell Interactions in the Tumor Microenvironment. Cancer Res. 2017;77(6):1271–82. Epub 2017/01/28. doi: 10.1158/0008-5472.CAN-16-2490. PubMed PMID: 28126714; PMCID: PMC5798883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dao D, Fraser AN, Hung J, Ljosa V, Singh S, Carpenter AE. CellProfiler Analyst: interactive data exploration, analysis and classification of large biological image sets. Bioinformatics. 2016;32(20):3210–2. Epub 2016/06/30. doi: 10.1093/bioinformatics/btw390. PubMed PMID: 27354701; PMCID: PMC5048071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caicedo JC, Singh S, Carpenter AE. Applications in image-based profiling of perturbations. Curr Opin Biotechnol. 2016;39:134–42. Epub 2016/04/19. doi: 10.1016/j.copbio.2016.04.003. PubMed PMID: 27089218. [DOI] [PubMed] [Google Scholar]
- 7.Young Hwan C, Thibault G, Azimi V, Johnson B, Jorgens D, Link J, Margolin A, Gray JW. Quantitative analysis of histological tissue image based on cytological profiles and spatial statistics. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:1175–8. Epub 2016/01/01. doi: 10.1109/EMBC.2016.7590914. PubMed PMID: 28324942. [DOI] [PubMed] [Google Scholar]
- 8.Tsujikawa T, Kumar S, Borkar RN, Azimi V, Thibault G, Chang YH, Balter A, Kawashima R, Choe G, Sauer D, El Rassi E, Clayburgh DR, Kulesz-Martin MF, Lutz ER, Zheng L, Jaffee EM, Leyshock P, Margolin AA, Mori M, Gray JW, Flint PW, Coussens LM. Quantitative Multiplex Immunohistochemistry Reveals Myeloid-Inflamed Tumor-Immune Complexity Associated with Poor Prognosis. Cell Rep. 2017;19(1):203–17. Epub 2017/04/06. doi: 10.1016/j.celrep.2017.03.037. PubMed PMID: 28380359; PMCID: PMC5564306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamentsky L, Jones TR, Fraser A, Bray MA, Logan DJ, Madden KL, Ljosa V, Rueden C, Eliceiri KW, Carpenter AE. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics. 2011;27(8):1179–80. Epub 2011/02/26. doi: 10.1093/bioinformatics/btr095. PubMed PMID: 21349861; PMCID: PMC3072555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogel I, Sagi D. Gabor filters as texture discriminator. Biological cybernetics. 1989;61(2):103–13. [Google Scholar]
- 11.Kesselring R, Thiel A, Pries R, Trenkle T, Wollenberg B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br J Cancer. 2010;103(8):1245–54. Epub 2010/09/30. doi: 10.1038/sj.bjc.6605891. PubMed PMID: 20877351; PMCID: PMC2967064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veta M, Pluim JP, van Diest PJ, Viergever MA. Breast cancer histopathology image analysis: a review. IEEE Trans Biomed Eng. 2014;61(5):1400–11. Epub 2014/04/25. doi: 10.1109/TBME.2014.2303852. PubMed PMID: 24759275. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz P, Nathanson R, Kastner T. Pertussis immunization patterns in special care nursery graduates. J Dev Behav Pediatr. 1991;12(1):38–41. Epub 1991/02/01. PubMed PMID: 2016401. [PubMed] [Google Scholar]
- 14.Gurcan MN, Boucheron LE, Can A, Madabhushi A, Rajpoot NM, Yener B. Histopathological image analysis: a review. IEEE Rev Biomed Eng. 2009;2:147–71. Epub 2009/01/01. doi: 10.1109/RBME.2009.2034865. PubMed PMID: 20671804; PMCID: PMC2910932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aswathy M, Jagannath M. Detection of breast cancer on digital histopathology images: present status and future possibilities. Informatics in Medicine Unlocked. 2017;8:74–9. [Google Scholar]
- 16.Rujuta O, Vyavahare A. Review of Nuclei Detection, Segmentation in Microscopic Images. J Bioengineer & Biomedical Sci. 2017;7(227):2. [Google Scholar]
