Abstract
Women with endometrial cancer (EC) frequently receive adjuvant paclitaxel and carboplatin (PC) chemotherapy. There is no standard first line chemotherapy at disease recurrence. Data extrapolated from ovarian cancer has suggested that patients with recurrent EC may benefit from further platinum-based chemotherapy. We performed a retrospective analysis of patients who were retreated with PC chemotherapy for recurrent EC at Memorial Sloan Kettering Cancer Center between January 2000 and December 2014. The median progression free survival (PFS) and overall survival (OS) were estimated using the Kaplan Meier method. Twenty patients were included in the analysis. Patients were re-treated with PC a median of 25 (8–79) months from their original PC. There were no complete responses, 10 (50%) patients had partial response (PR), 3 (15%) had stable disease, 2 (10%) had progression at best response and 5 (20%) were not evaluable by RECIST. A median of 6 cycles of PC were administered (2–9). Four patients (20%) transitioned to paclitaxel only due to carboplatin allergy. At the data cut off, one patient continued PC, and another was off therapy with PR. The remainder (N = 18, 90%) received a median of 2.5 (1–6) further lines of treatments. Median PFS and OS from re-treatment were 10 and 27 months respectively. Median OS from original diagnosis was 74 months. In this small retrospective study, selected patients with recurrent EC who are >6 months from completion of PC derive benefit from retreatment with PC with a response rate of 50%.
Keywords: Endometrial cancer, Carboplatin, Paclitaxel, Retreatment
Highlights
-
•
Re-exposure to paclitaxel and carboplatin chemotherapy resulted in tumor shrinkage in 50% of women with endometrial cancer.
-
•
15% of patients with recurrent endometrial cancer had stable disease upon re-exposure.
-
•
Re-using paclitaxel and carboplatin >6 months after adjuvant therapy is effective for disease control.
1. Introduction
The majority of EC patients present with early stage disease and are cured. Limited systemic treatment options exist for the fraction of patients who present with advanced or recurrent disease. For patients with chemotherapy naïve recurrent EC, paclitaxel and carboplatin (PC) chemotherapy is the front-line standard of care (Miller et al., 2012). However, for women who have previously received adjuvant PC, there is limited prospective data to guide systemic therapy in the recurrent disease setting. Small phase II studies have demonstrated that topotecan doxorubicin and bevacizumab in the first line metastatic setting have modest activity (Fleming, 2015). With the exception of pembrolizumab for mismatch repair deficient (MMR-D) tumors and megestrol acetate for the palliative treatment of recurrent endometrial cancer treatments, there are no FDA approved treatments in this setting (Fleming, 2015; Diaz et al., 2017).
Since PC chemotherapy is now frequently administered as adjuvant treatment for patients with high risk EC, it has become increasingly becoming more important to appropriately determine which patients will benefit from re-exposure to platinum-based chemotherapy. The concept of platinum sensitivity and retreating with platinum-based chemotherapy in recurrent EC has been explored in a number of retrospective and pooled analysis of prior prospective studies (Matoda et al., 2014; Nagao et al., 2013; Ueda et al., 2011; Nagao et al., 2015; Mazgani et al., 2008; Souza et al., 2016). These studies have largely evaluated a mix of platinum-based regimens, both as upfront therapy and at time of disease recurrence, reflective of the regimens in use over the periods of study. In this retrospective study, we examine the clinical outcomes of EC patients who received PC in the adjuvant setting and who were specifically re-treated with PC in the recurrent or metastatic disease setting.
2. Methods
Between January 2000 and December 2014, we identified through an institutional database at Memorial Sloan Kettering Cancer Center patients who had previously received PC in the adjuvant setting and were re-treated with PC at the time of recurrence. An IRB approved retrospective analysis of patient, tumor and treatment characteristics was performed. An independent radiologist, blinded to patients' clinical details assessed response per RECIST 1.1 criteria (Eisenhauer et al., 2009). The Kaplan Meier method was used to estimate the median progression free survival (PFS) to re-treatment with PC, the PFS and overall survival (OS) from re-treatment with PC (cycle 1, day 1) and the OS from original diagnosis. Statistical reporting was descriptive and Kaplan Meier curves were generated used Prism GraphPad version 6 software.
