Abstract
Oncolytic vaccinia viruses are currently in clinical development. However, the safety and the tumor selectivity of these oncolytic viruses must be improved. We previously constructed a first-generation oncolytic vaccinia virus by expressing the suicide gene FCU1 inserted in the J2R locus that encodes thymidine kinase. We demonstrated that the combination of this thymidine-kinase-deleted vaccinia virus and the FCU1/5-fluocytosine system is a potent vector for cancer therapy. Here, we developed a second generation of vaccinia virus, named TG6002, expressing FCU1 and with targeted deletions of the J2R gene and the I4L gene, which encodes the large subunit of the ribonucleotide reductase. Compared to the previously used single thymidine-kinase-deleted vaccinia virus, TG6002 is highly attenuated in normal cells, yet it displays tumor-selective replication and tumor cell killing. TG6002 replication is highly dependent on cellular ribonucleotide reductase levels and is less pathogenic than the single-deleted vaccinia virus. Tumor-selective viral replication, prolonged therapeutic levels of 5-fluorouracil in tumors, and significant antitumor effects were observed in multiple human xenograft tumor models after systemic injection of TG6002 and 5-fluorocytosine. TG6002 displays a convincing safety profile and is a promising candidate for treatment of cancer in humans.
Keywords: oncolytic vaccinia virus, ribonucleotide reductase, I4L, FCU1, TG6002
Introduction
The use of replication-competent viruses is an attractive strategy for tumor therapy after appropriate engineering so that they can preferentially and selectively propagate in cancer cells, thereby destroying tumor tissue, mainly via cell lysis, while leaving non-cancerous tissues unharmed.1 The efficient replication, cell lysis, and spread, as well as the broad host range of vaccinia virus (VACV), make it a very attractive vector for developing oncolytic viruses.2 Several strains of VACV are currently evaluated in preclinical and clinical trials, including the Wyeth, Western Reserve (WR), Copenhagen, and Lister strains.3, 4, 5, 6 Pexa-Vec (JX-594), the most advanced VACV oncolytic product, has now entered a randomized controlled phase 3 trial and was derived from a Wyeth strain engineered to express granulocyte/macrophage-colony-stimulating factor (GM-CSF).7 Like Pexa-Vec, most of the oncolytic VACVs reported to date harbor mutations that inactivate J2R, the gene encoding for viral thymidine kinase (TK).8 To ensure their own viral DNA synthesis, TK-deleted VACV strains (ΔJ2R VACV) depend on the cellular pool of thymidine triphosphate and thus on the expression of cellular TK, which is known to be overexpressed in tumor cells.9 The TK deletion maintains tumor targeting while displaying a reduced ability to replicate in other tissues,10 as demonstrated in numerous tumor models.2 In a previous study, we constructed a ΔJ2R VACV Copenhagen strain expressing the FCU1 gene by inserting the corresponding gene into the J2R locus, under the control of the p11K7.5 viral promoter.5 FCU1 encodes a bifunctional fusion protein combining cytosine deaminase (CDase) and uracil phosphoribosyltransferase activity (UPRTase). The CDase::UPRTase chimeric enzyme converts the non-toxic prodrug 5-fluorocytosine (5-FC) into the clinically approved chemotherapeutic compound 5-fluorouracil (5-FU), and further into 5-fluorouracil-monophosphate (5-FUMP), which ultimately inhibits DNA and protein syntheses.11 Because 5-FU is able to diffuse passively through cell membranes, it not only affects infected cells, but also neighboring cells, causing a bystander effect.11 The therapeutic effect of the ΔJ2R/FCU1 VACV combines both the VACV oncolytic activity and the cytotoxic effect of 5-FU. Expression of the FCU1 gene together with systemic administration of 5-FC, enhanced the therapeutic activity of VACV in subcutaneous and multi-focal liver metastasis models of human colorectal cancer.5 Nevertheless, increased safety and tumor selectivity could be achieved by additional modifications of the VACV genome. In fact, we have addressed this hypothesis by deletion of a second gene involved in the metabolism of DNA precursors—namely, a gene encoding a subunit of the viral ribonucleotide reductase (RR).
RR is an enzyme that catalyzes the conversion of ribonucleotide diphosphate (rNDPs) to deoxynucleotide diphosphate (dNDPs), which is further phosphorylated into deoxynucleotide triphosphate (dNTPs). dNTP is a direct substrate of DNA polymerases and therefore plays a central role in de novo DNA synthesis during cell replication, DNA repair, and cell growth.12 The RR enzyme primarily exists as a heterodimeric tetramer of a large catalytic subunit RRM1 with a small and regulatory subunit RRM2. Because of its critical role in DNA synthesis, RR is directly involved in neoplastic tumor growth, metastasis, and drug resistance. The proliferation of cancer cells requires excess dNTPs for DNA synthesis. Therefore, elevated RR expression is a characteristic of many primary and metastatic cancer cells.12 VACV encodes homologs of both the large and small subunits of RR, products of the I4L and F4L genes, respectively.13, 14 Biochemical studies showed that VACV and cellular RR enzymes share many features, including a similar tetrameric structure, allosteric modulation of activity by nucleotides, and comparable specific activities on most rNDP substrates.15, 16, 17 We hypothesized that combined deletion of the TK gene (J2R) and the large subunit of the RR gene (I4L) may enhance the tumor selectivity of the VACV Copenhagen strain. Although preliminary studies using such VACV deletion mutants have been reported by our team,18, 19, 20, 21, 22 the oncolytic potential was examined in a very limited number of cancer cell models and focused on the immune mechanisms of VACV oncolysis. Here, we report, compared to the singly deleted ΔJ2R/FCU1VACV, a more detailed investigation of the properties of the doubly deleted ΔI4LΔJ2R/FCU1 VACV (named TG6002) in an extensive range of human tumor cell lines, as well as healthy human cells. The replication and cytotoxicity of these viruses has been determined, and the biodistribution and antitumor effects have been studied in nude mice bearing subcutaneous human cancer xenografts. Viral pathogenicity and toxicity have been analyzed in vivo, in both nude and immunocompetent mice. We show that oncolytic and FCU1 activities are preserved in the doubly deleted TG6002 virus in vitro. This doubly deleted virus has similar antitumor activity against a human colorectal tumor model and displays enhanced tumor specificity. Furthermore, doubly deleted VACV is more highly attenuated in mouse challenge experiments and shows decreased side effects. Finally, we confirmed that TG6002 replication correlates with cellular RR levels. The data presented in this study demonstrate the advantage of deleting two viral genes involved in nucleotide metabolism for developing an oncolytic VACV that would be both very effective in tumor cells, and safe for normal cells.
Results
Deletion of Both J2R and I4L Genes Improves Safety and Efficacy of the VACV
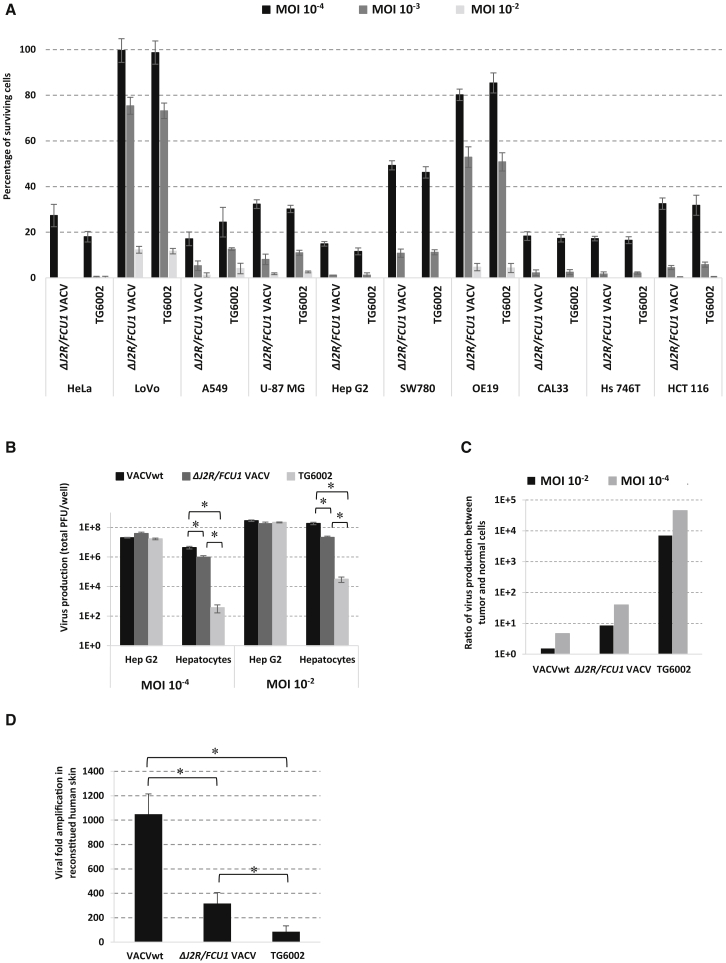
The oncolytic activities of the single (ΔJ2R)- and double (ΔJ2R/ΔI4L, or TG6002)-deleted VACV variants were compared in 10 human tumor cell lines from various origins: cervix (HeLa), colon (LoVo, HCT 116), lung (A549), brain (U-87 MG), liver (Hep G2), bladder (SW780), esophagus (OE19), head and neck (CAL33), and stomach (Hs 746T) (Figure 1A). Both VACV variants displayed comparable strong oncolytic activity, with less than 15% residual living cells at MOI 10−3 for 8 of the 10 cell lines tested and for all cells at MOI 10−2. We observed no major difference between ΔJ2R and TG6002 within each cell model. Thus, the additional deletion of the gene coding for the large subunit of RR (ΔI4L) has no impact on the infectivity and oncolytic activity of VACV in vitro. The second important question to be addressed in vitro was the confirmation of selective replication in tumor versus normal cells. We thus compared the replication of VACV variants in the hepatocarcinoma human cell line Hep G2 and in primary human hepatocytes at MOIs 10−2 and 10−4, respectively (Figure 1B). A similar pattern of replication was observed in tumor cells infected with wild-type VACV (VACVwt), ΔJ2R VACV, or TG6002 at both MOIs, showing that all variants were as active as the VACVwt in tumor cells. Compared with VACVwt, the production of ΔJ2R VACV was reduced 10-fold in human primary hepatocytes, in agreement with previous reports showing that TK gene deletion reduced replication in normal cells.23, 24 The diminution of replication in primary hepatocytes was improved with TG6002 with an approximately 4-log-fold decrease in virus production for the double-deleted TG6002 virus compared to the VACVwt and 3-log-fold decrease compared to ΔJ2R VACV (Figure 1B). The replication yield of the double-deleted TG6002 was about 3 in the hepatocytes, indicating no substantial replication of the double-deleted VACV in primary cells 48 h after infection. Consequently, the specificity index calculated from the ratio between the yield of viral progeny produced in tumor cells to that in primary cells was largely improved for TG6002, since a 10,000-fold ratio was observed for replication in Hep G2 versus normal cells (Figure 1C). This impressive increase in the specificity index represents major progress toward a safer oncolytic product. The pathogenesis of several different viruses, including papillomaviruses, adenoviruses, parvoviruses, poxviruses, and herpesviruses was previously tested with skin substitutes.25 For VACV, it has already been demonstrated that 3D multilayered skin cultures can be used as a predictive model for pock lesions.26 We compared the replication yields of wild-type, ΔJ2R VACVs, and TG6002 in a Phenion full-thickness 3D skin model. Note that in this model, compared with hepatocytes, replication of VACVwt was poorer. However, TG6002 appeared much less replicative than other VACVs, with 86-fold amplification for TG6002, compared with 1,050- and 320-fold amplification for wild-type and ΔJ2R-deleted viruses, respectively (Figure 1D). These results confirmed the benefit of deleting both the I4L and J2R genes to increase safety in non-cancerous tissues without affecting replication in tumor cells.
