Abstract
OBJECTIVES
This study aimed to evaluate the long-term prognostic value of serial assessment of coronary flow reserve (CFR) by rubidium Rb 82 (82Rb) positron emission tomography (PET) in heart transplantation (HT) patients.
BACKGROUND
Cardiac allograft vasculopathy is a major determinant of late mortality in HT recipients. The long-term prognostic value of serial CFR quantification by PET imaging in HT patients is unknown.
METHODS
A total of 89 patients with history of HT (71% men, 7.0 ± 5.7 years post-HT, age 57 ± 11 years) scheduled for dynamic rest and stress (dipyridamole) 82Rb PET between March 1, 2008 and July 31, 2009 (PET-1) were prospectively enrolled in a single-center study. PET myocardial perfusion studies were reprocessed using U.S. Food and Drug Administration-approved software (Corridor 4DM, version 2017) for calculation of CFR. Follow-up PET (PET-2) imaging was performed in 69 patients at 1.9 ± 0.3 years following PET-1. Patients were categorized based on CFR values considering CFR ≤1.5 as low and CFR >1.5 as high CFR.
RESULTS
Forty deaths occurred during the median follow-up time of 8.6 years. Low CFR at PET-1 was associated with a 2.77-fold increase in all-cause mortality (95% confidence interval [CI]: 1.34 to 5.74; p = 0.004). CFR decreased over time in patients with follow-up imaging (PET-1: 2.11 ± 0.74 vs. PET-2: 1.81 ± 0.61; p = 0.003). Twenty-five patients were reclassified based on PET-1 and PET-2 (high to low CFR: n = 18, low to high CFR: n = 7). Overall survival was similar in patients reclassified from high to low as patients with low to low CFR, whereas patients reclassified from low to high had similar survival as patients with high to high CFR. In multivariate Cox regression of patients with PET-2, higher baseline CFR (hazard ratio [HR] for a 0.73 unit (one standard deviation) increase: 0.36, 95% CI: 0.16 to 0.82) and reduction in CFR from PET-1 to PET-2 (HR for a 0.79 unit (one standard deviation) decrease: 1.50 to 7.84) were independent predictors of all-cause mortality.
CONCLUSIONS
Serial assessment of CFR by 82Rb PET independently predicts long-term mortality in HT patients.
Keywords: cardiac allograft vasculopathy, coronary flow reserve, heart transplantation, myocardial blood flow, positron emission tomography, prognosis
Cardiac allograft vasculopathy (CAV) is a pathologic process that affects transplanted hearts. CAV is characterized by diffuse, confluent luminal narrowing of the graft coronary vasculature secondary to marked concentric intimal hyperplasia and inadequate compensation by outward remodeling. The remodeling process involves the intramural coronaries as well as epicardial vessels of the graft vasculature.
The advent of new immunosuppressive strategies have contributed to reduction in graft rejection and acute graft mortality, however the incidence of CAV has not changed significantly over the past several decades (1). Registries report the cumulative incidence of CAV to be approximately 50% at 10 years follow-up (1). CAV remains a major determinant of mortality in transplant patients and is responsible for up to 15% of deaths annually beyond the first year following heart transplantation (HT) (1).
Conventional coronary angiography has limited sensitivity for the detection of CAV given the diffuse involvement of the coronary segments. Advanced intracoronary imaging techniques (e.g., intravascular ultrasound [IVUS] and optical coherence tomography) provide superior sensitivity for the detection of CAV, however their application is limited by extensive cost, limited expertise, and their restricted ability to evaluate only the epicardial vessels. In addition, both coronary angiography and advanced intracoronary imaging techniques require invasive instrumentation, which carry infrequent, but significant risk for complications. Furthermore, contrast administration during angiography can lead to renal dysfunction. Therefore, there has been an increasing demand to develop new noninvasive strategies for the identification of CAV.
Quantification of myocardial blood flow (MBF) and coronary flow reserve (CFR) using rubidium Rb 82 (82Rb) positron emission tomography (PET) is a promising noninvasive imaging strategy for the characterization of CAV, because it reflects the combination of flow in the epicardial arteries and the microcirculation. Previous large-scale clinical studies provided evidence that a severely reduced PET CFR (<1.5) is a powerful, independent predictor of mortality in a nontransplant patient population undergoing risk assessment for coronary artery disease (2). In HT patients, quantification of CFR with PET was able to predict short-term (~ 18 months) adverse events (3). However, whether PET CFR is able to predict long-term adverse events, or whether serial assessment of CFR carries additional prognostic value in HT patients is currently unknown. Therefore, we sought to investigate the long-term (>5 years) prognostic value of serial PET CFR quantification in predicting all-cause mortality in HT patients.
METHODS
Eighty-nine consecutive HT patients, who were on average 7 years post-orthotopic HT (interquartile range: 2.0 to 11.0 years) and scheduled for annual transplant evaluation by 82Rb PET from March 1, 2008 to July 31, 2009, were prospectively enrolled in a single-center study at Yale New Haven Hospital (New Haven, Connecticut). The institutional research ethics board approved the study and written and verbal informed consent was obtained from all study participants. A schematic of the study design and experimental procedures is depicted in Figure 1.
FIGURE 1. Study Design and Timeline.

PET = positron emission tomography; SPECT = single-photon emission computed tomography.
PET IMAGING PROTOCOL.
