Abstract
Intravenous (i.v.) drug self-administration remains the “gold standard” for assessing abuse potential of drugs. Failure of a drug to maintain self-administration might indicate merely the absence of positive reinforcing effects but might also indicate presence of aversive effects. Sensitivity to aversive effects is thought to affect the initiation and maintenance of drug use as well as relapse. Choice procedures are used to study positive reinforcing effects of drugs and to a much lesser extent to study punishing effects of drugs. Experiment 1 compared the mu opioid receptor agonist remifentanil (0.001–0.01 mg/kg/infusion), the kappa receptor agonist spiradoline (0.0056–0.056 mg/kg/infusion), and histamine (1.0 mg/kg/infusion) in rats choosing between a food pellet only and an intravenous (i.v.) infusion + a food pellet. To test whether a history with one punishing drug affects the punishing effects of a second drug, Experiment 2 compared sensitivity to spiradoline in rats with and without a history of histamine punishment. All rats predominantly chose a pellet alone when histamine + a pellet was the alternative, and they predominantly chose remifentanil + a pellet over a pellet alone. In Experiment 2, spiradoline was punishing in rats with a history of histamine punishment but not drug-naïve rats. This food choice procedure is sensitive to reinforcing and punishing effects of different drugs in the same subjects, suggesting that the procedure is well-suited for studying drug mixtures (e.g., mu and kappa agonists) and the impact of different physiological conditions (e.g., pain) on reinforcement and punishment.
Keywords: opioid, aversion, reinforcement, punishment, choice, operant behavior, drug history, rat
INTRODUCTION
Intravenous (i.v.) drug self-administration remains the gold standard for assessing abuse potential of drugs and candidate treatments for substance abuse (Hursh et al., 2005; Panlilio and Goldberg, 2007; Howell and Fantegrossi, 2009). For a broad range of drugs from different classes (with several noteworthy exceptions), there is a strong positive (predictive) relationship between positive reinforcing effects in nonhumans (i.e., self-administration) and the likelihood of abuse. Failure of a drug to maintain self-administration reflects the absence of positive reinforcing effects. However, most self-administration single- or concurrent schedule procedures, in which only positive reinforcing effects are measured, do not provide information on causal factors for the absence of drug-maintained responding. Other drug effects influencing self-administration might become apparent under conditions where both positive reinforcing and punishing effects of drugs can be studied in the same subjects.
The behavioral, including reinforcing, effects of drugs are impacted by a variety of factors, including drug and behavioral history as well as the particular environmental conditions under which they are studied. For example, squirrel monkeys have been shown to respond reliably on one lever to receive cocaine infusions and concurrently respond on a second lever to terminate scheduled cocaine infusions (Spealman, 1979). Monkeys self-administer i.v. nicotine alone but not nicotine paired with i.v. remifentanil when remifentanil alone is available (Koffarnus and Winger, 2015). These and other studies clearly demonstrate that all drugs have dose-dependent multiple effects suggesting that other (non-reinforcing or aversive) effects of a drug might contribute to or limit the expression of its reinforcing effects.
Choice procedures appear to be especially well suited for examining how the aversive effects of drugs might influence the extent to which a drug is self-administered and/or is likely to be abused (e.g., Riley, 2011; Verendeev and Riley, 2013). However, this approach has received little attention to date, with only a limited number of studies having been conducted to examine the potentially important aversive effects of drugs with reinforcing properties (Woolverton, 2003; Podlesnick et al., 2010; Woolverton et at., 2012; Podlesnick and Jimenez-Gomez 2013; Freeman et al., 2014; Koffarnus and Winger, 2015). A stimulus (e.g., drug) has punishing effects if response-dependent presentation of that stimulus decreases the frequency of responding (Azrin and Holz 1966; Skinner 1953); in choice procedures, a punishing effect is indicated by the re-allocation of behavior to an alternative response (e.g., lever) rather than the suppression of responding.
The present study used a food versus drug + food choice procedure in rats to compare opioid receptor agonists that act selectively at either kappa or mu receptors. Spiradoline (kappa agonist) was studied because it produces dysphoria in humans (Pfeiffer et al., 1986; Wadenberg, 2003) and conditioned place avoidance in rats (Rech et al., 2011), presumably reflecting its aversive properties. Remifentanil (mu agonist) was studied because it maintains responding in self-administration procedures under a broad range of conditions (e.g., Cooper et al., 2008; Sukhtankar et al., 2014), reflecting its positive reinforcing effects. Histamine also was studied because it has served as an effective punisher in choice procedures, presumably reflecting its aversive properties (Woolverton, 2003; Podlesnick et al., 2010; Woolverton et at., 2012; Podlesnick and Jimenez-Gomez 2013). It is well understood that behavioral or pharmacological history, as well as behavioral experiences during initial drug exposure, can determine subsequent drug effects (e.g., Hoffmeister and Schlichting, 1972; Smith et al., 1978; Stein et al., 2013, 2015). Thus, this study also tested whether exposure to one drug (i.e., spiradoline or histamine) alters the later exposure to a second drug (i.e., histamine or spiradoline, respectively).