3. Results
Twenty women were identified with a median age of 67 years (40–83 years). The median patient's BMI was 30 (23–44); only four patients had a BMI <25. Patient and tumor characteristics are outlined in Table 1. All patients received PC following their initial surgery with a median of 6 cycles (3–7). Just over half the patients (N = 11, 55%) received post-operative radiation therapy. Two thirds (N = 13, 65%) of the patients received additional therapy; hormonal therapy, surgery, or radiation prior to re-treatment with PC for recurrent disease as outlined in Table 2.
Table 1.
Patient and tumor characteristics at diagnosis.
| N = 20 | |
|---|---|
| Median age (range) | 67 (40–83) |
| Median BMI | 30 (23–44) |
| Comorbidities: | N (%) |
| Diabetes | 2 (10) |
| Hypertension | 12 (60) |
| Hyperlipidemia | 8 (40) |
| Coronary artery disease | 3 (15) |
| FIGO stage (2009): | N (%) |
| I | 5 (25) |
| II | 3 (15) |
| III | 7 (35) |
| IV | 5 (25) |
| Histology: | N (%) |
| Endometrioid | 3 (15) |
| Serous | 7 (35) |
| Carcinosarcoma | 7 (35) |
| Mixed endometrioid/serous | 3 (15) |
Table 2.
Post-operative therapy and Retreatment therapies with Best Overall Response.
| Post-operative therapy | |
|---|---|
| Adjuvant carboplatin and paclitaxel | Median 6 cycles (3–7) |
| Adjuvant radiation | N = 11 (55%) of these: external beam N = 4 (20%) IVRT N = 7 (35%) |
| Intervening therapies prior to retreating with carboplatin and paclitaxel for recurrent disease | Hormonal therapy N = 2 (10%) Further surgery N = 4 (20%) Further radiation N = 7 (35%) No additional therapy N = 7(35%) |
| Subsequent therapy | |
|---|---|
| Retreatment with carboplatin and paclitaxel | Median 6 cycles (2–9) |
| Therapy post completion of carboplatin and paclitaxel | N = 18 (90%) received median 2.5 (1–6) further lines of hormonal/chemotherapy N = 1 remains on therapy N = 1 remains off therapy |
| Best response: retreatment with carboplatin and paclitaxel as first line chemotherapy for recurrent disease N (%) | |
|---|---|
| Complete response | 0 |
| Partial response | 10 (50) |
| Stable disease | 3 (15) |
| Progression of disease | 2 (10) |
| Not eligible for RECIST 1.1 assessment | 5(25) |
Ten patients (50%) achieved a partial response (PR) and three patients (15%) achieved stable disease (SD). Best overall response (ORR) is demonstrated in Table 2. Five patients were not eligible for response assessment. Of those, two patients had no measurable target lesions and three patients did not have appropriately timed imaging, or their imaging was no longer available for review. All five patients not eligible for response assessment achieved clinical disease response or stability.
