Summary:
Due to the novelty of Percutaneous Ultrasonic Tenotomy (PUT), the risks and benefits of this minimally-invasive procedure for insertional Achilles tendinopathy (IAT) pain has only been examined in case studies and retrospective chart reviews for other diagnoses. This retrospective chart review over a 3.5 year period identified 34 patients with IAT who had percutaneous ultrasonic tenotomy (Age=52.2±11.6 years, BMI=32.9±7.5 kg/m2, 62% female). This procedure reduced the rate of moderate/severe pain from 68% at baseline to 15% at long-term follow-up and had a satisfaction rate of 70%. There was 1 minor complication out of 40 procedures in 34 patients.
Keywords: Enthesopathy, Tendinopathy, Tendonitis, Achilles tendon, Percutaneous Ultrasonic Tenotomy, Minimally-invasive procedure
Introduction
Insertional Achilles tendinopathy (IAT) is a painful condition that limits mobility, reduces work capacity, and impedes exercise participation.1 There are many non-operative treatment options for IAT, however the level of evidence guiding care remains low. Percutaneous Ultrasonic Tenotomy (PUT) allows patients to undergo a minimally invasive procedure and return to activity in a shorter period of time than an open operative procedure. Yet due to the novelty of this treatment option, the effectiveness and safety of this procedure for IAT is unknown.
The majority of data supporting the use of PUT for IAT is based on literature reporting positive outcomes for tendinopathy at the elbow. There are a number of studies supporting the use of PUT to reduce elbow tendinopathy pain with no complications reported.2–7 Rates of patient satisfaction were high and ranged from 79% to 100%, yet the sample sizes for each of these studies were small (≤ 20 patients).2–7 To date there is only one published study (n=6) on outcomes of PUT for chronic Achilles tendinopathy.8 This case series reported complications related to PUT, including deep vein thrombosis (n=1) and lack of pain relief or increased pain (n=6, 5 midportion AT, 1 insertional AT).8 Larger studies are needed to estimate the complication rate of PUT as a treatment option for IAT.
This retrospective chart review of a relatively larger sample is a needed first step toward advancing the level of evidence examining this minimally invasive procedure for tendinopathy. The risks of PUT for IAT need to be interpreted within the context of the potential benefits for pain relief, improved quality of life, and patient satisfaction. The primary aims of this study were to examine 1) changes in self-reported pain, quality of life and function, 2) patient satisfaction, and 3) complications with the procedure.
Methods
A retrospective review of charts dated between Sept 2013 and May 2017 of all patients who had a PUT procedure for IAT identified 34 patients (Mean±SD, Age= 52.2± 11.6 years, BMI= 32.9 ± 7.5 kg/m2, 62% female). The median duration of symptoms was 1.5 years (IQR 1 to 2.3 years). The majority of patients had tried other treatment options prior to PUT, including physical therapy (22/34) and cortisone injection (5/34). Six patients had the PUT procedure performed on both sides; therefore, a total of 40 procedures were performed during the chart review period.
Outcomes were assessed prior to the PUT procedure, at short-term follow-up (6 or 12 weeks), and long-term follow-up (median= 1.7 years, IQR= 11 to 36 months). The majority of outcomes were measured in clinic as part of routine care. In order to maximize the number of long-term follow-up responses, an online and phone survey was approved by our institution’s human subjects review board. Out of the 34 patients, 18 completed the long-term follow-up survey.
Pain was assessed using the 4-point scale from the American Orthopaedic Foot and Ankle Score (AOFAS) questionnaire as: 40-none, 30 mild/occasional, 20-moderate/daily, 0-severe/ almost always present. Quality of life was assessed with the Physical Component Summary (PCS) and the Mental Component Summary (MCS) subscales of the SF-12. The t-score of the PCS and MCS summary scales reference the general population with a mean t-score of 50 and standard deviation of 10.9 For function, patients were asked 1) if they had resumed their normal level of activity, and 2) if they had returned to work. Patient satisfaction was assessed by asking the patient, “Are you satisfied with the procedure and outcome?” and was graded on a 5-point scale: 1-very satisfied, 2-somewhat satisfied, 3-neutral, 4-somewhat dissatisfied, 5-very dissatisfied. Complications were evaluated based on clinic evaluation and patient report. As opioids were not routinely prescribed for post-procedure pain control, any prescription of an opioid for excessive procedure-related pain was also noted from chart review.
