Summary
Covalent organic frameworks (COFs), an emerging class of organic porous materials, have attracted intense attention due to their versatile applications. However, the deliberate fabrication of COF-based nanomaterials for nanomedical application remains challenging due to difficulty in their size- and structure-controlled synthesis and poor aqueous dispersibility. Herein, we report two boron-dipyrromethene (BODIPY)-decorated nanoscale COFs (NCOFs), which were prepared by the Schiff-base condensation of the free end –CHO (bonding defects in COFs) on the established imine-based NCOFs with the amino-substituted organic photosensitizer BODIPY via “bonding defects functionalization” approach. Thus BODIPY has been successfully nanocrystallized via the NCOF platform, and can be used for photodynamic therapy (PDT) to treat tumors. These NCOF-based PDT agents featured nanometer size (∼110 nm), low dark toxicity, and high phototoxicity as evidenced by in vitro and in vivo experiments. Moreover, the “bonding defects functionalization” approach might open up new avenues for the fabrication of additional COF-based platforms for biomedical treatment.
Subject Areas: Supramolecular Chemistry, Scaffolds in Supramolecular Chemistry, Cancer, Biomedical Materials
Graphical Abstract
Highlights
-
•
Covalent organic frameworks (COFs) are first used in tumor photodynamic therapy
-
•
BODIPY-decorated COFs are synthesized via bonding defects functionalization
-
•
BODIPY-decorated COFs have excellent anti-tumor efficacy in vitro and in vivo
-
•
COFs show great promise as nanoplatforms for biomedical applications
Supramolecular Chemistry; Scaffolds in Supramolecular Chemistry; Cancer; Biomedical Materials
Introduction
As is known, cancer is one of the greatest threats to human health (Fitzmaurice et al., 2018, Siegel et al., 2019). Photodynamic therapy (PDT) (Bolze et al., 2017, Guan et al., 2018a, Mallidi et al., 2016) is a promising clinical cancer treatment method in which a light-absorbing agent, referred to as a photosensitizer (PS), is interacted with light and oxygen to produce cytotoxic singlet oxygen (1O2). Compared with chemotherapy and radiotherapy, PDT generates less collateral damage to normal tissues, because 1O2 is produced only in the illuminated area where the PS accumulates. In this context, some organic dyes, such as boron-dipyrromethene (BODIPY)- and porphyrin-based species (Bertrand et al., 2018, Durantini et al., 2018, Josefsen and Boyle, 2012, Rajora et al., 2017), have been demonstrated to be the highly effective PSs owing to their high extinction coefficient and low dark toxicity in this minimally invasive cancer treatment. Their practical application, however, is often limited by the poor water solubility and instability, aggregation, sometimes photobleaching, and low cell permeability (Li et al., 2018a, Zhou et al., 2018).
Organic PS nanocrystallization has been proved to be an alternative approach to address the aforementioned issues (Abánades Lázaro and Forgan, 2019, Lismont et al., 2017). For example, recent studies revealed that organic PSs can be readily nanocrystallized via nanoscale metal-organic framework (NMOF) platform by either one-pot (Guan et al., 2018b, Lu et al., 2014) or post-synthetic modification (PSM) (Kan et al., 2018, Nian et al., 2017, Wang et al., 2016). In such a way, the molecular organic PSs are encapsulated in NMOF pores or are covalently attached to the established NMOF frameworks, and their aggregation and self-quenching are therefore effectively avoided due to their periodic arrangement within the MOF framework (Qin et al., 2019). In addition, nanocrystallization of the organic PSs significantly improves their endocytosis-based cellular uptake (Mosquera et al., 2018, Yoon and Rossi, 2018), and consequently, augments their cancer therapeutic efficacy.