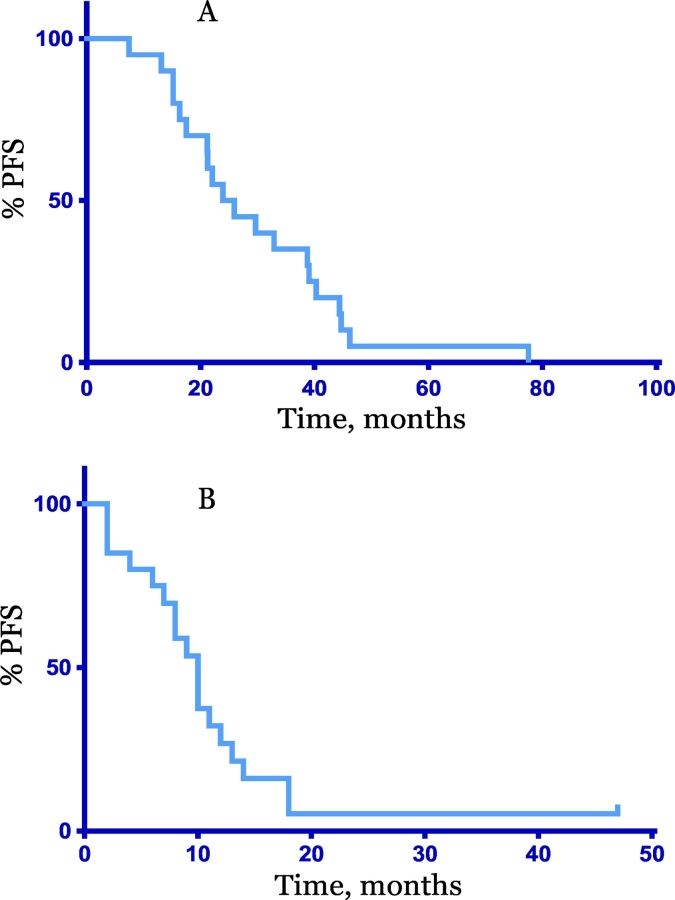
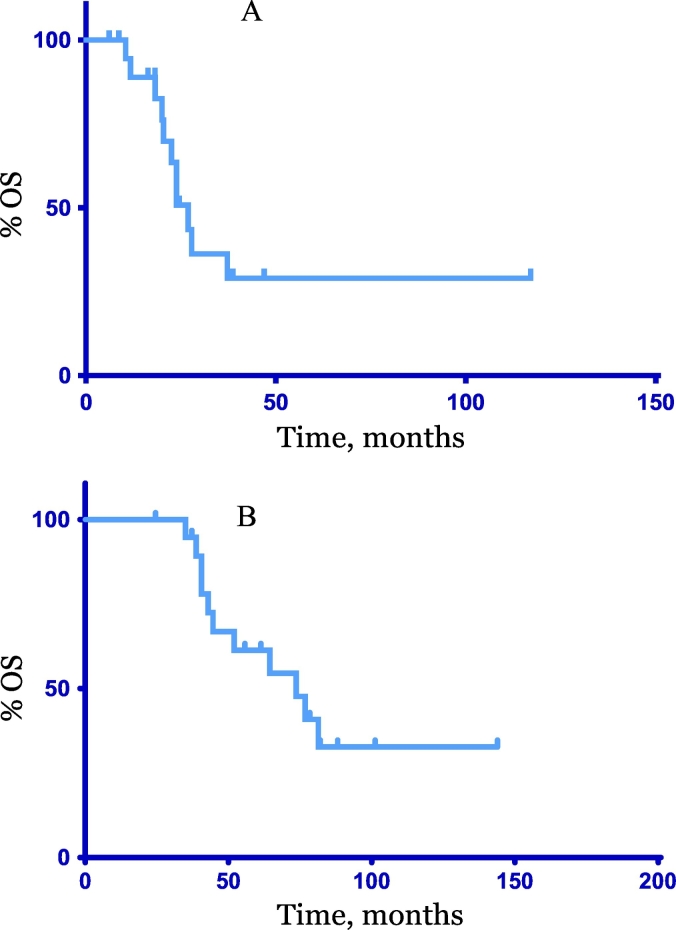
The median progression free survival (PFS) from the end of adjuvant PC to cycle 1 day 1 of PC retreatment was 25 months (7–78 months) Fig. 1. No patient had received PC within the 6 months prior to retreating. Patients received a median of six additional cycles of PC (2–9). Four patients (20%) transitioned to single agent paclitaxel while on therapy due to carboplatin allergy. Following PC, 18 (90%) patients received a median of 2.5 (1–6) lines of further systemic therapy (chemotherapy, hormonal therapy). At time of data cut off, one patient remained on PC therapy. One patient remained with no evidence of disease off all further systemic therapy (chemotherapy, hormonal therapy) four years after completion of PC for biopsy proven recurrent metastatic endometrial carcinosarcoma. The median PFS from cycle 1 day 1 of retreatment with carboplatin and paclitaxel was 10 months (2–47), Fig. 1. The median OS from retreatment was 27 months (6–117) and the median OS from original diagnosis was 74 months (25–144) Fig. 2.
Fig. 1.
Progression Free Survival (PFS). A. PFS from end of adjuvant carboplatin and paclitaxel to cycle 1 day 1 carboplatin and paclitaxel, median 25 months, (7–78). B: PFS from cycle 1 day 1 re-treatment with carboplatin and paclitaxel, median 10 months (2–47).
Fig. 2.
Overall Survival (OS). A: OS from cycle 1 day 1 re-treatment with carboplatin and paclitaxel, median 27 months, (6-117). B: Overall Survival from diagnosis, median 74 months, (25–144).
4. Discussion
Detailed genomic characterization of EC has revealed the diversity of this disease in terms of biology and behavior. Despite these advances, the management of recurrent EC remains challenging with limited therapeutic options available for the vast majority of patients and a median survival of approximately one year (Fleming, 2015). Recent progress has been made for patients with recurrent EC in the context of immunotherapy and further progress is anticipated in time with a myriad of targeted agents in development. However, there are unfortunately many patients with recurrent EC who are not eligible for novel therapies either due to lack of actionable molecular alterations or due to medical co-morbidities that would render immunotherapy and/or targeted therapies too toxic.