METHODS
Subjects
Twenty-six male Sprague-Dawley rats (Envigo, Indianapolis, Indiana, USA) weighed 250–275 g upon arrival and were individually housed in 45 × 24 × 20 cm plastic cages with rodent bedding and free access to water. Rats had free access to standard rodent chow (Envigo Teklad) for one week while habituated to handling. Thereafter rats were food restricted to 15 ± 5 g after daily sessions, and allowed to grow to a maximum body weight of 375 ± 5 g. A 14:10 h light:dark cycle was in effect (lights on at 06.30 h) with sessions conducted during the light period.
Ten rats were in Experiment 1 (due to loss of catheter patency or illness, 4 did not complete the study). Prior to the start of Experiment 1, rats responded under fixed-ratio schedules for different pellet amounts (i.e., 3 versus 1 and 2 versus 1) and different volumes, rates, and durations of histamine infusions. These initial parametric studies were conducted in order to optimize the experimental design; the terminal parameters and criteria described below were selected based on findings from these preliminary studies.
Sixteen additional rats were experimentally-naïve at the start of Experiment 2 and were randomly assigned to two groups of 8 after recovery from surgery; all 16 rats completed Experiment 2. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and in accordance with guidelines set forth by the Guide for the Care and Use of Laboratory Animals.
Apparatus
Sessions were conducted in commercially available operant conditioning chambers (31 × 24 × 21 cm; ENV-008CT; Med Associates, Inc., St Albans, Vermont, USA) enclosed in ventilated, sound-attenuating cubicles (ENV-022M; Med Associates, Inc.). The side panels of the chamber were Plexiglas, and the rear and front panels were aluminum. The front panel was equipped with two response levers horizontally aligned 11.5 cm apart. Above each lever was a 2.5-cm diameter stimulus light that could be trans-illuminated white with a 100 mA bulb (lever lights). A feeder dispensed 45-mg grain-based pellets (F0165; Bio-Serv, Frenchtown, New Jersey, USA) to a 5 × 5 cm food aperture centrally located between the two levers. The rear panel was equipped with a 100 mA house light centered 2 cm from the top of the chamber. A syringe pump (PHM-100, Med Associates, Inc.) delivered i.v. infusions through Tygon tubing connected to a 22-G single channel plastic swivel (375/22PS; Instech Laboratories, Inc., Plymouth Meeting, Pennsylvania, USA) and spring tether (VAB95T; Instech Laboratories, Inc.) that attached to a vascular access button (VAB95BS; Instech Laboratories, Inc.) on the back of the rat. MED-PC IV software and a PC-compatible interface (Med Associates, Inc.) controlled experimental events and recorded data.
Surgery
Rats were anesthetized with 2% isoflurane and prepared with a chronic indwelling catheter (CNC-3H-30–6/6.5; Access Technologies, Skokie, Illinois, USA) in the left femoral vein. The catheter was tunneled subcutaneously (s.c.) to the mid-scapular region, where it attached to a 22-G access port (VAB95BS; Instech Laboratories, Inc.) anchored in a s.c. pocket. Immediately after surgery, rats received s.c. injections of 0.5 ml penicillin (300,000 U/ml) and 1.0 mg/kg meloxicam and were allowed 7 days to recover before resuming sessions. Catheters were flushed with 0.5 ml of heparinized saline (100 U/ml) daily during recovery and after each session and flushed with 0.5 ml of sterile saline before each session. If catheter patency was compromised, as indicated by resistance in flushing, abnormal behavior, leakage of fluid, or failure to achieve sedation immediately after an infusion of 3.2 mg/kg of methohexital, another catheter was implanted in the right femoral vein or a jugular vein.
Procedure
Sessions comprised up to 100 trials in a maximum of 100 min. The first two trials were sampling trials in which either the left lever or right lever was active, signaled by illumination of the left or right stimulus light, respectively. The position (i.e., left or right) was selected at random for the first sampling trial, and the alternative lever was presented in the second sampling trial. The remaining trials (up to 98) were choice trials in which both levers were active, signaled by the illumination of both stimulus lights. A single response to an active lever extinguished the stimulus lights, resulted in a brief (0.1-s) flash of the house light, and delivered a food pellet. Food delivery was followed by a 10-s inter-trial interval, during which the stimulus lights were extinguished and lever presses had no scheduled consequence. For all trials, failure to respond within a 60-s limited hold was scored as an omission and initiated the inter-trial interval. Rats had a minimum of 7 consecutive sessions in which both sampling trials and at least 90% of choice trials were completed before surgery.
The procedure following surgery was identical to that described above, except that responding on one lever delivered a food pellet while responding on the other lever delivered an i.v. infusion + a food pellet. The lever designation (i.e., left or right) associated with the infusion + a pellet was switched twice (A-B-A reversal design) for each drug and dose studied. Lever reversals occurred after a minimum of 3 sessions according to the following criteria: 1) percent choice for the infusion + a pellet decreased to 25% or less for histamine and spiradoline tests (based on demonstrated and hypothesized aversive effects, respectively); 2) percent choice for the infusion + a pellet increased to at least 75% for remifentanil tests (based on demonstrated positive reinforcing effects); or 3) a maximum of 7 sessions. For saline tests, the lever reversals occurred after a minimum of 3 consecutive sessions in which both sampling trials and at least 90% of choice trials were completed. When drug or dose was changed, the infusion initially was paired with the preferred lever (i.e., the lever selected on more than 50% of choice trials) from the final session in the preceding condition; if a rat responded equally on both levers in the preceding condition the lever position was selected randomly.