Figure 1.
Oncolytic Activity and In Vitro Replication of Copenhagen-Based VACVs
(A) Oncolytic effect of ΔJ2R/FCU1 VACV and TG6002 on a panel of human tumor cells. Cells were infected at MOI 10−4, 10−3, or 10−2 with the indicated vectors, and cell viability was measured 5 days later by trypan blue exclusion. The results are presented as a mean of triplicate experiments ± SD. (B) Virus production of the different VACVs in primary normal cells and tumor cells. Normal human hepatocytes and tumor human Hep G2 hepatocarcinoma cells were infected by VACVwt, ΔJ2R/FCU1 VACV, and TG6002 at MOI 10−4 (102 PFU/well) or 10−2 (104 PFU/well). Viruses produced after 48 h were titered by plaque assay. The results are presented as a mean of triplicate experiments ± SD. The asterisks indicate a significant difference between groups (p < 0.05). (C) Ratio of virus yield in Hep G2 cells versus human hepatocytes 48 h after infection. Values are represented as the mean of three individual determinations. (D) Amplification factor of the different VACVs in Phenion full-thickness human skin model. 3D Phenion full-thickness human skin models were infected by VACVwt, ΔJ2R/FCU1 VACV, and TG6002 at 1 × 105 PFU. Seven days after infection, 3D skin and supernatant were collected and sonicated, and viral titers were determined by plaque assay. Results are expressed as viral fold increased (corresponding to output/input ratio). Values are represented as means ± SD. The asterisks indicate a significant difference between groups (p < 0.05).
Cellular RR Is Important for Replication of I4L-Deleted VACV
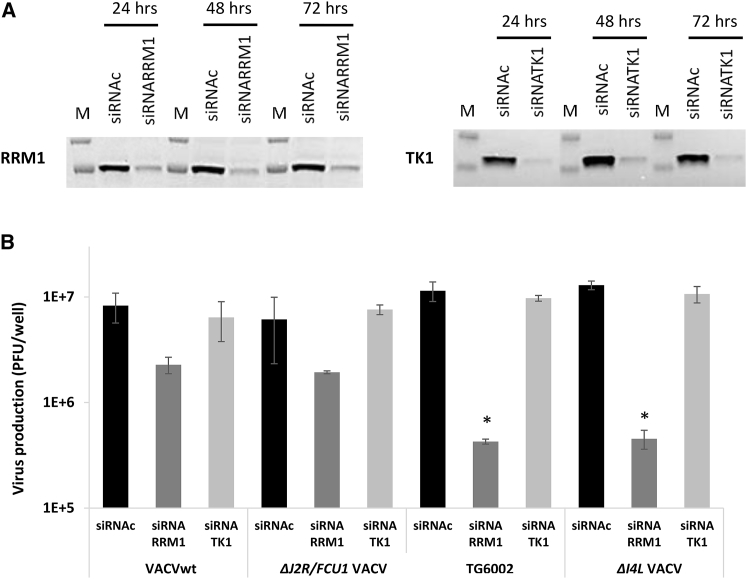
To evaluate the implication of cellular TK and RR in the replication of VACV, we measured the production of VACVs in LoVo cells after silencing of corresponding cellular genes TK1 and RRM1 by their specific small interfering RNAs (siRNAs). Western blot analysis showed that expression of TK1 and RRM1 proteins was significantly decreased following transfection with the specific siRNAs (Figure 2A), and this decline in protein expression persisted for over 3 days after transfection. After gene silencing, cells were infected with VACVwt, ΔJ2R/FCU1 VACV, ΔI4L/VACV, or TG6002 at MOI 10−3, then incubated for 48 h. Production of infectious VACV particles was evaluated by a viral plaque assay. As shown in Figure 2B, silencing TK1 had no effect on virus production, whatever the virus evaluated. These results confirm the data of others showing that a decrease in the TK level in tumor cells has little or no impact on the replication of VACVwt and JX-594.27 In contrast, treatment of LoVo cells with siRNA against RRM1 largely decreased virus yield, and this effect was particularly pronounced for the I4L-deleted virus, in association or not with the J2R deletion. In cells transfected with RRM1 siRNA and compared to cells transfected with non-targeting control siRNA, there was an ∼3-fold reduction in virus yield for the non-deleted I4L wild-type and single J2R-deleted viruses. In the same RRM1 silenced LoVo cells, there was a ∼27-fold decrease in the yield for the I4L-deleted TG6002 and ΔI4L VACV viruses (Figure 2B). These data demonstrate that the cellular RR gene plays a critical role in the replication of VACV and particularly argue for the dependence of TG6002 replication on cellular RR levels.
Figure 2.
Effects of siRNA-Mediated Knockdown of Cellular RRM1 and TK1 on Replication of VACVs
(A) Western blot detection of RRM1 (left) and TK1 (right) protein expression in LoVo cells 24, 48, and 72 h after transfection with specific siRNA and non-targeting control siRNA (siRNAc). Molecular weight standards are shown (M). The western blots are representative of three different experiments. (B) Virus production in RRM1 and TK1-knockdown LoVo cells following infection with the indicated VACVs at MOI 10−3 for 48 h before performing plaque assays. The data represent the average of three independent experiments and are shown as the mean ± SD. *p < 0.05 compared to cells transfected with the non-targeting siRNAc.
I4L Deletion Does Not Alter the FCU1 Expression
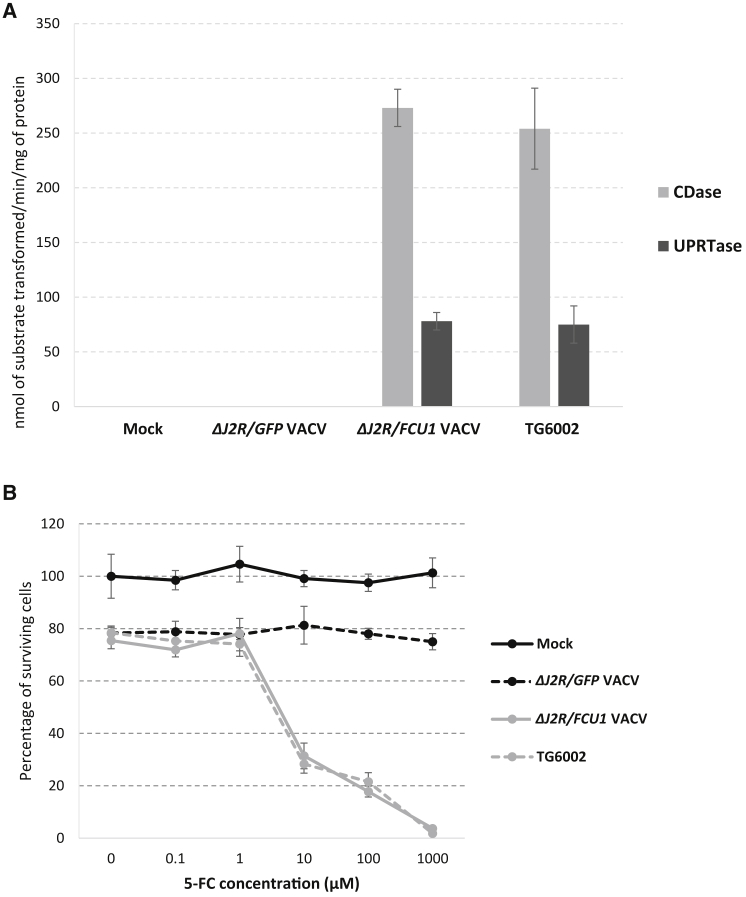
The expression of the transgene was examined by measuring the two enzymatic components of FCU1, i.e., the CDase and the UPRTase. These two activities were determined by observing the enzymatic conversion of 5-FC to 5-FU and 5-FU to 5-FUMP, respectively, 16 h after viral infection of human LoVo tumor cells. The cell lysates from mock-infected or ΔJ2R/GFP VACV-infected cells showed no detectable CDase and UPRTase activities, whereas cells infected with either ΔJ2R/FCU1 VACV or TG6002 displayed comparable high levels of CDase and UPRTase activities (Figure 3A). We previously showed that LoVo cells are rather poorly sensitive to the sole oncolytic activity of TG6002 (Figure 1A). To measure the contribution of prodrug conversion on tumor cytotoxicity, LoVo cells were infected at a low MOI (10−3) by ΔJ2R/GFP VACV, ΔJ2R/FCU1 VACV, and TG6002, then treated with 5-FC. As shown in Figure 3B, only 20% tumor cells were killed after 48 h at this very low MOI, with no difference between ΔJ2R/GFP, ΔJ2R/FCU1 VACVs, or TG6002 in the absence of 5-FC. As expected, the addition of 5-FC did not increase cytotoxicity in LoVo cells infected with control ΔJ2R/GFP VACV. In contrast, 5-FC largely killed LoVo cells infected by ΔJ2R/FCU1 VACV and TG6002 in a prodrug, dose-dependent manner. Consistent with the similar levels of FCU1 expression, both ΔJ2R/FCU1 VACV and TG6002-infected cells displayed a similar level of sensitivity to 5-FC, with a half-maximum inhibitory concentration (IC50) of around 3 μM. These data confirm that FCU1/5-FC cytotoxic activity was expressed by the double-deleted virus to the same extent as by the single-deleted parental virus.