Procedure Description
All procedures were performed by the senior author (MMH) who is a fellowship trained sports medicine physician with greater than 7 years of experience performing ultrasound guided musculoskeletal interventions. The procedures were performed in an outpatient setting (clinical procedure suite) under sterile conditions with local anesthesia only (i.e., no sedation). Patients were placed in the prone position with feet hanging free off the edge of the table. A pre-procedural ultrasound scan was performed with a high frequency linear array transducer (12–5 MHz, Philips Healthcare; Bothell, WA) to identify the location and extent of pathology which was used to individualize the technique for each case (Figure 1). The patient was then prepped and draped in usual sterile fashion; a sterile ultrasound transducer cover and sterile acoustic coupling gel were used for all procedures. Local anesthesia was obtained with 1% lidocaine without epinephrine infiltrated via a 25-gauge, 50mm needle under live ultrasound guidance first into the subcutaneous tissues and then proceeding into the Achilles tendon and down to the retro-calcaneal bursa when deep/anterior pathology was to be addressed. Approximately 5 – 10 mL of 1% lidocaine was used for each procedure without undue patient discomfort or need for additional anesthesia such as conscious sedation. A #11 blade was then used to make an approximately 5 mm incision down to the tendon to allow introduction of the cutting device. All incisions were made longitudinal (in line) with the Achilles tendon fibers to limit iatrogenic tissue damage (Figure 2). The TX (Tenex Health; Lake Forest, CA) ultrasonic cutting device was then introduced and first used to debride the more superficial/posterior retro-Achilles bursal tissue and thickened paratenon from the Achilles tendon until the device was free to move within this tissue plane unobstructed (Figure 2). After which the intra-tendinous pathology identified during the pre-procedural scan was addressed. Intra-tendinous calcifications were debrided as well as any regions of tendinopathic tissue represented by heterogenous hypoechoic tissue. If compressive pathology was appreciated at the deep/anterior surface (often in association with a Haglund deformity), the device was advanced to this location and further debridement performed. Examples of this subtype of compressive pathology are demonstrated in Figures 1a, 2c and 3c. A limited retro-calcaneal bursectomy was performed when bursal hypertrophy or hyperemia associated with the bursa was noted on Doppler imaging (suggesting active bursitis). In rare cases, if the Haglund deformity was felt to be amendable to limited debridement, the TX device was used off label to perform bony debridement (Figure 3). Any off label usage of the device was explicitly discussed with the patient prior to any procedure. Average energy time for all procedures was 7:18 minutes with a standard deviation of 4:00 (range 2:30 to 19:12 minutes). Once debridement was complete, the skin incision was closed with an adhesive bandage (Nexcare Steri-Strips; 3M, Minneapolis, MN), an occlusive film (Tegaderm; 3M, Minneapolis, MN), and a compressive sleeve.
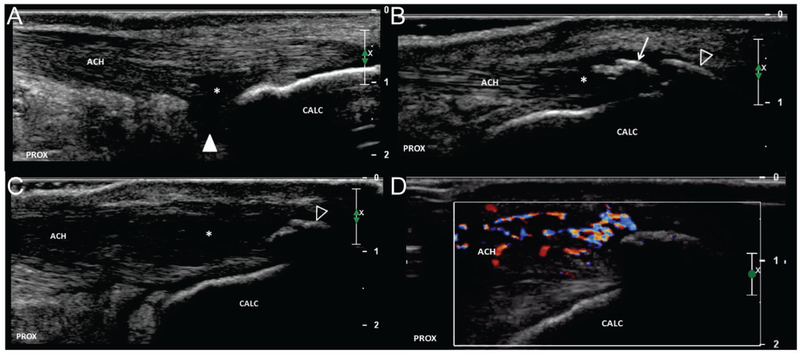
FIGURE 1. Variations in insertional Achilles tendon pathology.