- 17.Wang CW, Fennell D, Paul I, Savage K, Hamilton P. Robust automated tumour segmentation on histological and immunohistochemical tissue images. PLoS One. 2011;6(2):e15818 Epub 2011/03/10. doi: 10.1371/journal.pone.0015818. PubMed PMID: 21386898; PMCID: PMC3046129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. Epub 2012/06/30. doi: 10.1038/nmeth.2019. PubMed PMID: 22743772; PMCID: PMC3855844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otsu N A threshold selection method from gray-level histograms. IEEE transactions on systems, man, and cybernetics. 1979;9(1):62–6. [Google Scholar]
- 20.Najman L, Schmitt M. Geodesic saliency of watershed contours and hierarchical segmentation. IEEE Transactions on pattern analysis and machine intelligence. 1996;18(12):1163–73. [Google Scholar]
- 21.Young Hwan C, Thibault G, Madin O, Azimi V, Meyers C, Johnson B, Link J, Margolin A, Gray JW. Deep learning based Nucleus Classification in pancreas histological images. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:672–5. Epub 2017/10/25. doi: 10.1109/EMBC.2017.8036914. PubMed PMID: 29059962. [DOI] [PubMed] [Google Scholar]
- 22.Barber CB, Dobkin DP, Huhdanpaa H. The quickhull algorithm for convex hulls. ACM Transactions on Mathematical Software (TOMS) 1996;22(4):469–83. [Google Scholar]
- 23.Paz P, Piqueras JR, Tizado E. A program for the application of the radial distribution function to cluster analysis in cell biology. Bioinformatics. 1992;8(4):307–9. [DOI] [PubMed] [Google Scholar]
- 24.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276 Epub 2014/07/06. doi: 10.3389/fimmu.2014.00276. PubMed PMID: 24987392; PMCID: PMC4060300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geginat J, Paroni M, Maglie S, Alfen JS, Kastirr I, Gruarin P, De Simone M, Pagani M, Abrignani S. Plasticity of human CD4 T cell subsets. Front Immunol. 2014;5:630 Epub 2015/01/08. doi: 10.3389/fimmu.2014.00630. PubMed PMID: 25566245; PMCID: PMC4267263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurlow JK, Pena Murillo CL, Hunter KD, Buffa FM, Patiar S, Betts G, West CM, Harris AL, Parkinson EK, Harrison PR, Ozanne BW, Partridge M, Kalna G. Spectral clustering of microarray data elucidates the roles of microenvironment remodeling and immune responses in survival of head and neck squamous cell carcinoma. J Clin Oncol. 2010;28(17):2881–8. Epub 2010/05/12. doi: 10.1200/JCO.2009.24.8724. PubMed PMID: 20458058. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani S Human Th17 cells. Arthritis Res Ther. 2008;10(2):206 Epub 2008/05/10. doi: 10.1186/ar2392. PubMed PMID: 18466633; PMCID: PMC2453756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Li H. Generalizing cell segmentation and quantification. BMC Bioinformatics. 2017;18(1):189 Epub 2017/03/25. doi: 10.1186/s12859-017-1604-1. PubMed PMID: 28335722; PMCID: PMC5364575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Hematoxylin and cell segmentation images in support of Figure 1. Representative histological cancer types of head and neck squamous cell carcinoma (HNSCC) (A), malignant pleural mesothelioma (MPM) (B), and pancreatic ductal adenocarcinoma (PDAC) (C) are shown with hematoxylin-stained images and their nuclei segmentation label mask based on CellProfiler, ImageJ/FIJI-based segmentation and the proposed method. The boxes depict magnified areas. Scale bars = 250 μm (low magnification) and 100 μm (high magnification).