Experiment 1: Effects of histamine, spiradoline, and remifentanil on choice.
The purpose of this experiment was to use a choice procedure (Podlesnick et al. 2010) to test opioid receptor agonists for reinforcing and punishing effects in the same subjects. After testing saline, histamine (1.0 mg/kg/infusion) was tested to confirm sensitivity to a drug previously shown to be a punisher when paired with food in similar choice procedures (Woolverton 2003; Podlesnick et al. 2010; Podlesnick and Jimenez-Gomez 2013). Next, the kappa opioid receptor agonist spiradoline (0.056 and 0.0056 mg/kg/infusion) was tested. Kappa agonists produce dysphoria in humans and place avoidance conditioning as well as suppression of ongoing self-administration in nonhumans, presumably reflecting their aversive properties (Mucha and Herz 1985; Pfeiffer et al. 1986; Glick et al. 1995; Schenk et al. 1999; Wadenberg 2003). Spiradoline was tested to determine whether punishing effects were evident with a drug from a different pharmacological class (i.e., a kappa opioid receptor agonist compared with histamine used in previous studies). These doses of spiradoline were selected on the basis of our prior work with this compound in rats and its potency relative to the kappa agonist U50488 that has been studied extensively in rats (Di Chiara and Imperato 1988; Holtzman 2000; Morani et al. 2009). In contrast to kappa agonists, mu opioid receptor agonists are abused by humans and readily self-administered by nonhumans. Two doses of the mu opioid receptor agonist remifentanil (0.01 and 0.001 mg/kg/infusion) were tested to determine whether this procedure was sensitive to positive reinforcing effects in addition to punishing effects in the same subjects. These doses of remifentanil were selected on the basis of its potency as a positive reinforcer in other studies (O’Connor and Mead 2010; Panlilio and Schindler 2000). Histamine (1.0 mg/kg/infusion) was studied before and after remifentanil to test for possible changes in punishing effects that might result from experience with a drug having positive reinforcing effects.
Experiment 2:Drug history and the punishing effects of spiradoline.
Because the order in which drugs were tested was the same for all subjects in Experiment 1, it was unclear whether exposure to histamine affected the subsequent exposure to spiradoline. To test this possibility, a 2-sequence crossover design was used in Experiment 2 to examine possible differences in punishing effects when histamine was tested before spiradoline and, in a separate group of rats, when spiradoline was tested before histamine. Saline was studied first in all rats (n=16). Next, one group (n=8) was tested with i.v. histamine (1.0 mg/kg/infusion) while a second group (n=8) was tested with i.v. spiradoline (0.056 mg/kg/infusion). If a rat completed an average of fewer than 20 choice trials for both lever designations (see lever switching as described above), then the dose was decreased to 0.32 mg/kg/infusion for histamine (1 rat) and 0.0056 mg/kg/infusion for spiradoline (6 rats) before testing the second drug in the sequence. When spiradoline was tested in rats that initially received histamine, 6 rats required additional testing with a smaller dose (0.0056 mg/kg/infusion); and when histamine was tested in rats that initially received spiradoline, 2 rats required additional testing with a smaller dose (0.32 mg/kg/infusion).
Drugs
Histamine dihydrochloride (Sigma-Aldrich, St. Louis, MO), spiradoline mesylate (Upjohn, Kalamazoo, Michigan, USA), and remifentanil hydrochloride (National Institute on Drug Abuse Drug Supply Program, Bethesda, Maryland, USA) were dissolved in sterile 0.9% saline. I.V. infusions were in a volume of 1.0 ml/kg body weight at a rate of 3.36 ml/min. Methohexital sodium was generously provided by Eli Lilly and Company (Indianapolis, Indiana, USA), dissolved in sterile 0.9% saline, and administered in a volume of 1.0 ml/kg. All doses are expressed in terms of the salt.
Data Analyses
For each session, the number of choices for a pellet only and the number of choices for the infusion + a pellet were divided by the total number of choice trials completed to obtain percent choice for each alternative. Percent choice for a pellet only was subtracted from percent choice for the infusion + a pellet as a measure of relative choice (i.e., difference scores), with positive values indicating preference for the infusion + a pellet, negative values indicating preference for a pellet alone, and values not different from 0 indicating no preference (i.e., indifference). To construct dose-response functions for relative choice and number of trials completed, the last session from each condition (A-B-A) was included to obtain a mean value for each rat. A one-way repeated measures ANOVA with Bonnferoni’s post-hoc test (Experiment 1) and a two-way ANOVA with Bonferroni’s post-hoc test (Experiment 2) were used to analyze relative choice and the number of trials completed for each dose compared with saline; differences were considered significant for p<0.05. Analyses were conducted using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Experiment 1.