Figure 3.
In Vitro Evaluation of FCU1 Expression
(A) Specific CDase and UPRTase activities. LoVo cells were infected at MOI 10−2 with the indicated vectors. After 16 h, enzymatic activities were measured as described in Materials and Methods. CDase and UPRTase activities are expressed as the number of nanomoles 5-FC deaminated per minute per milligram of protein and the number of nanomoles 5-FU phosphorylated per minute per milligram of protein, respectively. Each value represents the average of three independent experiments ± SD. (B) Combination of oncolytic and prodrug activation cytotoxicity. LoVo cells were infected at MOI 10−3 with the indicated vectors. After 48 h, 5-FC was added in a range of concentrations, and cell survival was determined 3 days later, as described in the Materials and Methods section. Results were standardized against values for wells lacking virus and drug, which represented 100% viability. Values are represented as means ± SD of three individual determinations.
TG6002 Replicates Selectively in Xenografted Human Cancers
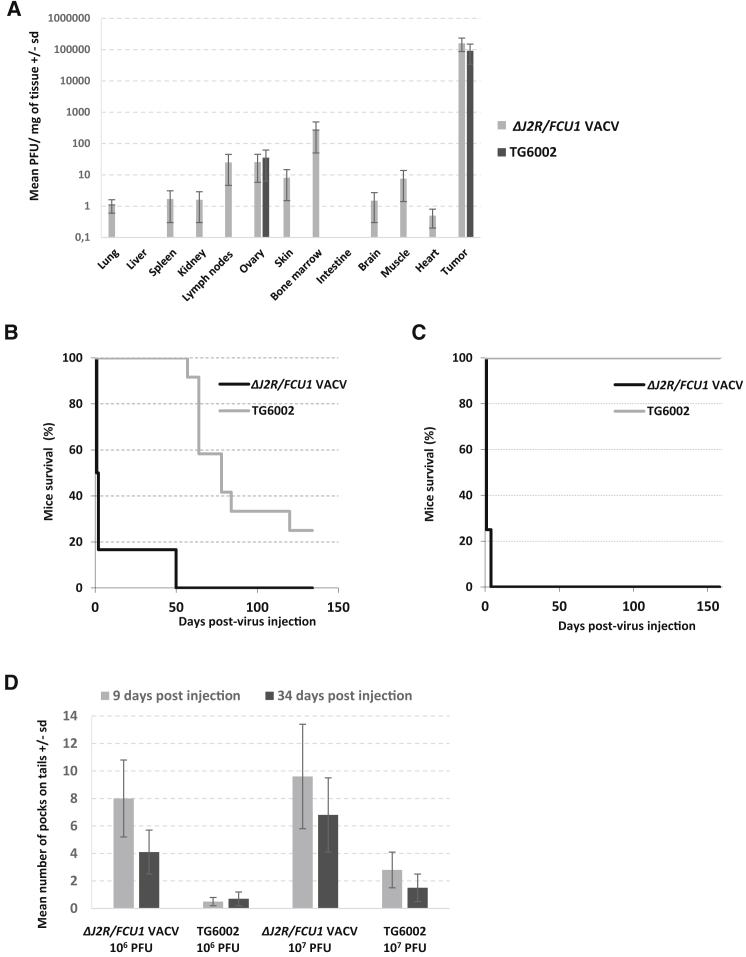
The impact of I4L deletion on the functional properties of VACV in vivo was approached in various tumor-bearing preclinical models. As a first challenging model, ΔJ2R/FCU1 VACV and TG6002 were injected intravenously at 1 × 106 PFU in nude mice bearing subcutaneous LoVo human tumors, and virus distribution was examined 14 days after injection. Similarly, high levels of both viruses were detected in tumors (Figure 4A). Both viruses generated approximately 108 PFU of infectious virus within the entire tumor after intravenous injection of 1 × 106 PFU. These results clearly demonstrated replication of the two viruses in the tumors. The injection of the single-deleted ΔJ2R/FCU1 VACV resulted in a broad distribution of the virus in healthy tissues, including lung, spleen, kidney, lymph node, ovaries, skin, bone marrow, brain, and muscle (Figure 4A), consistent with a previous study.5 The inactivation of the I4L gene led to significant reduction in the viral titers in healthy tissues. For TG6002-injected mice, viral particles were only recovered, and at low levels, from ovaries (Figure 4A). The reduced biodistribution of the double-deleted virus in various organs agreed with reduced replication in primary hepatocytes and in a reconstructed human skin model. These results confirm that combined deletion of two nonessential genes entails a decrease in replication in normal tissue but not in tumor cells. We then determined the kinetics of TG6002 distribution in organs and tumors, and the effect of 5-FC administration on virus biodistribution in view of the possibility that FCU1 expression in combination with 5-FC could interfere with viral replication and thereby reduce the overall antitumor efficacy.5 Female and male nude mice bearing established subcutaneous Hep G2 tumors were injected intravenously with 1 × 106 or 1 × 107 PFU of TG6002 and, starting at day 7 following viral infection, 5-FC was given or not for 3 weeks. The level of virus recovery from different organs and tumors was determined at day 7 (before the beginning of 5-FC treatment) and at days 14, 29, and 57 after infection (Table S1). The pattern of infectivity was the same for the mice treated at 1 × 106 or 1 × 107 plaque forming units (PFU) with a high titer of virus detected in the tumor at days 7 and 14 after infection. The lower viral titers in tumors at days 29 and 57 may be the result of tumor shrinkage. In a few animals treated at 1 × 106 or 1 × 107 PFU, low levels of virus were also found in some organs; however, most organs were negative for the virus. In groups of mice treated with 5-FC, an equivalent pattern was seen, with similar amounts of virus generated in the tumors (Table S1). These results indicate that initiation of 5-FC treatment, 7 days after infection, was not deleterious for replication of TG6002 in the tumor.
Figure 4.
Biodistribution and Pathogenicity of Single- and Double-Deleted VACVs
(A) Comparison of biodistribution of ΔJ2R/FCU1 VACV and TG6002 in tumor and normal tissues. Female nude mice bearing established subcutaneous LoVo tumors were infected intravenously with 1 × 106 PFU of ΔJ2R/FCU1 VACV or TG6002. On day 14 after virus administration, tumors and normal tissues were harvested and homogenized, and viral titers were determined by a standard plaque assay. Results are expressed in PFU per milligram tissue. Values are presented as means ± SD of three animals. (B) Survival of nude mice treated with 1 × 108 PFU of ΔJ2R/FCU1 VACV or TG6002 by one intravenous injection (n = 12/group). TG6002-infected mice had significantly prolonged survival (p < 0.01) compared with ΔJ2R/FCU1 VACV. (C) Survival of immunocompetent mice treated with 1 × 108 PFU of ΔJ2R/FCU1 VACV or TG6002 in one intravenous injection (n = 12/group). TG6002-infected mice had significantly prolonged survival (p < 0.001) compared with ΔJ2R/FCU1 VACV. (D) Mean number of pocks on tails after systemic injection of VACVs. Healthy nude mice were treated with one intravenous injection of ΔJ2R/FCU1 VACV and TG6002 at 1 × 106 PFU or 1 × 107 PFU. Pocks on tails were counted at days 9 and 34 after injection. Values represent the means ± SD of 12 animals.
The Double-Deleted VACV Is Less Toxic Than the Single-Deleted VACV
Immunodeficient mice were injected with ΔJ2R/FCU1 VACV or TG6002 at 1 × 108 PFU intravenously and then observed for survival (Figure 4B). The injection of 1 × 108 PFU of the single-deleted VACV resulted in the rapid death of 85% of the animals within 3 days (Figure 4B) and the remaining mice died at day 50 after infection. The administration of TG6002 was less pathogenic; most of the animals died between days 60 and 120 (p < 0.01), and 25% of the mice injected with the double-deleted virus survived and remained healthy until at least day 135, the day of sacrifice. For both viruses, the death of the animals was usually preceded by weight loss (data not shown). The pathogenicity of the viruses was also tested in immunocompetent mice (Figure 4C). Both viruses were injected intravenously into B6D2 mice at 1 × 108 PFU. All 12 mice injected with TG6002 remained healthy, without weight loss, until at least day 160, the day of sacrifice. Of the mice injected with ΔJ2R/FCU1 VACV, all mice died within 3 days. These results obtained for both nude and immunocompetent mice showed that TG6002 is much less pathogenic. The findings are in agreement with the observed reduction in the level of infection of various organs (Figure 4A). Another measure of the pathogenicity induced by VACVs is the number of tail lesions. Indeed, formation of pock lesions (red papules) on the tail is a typical symptom of systemic infection by VACVs.28 Usually these lesions appear 4–5 days after infection, with a maximum around 8–10 days after infection, then the number decreases over time. We observed an average of 9.6 pocks with ΔJ2R/FCU1 VACV compared with an average of 2.8 with TG6002 at an early time point (day 9) after infection with 1 × 107 PFU (Figure 4D). At a later time point after injection (day 34), tail lesions were reduced with ΔJ2R/FCU1 VACV (average of 6.8), whereas with TG6002, the number of lesions remained low (an average of 1.5). The number of pocks was smaller after injection of 1 × 106 PFU of ΔJ2R/FCU1 VACV, as compared to 1 × 107 PFU (an average of 8 at day 9 and 4.1 at day 34). However, at 1 × 106 PFU, we still observed a large difference in the number of pocks in mice infected with TG6002 (an average of 0.5 at day 9 and 0.7 at day 34). Whatever the dose of virus and the time point, the difference in pock number between the single- and double-deleted viruses was statistically significant (p < 0.01), in agreement with a decrease in pathogenicity and replication in healthy tissue after injection of TG6002. A low number of pock lesions on the tail were also seen after systemic injection of TG6002 in mice bearing subcutaneous LoVo tumors (Table S2). Moreover, we observed fewer pocks on tails in the group treated with 5-FC when compared to the group that was not treated with 5-FC, and this difference was statistically significant (p < 0.01), whatever the time after injection (Table S2).