Long axis images of Achilles tendon insertion demonstrating variability in location and extent of pathologic findings. a.) Hypoechoic changes (asterisks) are demonstrated adjacent to the posterosuperior calcaneus (arrow). The boundary with the retro-calcaneal bursa (arrowhead) is ill-defined. Note the relatively normal appearance of the superficial/posterior portion of the tendon. b.) The deep/anterior portion of the tendon is relatively normal; however, changes of tendinosis (asterisks) are appreciated adjacent to an intra-tendinous calcification (arrow). There is minimal posterior acoustic shadowing suggesting “soft” calcification which is amendable to percutaneous debridement. An enthesophyte (open arrowhead) demonstrates dense posterior acoustic shadowing consistent with cortical bone. c.) Hypoechoic changes of tendinosis (asterisks) are more extensive and pronounce. An enthesophyte is present (open arrowhead), but no intra-tendinous calcification is appreciated. d.) Corresponding Color Doppler imaging of figure 1c. There is hyperemia within the superficial/posterior tendon as well as paratenon. ACH = Achilles tendon, CALC = calcaneus, PROX = proximal.
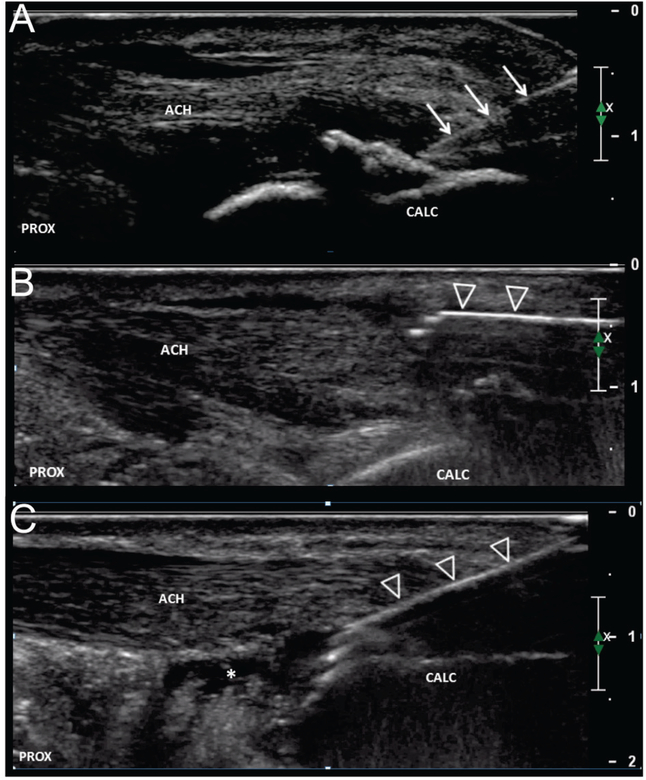
FIGURE 2. Procedural technique.
Long axis image of Achilles tendon demonstrating procedural technique. a.) After obtaining local anesthesia, a #11 blade (arrows) is used to make an incision down to the tendon. b.) The TX device (open arrowheads) is then introduced superficial/posterior to the tendon and the hypertrophied paratenon and connective tissue are debrided from the tendon. c.) The device is then guided into the tendon and the regions of tendinosis are debrided. In this example, there was concomitant retro-calcaneal bursitis (asterisks) and a limited bursectomy was performed. ACH = Achilles tendon, CALC = calcaneus, PROX = proximal.