When choosing between a pellet only and saline + a pellet, rats were generally indifferent to saline infusions and responded approximately equally on the two levers, yielding a relative choice value that was not significantly different from zero (Fig 1, triangle, upper panel). When choosing between a pellet only and histamine + a pellet, rats chose predominantly a pellet only (Fig 1, circle, upper panel; t=7.9, p<0.001,). Neither saline nor histamine decreased the number of trials completed (Fig 1, lower panel). When choosing between a pellet and remifentanil + a pellet, rats chose 0.01 mg/kg/infusion + a pellet over a pellet only (Fig 1, diamonds, upper panel; t=6.7, p<0.001,) despite a significant decrease in the number of trials completed (Fig 1, lower panel; t=4.2, p<0.002,). The effects on choice and trials completed with a 10-fold smaller dose of remifentanil (0.001 mg/kg/infusion) were not different from saline. When choosing between a pellet and 0.056 mg/kg/infusion spiradoline + a pellet, rats responded predominantly for a pellet only (Fig 1, squares, upper panel; t=6.0, p<0.001,); this dose of spiradoline significantly decreased the number of trials completed (Fig 1, lower panel; t=3.4, <0.02,). The effects on choice and trials completed with a 10-fold smaller dose of spiradoline (0.0056 mg/kg/infusion) were not different from saline. The effects of histamine in each of two re-determinations (data not shown) were not significantly different from the initial effects of histamine.
Figure 1.
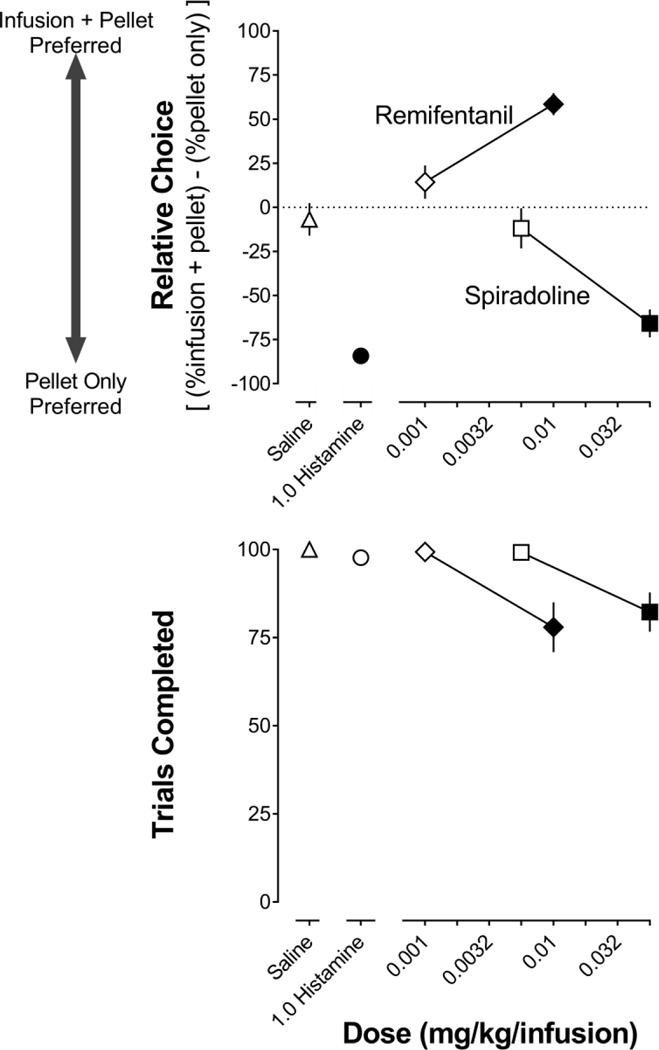
Mean (±1 SEM) relative choice for each alternative (upper) and the number of trials completed (lower) during saline (triangles), histamine (circles), remifentanil (diamonds), and spiradoline (squares) tests in Experiment 1. Ten rats completed tests with saline and histamine, 7 rats completed tests with spiradoline (0.056 mg/kg/infusion), and 6 rats completed the remaining tests (spiradoline [0.0056 mg/kg/infusion], remifentanil [0.001–0.01 mg/kg/infusion], and histamine redetermination). Data shown are for only the 6 rats that completed the study, with filled symbols denoting a significant (p<0.05) effect compared with saline. C: unit dose in mg/kg/infusion.
Fig 2 shows detailed patterns of responding across 3 consecutive sessions for a representative rat. The left panels show a summary measure (percent choice for the infusion + a pellet) for each session, while the right panels show a corresponding heat map depicting the response type for each of 100 trials. Omitted trials (no response within 60 sec) are indicated by white; responding for a pellet only is indicated by light gray shading; and responding for an infusion + a pellet is indicted by dark gray shading. Percent choice for an infusion of saline + a pellet neither increased nor decreased systematically over 3 consecutive sessions (Fig 2, upper left panel), with responses for each alternative evenly distributed within each session (Fig 2, upper right panel). Percent choice for an infusion of 1.0 mg/kg/infusion histamine or 0.056 mg/kg/infusion spiradoline + a pellet decreased progressively across 3 consecutive sessions to less than 25% (Fig 2, center left panels). Responses for the infusion (histamine or spiradoline) + a pellet were evenly distributed at the start of a session and later in the session, with many trials being omitted (Fig 2, center right panels). By the third session with histamine or spiradoline, fewer trials were omitted as the rat increasingly chose the pellet only; responding for the infusion + a pellet tended to be concentrated towards the beginning of the session (Fig 2, center right panels). Percent choice for an infusion of 0.01 mg/kg/infusion remifentanil + a pellet increased across 3 consecutive sessions to a maximum of greater than 90% (Fig 2, lower left panel). The relatively few choices for remifentanil + a pellet in the first session were evenly distributed across trials (Fig 2, lower right panel). Responding for remifentanil + a pellet increased in the second and third sessions (Fig 2, lower left panel) with responding for the pellet alone concentrated towards the start of the session (Fig 2, lower right panel).