TG6002 Antitumor Activity in Various Preclinical Models
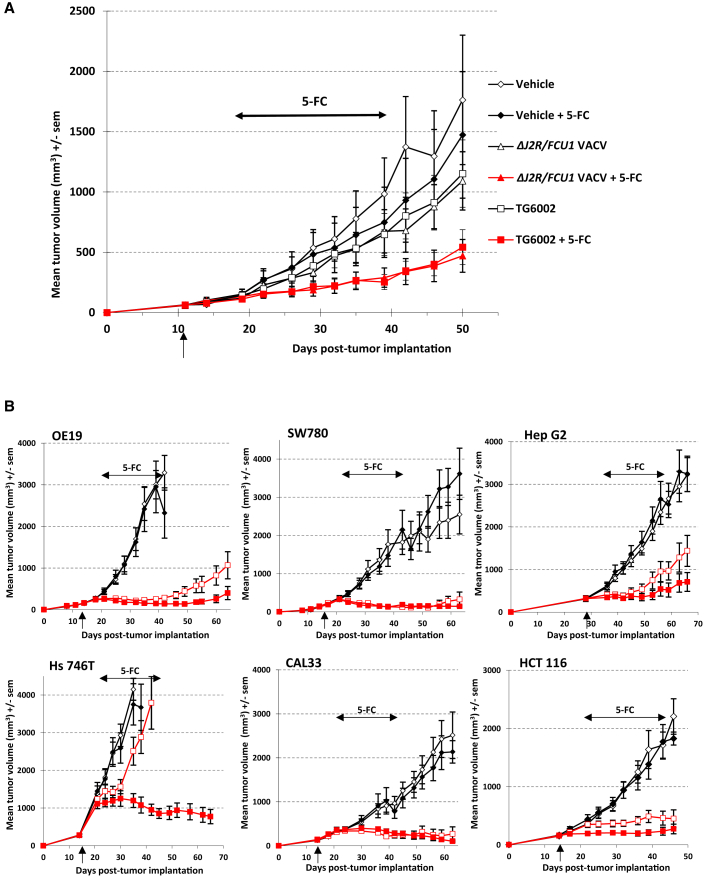
The ability of TG6002 to function as an oncolytic virus was examined in different models of human cancers. We first compared the oncolytic activity of the single- and double-deleted viruses in the colorectal LoVo model. As described in Figure 1A, the LoVo cell line is one of the most resistant cells to VACV oncolysis in vitro, with more than 70% surviving cells at MOI 10−3. Mice bearing LoVo tumors were injected intravenously with both viruses at 1 × 107 PFU. 5-FC or a mock treatment was given per os for 3 weeks starting on day 7 after viral delivery. As shown in Figure 5A, a single intravenous injection of both viruses resulted in a weak inhibition of tumor growth compared with the controls (no treatment or 5-FC alone). The administration of 5-FC alone had no effect on tumor growth. In contrast, the 5-FC treatment significantly improved the ΔJ2R/FCU1 VACV antitumoral activity when compared to virus alone (p < 0.05), indicating that the FCU1/5-FC approach compensated the lack of potent oncolytic activity of the virus in this model (Figure 5A). A similar potentiation was observed when 5-FC was co-administered with TG6002 (p < 0.05; Figure 5A). Comparable activity was observed regarding control of tumor growth induced by the two viruses alone or in combination with 5-FC. Thus, the deletion of the I4L gene did not impair the oncolytic activity of FCU1 expressing VACV. To further characterize the antitumoral efficacy of TG6002, we tested the oncolytic activity of this virus, with and without 5-FC administration in multiple human tumor xenograft models (Figure 5B). TG6002 was injected once intravenously at 1 × 107 PFU in models that are more resistant to VACV oncolysis in vitro (OE19 and SW780, Figure 1A), whereas the most sensitive models (CAL33, Hs 746T, HCT 116, and Hep G2, Figure 1A) were treated with one intravenous injection of the virus at 1 × 106 PFU. In all models tested, 5-FC was given per os for 3 weeks starting on day 7 after viral delivery. As shown in Figure 5B, in CAL33, SW780, and HCT 116 models, a single intravenous injection of TG6002 alone (no 5-FC administered) induced a strong inhibition of tumor growth compared with the controls (p < 0.001), resulting in complete response with tumor-free mice at the end of the experiment. In these models, the potent antitumor effect of the virus alone consequently hampered the evaluation of any 5-FC additional benefit. In Hep G2 and OE19 models, a single intravenous injection of TG6002 alone also resulted in strong inhibition of tumor growth compared with the controls (p < 0.001), and coadministration of the virus with 5-FC showed enhanced antitumor activity when compared to the virus alone. In the Hs 746T tumor model, intravenous injection of TG6002 induced a weak antitumor effect compared with the controls, and this effect was dramatically increased by 5-FC coadministration (TG6002 + 5-FC versus TG6002; p < 0.05). Control experiments were also performed to determine the in vivo antitumor effect of 5-FU treatment. Administration of the dose of 5-FU that was at the maximum tolerated concentration (intraperitoneal injection of 10 mg 5-FU/kg/day for 2 weeks), induced no statistically significant inhibition of tumor growth in all tumor models tested in this study (data not shown).
Figure 5.
In Vivo Antitumor Activity of VACVs
(A) Mean tumor volume after systemic treatment with 1 × 107 PFU of ΔJ2R/FCU1 VACV or TG6002 plus 5-FC administration. Mice bearing LoVo subcutaneous xenografts were treated with one intravenous administration of vehicle, ΔJ2R/FCU1 VACV or TG6002 (indicated by a vertical arrow). Seven days after virus injection, the animals were then treated twice daily with per os administration of water or 5-FC (200 mg/kg per day) for 3 weeks (indicated by a horizontal arrow). The data represent the mean of 12 animals. (B) Antitumor activity of TG6002 in multiple human tumor models. OE19 esophagus cancer cells, SW780 bladder cancer cells, Hep G2 hepatocarcinoma cells, Hs 746T stomach cancer cells, CAL33 head and neck cancer cells, and HCT 116 colorectal carcinoma cells were implanted subcutaneously in mice. At the day indicated by the vertical arrow, the mice were treated with one intravenous administration of vehicle (open diamonds, vehicle + water; solid diamonds, vehicle + 5-FC) or with one intravenous administration of TG6002 at the dose indicated in Materials and Methods (open red squares, TG6002 + water; solid red squares, TG6002 + 5-FC). Seven days after virus injection, the animals were treated twice daily with per os administrations of water or 5-FC (200 mg/kg per day) for 3 weeks (indicated by a horizontal arrow). The data represent the mean ± SD of 12 animals.
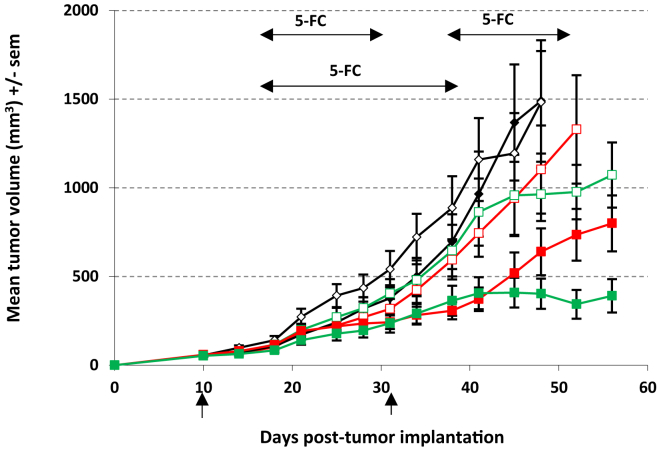
Finally, we evaluated in our well-documented LoVo model, the efficacy of multiple cycles of TG6002/5-FC (Figure 6). As before, we first performed a single intravenous injection of TG6002 (1 × 107 PFU), and 5-FC (or control) was given per os for 3 weeks starting on day 7 after viral delivery. In the multiple cycles of TG6002/5-FC treatment, at the end of the first 5-FC regimen, mice received an additional dose of virus at 1 × 107 PFU, and 5-FC was administered 7 days later for 2 consecutive weeks. The group receiving a single injection of the virus alone showed no inhibition of tumor growth, and the addition of 5-FC resulted in a benefit (Figure 6), similar to the previous experiment (Figure 5A). Without 5-FC administration, the second injection of TG6002 at day 31 after tumor implantation also had no immediate antitumor effect but a reduction of tumor growth was observed between days 45 and 52 after tumor implantation. The tumor growth for the group treated with multiple cycles of 5-FC was markedly suppressed in comparison with that of the control groups (p < 0.01). For mice treated with 5-FC, from day 48 after tumor implantation on, there was a significant difference in tumor size between the single-cycle group and the two-cycle group (two cycles TG6002/5-FC versus one cycle TG6002/5-FC; p < 0.05). Overall, in terms of antitumor activity, multiple cycles of TG6002/5-FC therapy had an advantage over a single cycle.
Figure 6.