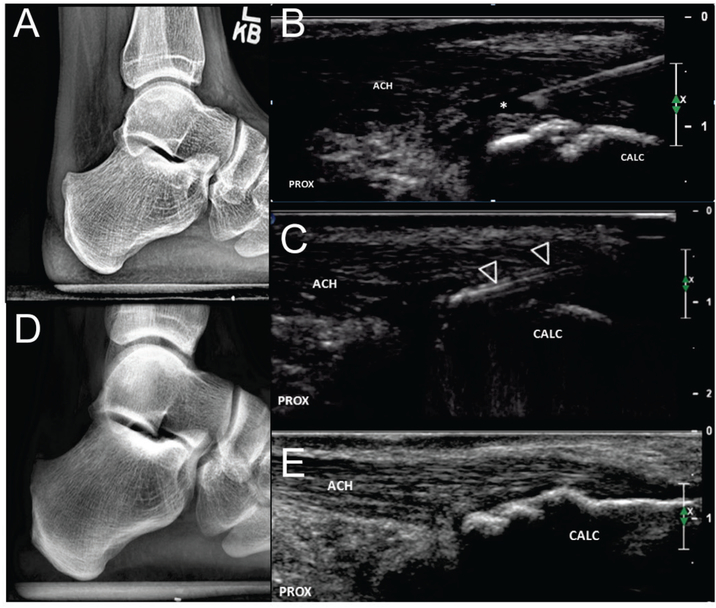
FIGURE 3. Haglund bony debridement.
a.) Pre-procedural X-ray demonstrates a posteriorly projecting bony protuberance at the posterosuperior calcaneus which correlated with location of patient’s maximal pain. b.) Procedural long axis ultrasound image during local anesthesia demonstrates partial thickness tear (asterisks) adjacent to the region of cortical irregularity at the posterosuperior calcaneus (arrowhead). c.) The TX device (open arrowheads) is used to shave down the posteriorly projecting bony protuberance using a layer by layer technique working from superficial to deep. Follow up radiograph at 6 weeks (d.) demonstrates decreased prominence of the previously noted bony protuberance while follow up ultrasound at 3 years (e) is consistent with bony remodeling and complete healing of the debrided partial tendon tear. Patient reports no pain or functional limitation at 3 year follow up. ACH = Achilles tendon, CALC = calcaneus, PROX = proximal.
Post-procedure protocol included 2 weeks of protected weight bearing in a walking boot after which patients were allowed to wean out of the boot and transition to a self-selected shoe as tolerated. Patients were allowed to come out of the boot to perform pain free active range of motion starting post-procedure day 1 and were not required to sleep in the boot. Strengthening exercises were started at 2 weeks post-procedure and patients were allowed to return to daily activities as tolerated. No heavy use (prolonged walking, running, jumping, change of direction, etc) was allowed until a minimum 6 weeks.
Analysis
For the 6 patients who had PUT on both sides, only one side per patient was used in the statistical analysis in order to maintain independence of observations. Data corresponding to the second time a patient had a procedure (on the opposite side) was analyzed when available (4/6), otherwise data for the first side was used (2/6). The Wilcoxon Signed Rank test was used to compare baseline to short-term and long-term follow-up time points. Pairwise deletion was used, so that participants with baseline data yet missing short-term data could be included in the analysis of long-term follow-up compared to baseline (and vice-versa with missing long-term data). Therefore the sample size for the statistical analyses are smaller than the sample size with data at any single time point. While most people with missing baseline data are also missing subsequent follow-up data (n=4, AOFAS Pain Scale), there are a couple people with missing baseline data yet available follow-up data who are included in the data presented in the tables but not the statistical comparisons to baseline (n=2, AOFAS Pain Scale). Paired t-tests were used to compare quality of life from baseline to short-term follow-up. Descriptive statistics were used for function, patient satisfaction, and complication rate. In order to minimize sample bias and maximize the inclusion of the patients from all identified in the retrospective review, data on complications were included from any time point.