Figure 2.
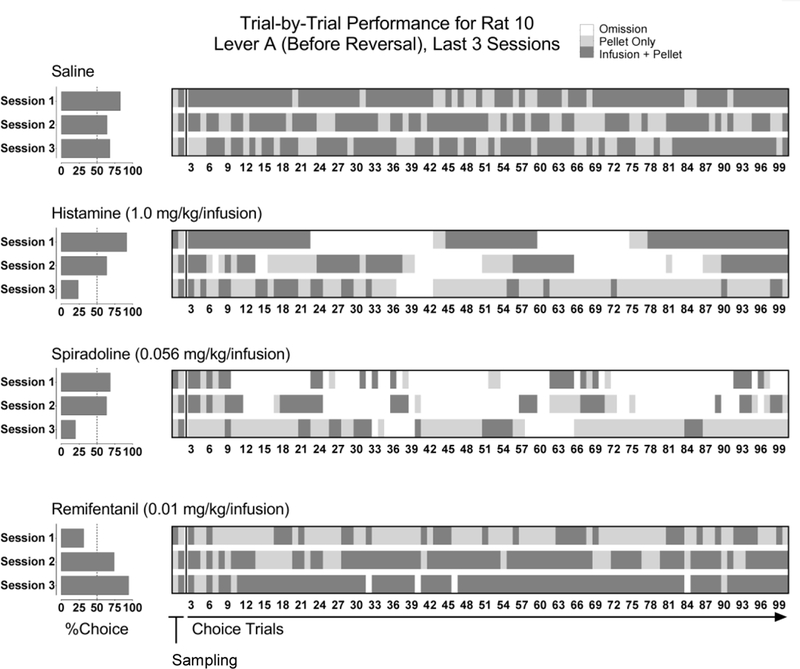
Representative patterns of responding for an individual rat across 3 consecutive sessions of saline, histamine, spiradoline, or remifentanil paired with one lever (Lever A; i.e., before a lever reversal). Percent choice for the infusion + a pellet (left panels) and corresponding heat maps depicting response type for each trial (right panels). Trial-by-trial performance is color-coded as follows: white represents no response (an omission); light gray represents a response for a pellet only; and dark gray represents a response for the infusion + a pellet. The preceding condition to saline was no infusion (i.e., before surgery). The preceding condition to histamine (1.0 mg/kg/infusion) was saline (paired with Lever A, reversal). The preceding condition to both spiradoline (0.056 mg/kg/infusion) and remifentanil (0.01 mg/kg/infusion) was histamine (paired with Lever A, reversal).
Experiment 2.
When choosing between a pellet only and saline + a pellet, all rats were generally indifferent to saline infusions, responding on the two levers approximately equally (Fig 3, upper panel). The rats that were tested first with histamine chose a pellet alone over histamine + a pellet (Fig 3, black circles, upper panel; t=5.0, p<0.001 for 1.0 mg/kg/infusion). Rats that received histamine first also chose predominantly a pellet alone when the alternative was spiradoline + a pellet (Fig 3, black squares, upper panel; t=3.2 for 0.0056 and 0.056 mg/kg/infusion, p<0.01). In contrast, rats that were tested first with spiradoline responded approximately equally on each lever with relative choice not being different from saline (Fig 3, gray squares, upper panel; t=0.51 for 0.0056 mg/kg/infusion and t=0.75 for 0.056 mg/kg/infusion, NS); however, rats tested first with spiradoline subsequently chose a pellet alone when the alternative was histamine + a pellet (Fig 3, gray circles, upper panel; t=2.9, p<0.02 for 0.32 mg/kg/infusions and t=4.2, p<0.001 for 1.0 mg/kg/infusion). In both groups of rats, spiradoline and histamine decreased the number of trials completed in a dose-related manner (Fig 3 lower panel).
Figure 3.