Antitumor Activity of Multiple Cycles of TG6002/5-FC
Mean tumor volume after multiple cycles of TG6002/5-FC. Mice bearing LoVo subcutaneous xenografts were treated with one or two cycles of TG6002/5-FC. In the single cycle of treatment, at day 10 (indicated by vertical arrow), mice were treated with one intravenous administration of vehicle (open diamonds, vehicle + water; solid diamonds, vehicle + 5-FC) or with one intravenous administration of TG6002 at 1 × 107 PFU (open red squares, TG6002 + water; closed red squares, TG6002 + 5-FC). Seven days after viral injection, 5-FC (200 mg/kg per day) was administered for 3 weeks (indicated by a horizontal arrow). In the multiple cycles of TG6002/5-FC treatment, at days 10 and 31 (indicated by vertical arrows), mice were treated with one intravenous administration of TG6002 at 1 × 107 PFU (open green squares, TG6002 + water; closed green squares, TG6002 + 5-FC). Seven days after each viral injection, 5-FC (200 mg/kg per day) was administered for 2 weeks (indicated by horizontal arrows). The data represent the mean ± SD of 12 animals.
The Combination of TG6002 and 5-FC Produces a High Level of 5-FU in Tumor Tissues
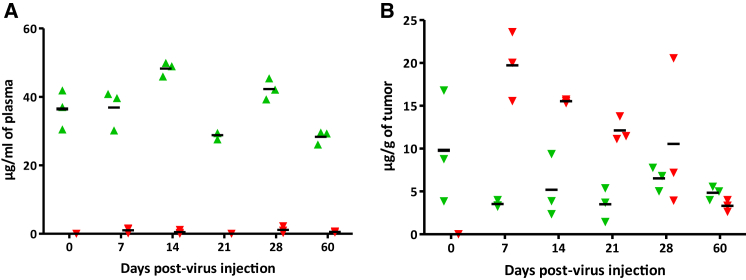
A sensitive and specific high-performance liquid chromatography (HPLC) assay was set up to measure the concentrations of 5-FC and 5-FU in tumor tissue and plasma samples obtained 7–60 days after a single intravenous injection of TG6002 and oral administration of 5-FC for several days into nude mice bearing well developed subcutaneous Hep G2 tumors. The mean plasma concentration of 5-FC after oral administration ranged from 27.44 ± 1.37 (day 60) to 48.30 ± 1.69 (day 14) μg/mL (Figure 7A). Intratumoral concentrations of 5-FC, resulting from passage of the prodrug from blood to the tumor, ranged from 3.21 ± 0.14 (day 7) to 9.80 ± 5.32 (day 0, non-infected mice) μg/g (Figure 7B). 5-FU was present at measurable concentrations in all tumor samples of mice treated with TG6002 and 5-FC. The mean intratumoral concentration of 5-FU decreased progressively from 19.72 ± 0.14 μg/g at day 7 to 10.56 ± 7.19 μg/g at day 28. At 60 days after infection, 5-FU was still detectable in the tumor tissues at a concentration of 3.32 ± 0.56 μg/g (Figure 7B). The concentration of 5-FU in plasma was not measurable (i.e., below 1 μg/mL) in all collected samples and was undetectable in one-third of the samples (Figure 7A), arguing for the safety of the targeted prodrug conversion approach.
Figure 7.
Mouse Pharmacokinetic Studies
Concentrations of 5-FC and 5-FU in plasma and tumor tissue samples. TG6002 was injected intravenously at 1 × 106 PFU in nude mice bearing subcutaneous human Hep G2 tumors. Before virus injection (day 0) and at 7, 14, 21, 28, and 60 days after viral injection, blood and tumor tissues were collected 1 h after administration of 5-FC (per os at 100 mg/kg) and the concentrations of 5-FC and 5-FU were measured in plasma (A) or in tumors (B) as described in Materials and Methods. Samples were obtained from groups of three mice at each sample time. Concentrations determined in samples from individual animals are indicated as 5-FC (green triangle) and 5-FU (red downward triangle). Symbols drawn through the x axis denote samples with concentrations below the limit of quantitation. Horizontal bars represent mean concentrations.
Discussion
We demonstrated in a series of experiments that, in comparison with the parental single-deleted TK-deficient virus, the double-deleted virus TG6002 displayed reduced multiplication in primary non-transformed human cells (human primary hepatocytes and 3D skin model). Moreover, we have demonstrated the ability of this virus to selectively infect and replicate in tumor tissues, compared with normal tissue. ΔJ2R/FCU1 VACV and the double-deleted virus were detected at a level 3 to 4 logs higher in tumors than in normal mouse tissues in a subcutaneous human colorectal cancer model after systemic administration of virus at 1 × 106 PFU. Moreover, the distribution of the double mutant virus was more restricted to the tumor. The biodistribution results were reflected in the mouse survival experiments. At a supra-pharmacological concentration of 1 × 108 PFU, nude and immunocompetent mice infected intravenously with TG6002 had significantly increased survival compared with mice infected with ΔJ2R/FCU1 VACV. A striking finding is the survival of immunocompetent mice injected with the double-deleted virus (100% survival), whereas all mice injected with the parental virus died very rapidly after injection. We showed that a consequence of this double deletion is the significant reduction of tail lesions (a common side effect of VACVs in mice). In previously described clinical studies using oncolytic J2R-deleted VACVs, about 10%–20% of the patients developed pustular lesions consistent with vaccinia-related pustules on the skin.6, 29, 30 Thus, one may postulate that double-deleted ΔI4LΔJ2R VACV would induce fewer pustular lesions in treated patients. We also observed here that 5-FC can inhibit the development of tail lesions caused by TG6002. The protective activity of 5-FC against the virus expressing FCU1 was likely due to the formation of small amounts of 5-FU in skin which inhibits the limited replication at this location. In a recent preclinical study, Potts et al.31 described an oncolytic WR strain bearing a deletion in F4L, which encodes the small subunit of RR. This F4L-deficient VACV promotes oncolysis in immune-compromised mice bearing either subcutaneous or orthotopic human bladder cancer xenografts. Oncolytic activity was also observed in immune-competent rats bearing orthotopic bladder tumors.31 This F4L-deficient WR strain also appears to be safer than the TK-deficient WR strain. When the TK-deficient virus was administered by intravenous or intratumoral routes, weight loss and pock lesions were observed. In contrast, F4L-deficient virus did not cause these classic signs of poxvirus virulence.31 The combination of F4L and J2R deletions produces a virus that lacks the virulence of a TK-deficient strain in immune-compromised animals while still being able to cure xenografted tumors.31
We hypothesized that cellular RR levels should influence the replication of a double-deleted ΔI4LΔJ2R VACV. This vector, defective in RR activity, should replicate in cancer cells where RR is available. In our study, when cellular RRM1 levels were reduced using siRNA silencing, the yields of I4L-deleted VACV mutants were greatly reduced, whereas growth of wild-type VACV was not significantly affected. In contrast, a decrease in the level of cellular TK had no significant effect on J2R-deleted VACV. These results indicate the essential role of cellular RR activity in the regulation of VACV replication, the impact of this RR activity on VACV replication being greater than that of TK. Many studies have established that RR activity is highly correlated with the rate of cancer growth. Carroll et al.32 examined RR expression in biopsies of colon carcinoma liver metastases and adjacent normal liver from patients by western blot analysis. RRM1 expression in the relatively quiescent liver was extremely low compared with the high levels detected in the colon carcinoma metastasis.32 RRM2 overexpression has been observed in gastric, ovarian, bladder, and colorectal cancers.33, 34, 35, 36 RRM2 expression is correlated with tumor grade for both breast and epithelial ovarian cancers, suggesting a role for RR in supporting rapid cell division of high-grade tumors.37, 38 Using the ONCOMINE cancer gene expression database (https://www.oncomine.com), Aye et al.12 found that a significant fraction of cancer specimens exhibited increased RRM1 and RRM2 levels. These data support the feasibility of selectively targeting tumors with VACV defective in RR activity. As previously reported,39 deleting I4L had no effect on viral replication on tumor cells. In contrast, F4L deletion reduces the yield of VACV ∼50-fold compared to VACVwt, when grown in tumor cells,39 suggesting a potential advantage of ΔI4L over ΔF4L.
Despite the efficiency of the recombinant virus in vitro, complete infection and lysis of the entire tumor is difficult. Therefore, oncolytic viruses are often armed. The combination of oncolytic VACV and enzyme-prodrug systems has been considered an attractive strategy.2 The FCU1/5-FC enzyme-prodrug system has been extensively investigated in vitro and in preclinical models of xenografts.11, 40, 41, 42, 43 The proof of this suicide gene concept has also been demonstrated in humans, by using TG4023, a non-propagative VACV (modified vaccinia virus Ankara [MVA]) hosting the FCU1 gene.44 In this phase I study, after a single percutaneous intratumoral injection of TG4023 in primary or metastatic liver tumors in combination with systemic administration of 5-FC, therapeutic 5-FU concentrations in tumors were detected without significant systemic exposure to the cytotoxic anticancer drug. We have shown here that the additional I4L deletion did not interfere with the expression of FCU1, as clearly shown in enzymatic assays and in in vitro 5-FC sensitivity. In vivo, in combination with 5-FC, a similar antitumor effect was obtained after injection of the double-deleted virus in comparison to the single-deleted parental virus. TG6002 in vivo antitumor activity studies were performed in several human cancer models, including colorectal, bladder, esophagus, stomach, head and neck, and liver cancers. In our experimental conditions, TG6002 alone was able to provide an efficient antitumoral effect, and we have shown that the combination of TG6002 with 5-FC had usually a more pronounced effect on antitumor activity than TG6002 alone. We also noted an increase in the antitumor activity with multiple cycles of TG6002/5-FC. However, in some models, such as LoVo or Hep G2 models, even with 5-FC administration, a partial antitumor effect was obtained, indicating that it would therefore probably be necessary to reduce tumor size by some other means before or during virus treatment. Our laboratory has also generated a surrogate product of TG6002, a VACV WR strain bearing deletions in J2R and I4L and expressing the FCU1 gene.19 The VACV Copenhagen strain is weakly effective in murine tumor cells (personal observation) and the VACV WR strain, originally adapted for growth in mice, constituted an attractive model to evaluate antitumor efficacy in immunocompetent preclinical models. The combination of 5-FC with this J2R-I4L-deficient WR strain, resulted in more sustained control of syngenic renal carcinoma tumors,19 and this virus was shown to induce tumor growth control of distant untreated tumors in immunocompetent mice.22 These VACV-mediated abscopal effects were greatly amplified when tumors were defective for the type I interferon receptor (IFNAR) signaling pathway, and these effects were further enhanced when oncolytic treatment was combined with immunogenic chemotherapy or with immune checkpoint blockade.22 In xenografted mice that had been treated by a single intravenous injection of TG6002 followed by per os 5-FC administration, high levels of 5-FU were detected in tumors and persist in tumors for a minimum of 60 days after virus administration. Previously, we have shown that in mice treated with a single injection of 5-FU at the maximum tolerated dose (10 mg/kg administered intraperitoneally), the blood concentration of 5-FU reached a maximum of 5 μg/mL 5 min after injection of 5-FU and the highest level of 5-FU in tumor tissues was detected 10 min after injection with a concentration of 0.6–0.7 μg/g of tumor.40 When a single injection of 5-FU at 200 mg/kg (20 times the maximum tolerated dose) was given, the 5-FU level reached a maximum of 90 μg/mL of plasma and 10 μg/g of tumor 10 min after 5-FU administration, and 5-FU became undetectable in tumors 1 h after injection.40 Here, we demonstrated that the intratumoral 5-FU level resulting from systemic injection of TG6002 was equivalent to the maximum level attained with systemically administered 5-FU at 20-fold the maximum tolerated dose. In addition, we showed that after production of 5-FU at the tumor site using TG6002, 5-FU was nearly undetectable in plasma. These results demonstrate that combination therapy with TG6002 and the prodrug 5-FC achieves therapeutic intratumoral concentrations of 5-FU without significant systemic exposure to the cytotoxic anticancer drug.