Dynamic rest-stress 82Rb PET myocardial perfusion imaging was performed on a whole body 2-dimensional (2D)/3D PET camera equipped with a 16-slice computed tomography (CT) scanner (Discovery ST, GE Healthcare, Waukesha, Wisconsin). Patients refrained from caffeine- and methylxanthine-containing substances and drugs for 24 h prior to the study. Following a scout image, a low-energy CT (120 kVp, 13 mA) was acquired for attenuation correction. Dynamic rest PET imaging was performed after intravenous injection of 46 ± 7 mCi of 82Rb. The dose of 82Rb was adjusted based on the age of the generator (Bracco Diagnostic Inc., Melville, New York). Twenty-seven dynamic frames were acquired in list mode with the following time sequences: 14 × 5-s frames; 6 × 10-s frames; 3 × 20-s frames; 3 × 30-s frames; and 1 × 90-s frame. After the rest PET scan, the patients underwent pharmacological stress with a slow infusion of dipyridamole (0.56 mg/kg) over 4 min. Seven minutes after the start of dipyridamole infusion, 46 ± 6 mCi of 82Rb was administered intravenously and dynamic PET images were acquired in an identical fashion to rest imaging. Following stress PET imaging, another low-energy CT (120 kVp, 8 mA) scan was acquired for attenuation correction of stress images to account for any patient movement between the scans. Heart rate and rhythm (12-lead electrocardiography) and noninvasive blood pressure were recorded at rest, at peak stress, and in recovery. Dynamic images were reconstructed with attenuation and scatter corrections but without prompt gamma correction.
FOLLOW-UP PET.
Follow-up PET (PET-2) imaging was performed in 69 patients at 1.9 ± 0.3 years following the original PET study. Follow-up imaging was performed on a whole body 2D/3D PET scanner equipped with a 16-slice (GE Discovery Rx, GE Healthcare) or 64-slice CT scanner (GE Discovery 690; GE Healthcare). During the follow-up study, patients underwent pharmacological stress with dipyridamole (n = 63 of 69), regadenoson (n = 5 of 69), or dobutamine (n = 1 of 69). Dipyridamole was dosed similarly to baseline PET, regadenoson was administered as a bolus (0.4 mg over 40 s), whereas dobutamine was given as a continuous infusion at a maximum rate of 40 mg/kg/min. Dynamic PET imaging was performed after 82Rb was intravenously administered at rest (44 ± 11 mCi) and stress (44 ± 11 mCi). As was already stated, the dose of 82Rb was adjusted based on the age of the generator (Bracco Diagnostic Inc.). The attenuation CT and PET acquisition, reconstruction, and correction methods were identical to those used in the baseline PET study.
PET DATA ANALYSIS.
Reconstructed dynamic images were reprocessed using a U.S. Food and Drug Administration-approved software (Corridor 4DM, version 2017, INVIA, Ann Arbor, Michigan). Regional and global rest and peak stress MBF were calculated by fitting the 82Rb time-activity curves to a singlecompartment tracer kinetic model as described previously (4). A weak, but significant correlation was observed between both rest MBF and rate-pressure product (RPP) (r = 0.378, p = 0.003) (Online Figure 1A) and between stress MBF and RPP (r = 0.25, p = 0.018) (Online Figure 1B), and also with combined rest and stress MBF values and RPP (r = 0.13, p = 0.04) (Online Figure 1B) suggesting a statistically significant but weak coupling between RPP and MBF during both rest and stress conditions. Therefore, to control for the difference in hemodynamic loading conditions, both rest and stress flows were corrected for the rate pressure product (heart rate × systolic blood pressure) as follows: rest and stress flows were multiplied by the reference RPP (9,000) and then divided by the respective rest or peak stress RPP. CFR was calculated as the ratio of stress to rest MBF (Figure 2). In addition, perfusion defect size was quantified and expressed as a percentage of the left ventricle (LV) for both PET-1 and PET-2 as described previously using Wackers-Liu CQ software (Yale University, New Haven, Connecticut) (5).
FIGURE 2. Representative PET Perfusion Images for a Patient With Heart Transplant.
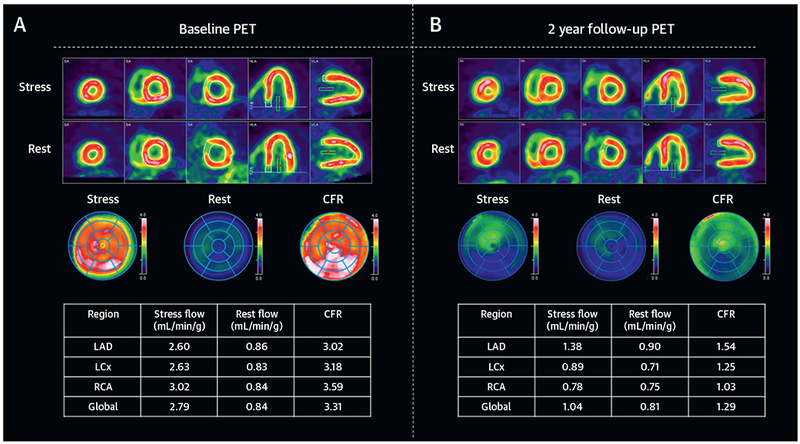
Representative relative perfusion images, polar maps, and rate pressure product–corrected myocardial blood flow and coronary flow reserve (CFR) values for a 48-year-old male patient who underwent dynamic positron emission tomography (PET) perfusion imaging 6 months after heart transplantation (A). Both stress (top) and rest (bottom) images demonstrate normal relative perfusion. Polar maps of stress and rest images and CFR demonstrate homogenously normal perfusion with normal global CFR of 3.31. Follow-up PET scan 2 years later showed a small equivocal inferolateral perfusion defect (B). Polar map of CFR at this time showed severe, diffuse reduction in CFR (mean global CFR: 1.29), which was due to reduction in stress myocardial blood flow, whereas rest flow was unchanged. Coronary angiography after PET-2 revealed severe triple vessel allograft vasculopathy. LAD = left anterior descending artery; LCX = left circumflex artery; RCA = right coronary artery.
CLINICAL SURVEILLANCE AND OUTCOMES.