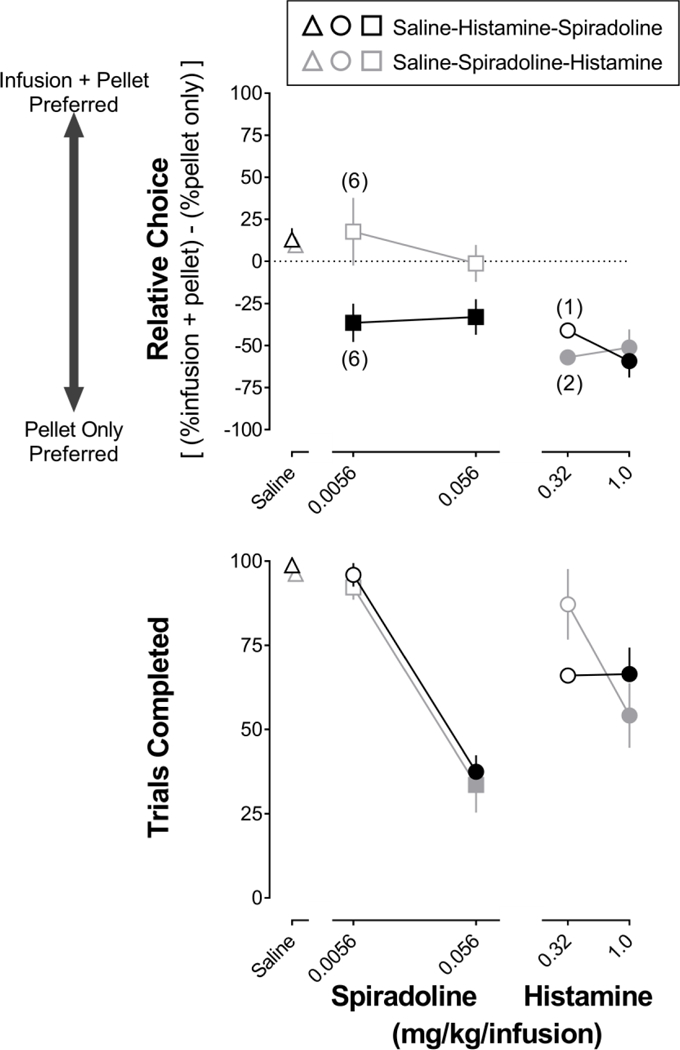
Mean (±1 SEM) relative choice for each alternative (upper) and the number of trials completed (lower) during saline (triangles), histamine (circles), and spiradoline (squares) tests in Experiment 2. Black outlined symbols represent 8 rats that were tested with histamine prior to spiradoline. Gray outlined symbols represent 8 rats that were tested with spiradoline prior to histamine. Filled symbols denote a significant (p<0.05) effect compared with saline. Each data point represents 8 rats, except where noted by numbers in parentheses. Horizontal axes: unit dose in mg/kg/infusion.
DISCUSSION
One purpose of the current study was to test the sensitivity of a food versus drug + food choice procedure to reinforcing and punishing effects of mu and kappa opioid receptor agonists, respectively. This study also compared the effects of spiradoline (kappa receptor agonist) in rats with and without histamine experience to test the hypothesis that exposure to one drug affects the response to subsequent exposure to second drug. Histamine had punishing effects in all rats, consistent with previous reports using food choice in rats (Podlesnick et al. 2010) and monkeys (Woolverton 2003) and drug choice in monkeys (Woolverton et al. 2012). Remifentanil was reinforcing (i.e., rats chose predominantly a food pellet that was paired with an infusion of remifentanil over a food pellet alone) whereas spiradoline was punishing (i.e., rats chose predominantly a food pellet alone over a food pellet that was paired with an infusion of spiradoline) in rats that previously received histamine; however, spiradoline was not punishing in drug-naïve rats.
Overall, this choice procedure was sensitive to both reinforcing effects of remifentanil (mu) and punishing effects of spiradoline (kappa) in the same subjects. The ability to study reinforcing and punishing effects in the same subjects offers an advantage over single- or concurrent-schedule procedures in which animals do or do not respond for drug. Under those conditions, the absence of self-administration is typically interpreted as merely the absence of positive reinforcing effects since those procedures are not sensitive to possible aversive effects of drugs. Hoffmeister and Wuttke (1973) distinguished between positive reinforcing and negative reinforcing effects of various opioids in monkeys by using an escape/avoidance protocol in which responses to a lever either terminated a drug infusion (escape) or postponed scheduled infusions (avoidance). Nalorphine and cyclazocine, which both have actions at kappa receptors, engendered high levels of escape and avoidance responding compared to saline, whereas the mu agonist codeine was well-tolerated, as evidenced by complete extinction of escape and avoidance responding. Note that the absence of responding is interpreted as a positive reinforcing effect, whereas the reallocation of responding among two or more alternatives is the primary measure in choice procedures. Place conditioning procedures can distinguish between rewarding and aversive effects; however, as each subject typically is exposed to only a single condition (Cunningham et al. 2006), adequately powered studies that include dose-response determinations studies for multiple drugs requires a very large number of subjects.
The relative choice for a pellet alone was approximately −80 when histamine + a pellet was the alternative and −65 when spiradoline + a pellet was the alternative. This difference might result from the criteria used for changing experimental conditions (i.e., changing conditions when choice for one alternative was greater than 75%); if the same condition was studied for more sessions, the effects of histamine and spiradoline might have been more similar. However, that spiradoline was punishing in rats that had received histamine but not in drug-naïve rats suggests that histamine is a more effective punishing stimulus, perhaps due to its very rapid onset and offset of action compared with spiradoline (e.g., Bovet et al. 1958; Chang et al. 2011). It will be important to understand how variations on onset and duration of action impact the reinforcing and punishing effects of drugs under procedures such as the one used in this study.