Results
There were statistically significant decreases in pain at short-term and long-term follow-up after PUT (Table 1, p<0.05). Quality of life, as measured by the PCS component of the SF-12, improved at short-term follow-up (p=0.03, n=23 patients), yet there was not a significant change in the MCS (Table 2, p=0.96). At short-term follow-up 11/21 (n=7 at 6 weeks, n=14 at 12 weeks) had resumed normal activity and 14/14 had resumed work (n=11 at 6 weeks, n=3 at 12 weeks). At short-term follow-up 70% of patients were satisfied with their treatment (14 reported “very satisfied” and 10 reported “somewhat satisfied”). Some patients were neutral (n=5), somewhat dissatisfied (n=2), or had missing data for this outcome (n=3). For complications, one patient reported a superficial skin infection that resolved with oral antibiotics. No patients were prescribed opioids for pain management at any point. No other complications related to the PUT procedure were reported by the other 33 patients or documented in their medical record.
TABLE 1.
Pain level reported on the AOFAS Pain scale (N=34)
| Baseline | Short-term follow-upα | Long-term follow-upβ | |
|---|---|---|---|
| None | 0 (0%) | 3 (9%) | 4 (12%) |
| Mild/ Occasional | 5 (15%) | 13 (39%) | 13 (39%) |
| Moderate/ Daily | 18 (55%) | 8 (24%) | 2 (6%) |
| Severe/ Almost always present | 4 (12%) | 2 (6%) | 1 (3%) |
| Missing | 6 (18%) | 7 (21%) | 13 (39%) |
Values presented as number (% of sample)
Short-term: 6 week follow-up (n=13), 12 week follow-up (n=14)
Long-term follow-up (n= 22): median = 1.7 years [IQR= 11 to 36 months]
Wilcoxon Signed Rank test, n=23, short-term follow-up compared to baseline, p< 0.01
Wilcoxon Signed Rank test, n=17, long-term follow-up compared to baseline, p= 0.01
TABLE 2.
PCS and MCS components of the SF-12
| Baseline | Short-term follow-up | |
|---|---|---|
| PCS | 40.8± 9.4 | 44.0± 7.1α |
| MCS | 59.4± 5.2 | 59.8± 3.7 |
Values presented as mean ± standard deviation
Prior to PUT, n=24
Short-term: 6-week follow-up (n=9), 12-week follow-up (n=14)
Paired t-test, n=17, short-term follow-up compared to baseline, p= 0.03
Discussion
To date this sample of 40 procedures in 34 patients with IAT is the largest that has been used to examine the efficacy and safety of PUT. We found that PUT can decrease pain, improve quality of life, and have a high satisfaction rate for patients with chronic IAT. In addition, there was a low complication rate (1/40 had superficial skin infection that resolved with antibiotics) over the period of time when complications are most likely to arise. The low severity and complication rate (3%) in this study contrasts the report of severe complications and increased pain with PUT in a case series of 6 patients with Achilles tendinopathy.8 Larger studies are needed to resolve this current level of conflicting evidence on the severity and rate of complications of PUT for chronic Achilles tendinopathy pain.
While the findings of this preliminary study need to be further examined, we found that PUT decreases chronic IAT pain (p< 0.05, Table 1). Of the 28 patients with baseline data, only 5 reported none to mild, occasional pain at baseline. By long-term follow-up 17 patients reported none to mild pain. A limitation of this retrospective study is missing data that was not collected during routine care, which resulted in missing 18% (at baseline) to 39% (at long-term follow-up) of the data for this key self-reported outcome measure. Despite missing follow-up data for up to 12 people, we know that at least half (n=17) of the original sample achieved mild to no pain after the PUT procedure. There was only one patient who reported worse pain (severe/ always present at 12 week follow-up) compared to baseline (moderate/ daily) pain rating. Upon further chart review the patient saw an orthopaedic surgeon 7 months after PUT, reported decreased pain, and decided not to pursue surgical intervention at that time. Based on clinical experience, our interpretation of this finding is that it may take up to a year to achieve maximum pain relief from the procedure.