All patients underwent standard post-transplantation surveillance for rejection by right heart catheterization and endomyocardial biopsy. Coronary angiography was performed in 43 patients within 19 ± 46 days of PET-1. Coronary vessels were classified according to International Society for Heart and Lung Transplantation recommendations (6). Of note, coronary angiography was performed in all patients who were within 5 years of HT at the time of the baseline PET study (n = 39). Although IVUS was performed in some patients, the images were not available for analysis. Baseline patient characteristics including age, sex, body mass index, race, time of HT, indication for HT, comorbidities, cardiovascular and immunosuppressive medications, and standard laboratory values were recorded at the time of enrollment. Patient follow-up data were obtained from heart failure clinic visits, hospital admissions, and the United Network for Organ Sharing database. The primary outcome was all-cause mortality. In addition, information regarding myocardial infarction (MI) and any revascularization was collected and pooled with all-cause mortality as a secondary outcome. The cause of death was identified by chart review. In patients with unknown cause of death, the cause of death was assumed to be cardiovascular based on recently published guidelines (7). Median survival after transplantation was higher in our patients (16.2 years) than the median survival in adult HT registries (8.3 to 12.6 years) (1). However, our study included patients who were further out from HT, and therefore likely underrepresented patients who died in the early post-transplantation period.
STATISTICAL ANALYSES.
Differences between dichotomous and categorical variables were assessed using Fisher exact and chi-square tests, respectively. Continuous variables were compared using a 2-tailed t-test for normally distributed data. Non-normally distributed continuous variables were compared with Mann-Whitney U or Wilcoxon signed-rank tests for unmatched and matched data, respectively. Analysis of variance or Kruskal-Wallis test was used to compare differences among more than 2 groups for normally and nonnormally distributed variables, respectively. Pearson correlation coefficient with 95% confidence interval (CI) was used to evaluate the correlation between dependent variables of interest with CFR. For data presented as box and whiskers plots, the boxes represent the 25th to 75th percentiles, the midlines represent the median values, and the whiskers indicate minimal and maximal values.
To assess the impact of CFR on mortality, the Cox proportional hazards model was used after controlling for the effects of other variables that are known to affect mortality recorded at the time of PET-2 (1). The risk represented by CFR was included as a continuous variable in 2 separate ways in Cox regression modeling as follows: the hazard ratio (HR) for baseline PET (PET-1) CFR was presented for 1-SD increase in CFR and the HR for change in CFR from PET-1 to PET-2 was presented for 1-SD decrease in CFR from PET-1 to PET-2. The time of PET-2 served as the reference time point for outcomes in all analyses including the change in CFR from PET-1 to PET-2. Stepwise Cox regression modeling with backward selection was performed to determine independent predictors of all-cause mortality. Parameters showing a p ≤ 0.10 in the univariate analysis were considered eligible for entry into the multivariate model.
Standard survival analysis strategies were used to determine long-term (>5 years) all-cause mortality. Unadjusted survival analysis was conducted using the Kaplan-Meier method, and statistical comparisons were drawn using the log-rank test. Patients were categorized for unadjusted survival curves based on previously published CFR cutoff values (2).
All statistical analyses were performed using STATA 14 (StataCorp LLC, College Station, Texas) and statistical significance was defined as p < 0.05 for all analyses unless otherwise noted.
RESULTS
PATIENT CHARACTERISTICS.
A total of 89 HT patients underwent rest and dipyridamole stress 82Rb PET between March 2008 and July 2009. The baseline characteristics are depicted in Table 1, rest and stress hemodynamics are depicted in Online Table 1. There was a weak, but significant inverse correlation between CFR values and the time interval between transplantation and PET-1 (r = −0.28; 95% CI: −0.46 to −0.07; p = 0.009) (Online Figure 2A), whereas there was no significant correlation between CFR and the age at the time of PET-1 (r = −0.14; 95% CI: −0.34 to 0.07; p = 0.18) (Online Figure 2B). CFR was significantly lower in all coronary territories and globally in patients who underwent transplantation more than 10 years before PET-1 compared with patients who underwent transplantation within 1 year (Online Table 2). In addition, in the right coronary artery territory, CFR was significantly lower in patients who underwent transplantation within 5 to 10 years when compared with patients who underwent transplantation within 1 year. There were no other differences observed between the groups.
TABLE 1.
Baseline Patient Characteristics
| CFR ≤1.5 (n = 26) | CFR >1.5 (n = 63) | Total (N = 89) | p Value | |
|---|---|---|---|---|
| Age, yrs | 60.8 (54.5-65.5) | 59.3 (49.3-64.9) | 59.8 (51.4-65.1) | 0.44 |
| Female | 5 (19) | 21 (33) | 26 (29) | 0.21 |
| Body mass index, kg/m2 | 27.0 (24.0-31.0) | 28.7 (25.8-33.1) | 28.0 (25.7-33.0) | 0.18 |
| Race | 0.79 | |||
| Caucasian | 22 (85) | 48 (76) | 70 (79) | |
| African American | 2 (8) | 9 (14) | 11 (12) | |
| Hispanic | 1 (4) | 4 (6) | 5 (6) | |
| Other/unknown | 1 (4) | 2 (3) | 3 (3) | |
| Transplantation factors | ||||
| Time since transplantation, yrs | 7.5 (3.74-13.59) | 5.94 (1.01-9.00) | 6.98 (2.04-11.01) | 0.13 |
| Indication for transplantation | 0.17 | |||
| Ischemic cardiomyopathy | 9 (35) | 28 (44) | 37 (42) | |
| Dilated cardiomyopathy | 16 (62) | 27 (43) | 43 (48) | |
| Other | 1 (4) | 9 (14) | 10 (11) | |
| Comorbidities | ||||
| Previously documented CAV | 4 (15) | 1 (2) | 5 (6) | 0.024 |
| Revascularization post-HT | 1 (4) | 1 (2) | 2 (2) | 0.50 |
| Previous rejections | 1.93 ± 0.66 | 0.51 ± 0.10 | 0.77 ± 0.13 | 0.005 |
| Diabetes mellitus | 11 (42) | 21 (33) | 32 (36) | 0.47 |
| Peripheral artery disease | 2 (8) | 3 (5) | 5 (6) | 0.63 |
| Prior transient ischemic attack/stroke | 2 (8) | 4 (6) | 6 (7) | 1.00 |
| Chronic kidney disease | 11 (42) | 19 (30) | 30 (34) | 0.33 |
| Immunosuppressants | ||||
| Cyclosporine | 13 (50) | 32 (51) | 45 (51) | 1.00 |
| Tacrolimus | 6 (23) | 18 (29) | 24 (27) | 0.79 |
| Mycophenolate mofetil | 20 (77) | 55 (87) | 75 (84) | 0.34 |
| Azathioprine | 1 (4) | 3 (5) | 4 (4) | 1.00 |
| Sirolimus | 8 (31) | 15 (24) | 23 (26) | 0.60 |
| Cardiovascular medications | ||||
| Beta-blocker | 8 (31) | 17 (27) | 25 (28) | 0.80 |
| Calcium-channel blocker | 11 (42) | 35 (56) | 46 (52) | 0.35 |
| Angiotensin-converting enzyme inhibitor | 6 (23) | 13 (21) | 19 (21) | 0.78 |
| Angiotensin-receptor blocker | 7 (27) | 20 (32) | 27 (30) | 0.80 |
| Diuretics | 17 (65) | 28 (44) | 45 (51) | 0.10 |
| Aspirin | 15 (58) | 44 (70) | 59 (66) | 0.33 |
| Clopidogrel | 1 (4) | 4 (6) | 5 (6) | 1.00 |
| Statins | 19 (73) | 55 (87) | 74 (83) | 0.12 |
| Insulin | 9 (35) | 14 (22) | 23 (26) | 0.29 |
| Laboratory values | ||||
| Creatinine | 1.6 (1.3-2.0) | 1.4 (1.2-1.9) | 1.5 (1.2-1.85) | 0.29 |
| Low-density lipoprotein | 93 (65-114) | 78 (58-101) | 80 (61-104) | 0.16 |
Values are median (interquartile range), n (%), or mean ± SEM. Bold values are statistically significant. CAV = cardiac allograft vasculopathy; CFR = coronary flow reserve; HT = heart transplantation.