Spiradoline likely accumulated within a session, potentially impacting performance across trials: spiradoline might be a more effective punisher in drug-naïve rats if, for example, infusions were available less frequently (e.g., long inter-trial intervals). The onset of the aversive stimulus is critical for establishing a punishment effect with non-drug stimuli (e.g., shock). Delaying an aversive stimulus like shock or a histamine infusion, even by a few seconds, reduces the effectiveness substantially (Banks and Vogel-Sprott 1965; Woolverton et al. 2012). Experience with histamine appeared to be important in establishing a punishing effect for spiradoline, but it is not the case that experience with histamine resulted in punishing effects for all subsequently studied drugs. In Experiment 1, rats received histamine before (and after) remifentanil, which had robust positive reinforcing effects. The possibility also remains that longer acting mu agonists, such as morphine, might produce less robust preference as compared to remifentanil.
In the current experiments, there were marked differences in drug effects between rats with different histories. In Experiment 1, 0.0056 mg/kg/infusion spiradoline + a pellet was without effect on choice or trials completed; however, this dose of spiradoline had significant effects on choice (punishing) in Experiment 2. Moreover, 1.0 mg/kg/infusion of histamine decreased the number of trials completed in Experiment 2 but not in Experiment 1. Spiradoline was an effective punisher in histamine-experienced but not drug-naïve rats. These difference in effectiveness of spiradoline as a punisher might be related to a global difference in sensitivity of rats in the two studies to punishment. Hoffmeister and Wuttke (1973) showed that monkeys readily responded to escape and avoid infusions of nalorphine and cyclazocine after extensive training with shock avoidance, although it is not known whether experience with shock avoidance was necessary to establish the negative reinforcing effects of nalorphine and cyclazocine. Likely, many factors affect punishing effects of drugs just as many factors affect positive reinforcing effects of drugs. For drug-maintained responding, subjects with an extensive history of reinforcement respond at higher rates and for lower doses of drug, compared with subjects that have a more limited history of drug taking (Goldberg 1973). Systematic investigation of a wide range of parameters and a variety of drugs will be necessary in order to understand the complex relationships among positive reinforcement, punishment, drug history, behavioral history, and environmental conditions.
One apparent advantage of this food versus drug + food choice procedure, compared with single- or concurrent-schedule procedures (Maguire et al., 2013; Weed et al., 2017), is that it can distinguish between reinforcing effects, punishing effects, and the general suppression of behavior. These features will be useful for studying interactions between reinforcing and punishing effects of drugs administered alone and in mixtures as well as any experimental manipulation (i.e., treatment) that might influence the relative contribution of each effect. Pain can modulate reinforcing and aversive effects of drugs (Shippenberg et al., 1988; Martin et al., 2006; Martin and Ewan, 2008; Li, 2013; Wade et al., 2013). One possibility is that titrating the doses of one drug that is a positive reinforcer and another drug that is a punisher might yield a mixture that effectively treats pain with little to no abuse potential (i.e., is indifferent in this choice procedure). The food choice procedure used in the present study likely would be appropriate for evaluating drug mixtures. Punishing effects of kappa agonists and reinforcing effects of mu agonists might be mutually antagonized in a drug mixture (e.g., mu/kappa) or by a physiological state (e.g., pain). If unwanted effects of each drug can be significantly reduced or avoided, then mu/kappa mixtures might be advantageous to mu opioids alone for treating pain.
ACKNOWLEDGEMENTS
The authors thank Christopher Podlesnick for helpful comments and suggestions, Yong-Gong Shi for excellent technical assistance, and James H Woods and Gail Winger for insightful discussions about the data.
Funding: This work was supported by USPHS Grants F32DA043348, R25NS080684, and T32DA031115, and the Welch Foundation [AQ-0039]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: None declared.