Figure S2. Manual cell identification toward validation of cell segmentation. Manually identified nuclei objects by three independent physicians (Red: physician #1, green: physician #2, blue: physician #3) by dilation with different radii to evaluate matching and discrepancy of cell identification.
Figure S3. Image cytometry analysis for quantification of TH17 cell sub-populations. Gating strategies of image cytometry analysis to identify TH17 cell populations were shown. Gating thresholds for qualitative identification were determined based on data in negative controls. The x and y axes are shown on logarithmic scales.
Figure S4. Cell composition comparison using image cytometry analysis based on both of CP-based and our new segmentation method. (A) Spearman correlation coefficient was utilized for comparing various cell percentages of total CD45+ leukocytes quantified by the proposed method (vertical axes) and CellProfiler (horizontal axes). Overall immune cells composition was preserved and highly correlated with each other. (B) A heat map according to a color scale (right) was shown with immune cell compositions across individual patient samples (N=38).
Figure S5. TH17-associated tissue immunological characteristics are analyzed via image cytometry. (A) Dot plots represent distribution of CD66b+ granulocytes (black dots) and TH2-like (cyan circles), but not TH1-like (red circles) TH17 cells. (B) Cell to cell distances from CD66b+ granulocyte to nearest TH1-like TH17 versus TH2-like TH17 cells were compared. Statistical significance was determined via a Wilcoxon signed rank test. (C) Microregional cell densities of TH1-like TH17 and TH2-like TH17 cells were shown depending on distance from nearest CD66b+ granulocytes (x axis).
Table S1. Manually identified nuclei objects and inter-observer variability by three independent physicians. Three physicians marked the centroids of all visually recognizable cells in assigned images derived from different histological cancer types of squamous cell carcinoma, adenocarcinoma, and malignant mesothelioma where “P_i only” represents cells which only i-physician annotated. “P_ij only” represents cells which physician-i and physician-j annotated. “P_ik only” represents cells which physician-i and physician-k annotated. “P_ij or P_ik” represents union of “P_ij only” and “P_ik only.” “P_ijk only” represents cells which all physicians annotated. “N_total” represents the number of cells which physician annotated. “All agree” represents “P_ijk only” divided by “N_total” x 100. “R_ij” represents “P_ij only” divided by “N_total” x 100. “R_jk” represents “P_jk only” divided by “N_total” x 100. “Only 2 agree” represents “P_ij or P_ik” divided by “N_total” x 100. “Mismatch” represents “P_i” divided by “N_total” x 100. “R” represents dilated radius, and “P1”, “P2” and “P3” represent anonymize physicians.
Table S2. Comparison of cell segmentation methods with manual cell annotations. “P1 only” represents the number of segmented cells which only Pathologist 1 annotated. “P2 only” represents the number of segmented cells which only Pathologist 2 annotated. “P3 only” represents the number of segmented cells which only Pathologist 3 annotated. “P12” represents the number of segmented cells which Pathologist 1 and Pathologist 2 annotated. “P23” represents the number of segmented cells which Pathologist 2 and Pathologist 3 annotated. “P13” represents the number of segmented cells, which Pathologist 1 and Pathologist 3 annotated. “P123” represents the number of segmented cells, which all Pathologists annotated. “FALSE” represents the number of segmented cells, which do not have Pathologist’s annotation. “Discrepancy” represents sum of “P1 only,” “P2 only” and “P3 only.” “F2” represents sum of “FALSE” and “Discrepancy.” “TRUE (P12 + P23 + P13 + P123)” represents the number of segmented cells, which at least two pathologists annotated. “TRUE(ALL)” represents the number of segmented cells, which any pathologists annotated. “Total” represents sum of “P1 only,” “P2 only,” “P3 only,” “P12,” “P23,” “P13,” “P123,” and “FALSE.” We also compared with the recent method proposed by Wang et al. (28), because the authors propose a general framework able to segment cells acquired from different imaging modalities, and claim “a higher accuracy compared to past work.” We used the code provided by the authors, and the results presented are the best obtained after parameters tuning.