Moderate (≥5% LV to <10% LV) and large (≥10% LV) perfusion defects were uncommon in our patient population. At the time of PET-1, the average perfusion defect size was very small, with only 0.35 ± 0.90% of the LV at rest and 0.70 ± 1.6% of the LV at stress. At baseline imaging, no patient had a moderate-sized perfusion defect and only 1 patient had a large size perfusion defect. On repeat imaging (PET-2), the average perfusion defect size was 0.93 ± 2.97% of the LV at rest and 0.99 ± 2.37% of the LV at stress, with 2 patients showing moderate-sized and 3 patients showing large-sized perfusion defects.
Consistent with previous observations, there were no differences in CFR among coronary territories, which reflect the diffuse involvement of coronary vascular beds in CAV (CFR values with interquartile range [IQR] left anterior descending artery: 1.97 [IQR: 1.44 to 2.51], left circumflex artery: 1.91 [IQR: 1.41 to 2.44], right coronary artery: 1.81 [IQR: 1.38 to 2.51]). Stress MBF was significantly higher in patients with severely reduced CFR (CFR ≤1.5) than in the patients without severely reduced CFR (CFR >1.5), whereas there was no difference in rest MBF between the 2 groups (Figure 3). Previously diagnosed CAV was more common in the group with severely reduced CFR than in the group without severely reduced CFR. In addition, the patients with severely reduced CFR had significantly higher number of rejection episodes prior to enrollment. In agreement with this, CFR was significantly lower in patients who had 2 or ≥3 rejection episodes prior to enrollment when compared with patients with no rejections (Figure 4). Compared with patients with no rejections, patients with 1 rejection episode also had a nonsignificant (p = 0.075) reduction in CFR. There were no other differences between patients with or without severely reduced CFR with respect to baseline characteristics including age, sex, body mass index, race, time of HT, indication for HT, comorbidities, cardiovascular and immunosuppressive medications, and baseline laboratory values (Table 1).
FIGURE 3. Baseline MBF and CFR.
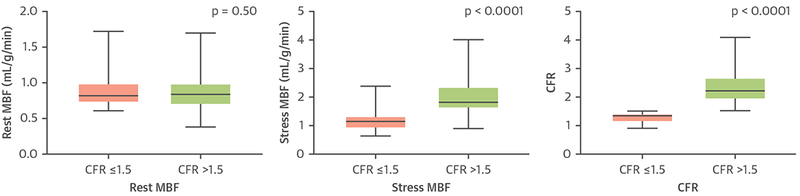
Rest and stress myocardial blood flow (MBF) and coronary flow reserve (CFR) values at baseline positron emission tomography based on CFR.
FIGURE 4. CFR and Rejection Episodes.
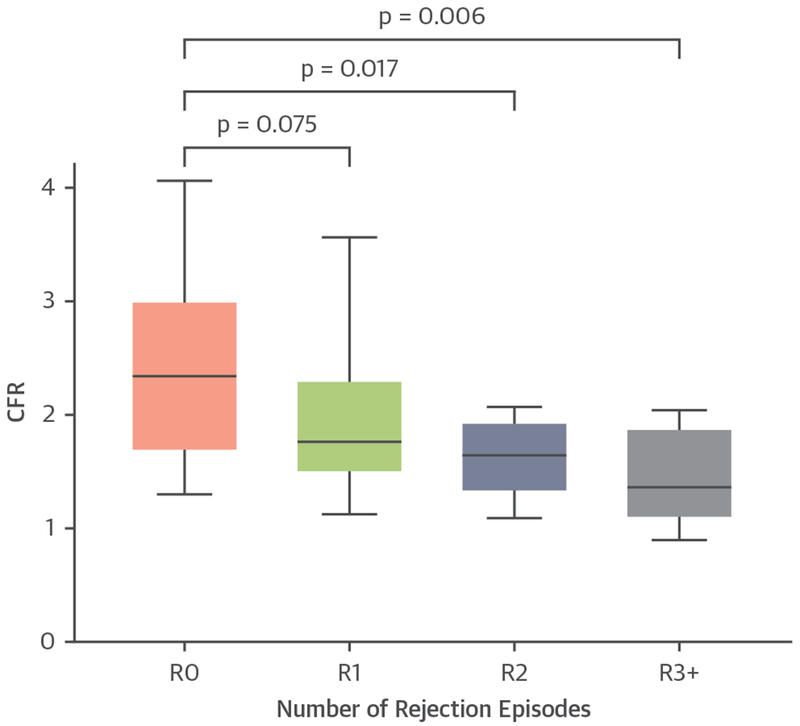
CFR values at baseline positron emission tomography imaging grouped based on the number of rejection (R) episodes prior to imaging. R0 = no rejection prior to PET-1 (baseline); R1 = 1 rejection episode; R2 = 2 rejection episodes; R3+ = 3 or more rejection episodes; other abbreviations as in Figures 1 and 2.