In 2017, the United States Food and Drug Administration (FDA) granted accelerated agnostic approval for pembrolizumab for patients with unresectable or metastatic, microsatellite instability- high (MSI-H) or mismatch repair deficient (MMR-D) solid tumors including MSI-H endometrial cancers. This was based on an impressive response rate of 40% and a response duration of >6 months in 80% of those who responded (Diaz et al., 2017). Given that approximately 20–30% of EC across all stages exhibit MMR-D, this is an additional and important therapeutic option (Cancer Genome Atlas Research N et al., 2013). Subsequently, the FDA granted breakthrough designation for the combination of lenvatinib and pembrolizumab for all patients with EC regardless of MSI status who have progressed after first line treatment, based on an impressive ORR by independent radiology review of nearly 50% exhibited in a phase 1b/II trial (Makker et al., 2018). It is important to note that in this trial, 80% (43/54) patients enrolled had microsatellite stable (MSS) disease. There is currently a phase III study underway evaluating the efficacy of this promising combination (ClinicalTrials.gov #NCT03517449) versus physician's choice chemotherapy.
In addition to these immunotherapy advances, there are several targeted therapies under investigation. Given the high rate of PI3K pathway alterations in EC, a number of studies have explored the role of inhibiting this pathway and demonstrated modest activity response rates but, unfortunately, ORR are low (<10%) and therapy delivery has been limited by hyperglycemia (Makker et al., 2016). Likewise, the use of mTOR inhibitors as single agents or in combination with other therapies have also shown modest response rates (Alvarez et al., 2013). Additionally, for a subset of serous and serous like EC with HER2 overexpression or ERBB2 amplifications, anti-HER2 therapy targeting agents represent an additional therapeutic option (Jhaveri et al., 2018; Li et al., 2018). Other second line standard options in recurrent EC include anti estrogen hormone therapy, mTOR inhibitors and bevacizumab, all of which have response rates <13.5% (Fleming, 2015).
For the exception of clinical trials, chemotherapy remains the standard of care for patients who are not candidates for these approaches. This leads to many unanswered questions regarding optimal chemotherapy treatment at the time of recurrence. Previous trials investigating second line treatment for EC with docetaxel (ORR 7.7%), gemcitabine (ORR 4%), topotecan (ORR 9%), ixabepilone (ORR 12%), and pegylated liposomal doxorubicin (ORR 9.5%) have demonstrated modest activity and remain as possible cytotoxic therapeutic options (Fleming, 2015). In keeping with prior data, we have shown that re-challenging patients with PC is also a viable treatment strategy for select patients with recurrent EC (Matoda et al., 2014; Nagao et al., 2013; Ueda et al., 2011; Nagao et al., 2015; Mazgani et al., 2008; Souza et al., 2016). In this small series, 50% of evaluable patients had a PR and an additional 15% had SD with clinical benefit rate of 65%. Additionally, the median PFS and OS from the time of retreatment was 10 months and 27 months respectively. Similar, data has been reported in abstract form from Brazil where 36 patients re-exposed to PC in the second line setting showed benefit from PC re-treatment (Souza et al., 2016). In that series, only 67% of patients had a platinum free interval of >6 months, nonetheless OS (from second line therapy) in the platinum re-challenge arm was 13.9 months compared to 7.9 months with other chemotherapeutic regimens. Additionally, re-exposure to platinum based chemotherapy in the second line setting was associated with higher ORR (43% vs 13%) and PFS (from second line therapy) (5.2 vs 3.2 months) (Souza et al., 2016). Other retrospective studies have also suggested that the platinum free interval may be equally important in this disease as in ovarian cancer in dictating the likelihood of response from further platinum treatment. In a study by Nagao and colleagues re-challenge with a platinum containing chemotherapy <6 months versus re-challenge >12 months since last platinum resulted in a 25% ORR versus 61% ORR respectively; the authors subsequently reported ORR of 67% for patients retreated >12 months post platinum compared to 40% in patients who were retreated within 12 months of completion of prior regimen (Nagao et al., 2013; Nagao et al., 2015). A further retrospective study from British Columbia suggested that EC patients who progressed after adjuvant PC had an improved OS from diagnosis (42 months vs 19 months) when they were re-exposed to PC versus other chemotherapy, albeit this may be driven partially by selection bias (Mazgani et al., 2008).