As a promising alternative to MOFs, metal-free covalent organic frameworks (COFs) (Bisbey and Dichtel, 2017, Lohse and Bein, 2018) should be more biocompatible and suitable for biomedical treatment because of their pure organic nature (Figure S1, see Normal Tissue Cytotoxicity Test in Transparent Methods for experimental details) (Guan et al., 2018b, Jiang et al., 2019, Ruyra et al., 2015, Tamames-Tabar et al., 2014). Some examples of COF-based biomedical applications have been reported, such as fluorescence bioimaging (Das et al., 2018, Li et al., 2017, Liu et al., 2019b, Wang et al., 2018a), drug delivery (Bai et al., 2016, Fang et al., 2015, Liu et al., 2019a, Mitra et al., 2017, Vyas et al., 2016b, Zhang et al., 2018b), antibacterial therapy (Hynek et al., 2018, Liu et al., 2017, Mitra et al., 2016), enzyme immobilization (Kandambeth et al., 2015, Sun et al., 2018c), biochemical analysis (Liu et al., 2019c, Yan et al., 2019, Zhang et al., 2018e, Zhang et al., 2018f, Zhou et al., 2019), and so on. In addition, some conceptual therapeutic models, such as 1O2 generation (Feng et al., 2016, Lin et al., 2017, Nagai et al., 2013), photothermal conversion (Tan et al., 2016), and apoptosis induction (Bhanja et al., 2017), have also been reported. To the best of our knowledge, nanoscale COF (NCOF)-based PSs for tumor PDT, have not been reported so far, although they are conceptually practicable. This might be limited by the following issues: (1) COFs are usually obtained as microscale particles with low aqueous dispersibility, which are not conducive to cell uptake; (2) in situ one-pot synthesis of NCOF-based PSs from PS-attached building blocks might not be versatile because it is difficult to ensure that the PSs are intact during COF synthesis; (3) covalent decoration of the molecular PSs on the established COF framework by the existing PSM approach might also be limited by the stability and activity of the pre-embedded active precursors under the harsh COF synthetic conditions, and moreover, the incoming bulky PS molecules probably lead to crystallinity decrease, structural transformation, and even structure collapse of the pristine COFs.
Based on recent reports, NCOF synthesis can be realized by the polymer-assisted solvothermal method (Zhao et al., 2017) or with the help of ultrasonic stripping (Chandra et al., 2013, Zhang et al., 2017a). On the other hand, COFs, which are composed of organic building partners via the covalent bonds, contain the unbonded functional groups (bonding defects) at the end of COF matrix (Nguyen and Grünwald, 2018), which should be a heaven-sent opportunity to graft small organic PSs onto the established NCOF with a known structure via bonding defects functionalization (BDF). In this way, the isostructural but PS-attached NCOFs that cannot be directly prepared by the conventional one-pot synthesis and existing PSM would be readily achieved.
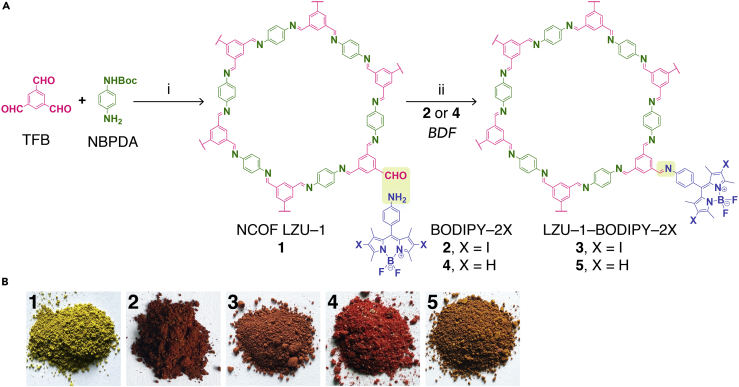
In this contribution, we report, the first of its kind, two BODIPY-decorated NCOFs, which were generated from the NCOF LZU-1 (1) (Ding et al., 2011) and two amino-decorated BODIPY molecules, termed BODIPY-2I (2) and BODIPY-2H (4), by the BDF approach under the given conditions (Figure 1A). Markedly, the obtained nanoscale LZU-1-BODIPY-2I (3) and LZU-1-BODIPY-2H (5) are highly crystalline and isostructural to pristine 1, and they both feature low cytotoxicity, good biocompatibility, high cancer cell uptake, and highly efficient 1O2 generation. Therefore, they can be used as high-performing PDT agents for cancer treatment under the given in vitro and in vivo conditions.