In the 43 patients undergoing left heart catheterization, 28 patients had no detectable CAV, 11 patients had mild CAV, and 4 patients had severe CAV. CFR was significantly lower in patients with severe CAV than in patients with mild or no detectable CAV (CFR: 1.43 ± 0.47 vs. 2.29 ± 0.69, respectively; p = 0.013). There was no significant difference between the CFR of patients with any detectable CAV versus CFR of patients with no detectable CAV (CFR: 2.08 ± 0.91 vs. 2.30 ± 0.63, respectively; p = 0.25).
FOLLOW-UP IMAGING.
CFR corrected for both rest and stress RPP was significantly reduced on PET-2 compared with PET-1, whereas there was a trend toward increasing RPP-corrected resting MBF and a trend toward decreasing RPP-corrected stress MBF (Figure 5). Patients were categorized as having low (≤1.5) or high (>1.5) CFR for both PET-1 and PET-2. A total of 35 patients had normal CFR on both exams (high to high CFR), whereas 7 patients demonstrated an increase in CFR over time (low to high CFR). Patients with low to high CFR showed no change in rest MBF (PET-1: 1.06 ± 0.41 ml/g/min vs. PET-2: 0.96 ± 0.18 ml/g/min; p = 0.58), whereas they demonstrated significant increase in stress MBF (PET-1:1.35 ± 0.53 ml/g/min vs. PET-2:1.92 ± 0.51 ml/g/min; p = 0.04). Nine patients had low CFR on both PET examinations (low to low CFR), whereas 18 patients demonstrated a reduction in CFR over time (high to low CFR).
FIGURE 5. Change in MBF and CFR Over Time.
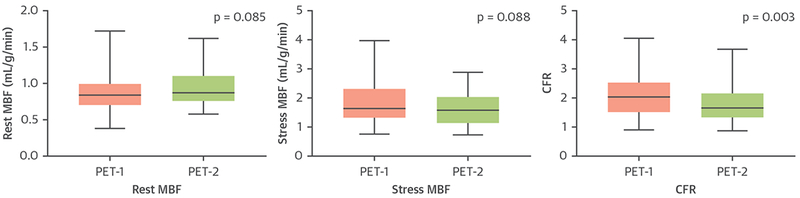
Rest and stress MBF and CFR values derived from PET-1 and PET-2. PET-1 = baseline positron emission tomography; PET-2 = follow-up positron emission tomography; other abbreviations as in Figures 1 and 2.
PATIENT OUTCOMES.
During the median follow-up time of 8.6 years, 40 deaths occurred, 4 patients suffered MI, and 14 patients underwent revascularization. The cause of death was MI in 1 patient, heart failure or severe CAV in 14 patients, sudden cardiac death in 5 patients, stroke in 2 patients, and other cardiovascular causes, such as cardiovascular procedure-related or pulmonary embolism, in 3 additional patients. We were unable to determine the cause of death in 9 patients based on this method. Importantly, prior records indicated that most of these patients had cardiovascular abnormalities preceding their deaths, such as ventricular arrhythmias, impaired LV function, and recurrent hospitalizations for cardiovascular problems.
On Kaplan-Meier survival analysis, patients with a CFR ≥1.5 had an HR of 2.77 (95% CI: 1.34 to 5.74) for death (Figure 6A) and an HR of 2.51 (95% CI: 1.23 to 5.10) for the combined endpoint of death, MI, and revascularization (Figure 6C). Interestingly overall survival was similar in patients reclassified from high to low as patients with low to low CFR, whereas patients reclassified from low to high had similar survival as patients with persistent high CFR (Figure 6B). A similar relationship was observed for the combined endpoint (Figure 6D). In addition, similar results were observed regarding cardiovascular mortality, where patients with a CFR ≥1.5 had an HR of 3.04 (95% CI: 1.38 to 6.69; p = 0.006) for cardiovascular death (Online Figure 3).
FIGURE 6. Mortality and Composite Outcomes.
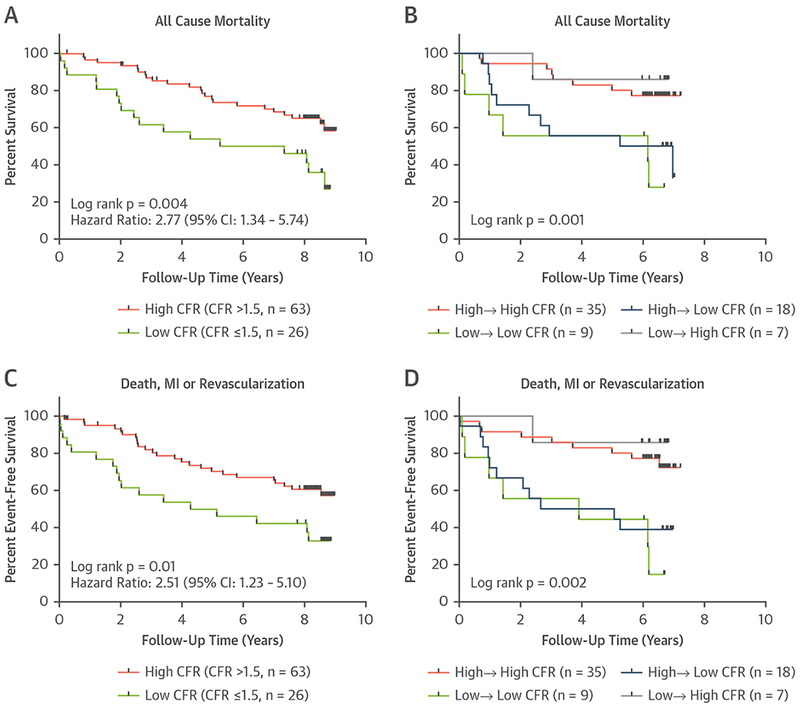
Kaplan-Meier plots for cumulative survival in the entire cohort based on baseline PET-1 CFR (A) and in patients with available follow-up imaging based on PET-1 and PET-2 CFR values (B). High → high represents patients with CFR >1.5 at both PET-1 and PET-2. Low → high CFR represents patients with CFR ≤1.5 at PET-1 and CFR >1.5 at PET-2. Low → low CFR represents patients with CFR ≤1.5 at both PET-1 and PET-2. High → low represents patients with CFR >1.5 at PET-1 and CFR ≤1.5 at PET-2. (C, D) Similar Kaplan-Meier plots are presented for the combined endpoints of death, myocardial infarction (MI), and revascularization. CI = confidence interval, other abbreviations as in Figures 2 and 5.