REFERENCES
- Azrin NH, Holz WC (1966). Punishment. In: Honig WK (Ed.), Operant behavior: Areas of research and application (pp. 380–447). New York: Appleton-Century-Crofts. [Google Scholar]
- Banks ML, Negus SS (2012). Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Advances in Pharmacological Sciences2012: Article ID 281768, 17 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovet D, Kohn R, Marotta M, Silvestrini B (1958). Some effects of histamine in the normal and Haemophilus pertussis vaccinated rat. Brit J Pharmacol 13: 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Byon W, Lu Y, Jacobsen LK, Badura LL, Sawant-Basak A, Miller E, Liu J, Grimwood S, Wang EQ, Maurer TS (2011). Quantitative PK-PD model-based translational pharmacology of a novel kappa opioid receptor antagonist between rats and humans. AAPS J 13: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Truong YN-T, Shi Y-G, Woods JH (2015). Morphine deprivation increases self-administration of the fast- and short-acting μ-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther 326: 920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA (2006). Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1: 1662–1670. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988). Opposite effects of my and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244: 1067–1080. [PubMed] [Google Scholar]
- Freeman KB, Naylor JE, Prisinzano TW, Woolverton WL (2014). Assessment of thekappa opioid agonist, salvinorin A, as a punisher of drug self-administration in monkeys. Psychopharmacology (Berl) 231: 2751–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S (1995). Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res 681: 147–152. [DOI] [PubMed] [Google Scholar]
- Goldberg SR (1973). Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J Pharmacol Exp Ther 186: 18–30. [PubMed] [Google Scholar]
- Hoffmeister F, Schlichting UU (1972). Reinforcing properties of some opiates and opioids in rhesus monkeys with histories of cocaine and codeine self-administration. Psychopharmacology (Berl) 23: 55–74. [DOI] [PubMed] [Google Scholar]
- Hoffmeister F, Wuttke W (1973). Negative reinforcing properties of morphine-antagonists in naïve rhesus monkeys. Psychopharmacology (Berl) 33: 247–258. [DOI] [PubMed] [Google Scholar]
- Holtzman SG (2000). Further characterization of the discriminative stimulus effects of spiradoline. Pharmacol Biochem Behav 66: 517–522. [DOI] [PubMed] [Google Scholar]
- Howell LL, Fantegrossi WE (2009). Intravenous drug self-administration in nonhuman primates In: Methods of behavior analysis in neuroscience. Buccafusco JJ (editor). Boca Raton: CRC Press; /Taylor & Francis; Chapter 9. [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH (2005). The economics of drug abuse: a quantitative assessment of drug demand. Mol Interv 5: 20–28 [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Winger G (2015). Individual differences in the reinforcing and punishing effects of nicotine in rhesus monkeys. Psychopharmacology (Berl) 232: 2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX (2013). The application of conditioning paradigms in the measurement of pain. Eur J Pharmacol 716: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP (2013). Effect of delay on self-administration of remifentanil under a drug versus drug choice procedure in rhesus monkeys. J Pharmacol Exp Ther 347: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Ewan E (2008). Chronic pain alters drug self-administration: implications for addiction and pain mechanisms. Exp Clin Psychopharmacol 16: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Eisenach JC (2006). Clonidine maintains intrathecal self-administration in rats following spinal nerve ligation. Pain 125: 257–226. [DOI] [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S (2009). Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline, and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav 94: 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A (1985). Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 86: 274–280. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR (2007). Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction 102: 1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW (2000). Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology (Berl) 150: 61–66. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Mead AN (2010). Tramadol acts as a weak reinforcer in the rat self-administration model, consistent with its low abuse liability in humans. Pharmacol Biochem Behav 96: 279–286. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM (1986). Psychotomimesis mediated by kappa opiate receptors. Science 233: 774–776. [DOI] [PubMed] [Google Scholar]
- Podlesnick CA, Jimenez-Gomez C, Woods JH (2010). A choice procedure to assess the aversive effects of drugs in rodents. J Exp Anal Behav 93: 203–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnick CA, Jimenez-Gomez C (2013). Punishing and cardiovascular effects of intravenous histamine in rats: pharmacological selectivity. J Exp Anal Behav 100: 333–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech RH, Mokler DJ, Briggs SL (2011). Effects of combined opioids on pain and mood in mammals. Pain Research and Treatment2012: Article ID 145965, 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley AL (2011). The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav 103: 69–78. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS (1999). U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 144: 339–346. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Stein C, Huber A, Millan MJ, Herz A (1988). Motivational effects of opioids in an animal model of prolonged inflammatory pain: alteration in the effects of kappa- but not of mu-receptor agonists. Pain 35: 179–186. [DOI] [PubMed] [Google Scholar]
- Skinner BF (1953). Science and human behavior. New York: MacMillan [Google Scholar]
- Smith JB, Branch MN, McKearney JW (1978). Changes in the effects of d-amphetamine on escape responding by its prior effects on punished responding. J Pharmacol Exp Ther 207: 159–164. [PubMed] [Google Scholar]
- Spealman RD (1979). Behavior maintained by termination of a schedule of self-administered cocaine. Science 204: 1231–1233. [DOI] [PubMed] [Google Scholar]
- Stein JS, Johnson PS, Renda CR, Smits RR, Liston KJ, Shahan TA, Madden GJ (2013). Early and prolonged exposure to reward delay: effects on impulsive choice and alcohol self-administration in male rats. Exp Clin Psychopharmacol 21: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Renda CR, Hinnenkamp JE, Madden GJ (2015). Impulsive choice, alcohol consumption, and pre-exposure to delayed rewards: II. Potential mechanisms. J Exp Anal Behav 103: 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Lagorio CH, Ko MC (2014). Effects of the NOP agonist SCH221510 on producing and attenuating reinforcing effects as measured by drug self-administration in rats. Eur J Pharmacol 745: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verendeev A, Riley AL (2013). The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behav Pharmacol 24: 363–374. [DOI] [PubMed] [Google Scholar]
- Wade CL, Krumenacher P, Kitto KF, Peterson CD, Wilcox GL, Fairbanks GA (2013). Effect of chronic pain on fentanyl self-administration in mice. PLoS One 8: e79239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg ML (2003). A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev 9: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed PF, France CP, Gerak LR (2017). Preference for an opioid/benzodiazepine mixture over an opioid alone using a concurrent choice procedure in rhesus monkeys. J Pharmacol Exp Ther 362: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL (2003). A novel choice method for studying drugs as punishers. Pharmacol Biochem Behav 76: 125–131. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Freeman KB, Myerson J, Green L (2012). Suppression of cocaine self-administration in monkeys: effects of delayed punishment. Psychopharmacology (Berl) 220: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]