Several wild-type VACV strains differing in pathogenicity and host range exist mainly due to the evolution of the virus during propagation of the smallpox vaccine in different parts of the world.45 In Europe, different strains were used in different countries, regions, and periods. The Copenhagen strain of VACV was originally used as a smallpox vaccine in Denmark and the Netherlands. Probably due to variations in the expression or functionality of different virulence genes between strains, in vitro and in vivo preclinical studies have recently shown that the Copenhagen strain has a more potent oncolytic viral activity against human tumor cells than the Wyeth and the WR strains,46, 47 the original strains of oncolytic VACVs that are currently in clinic. These results suggest that Copenhagen-based VACV should be more effective at controlling tumor growth in the clinic. In summary, we have shown here that the doubly deleted VACV TG6002 is able to induce notable antitumor effects through viral replication and subsequent cell death. J2R-I4L-deletions can further reduce virulence as well as enhance tumor targeting. Nevertheless, there was some variation in the sensitivity of cells to TG6002. For example, the two colon cancer cell lines tested, LoVo and HCT 116, show significantly different sensitivity toward TG6002 in vitro (Figure 1A) and in vivo (Figure 5). The characterization of cells factors influencing this sensitivity would be of high translational relevance in terms of biomarkers for patient selection. Furthermore, we have shown that the expression of the FCU1 suicide gene together with systemic administration of 5-FC, enhanced the therapeutic activity of the doubly deleted VACV. TG6002 has recently entered into clinical development in patients with recurrent glioblastoma (NCT03294486) and in patients with advanced gastrointestinal tumors (NCT03724071). These clinical trials are phase 1 studies evaluating the safety and tolerability of multiple-ascending doses of TG6002 administered intravenously in combination with oral 5-FC. In conclusion, the present study confirms that the doubly deleted Copenhagen strain is a promising candidate for use as a highly selective oncolytic virus or gene therapy vector.
Materials and Methods
Cell Culture
The human colon cancer cell lines LoVo and HCT 116, human stomach cancer cell line Hs 746T, human lung cancer cell line A549, hepatocarcinoma human cell line Hep G2, glioblastoma human cancer cell line U-87 MG, human bladder cancer cell line SW780, and human cervical cancer cell line HeLa were obtained from American Type Culture Collection (ATCC, Rockville, MD). The human esophageal cancer cell line OE19 was obtained from the European Collection of Cell Culture (ECACC, Salisbury, UK). The human head and neck squamous cell carcinoma cell line CAL33 was isolated in the Centre Antoine-Lacassagne (Nice, France).48 All cell lines were grown in DMEM supplemented with 10% fetal bovine serum (FBS). Fresh human hepatocytes were purchased from Biopredic International (Rennes, France) and maintained in hepatocyte medium according to the manufacturer’s instructions. The Phenion full-thickness skin model, a 3D tissue construct that simulates histological and physiological properties of human skin, was purchased from Henkel (Düsseldorf, Germany). This organotypic epithelial raft culture model was maintained in tissue culture medium according to the manufacturer’s instructions. Primary chicken embryonic fibroblasts (CEFs) were used for recombination, amplification and titration of viral vectors. CEF cells were prepared as previously described40 and maintained in basal medium eagle (BME) supplemented with 5% FBS.
Generation of Recombinant VACVs
All recombinant VACVs were derived from the Copenhagen strain. TK gene-deleted VACVs, expressing either the fusion gene FCU1 (ΔJ2R/FCU1 VACV) or the GFP gene (ΔJ2R/GFP VACV) under the control of the p11K7.5 promoter, were constructed and have been characterized previously.5 For generation of the double-deleted ΔI4LΔJ2R/FCU1 VACV, designated TG6002, we used the pΔI4L shuttle plasmid containing the selection cassette GFP/GPT, a fusion of the gene encoding the GFP and the gene encoding guanine phosphoribosyltransferase (GPT), surrounded by the flanking sequences of VACV I4L gene. Recombination was performed into ΔJ2R/FCU1 VACV using the pΔI4L plasmid and induced the deletion of 141 amino acids of the central domain of I4L. The generation of recombinant virus based on GPT selection was previously described in detail.40 The same methods were used to generate the ΔI4L VACV (single-deleted I4L VACV without transgene) by homologous recombination between the shuttle plasmid pΔI4L and the Copenhagen wild-type VACV (VACVwt). VACVwt and recombinant VACVs were amplified in CEF and purified, and virus stocks were titrated on CEFs by plaque assay.
In Vitro FCU1 Enzymatic Assay
CDase and UPRTase activities in LoVo cells were determined using 5-FC (Toronto Research Chemicals, North York, ON, Canada) and 5-FU (Sigma-Aldrich, Saint-Quentin Fallavier, France) as substrates. LoVo human tumor cells (5 × 106 cells) were infected with each VACV vector at MOI 10−2. Sixteen hours later, enzymatic assays in cell lysate were determined, as previously described.40 All samples were measured in triplicate.
In Vitro Cytotoxicity Assay
Human tumor cells were transduced in suspension by respective recombinant VACVs at a MOIs of 10−2, 10−3, and 10−4. A total of 3 × 105 infected cells/well were plated in 6-well culture dishes. At 5 days after infection, cell viability was determined by trypan blue exclusion, using a Vi-CELL Cell Counter (Beckman Coulter, Brea, CA). For sensitivity to 5-FC, LoVo tumor cells were transduced in suspension by the respective recombinant VACVs at MOI 10−3. A total of 3 × 105 infected cells/well were plated in 6-well culture dishes. At 48 h after infection, the cells were exposed to various concentrations of 5-FC for 3 days before determination of cell viability by trypan blue exclusion. All samples were analyzed in triplicate.
In Vitro Virus Yield
To evaluate viral replication in human tumor cells and human primary cells, human hepatocarcinoma cells Hep G2 and human primary hepatocytes were infected in 6-well plates (1 × 106 cells/well) by VACVwt, ΔJ2R/FCU1 VACV, and TG6002 at MOI 10−4 (102 PFU/well) or 10−2 (104 PFU/well). Cells were incubated in fresh growth medium until harvesting. At 48 h after infection, supernatants and cells were collected, freeze thawed, and sonicated, and viral progeny was quantified on CEFs by plaque assay. To evaluate viral replication in a reconstructed human skin model, culture of Phenion full-thickness skin model was infected with VACVwt, ΔJ2R/FCU1 VACV and TG6002 at 105 PFU. Cultures were incubated for 7 days at 37°C and medium was changed every other day. Viral progeny in reconstructed skin were quantified on CEF by plaque assay after 2 cycles of sonication in PBS.
siRNA-Silencing Experiment and Virus Infection
TK1, RRM1, and non-targeting control siRNA duplexes (ON-TARGET plus siRNA-SMART pool) were purchased from Dharmacon (Cambridge, UK). LoVo cells were seeded in 6-well plates at 2 × 106 cells/well in complete medium without antibiotic. After 24 h, LoVo cells were transfected with 20 nM of siRNA duplexes using Dharmafect according to Dharmacon’s instructions. Twenty-four hours after transfection, cells were infected at MOI 10−3 by the indicated VACVs. Supernatants and cells collected 48 h after infection were submitted to a quick freeze-thaw cycle and sonication to release intracellular viral particles, and viral progeny was quantified on CEF by plaque assay.
Western Blotting
The TK1 and RRM1 knockdown by specific siRNA were confirmed by western blot analysis. One, two and three days after siRNA transfection, LoVo cells were washed with PBS and 200 μL of lysis buffer was used to harvest the cells for Western blot analysis. Cell lysate proteins (20 μg) were used for immunoblot analyses using antibodies against RRM1 (sc-11733; Santa Cruz Biotechnology, Heidelberg, Germany) and TK1 (ab76495; Abcam, Paris, France).
Animal Experiments
All animal protocols were carried out according to standard operating procedures of the Federation of European Laboratory Animal Science Associations (FELASA) and were approved by the French Research and Education Ministry. Swiss nude mice and immunocompetent B6D2 mice were obtained from Charles River Laboratories (Saint-Germain-Nuelles, France). Animals used in the studies were uniform in age (6 weeks) and body weight (20–23 g).
Subcutaneous Tumor Models
To evaluate both biodistribution and therapeutic activity of VACVs in human xenograft tumor models, 5 × 106 human cancer cells were injected subcutaneously into the flank of the mice. When tumors reached a diameter of 100–200 mm3, the mice were randomized in a blinded manner and treated with the indicated vectors.