UNIVARIATE AND MULTIVARIATE PREDICTORS OF MORTALITY.
In univariate Cox proportional hazards regression models performed in patients with available follow-up imaging, higher baseline CFR, represented by a 0.73-U (1 SD) increase, was a negative predictor of overall mortality (Figure 7A). In addition, a decrease in CFR over time, presented as a 0.79-U (1 SD) decrease in CFR from PET-1 to PET-2, was associated with increased overall mortality in univariate analyses. Other significant univariate predictors (p < 0.10) of increased risk of overall mortality included the number of previous rejections, creatinine level, PET-1 stress RPP, PET-1 LV perfusion defect size, and an increase in LV perfusion defect size over time (PET-1 to PET-2). Only higher baseline CFR (per 1-SD increase) and a decrease in CFR from PET-1 to PET-2 (per 1-SD decrease) remained independent predictors of overall mortality in the multivariate models (Figure 7B). In a separately performed analysis, including stress MBF instead of CFR, baseline stress MBF and decline in stress MBF were univariate predictors of mortality (Online Figure 4A), whereas in multivariate models, only the decline of stress MBF remained a strong independent predictor of overall mortality (HR: 5.07; 95% CI: 1.68 to 15.28; p = 0.004) and baseline stress MBF was no longer an independent predictor of mortality (HR: 0.49; 95% CI: 0.20 to 1.16; p = 0.10) (Online Figure 4B).
FIGURE 7. Forest Plot of HR for Overall Survival.
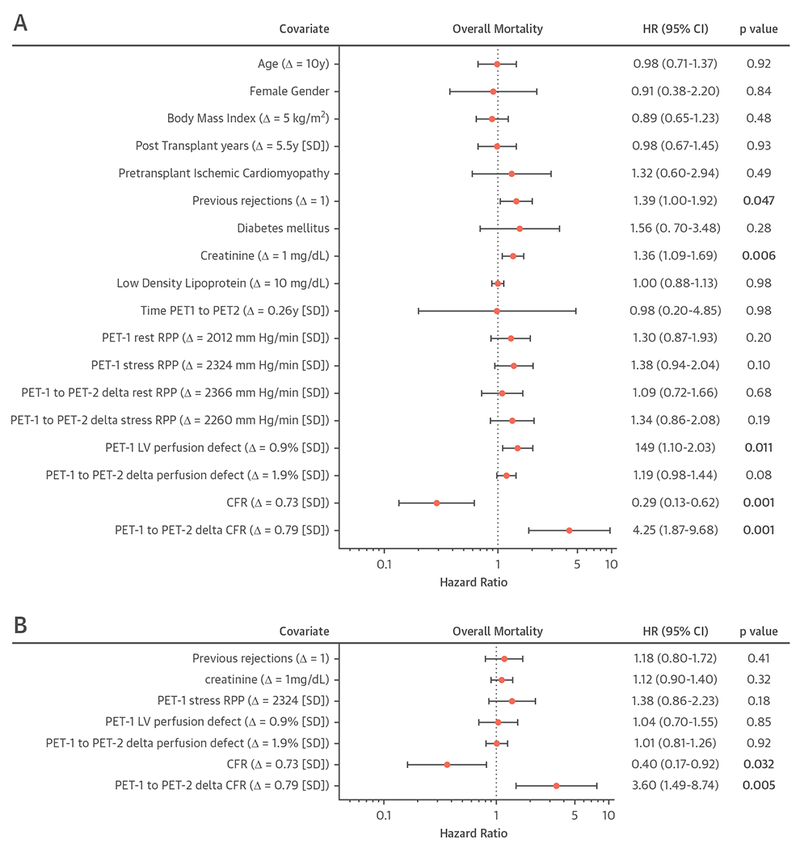
Forest plot of hazard ratios (HR) of overall mortality in univariate (A) and multivariate (B) Cox proportional hazards regression models in patients with available follow-up imaging. The risk represented by CFR is presented in 2 separate ways in Cox regression modeling: the hazard HR for PET-1 CFR is presented for a 0.73 ± 1.00-U increase in CFR, the HR for change in CFR from PET-1 to PET-2 is presented for a 0.79 ± 1.00-U decrease in CFR from PET-1 to PET-2. For other continuous variables, HR are presented as an SD increase for rate pressure product (RPP) and left ventricular (LV) perfusion defect size, a 10-year increase in age, a 5-kg/m2 increase for body mass index, a 5.7 ± 1-year increase for post-transplantation years, a 1 mg/dl increase in creatinine, and a 10 mg/dl increase in low-density lipoprotein. HR of previous rejections is presented as an increase of 1 in the number of prior rejections. Abbreviations as in Figures 2, 5, and 6.
DISCUSSION
The major finding of the current study is that we show for the first time that CFR assessed by dynamic 82Rb PET predicts long-term mortality (>5 years) in HT patients. In addition, we present the first evidence of the incremental prognostic value offered by serial PET CFR assessment in predicting overall mortality risk in HT recipients.