While this is a small single institution retrospective review with an inherent selection bias, our findings support previously published data showing that retreating patients with recurrent EC who are >6 months from completion of adjuvant therapy with PC is an effective and tolerated therapeutic option.
Conflict of interest statement
None of the authors have conflicts of interested pertaining to this publication.
Author contributions
Study concept and design: Cadoo.
Acquisition, analysis, or interpretation of data: Rubinstein, Halpenny, Makker, Grisham, Aghajanian and Cadoo.
Drafting of the manuscript: Rubinstein, Cadoo.
Critical revision of the manuscript for important intellectual content: Rubinstein, Halpenny, Makker, Grisham, Aghajanian and Cadoo.
Statistical analysis: Cadoo.
Acknowledgements
The authors are supported in part by the Memorial Sloan Kettering Cancer Center iNIH/NCI core grant P30 CA008748.
References
- Alvarez E.A., Brady W.E., Walker J.L. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a gynecologic oncology group study. Gynecol. Oncol. 2013;129:22–27. doi: 10.1016/j.ygyno.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N, Kandoth C., Schultz N. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz L.A., Marabelle A., Delord J.-P. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. J. Clin. Oncol. 2017;35 (3071–3071) [Google Scholar]
- Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Fleming G.F. Second-line therapy for endometrial cancer: the need for better options. J. Clin. Oncol. 2015;33:3535–3540. doi: 10.1200/JCO.2015.61.7225. [DOI] [PubMed] [Google Scholar]
- Jhaveri K.L., Makker V., Wang X.V. Ado-trastuzumab emtansine (T-DM1) in patients (pts) with HER2 amplified (amp) tumors excluding breast and gastric/gastro-esophageal junction (GEJ) adenocarcinomas: results from the National Cancer Institute (NCI) Molecular Analysis for Therapy Choice (MATCH) trial. J. Clin. Oncol. 2018;36 (100–100) [Google Scholar]
- Li B.T., Makker V., Buonocore D.J. Vol. 36. 2018. A Multi-Histology Basket Trial of ado-Trastuzumab Emtansine in Patients with HER2 Amplified Cancers. (2502–2502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makker V., Rasco D.W., Vogelzang N.J. Lenvatinib + pembrolizumab in patients with advanced endometrial cancer: updated results. J. Clin. Oncol. 2018;36 (5596–5596) [Google Scholar]
- Makker V., Recio F.O., Ma L. A multicenter, single-arm, open-label, phase 2 study of apitolisib (GDC-0980) for the treatment of recurrent or persistent endometrial carcinoma (MAGGIE study) Cancer. 2016;122(22):3519–3528. doi: 10.1002/cncr.30286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoda M., Omatsu K., Yamamoto A. Importance of platinum-free interval in second-line chemotherapy for advanced or recurrent endometrial cancer. Eur. J. Gynaecol. Oncol. 2014;35:224–229. [PubMed] [Google Scholar]
- Mazgani M., Le N., Hoskins P.J. Reuse of carboplatin and paclitaxel in patients with relapsed endometrial cancer—the British Columbia Cancer Agency experience. Gynecol. Oncol. 2008;111:474–477. doi: 10.1016/j.ygyno.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Miller D., Filiaci V. 2012 Society of Gynecologic Oncology Annual Meeting. Austin, TX. 2012. Late-Breaking Abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A gynecologic oncology group study. [Google Scholar]
- Nagao S., Nishio S., Michimae H. Applicability of the concept of "platinum sensitivity" to recurrent endometrial cancer: the SGSG-012/GOTIC-004/intergroup study. Gynecol. Oncol. 2013;131:567–573. doi: 10.1016/j.ygyno.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Nagao S., Nishio S., Okada S. What is an appropriate second-line regimen for recurrent endometrial cancer? Ancillary analysis of the SGSG012/GOTIC004/intergroup study. Cancer Chemother. Pharmacol. 2015;76:335–342. doi: 10.1007/s00280-015-2793-9. [DOI] [PubMed] [Google Scholar]
- Souza R.P., Soares G.P., Lage L.V. The role of platinum rechallenge as second line chemotherapy for metastatic endometrial carcinoma. J. Clin. Oncol. 2016;34 (e17108-e17108) [Google Scholar]
- Ueda Y., Miyake T., Egawa-Takata T. Second-line chemotherapy for advanced or recurrent endometrial carcinoma previously treated with paclitaxel and carboplatin, with or without epirubicin. Cancer Chemother. Pharmacol. 2011;67:829–835. doi: 10.1007/s00280-010-1384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]