Biodistribution of the Viruses
To compare the biodistribution of TG6002 versus ΔJ2R/FCU1 VACV, 1 × 106 PFU of the indicated vectors were injected intravenously by tail vein injection into female nude mice bearing established subcutaneous LoVo. To further characterize the biodistribution of the double-deleted virus, 1 × 106 and 1 × 107 PFU of TG6002 were injected intravenously into female and male nude mice bearing established subcutaneous Hep G2 tumors. In the Hep G2 tumor model, treatment with 5-FC began 7 days after virus injection and was given by oral gavage for 3 weeks at 100 mg/kg (0.5 mL 5-FC 0.5% in water) twice a day. Mice were sacrificed at the indicated time points (Figure 4A; Table S1). Before the different organs were collected, mice were perfused intracardially with an exsanguinating solution (0.9% NaCl with heparin 50 IU/mL) until all the blood was removed. Tumors and other organs were collected and weighed, homogenized in PBS, and sonicated, and titers were determined on CEFs by plaque assay. Viral titers were standardized to milligrams of tissue.
Viral Pathogenicity
Viral pathogenicity was assessed by survival studies on both Swiss nude mice and immunocompetent B6D2 mice. High doses (1 × 108 PFU) of the indicated vectors were injected intravenously by tail vein injection. Mice were observed daily throughout the course of the experiment. Pocks on tails were also measured and recorded after intravenous injection of 1 × 106 or 1 × 107 PFU of each virus in Swiss nude mice.
In Vivo Antitumor Activity
In the first experiment, nude mice bearing established subcutaneous LoVo tumors were infected once intravenously (tail vein injection) with the indicated vectors at a dose of 1 × 107 PFU. In the second series of experiments, nude mice bearing established s.c Hep G2, HCT 116, SW780, Hs 746T, OE19, or CAL33 were infected once intravenously with 1 × 106 PFU TG6002 (Hep G2, HCT 116, Hs 746T, and CAL33) or 1 × 107 PFU (SW780 and OE19). In the two sets of experiments, starting at day 7 following viral injection, 5-FC was given by oral gavage for 3 weeks at 100 mg/kg (0.5 mL 5-FC 0.5% in water) twice a day. The antitumor effect of multiple cycles of TG6002/5-FC versus a single cycle were also evaluated in a subcutaneous LoVo model. In the single-cycle group, TG6002 was injected intravenously at 1 × 107 PFU and 5-FC was given per os for 3 weeks, starting on day 7 after virus injection. In the multiple cycle group, TG6002 was injected intravenously at 1 × 107 PFU, and 5-FC was given per os for 2 weeks, starting on day 7 after virus injection. At the end of the 5-FC regimen in the first cycle, TG6002 was injected again at 1 × 107 PFU, and 7 days later 5-FC was given again for 2 weeks.
Tumor size was measured twice a week with calipers. Tumor volumes were calculated in cubic millimeters using the formula Π/6 × length × width2.
Determination of Tumor and Blood Concentration of 5-FC and Converted 5-FU
Swiss nude mice bearing established s.c Hep G2 were infected once intravenously with 1 × 106 PFU of TG6002. Animals were sacrificed 1 h after a single per os 5-FC administration at 100 mg/kg (0.5 mL 5-FC 0.5% in water) on day 0 (before virus injection) and on days 7, 14, 21, 28, and 60 after virus injection. Blood samples and tumors were collected. Plasma was separated by centrifugation from blood collected via the retro-orbital sinus in heparinized tubes. Tumors were homogenized in a Polytron homogenizer. Tumor or plasma samples were quenched with 1 mL ethyl acetate/2-propanol/0.5 M acetic acid solution (84:15:1). The organic supernatant was reconstituted in 50 μL of water and analyzed by HPLC using 50 mM phosphoric acid adjusted to pH 2.1 as the mobile phase.
Statistical Analyses
To analyze the impact of the TK1 and RRM1 knockdown by specific siRNA on virus replication, a mixed model was applied on log-transformed data. Post hoc comparisons of the interactions were performed with Tukey’s correction, to estimate the impact of siRNA for each virus independently. Graphical representations of overall survival were displayed using a Kaplan-Meier curve. Log-rank test was applied to compare treatment effect on overall survival.
For statistical analysis of the in vitro viral replication in tumor and healthy human cells, a three-way ANOVA with Tukey’s test was performed. Statistical analyses of tumor volume growth were performed, using a mixed model to take the longitudinal aspect into account. The inter-individual heterogeneity was considered as a random effect in the model. Tumor sizes were converted in equivalent diameters to comply with the mixed model assumption and to get a better fit to the statistical model.49 Comparisons between treatments were also performed using least-squares means. To study non-inferiority relations, overall estimation of tumor size growth with the associated interval of measurement uncertainty was compared. Comparison of pock numbers was done using a non-parametric Wilcoxon test with normal approximation. Analysis were performed with the software SAS 9.4. Differences were considered significant at p < 0.05.
Author Contributions
Conception and design, J.F. and P.E.; development of methodologies and performance of experiments, J.F., J.K., N.F., J.K., P.C., C.P., A.F. and P.E.; analysis and interpretation of data, J.F. and P.E.; statistical analysis of data, J.F., F.V. and P.E.; writing, review, and/or revision of the manuscript, J.F., E.Q and P.E.; and study supervision, P.E.
Conflicts of Interest
All authors were employees of Transgene SA when the work was performed. Transgene SA is a publicly traded French biopharmaceutical company, with Institut Merieux as the major shareholder. The authors declare no other competing interests.
Acknowledgments
We thank Chantal Hoffmann for preparation of the primary chicken embryo fibroblasts.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2019.03.005.
Supplemental Information
References
- 1.Miest T.S., Cattaneo R. New viruses for cancer therapy: meeting clinical needs. Nat. Rev. Microbiol. 2014;12:23–34. doi: 10.1038/nrmicro3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddad D. Genetically Engineered Vaccinia Viruses As Agents for Cancer Treatment, Imaging, and Transgene Delivery. Front. Oncol. 2017;7:96. doi: 10.3389/fonc.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heo J., Reid T., Ruo L., Breitbach C.J., Rose S., Bloomston M., Cho M., Lim H.Y., Chung H.C., Kim C.W. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeh H.J., Downs-Canner S., McCart J.A., Guo Z.S., Rao U.N., Ramalingam L., Thorne S.H., Jones H.L., Kalinski P., Wieckowski E. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol. Ther. 2015;23:202–214. doi: 10.1038/mt.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foloppe J., Kintz J., Futin N., Findeli A., Cordier P., Schlesinger Y., Hoffmann C., Tosch C., Balloul J.M., Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- 6.Mell L.K., Brumund K.T., Daniels G.A., Advani S.J., Zakeri K., Wright M.E., Onyeama S.J., Weisman R.A., Sanghvi P.R., Martin P.J., Szalay A.A. Phase I Trial of Intravenous Oncolytic Vaccinia Virus (GL-ONC1) with Cisplatin and Radiotherapy in Patients with Locoregionally Advanced Head and Neck Carcinoma. Clin. Cancer Res. 2017;23:5696–5702. doi: 10.1158/1078-0432.CCR-16-3232. [DOI] [PubMed] [Google Scholar]
- 7.Breitbach C.J., Burke J., Jonker D., Stephenson J., Haas A.R., Chow L.Q., Nieva J., Hwang T.H., Moon A., Patt R. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 8.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengstschläger M., Knöfler M., Müllner E.W., Ogris E., Wintersberger E., Wawra E. Different regulation of thymidine kinase during the cell cycle of normal versus DNA tumor virus-transformed cells. J. Biol. Chem. 1994;269:13836–13842. [PubMed] [Google Scholar]
- 10.Puhlmann M., Brown C.K., Gnant M., Huang J., Libutti S.K., Alexander H.R., Bartlett D.L. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther. 2000;7:66–73. doi: 10.1038/sj.cgt.7700075. [DOI] [PubMed] [Google Scholar]
- 11.Erbs P., Regulier E., Kintz J., Leroy P., Poitevin Y., Exinger F., Jund R., Mehtali M. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- 12.Aye Y., Li M., Long M.J., Weiss R.S. Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene. 2015;34:2011–2021. doi: 10.1038/onc.2014.155. [DOI] [PubMed] [Google Scholar]
- 13.Tengelsen L.A., Slabaugh M.B., Bibler J.K., Hruby D.E. Nucleotide sequence and molecular genetic analysis of the large subunit of ribonucleotide reductase encoded by vaccinia virus. Virology. 1988;164:121–131. doi: 10.1016/0042-6822(88)90627-7. [DOI] [PubMed] [Google Scholar]
- 14.Slabaugh M., Roseman N., Davis R., Mathews C. Vaccinia virus-encoded ribonucleotide reductase: sequence conservation of the gene for the small subunit and its amplification in hydroxyurea-resistant mutants. J. Virol. 1988;62:519–527. doi: 10.1128/jvi.62.2.519-527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slabaugh M.B., Mathews C.K. Vaccinia virus-induced ribonucleotide reductase can be distinguished from host cell activity. J. Virol. 1984;52:501–506. doi: 10.1128/jvi.52.2.501-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendricks S.P., Mathews C.K. Allosteric regulation of vaccinia virus ribonucleotide reductase, analyzed by simultaneous monitoring of its four activities. J. Biol. Chem. 1998;273:29512–29518. doi: 10.1074/jbc.273.45.29512. [DOI] [PubMed] [Google Scholar]