An improvement in symptoms was also demonstrated by an increase in the PCS of the SF-12 at short-term follow-up (p=0.03, n=23). Yet we failed to detect a change in the MCS component of the SF-12, indicating that PUT is more effective at improving the physical component rather than mental component of quality of life. Despite only having short-term follow-up data at 6 or 12 weeks, the 70% rate of patient satisfaction with PUT for IAT in this study was similar to other studies for tendinopathy at the elbow reporting ranges from 75% to 100%.2, 4, 7 In comparison, the satisfaction rate for operative debridement of IAT is generally greater than 87 percent.10–18 Yet this high patient satisfaction rate also has a complication rate ranging from 6% to over 30%, which includes wound healing issues (superficial would infection, skin necrosis, hematoma, delayed wound healing), scar abnormalities (hypersensitivity, hypertrophy, numbness), sural nerve injury, tendon avulsion, deep vein thrombosis, and recurrence of pain.10, 11, 13–16
There are several advantages of PUT relative to an open or endoscopic operative procedure. The cost associated with PUT is a small fraction of the cost of a traditional operation. For example, at our institution, an open Achilles debridement costs greater than $18,000 more than PUT. An additional benefit, and partial reason for reduced cost, is that there are less resources and time required for PUT performed in an outpatient clinical setting compared to a procedure performed in a surgical center or operative theatre. There are also reduced risks associated with anesthesia. We have demonstrated that PUT is well tolerated and safely performed using only local anesthesia. Furthermore, no patient required opioid pain medication at any point post-procedure demonstrating a lack of excessive post-procedure pain and decreased risk of opioid abuse or future dependence. Patients are also able to return to their previous level of activity much sooner after PUT. We found that half of patients were able to return to their previous level of activity by 12 weeks and all returned to work at 12 week follow-up.
This case series has several limitations due to study design. Because the data was collected by chart review, there is missing baseline and follow-up data that make it more difficult to estimate the effect size of PUT for self-reported pain, quality of life, function, and patient satisfaction. We have indicated when data was missing for particular variables to indicate the potential for drop out bias. We also attempted to contact all participants by email and phone to maximize long-term follow-up and capture any complications that could have been overlooked in the chart review. This is also a small sample size that includes a variety of different types of tissue pathology at the tendon insertion (e.g. variable involvement of Haglund deformity, size of enthosphytes, superficial vs deep tendon degeneration, etc) as well as systemic factors, such as obesity, which could impact outcomes. Due to the heterogeneity of our sample, generalization of these study findings to all patients with IAT may not be appropriate. An additional limitation of this study is that it only reflects outcomes of a single physician in a single geographical area. A prospective, multi-site study with a large sample size is needed to more accurately evaluate the relative risk to benefit ratio of PUT for IAT.
Conclusions
Percutaneous ultrasonic tenotomy has the benefits of reducing the rate of moderate/severe IAT pain from 68% at baseline to 15% at long-term follow-up and had a short-term satisfaction rate of 70%. Additional benefits of this procedure include reduced cost and time on behalf of the provider and patient compared to an open or endoscopic operation. The identified risk in this study was low with 1 minor complication out of 40 procedures. This study supports the safety of PUT performed in a clinical setting and the need for larger studies to further investigate the long-term efficacy.
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K99AR071517 and National Institute of Neurological Disorders and Stroke under Award Number T32 NS045549-12. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the American Physical Therapy Association, Orthopeadic Section Grant. Dr. Hall reports royalties from UpToDate, consulting fees from Tenex Health, and stock in Sonex Health.