PRIOR STUDIES: MBF QUANTIFICATION BY PET.
The knowledge provided by previous small-sized studies evaluating PET-derived MBF in HT recipients contributed significantly to the understanding of the pathophysiology of CAV. Elevated resting MBF has been demonstrated in HT patients, which is partially explained by elevated resting heart rates due to allograft vagal denervation (8). In accordance with our findings, prior studies have described a homogenous distribution of CFR across all coronary territories, indicating the diffuse involvement of CAV (8,9). Also, in line with our results, multiple groups have reported a significant inverse relationship between global CFR and time from cardiac transplantation (3,10). In addition, Kushwaha et al. (11) described increased hyperemic vascular resistance (calculated as mean arterial pressure over hyperemic blood flow) in patients >3 years post-HT compared with patients within 3 years of HT, a finding suggestive of microvascular dysfunction.
An early study by Zhao et al. (10) showed that serial MBF quantification using nitrogen N 13 ammonia PET in 35 HT patients allowed for the detection of changes in resting MBF over time in this population. Despite serially quantifying MBF with PET, this study protocol did not incorporate stress testing, thus CFR calculations were not available and the added prognostic value of serially measuring CFR remained unclear. Our results extend these previous findings, as we report for the first time that global CFR decreases on serial assessment in HT patients, and provide compelling evidence of an incremental prognostic value of serial CFR evaluation in this patient population in terms of predicting all-cause mortality. Our data suggest that the temporal decrease in peak hyperemic MBF might be responsible for the predictive value of the CFR reduction. Interestingly, patients that had a change in CFR from low to high or high to low on repeat imaging carried the same risk as patients with continual high or low CFR values on repeat imaging, respectively. These data indicate that serial CFR assessment with PET may also be useful in reclassifying patient risk for all-cause mortality. Of note, the list of available treatment options for CAV remains very limited; however, recent studies suggest that angiotensin-converting enzyme inhibitors or mammalian target of rapamycin inhibitors may be useful for the prevention and/or treatment of CAV (12,13). Importantly, a reduction in CFR over time may indicate higher risk for CAV and warrant closer monitoring for these patients.
We showed increasing stress MBF in a subset of patients that we classified with low to high CFR; however, our study does not provide insight into the underlying mechanism of this change. We can speculate that such an increase can be related to changes in cardiovascular and immunosuppressive regimens, linked to sympathetic reinnervation, or related to subclinical rejection episodes at the time of PET-1 that were resolved by time PET-2. Of note, MBF values were normalized to the RPP, and therefore changes in rest and stress hemodynamic status are less likely responsible for this finding.
More recently, Mc Ardle et al. (3) reported that reduced 82Rb PET CFR in HT patients was associated with a higher risk for the combined endpoint of death, acute coronary syndrome, and heart failure hospitalization during a 1.5-year median follow-up time. Although this study included 140 patients, the number of adverse events compared with the sample size was relatively low (n = 15, 11%), most likely secondary to the short follow-up time. Along these lines, CFR was only associated with a higher risk of a composite endpoint and independent predictors of adverse outcomes were not reported. Our results extend the findings of these prior studies, as we provide evidence of the long-term, independent predictive value of PET CFR for all-cause mortality that persists with a median follow-up time beyond 8 years.
CORRELATION OF PET MBF AND REJECTION EPISODES.
Growing evidence indicates an association between acute rejection and CAV (14,15). Severe impairment in CFR has been demonstrated during acute rejection episodes by using serial nitrogen N 13 ammonia PET imaging (15). In this study, whereas CFR was significantly depressed during acute rejection episodes, it improved significantly after recovery (15). Our results extend these findings and suggest that despite recovery of CFR after an acute rejection, rejection episodes may contribute cumulatively to long-term reduction in CFR. In support of this finding, evidence suggests that acute allograft rejection episodes are strongly associated with the future incidence of CAV, especially within first year of HT (14).
STUDY LIMITATIONS.
Despite the fact that this was one of the largest studies reporting on PET CFR in HT recipients to date, this study was a single-center, nonrandomized, observational study, which carries all the inherent limitations of such a study design. Therefore, we cannot exclude the presence of confounders, despite carefully controlling for numerous covariables reported to be associated with mortality and CAV in the HT population.
All patients within 5 years of HT had undergone coronary angiographic assessment for CAV, however coronary angiography was not routinely performed for study participants beyond this time frame. In addition, IVUS was not routinely performed, and therefore it was not included in our analyses. Nevertheless, prior studies have shown only modest correlation between PET-derived CFR and IVUS indices likely related to the fact that IVUS is only able to interrogate the large epicardial vessels, but not the microvasculature. In addition, the absence of moderate-sized or large-sized perfusion defects in the vast majority of our patients suggests that diffuse large vessel disease or impaired microvascular function is responsible for reduced CFR.
We applied a broad inclusion criterion for participation in the study that included all consecutive HT patients regardless of their time interval post-transplantation. This resulted in wide variation in the time between HT and imaging studies. Despite this, by including patients with a wide range of post-transplantation years, we were able to detect a weak, but significant inverse correlation between PET CFR and post-transplantation years. Importantly, CFR and change in CFR over time remained significant predictors of overall mortality, even after controlling for post-transplantation years.
Follow-up PET was not available in all patients enrolled in the study; however, follow-up PET scan rate was considerably high (90%), when not accounting for the patients who died before follow-up could have been performed. On repeat imaging, the same stressor agent (dipyridamole) was employed in most of the cases (91%), whereas regadenoson and dobutamine were used in the remaining patients. As previously reported, regadenoson stress is only able to achieve 80% of dipyridamole hyperemia as determined by quantitative 82Rb PET (16). Theoretically, the decrease in CFR over time in patients administered regadenoson at follow-up PET could represent a lower level of hyperemia achieved with this stressor than that observed at baseline with dipyridamole. However, in the 6 patients with nondipyridamole PET-2, only 1 patient showed a reduction in CFR from PET-1 to PET-2. Of note, a recently published study demonstrated that dipyridamole hyperemia can be 10% lower when PET imaging is performed at 7 to 8 min versus at 12 min after the start of dipyridamole infusion, suggesting that our results might have underestimated hyperemic MBF and hence CFR (17).