Figure 1.
Design of BODIPY-Decorated Nanoscale COFs for PDT
(A) The preparation of 1, 3, and 5. (i) PVP, EtOH, CF3COOH, 120°C, 12 h; (ii) EtOH, HOAc, 75°C, 4 h. Boc, tert-butyloxycarbonyl; BDF, bonding defects functionalization. See Synthesis of NCOF LZU-1 (1), Synthesis of BODIPY-2I (2), Synthesis of LZU-1-BODIPY-2I (3), Synthesis of BODIPY-2H (4), and Synthesis of LZU-1-BODIPY (5) in Transparent Methods for experimental details.
(B) Digital photographs of 1, 2, 3, 4, and 5.
Results
Synthesis and Characterization
With the aid of nonionic surfactant polyvinyl pyrrolidone, the NCOF of LZU-1 (1) was prepared by the combination of benzene-1,3,5-tricarbaldehyde and tert-butyl (4-aminophenyl)carbamate (NBPDA) under solvothermal conditions (EtOH, CF3COOH, 120°C, 12 h) (Zhao et al., 2017). Furthermore, the BDF of 1 via Schiff-base condensation between its free end aldehyde groups and two amino-decorated BODIPY molecules, termed as BODIPY-2I (2) and BODIPY-2H (4), afforded nanoscale LZU-1-BODIPY-2I (3) and LZU-1-BODIPY-2H (5) under solvothermal conditions (EtOH, HOAc, 75°C, 4 h) (Figure 1A, see Synthesis of NCOF LZU-1 (1), Synthesis of BODIPY-2I (2), Synthesis of LZU-1-BODIPY-2I (3), Synthesis of BODIPY-2H (4), and Synthesis of LZU-1-BODIPY (5) in Transparent Methods for experimental details). The colors of 3 and 5 were significantly different from those of their precursors after the reaction (Figure 1B). The standard curve method (Figure S2, see BODIPY Contents Determination in Transparent Methods for experimental details) revealed decorated BODIPY amounts in 3 and 5 of 0.1360 ± 0.0312 mmol/g and 0.1545 ± 0.0220 mmol/g, respectively. This result was further confirmed by inductive coupled plasma optical emission spectrometry (ICP-OES) measurement (0.1218 ± 0.0137 mmol/g, 0.1492 ± 0.0278 mmol/g, respectively). Owing to their structural similarity, we took 3 as an example to discuss its structural characterization in detail herein, and the corresponding detailed characterization data for 5 are provided in Figures S3 and S4.
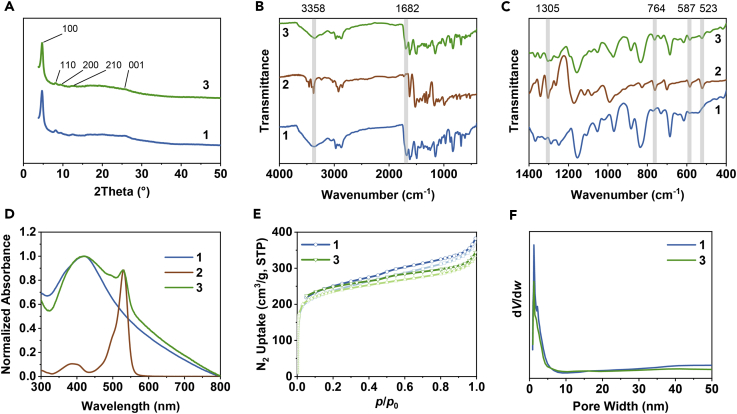
The obtained 1 and 3 are isostructural and possess good crystallinity, as revealed by their experimental powder X-ray diffraction (PXRD). As indicated in Figure 2A, the most intense peak at 2θ = 4.7° was attributed to the (100) crystal facet, and other diffraction peaks at 2θ = 8.2, 9.5, and 12.4° were assigned to the (110), (200), and (210) facets, respectively. The broad peak at 2θ = 25.7° could be indexed to the π–π stacked planes (001) of 1 (Peng et al., 2016). This suggested that the BDF of 1 with 2 did not change its structural integrality and crystallinity under the given conditions.