References
- 1.Chimenti RL, Cychosz CC, Hall MM, Phisitkul P. Current Concepts Review Update: Insertional Achilles Tendinopathy. Foot Ankle Int. October 2017;38(10):1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seng C, Mohan PC, Koh SB, et al. Ultrasonic Percutaneous Tenotomy for Recalcitrant Lateral Elbow Tendinopathy: Sustainability and Sonographic Progression at 3 Years. Am. J. Sports Med February 2016;44(2):504–510. [DOI] [PubMed] [Google Scholar]
- 3.Williams RC, Pourcho AM. Percutaneous Ultrasonic Tenotomy for Refractory Common Extensor Tendinopathy After Failed Open Surgical Release: A Report of Two Cases. PM R. March 2018;10(3):313–316. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DE, Beckley JM, Smith J. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J. Shoulder Elbow Surg. January 2015;24(1):67–73. [DOI] [PubMed] [Google Scholar]
- 5.Battista CT, Dorweiler MA, Fisher ML, Morrey BF, Noyes MP. Ultrasonic Percutaneous Tenotomy of Common Extensor Tendons for Recalcitrant Lateral Epicondylitis. Tech. Hand Up. Extrem. Surg March 2018;22(1):15–18. [DOI] [PubMed] [Google Scholar]
- 6.Hall MM, Woodroffe L. Ultrasonic Percutaneous Tenotomy for Recalcitrant Calcific Triceps Tendinosis in a Competitive Strongman: A Case Report. Curr. Sports Med. Rep May-Jun 2017;16(3):150–152. [DOI] [PubMed] [Google Scholar]
- 7.Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am. J. Sports Med March 2013;41(3):636–644. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez PJ, Grady JF, Saxena A. Percutaneous Ultrasonic Tenotomy for Achilles Tendinopathy Is a Surgical Procedure With Similar Complications. J. Foot Ankle Surg Sep-Oct 2017;56(5):982–984. [DOI] [PubMed] [Google Scholar]
- 9.Ware JEK M; Keller SD SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, Massachusetts: The Health Insitute, New England Medical Center; 1995. [Google Scholar]
- 10.Lin HA, Chong HA, Yeo W. Calcaneoplasty and reattachment of the Achilles tendon for insertional tendinopathy. Journal of orthopaedic surgery (Hong Kong). April 2014;22(1):56–59. [DOI] [PubMed] [Google Scholar]
- 11.Lim S, Yeap E, Lim Y, Yazid M. Outcome of calcaneoplasty in insertional achilles tendinopathy. Malaysian orthopaedic journal. June 2012;6(SupplA):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KW, Zalavras C, Thordarson DB. Surgical management of insertional calcific achilles tendinosis with a central tendon splitting approach. Foot & ankle international. April 2006;27(4):245–250. [DOI] [PubMed] [Google Scholar]
- 13.Hunt KJ, Cohen BE, Davis WH, Anderson RB, Jones CP. Surgical Treatment of Insertional Achilles Tendinopathy With or Without Flexor Hallucis Longus Tendon Transfer: A Prospective, Randomized Study. Foot & ankle international. September 2015;36(9):998–1005. [DOI] [PubMed] [Google Scholar]
- 14.Greenhagen RM, Shinabarger AB, Pearson KT, Burns PR. Intermediate and long-term outcomes of the suture bridge technique for the management of insertional achilles tendinopathy. Foot & ankle specialist. June 2013;6(3):185–190. [DOI] [PubMed] [Google Scholar]
- 15.Gillis CT, Lin JS. Use of a Central Splitting Approach and Near Complete Detachment for Insertional Calcific Achilles Tendinopathy Repaired With an Achilles Bridging Suture. J. Foot Ankle Surg Mar-Apr 2016;55(2):235–239. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger S, Razzaq R, Waizy H, et al. Operative Treatment of the Insertional Achilles Tendinopathy Through a Transtendinous Approach. Foot & ankle international. March 2016;37(3):288–293. [DOI] [PubMed] [Google Scholar]
- 17.Elias I, Raikin SM, Besser MP, Nazarian LN. Outcomes of chronic insertional Achilles tendinosis using FHL autograft through single incision. Foot & ankle international. March 2009;30(3):197–204. [DOI] [PubMed] [Google Scholar]
- 18.Ahn JH, Ahn CY, Byun CH, Kim YC. Operative Treatment of Haglund Syndrome With Central Achilles Tendon-Splitting Approach. The Journal of foot and ankle surgery : official publication of the American College of Foot and Ankle Surgeons. Nov-Dec 2015;54(6):1053–1056. [DOI] [PubMed] [Google Scholar]