CONCLUSIONS
Our results indicate that CFR derived from dynamic 82Rb PET predicts long-term overall mortality and adverse cardiovascular outcomes in HT recipients. In addition, temporal change in CFR was demonstrated to be an additional independent risk factor for all-cause mortality. Large, prospective studies are needed to validate the predictive value of PET CFR in the HT population and to assess whether PET-derived CFR can direct HT patient management.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
CAVisa major determinant of late mortality in HT recipients. Quantification of MBF and CFR by PET holds great promise for evaluation of CAV by assessing abnormalities in epicardial flow and microvascular function. Our study provides evidence that baseline CFR and reduction in CFR over time are independent predictors of all-cause mortality.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Quantitative assessment in myocardial perfusion and determination of absolute MBF can be achieved noninvasively using dynamic PET imaging. A widespread application of this technique in HT patients would decrease the number of invasive coronary angiography procedures that carry infrequent, but significant risk for complications. Furthermore, this would reduce contrast administration during angiography, which can lead to renal dysfunction.
TRANSLATIONAL OUTLOOK:
Further large-scale, randomized-controlled studies are warranted to determine the predictive value of PET-derived CFR in transplant patients and to assess whether PET-derived CFR can direct medical management in the HT population.
Acknowledgments
This study was supported by a National Institutes of Health T32 training grant (HL098069 to Dr. Sinusas), by the American Society of Nuclear Cardiology (2009 Nuclear Cardiology Foundation Pilot and Feasibility Award to Dr. Srivastava), and by the British Heart Foundation-Fulbright scholar award (FS/16/28/32327 to Dr. Quail). Dr. Miller is a consultant and for Bracco, Inc. and GE Healthcare, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CAV
cardiac allograft vasculopathy
- CFR
coronary flow reserve
- CI
confidence interval
- CT
computed tomography
- 2D
2-dimensional
- IQR
interquartile range
- IVUS
intravascular ultrasound
- HR
hazard ratio
- HT
heart transplantation
- LV
left ventricle
- MBF
myocardial blood flow
- MI
myocardial infarction
- PET
positron emission tomography
- 82Rb
rubidium Rb 82
- RPP
rate-pressure product
Footnotes
APPENDIX For supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Lund LH, Edwards LB, Dipchand AI, et al. , for the International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: thirtythird adult heart transplantation report–2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35:1158–69. [DOI] [PubMed] [Google Scholar]
- 2.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mc Ardle BA, Davies RA, Chen L, et al. Prognostic value of rubidium-82 positron emission tomography in patients after heart transplant. Circ Cardiovasc Imaging 2014;7:930–7. [DOI] [PubMed] [Google Scholar]
- 4.Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765–74. [DOI] [PubMed] [Google Scholar]
- 5.Liu YH. Quantification of nuclear cardiac images: the Yale approach. J Nucl Cardiol 2007;14:483–91. [DOI] [PubMed] [Google Scholar]
- 6.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy–2010. J Heart Lung Transplant 2010;29:717–27. [DOI] [PubMed] [Google Scholar]
- 7.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 8.Allen-Auerbach M, Schoder H, Johnson J, et al. Relationship between coronary function by positron emission tomography and temporal changes in morphology by intravascular ultrasound (IVUS) in transplant recipients. J Heart Lung Transplant 1999;18:211–9. [DOI] [PubMed] [Google Scholar]
- 9.Wu YW, Chen YH, Wang SS, et al. PET assessment of myocardial perfusion reserve inversely correlates with intravascular ultrasound findings in angiographically normal cardiac transplant recipients. J Nucl Med 2010;51:906–12. [DOI] [PubMed] [Google Scholar]
- 10.Zhao XM, Delbeke D, Sandler MP, Yeoh TK, Votaw JR, Frist WH. Nitrogen-13-ammonia and PET to detect allograft coronary artery disease after heart transplantation: comparison with coronary angiography. J Nucl Med 1995;36:982–7. [PubMed] [Google Scholar]
- 11.Kushwaha SS, Narula J, Narula N, et al. Pattern of changes over time in myocardial blood flow and microvascular dilator capacity in patients with normally functioning cardiac allografts. Am J Cardiol 1998;82:1377–81. [DOI] [PubMed] [Google Scholar]
- 12.Asleh R, Briasoulis A, Kremers WK, et al. Longterm sirolimus for primary immunosuppression in heart transplant recipients. J Am Coll Cardiol 2018;71:636–50.29420960 [Google Scholar]
- 13.Fearon WF, Okada K, Kobashigawa JA, et al. Angiotensin-converting enzyme inhibition early after heart transplantation. J Am Coll Cardiol 2017;69:2832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raichlin E, Edwards BS, Kremers WK, et al. Acute cellular rejection and the subsequent development of allograft vasculopathy after cardiac transplantation. J Heart Lung Transplant 2009;28:320–7. [DOI] [PubMed] [Google Scholar]
- 15.Chan SY, Kobashigawa J, Stevenson LW, Brownfield E, Brunken RC, Schelbert HR. Myocardial blood flow at rest and during pharmacological vasodilation in cardiac transplants during and after successful treatment of rejection. Circulation 1994;90:204–12. [DOI] [PubMed] [Google Scholar]
- 16.Johnson NP, Gould KL. Regadenoson versus dipyridamole hyperemia for cardiac PET imaging. J Am Coll Cardiol Img 2015;8:438–47. [DOI] [PubMed] [Google Scholar]
- 17.Kitkungvan D, Johnson NP, Roby AE, Patel MB, Kirkeeide R, Gould KL. Routine clinical quantitative rest stress myocardial perfusion for managing coronary artery disease: clinical relevance of testretest variability. J Am Coll Cardiol Img 2017;10:565–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