- 17.Chimploy K., Mathews C.K. Mouse ribonucleotide reductase control: influence of substrate binding upon interactions with allosteric effectors. J. Biol. Chem. 2001;276:7093–7100. doi: 10.1074/jbc.M006232200. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich B., Klein J., Delic M., Goepfert K., Engel V., Geberzahn L., Lusky M., Erbs P., Preville X., Moehler M. Immunogenicity of oncolytic vaccinia viruses JX-GFP and TG6002 in a human melanoma in vitro model: studying immunogenic cell death, dendritic cell maturation and interaction with cytotoxic T lymphocytes. OncoTargets Ther. 2017;10:2389–2401. doi: 10.2147/OTT.S126320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fend L., Remy-Ziller C., Foloppe J., Kempf J., Cochin S., Barraud L., Accart N., Erbs P., Fournel S., Préville X. Oncolytic virotherapy with an armed vaccinia virus in an orthotopic model of renal carcinoma is associated with modification of the tumor microenvironment. OncoImmunology. 2015;5:e1080414. doi: 10.1080/2162402X.2015.1080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delaunay T., Violland M., Boisgerault N., Dutoit S., Vignard V., Münz C., Gannage M., Dréno B., Vaivode K., Pjanova D. Oncolytic viruses sensitize human tumor cells for NY-ESO-1 tumor antigen recognition by CD4+ effector T cells. OncoImmunology. 2017;7:e1407897. doi: 10.1080/2162402X.2017.1407897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinpeter P., Fend L., Thioudellet C., Geist M., Sfrontato N., Koerper V., Fahrner C., Schmitt D., Gantzer M., Remy-Ziller C. Vectorization in an oncolytic vaccinia virus of an antibody, a Fab and a scFv against programmed cell death -1 (PD-1) allows their intratumoral delivery and an improved tumor-growth inhibition. OncoImmunology. 2016;5:e1220467. doi: 10.1080/2162402X.2016.1220467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fend L., Yamazaki T., Remy C., Fahrner C., Gantzer M., Nourtier V., Préville X., Quéméneur E., Kepp O., Adam J. Immune Checkpoint Blockade, Immunogenic Chemotherapy or IFN-α Blockade Boost the Local and Abscopal Effects of Oncolytic Virotherapy. Cancer Res. 2017;77:4146–4157. doi: 10.1158/0008-5472.CAN-16-2165. [DOI] [PubMed] [Google Scholar]
- 23.McCart J.A., Ward J.M., Lee J., Hu Y., Alexander H.R., Libutti S.K., Moss B., Bartlett D.L. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 24.Thorne S.H., Hwang T.H., O’Gorman W.E., Bartlett D.L., Sei S., Kanji F., Brown C., Werier J., Cho J.H., Lee D.E. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J. Clin. Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrei G., Duraffour S., Van den Oord J., Snoeck R. Epithelial raft cultures for investigations of virus growth, pathogenesis and efficacy of antiviral agents. Antiviral Res. 2010;85:431–449. doi: 10.1016/j.antiviral.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Snoeck R., Holý A., Dewolf-Peeters C., Van Den Oord J., De Clercq E., Andrei G. Antivaccinia activities of acyclic nucleoside phosphonate derivatives in epithelial cells and organotypic cultures. Antimicrob. Agents Chemother. 2002;46:3356–3361. doi: 10.1128/AAC.46.11.3356-3361.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parato K.A., Breitbach C.J., Le Boeuf F., Wang J., Storbeck C., Ilkow C., Diallo J.S., Falls T., Burns J., Garcia V. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 2012;20:749–758. doi: 10.1038/mt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Clercq E., Holý A., Rosenberg I. Efficacy of phosphonylmethoxyalkyl derivatives of adenine in experimental herpes simplex virus and vaccinia virus infections in vivo. Antimicrob. Agents Chemother. 1989;33:185–191. doi: 10.1128/aac.33.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cripe T.P., Ngo M.C., Geller J.I., Louis C.U., Currier M.A., Racadio J.M., Towbin A.J., Rooney C.M., Pelusio A., Moon A. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 2015;23:602–608. doi: 10.1038/mt.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kung C.H., Kuo S.C., Chen T.L., Weng W.S. Isolation of vaccinia JX594 from pustules following therapy for hepatocellular carcinoma. BMC Cancer. 2015;15:704. doi: 10.1186/s12885-015-1753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potts K.G., Irwin C.R., Favis N.A., Pink D.B., Vincent K.M., Lewis J.D., Moore R.B., Hitt M.M., Evans D.H. Deletion of F4L (ribonucleotide reductase) in vaccinia virus produces a selective oncolytic virus and promotes anti-tumor immunity with superior safety in bladder cancer models. EMBO Mol. Med. 2017;9:638–654. doi: 10.15252/emmm.201607296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll N.M., Chiocca E.A., Takahashi K., Tanabe K.K. Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virus. Ann. Surg. 1996;224:323–329, discussion 329–330. doi: 10.1097/00000658-199609000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morikawa T., Maeda D., Kume H., Homma Y., Fukayama M. Ribonucleotide reductase M2 subunit is a novel diagnostic marker and a potential therapeutic target in bladder cancer. Histopathology. 2010;57:885–892. doi: 10.1111/j.1365-2559.2010.03725.x. [DOI] [PubMed] [Google Scholar]
- 34.Morikawa T., Hino R., Uozaki H., Maeda D., Ushiku T., Shinozaki A., Sakatani T., Fukayama M. Expression of ribonucleotide reductase M2 subunit in gastric cancer and effects of RRM2 inhibition in vitro. Hum. Pathol. 2010;41:1742–1748. doi: 10.1016/j.humpath.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Wang L.M., Lu F.F., Zhang S.Y., Yao R.Y., Xing X.M., Wei Z.M. Overexpression of catalytic subunit M2 in patients with ovarian cancer. Chin. Med. J. (Engl.) 2012;125:2151–2156. [PubMed] [Google Scholar]
- 36.Liu X., Zhang H., Lai L., Wang X., Loera S., Xue L., He H., Zhang K., Hu S., Huang Y. Ribonucleotide reductase small subunit M2 serves as a prognostic biomarker and predicts poor survival of colorectal cancers. Clin. Sci. (Lond.) 2013;124:567–578. doi: 10.1042/CS20120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma X.J., Salunga R., Tuggle J.T., Gaudet J., Enright E., McQuary P., Payette T., Pistone M., Stecker K., Zhang B.M. Gene expression profiles of human breast cancer progression. Proc. Natl. Acad. Sci. USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aird K.M., Li H., Xin F., Konstantinopoulos P.A., Zhang R. Identification of ribonucleotide reductase M2 as a potential target for pro-senescence therapy in epithelial ovarian cancer. Cell Cycle. 2014;13:199–207. doi: 10.4161/cc.26953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gammon D.B., Gowrishankar B., Duraffour S., Andrei G., Upton C., Evans D.H. Vaccinia virus-encoded ribonucleotide reductase subunits are differentially required for replication and pathogenesis. PLoS Pathog. 2010;6:e1000984. doi: 10.1371/journal.ppat.1000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erbs P., Findeli A., Kintz J., Cordier P., Hoffmann C., Geist M., Balloul J.M. Modified vaccinia virus Ankara as a vector for suicide gene therapy. Cancer Gene Ther. 2008;15:18–28. doi: 10.1038/sj.cgt.7701098. [DOI] [PubMed] [Google Scholar]
- 41.Ricordel M., Foloppe J., Pichon C., Sfrontato N., Antoine D., Tosch C., Cochin S., Cordier P., Quemeneur E., Camus-Bouclainville C. Cowpox Virus: A New and Armed Oncolytic Poxvirus. Mol. Ther. Oncolytics. 2017;7:1–11. doi: 10.1016/j.omto.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dias J.D., Liikanen I., Guse K., Foloppe J., Sloniecka M., Diaconu I., Rantanen V., Eriksson M., Hakkarainen T., Lusky M. Targeted chemotherapy for head and neck cancer with a chimeric oncolytic adenovirus coding for bifunctional suicide protein FCU1. Clin. Cancer Res. 2010;16:2540–2549. doi: 10.1158/1078-0432.CCR-09-2974. [DOI] [PubMed] [Google Scholar]
- 43.Kaufmann J.K., Bossow S., Grossardt C., Sawall S., Kupsch J., Erbs P., Hassel J.C., von Kalle C., Enk A.H., Nettelbeck D.M., Ungerechts G. Chemovirotherapy of malignant melanoma with a targeted and armed oncolytic measles virus. J. Invest. Dermatol. 2013;133:1034–1042. doi: 10.1038/jid.2012.459. [DOI] [PubMed] [Google Scholar]
- 44.Husseini F., Delord J.P., Fournel-Federico C., Guitton J., Erbs P., Homerin M., Halluard C., Jemming C., Orange C., Limacher J.M., Kurtz J.E. Vectorized gene therapy of liver tumors: proof-of-concept of TG4023 (MVA-FCU1) in combination with flucytosine. Ann. Oncol. 2017;28:169–174. doi: 10.1093/annonc/mdw440. [DOI] [PubMed] [Google Scholar]
- 45.Kretzschmar M., Wallinga J., Teunis P., Xing S., Mikolajczyk R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS Med. 2006;3:e272. doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y.P., Wang J., Avanzato V.A., Bakkum-Gamez J.N., Russell S.J., Bell J.C., Peng K.W. Oncolytic vaccinia virotherapy for endometrial cancer. Gynecol. Oncol. 2014;132:722–729. doi: 10.1016/j.ygyno.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricordel M., Foloppe J., Antoine D., Findeli A., Kempf J., Cordier P., Gerbaud A., Grellier B., Lusky M., Quemeneur E., Erbs P. Vaccinia Virus Shuffling: deVV5, a Novel Chimeric Poxvirus with Improved Oncolytic Potency. Cancers (Basel) 2018;10:E231. doi: 10.3390/cancers10070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magné N., Fischel J.L., Dubreuil A., Formento P., Poupon M.F., Laurent-Puig P., Milano G. Influence of epidermal growth factor receptor (EGFR), p53 and intrinsic MAP kinase pathway status of tumour cells on the antiproliferative effect of ZD1839 (“Iressa”) Br. J. Cancer. 2002;86:1518–1523. doi: 10.1038/sj.bjc.6600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bastogne T., Samson A., Vallois P., Wantz-Mézières S., Pinel S., Bechet D., Barberi-Heyob M. Phenomenological modeling of tumor diameter growth based on a mixed effects model. J. Theor. Biol. 2010;262:544–552. doi: 10.1016/j.jtbi.2009.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.