Figure 2.
Spectroscopic Characterization of 1 and 3
(A) PXRD patterns of 1 and 3.
(B and C) Fourier transform infrared spectra (B) and their fingerprint regions (C) of 1, 2, and 3.
(D) UV-vis absorption spectra of 1, 2, and 3 in DMF.
(E) N2 adsorption-desorption isotherms (77 K) of 1 and 3.
(F) Pore width distribution plots of 1 and 3.
The successful formation of 1 and 3 was assessed by Fourier transform infrared spectroscopy. As shown in Figure 2B, the strong stretching band of C=N in 1 appeared at 1,621 cm−1, clearly indicating the formation of the imine linkage (Yuan et al., 2018). Meanwhile, the peaks at 1,682 and 3,358 cm−1 associated with aldehyde and amino groups, respectively, in 1 significantly decreased, but did not completely disappear, suggesting the existence of bonding defects. After BDF, the residual peak for the aldehyde group still existed, which demonstrated the BDF between 1 and 2 was not quantitative, which might result from the inherent imperfection of the heterogeneous solid-liquid reaction. Notably, as shown in Figure 2C, the characteristic peaks of 2 at 1,305, 764, 587, and 523 cm−1 were found in the fingerprint region of 3, which could be direct evidence for the existence of BODIPY species in 3. The formation of 1 and 3 was further confirmed by 13C solid-state nuclear magnetic resonance (Figure S4). It is worth noting that the changes observed for the resonance signals at δ = 17.3 ppm for 3 unequivocally supported the existence of BODIPY species.
The ultraviolet-visible (UV-vis) absorption spectra of 1, 2, and 3 were recorded in N,N-dimethylformamide (DMF). As shown in Figure 2D, 3 displayed the characteristic absorption bands of both 1 and 2. A broadband absorption of 1 and 3 throughout the whole visible light region was observed, indicating their stacked layer structures with enhanced light-harvesting capability in a wide range of visible light region due to the delocalized π electrons in the COFs. Compared with 2, the broadened characteristic absorption peak of BODIPY at 530 nm in 3 was also observed but without band-shift, indicating that the involved BODIPY species in 3 were well dispersed, as we know that aggregated BODIPY would cause the adsorption band to be significantly red-shifted (Li et al., 2018d, Liu et al., 2019d, Zhang et al., 2016). More importantly, this observation demonstrated that 3 is a COF-BODIPY covalently bonded species rather than a simply physical blend or a host-guest complex.
To examine the permanent porosity, N2 adsorption properties of 1 and 3 were measured at 77 K. As shown in Figure 2E, the Brunauer-Emmett-Teller (BET) surface areas of 1 and 3 were 822 m3/g and 805 m3/g, respectively. The slightly decreased surface area in 3 should be caused by the introduced BODIPY species because the involved BODIPY species certainly enhanced the material weight, thus resulting in a corresponding decrease of dV/dw. Density functional theory fitting of the adsorption branches (Figure 2F) showed the average pore size distributions of both 1 and 3 centered at ca. 1.2 nm, implying that the covalently bonded BODIPY species should be located at the end of the COF matrix.
To further verify the covalent bonding of BODIPY species of 2 and 4 to the COF framework of 1, a control experiment was designed and conducted (Figure S5, see Control Experiments in Transparent Methods for experimental details). When 1 was separately impregnated in an EtOH solution of 2 and 4 for 4 h, the host-guest complexes of BODIPY-2I⊂LZU-1 (3′) and BODIPY-2H⊂LZU-1 (5′) were generated. As determined by the standard curve method, the loading amounts of BODIPY in 3′ and 5′ were up to 0.2149 ± 0.0875 mmol/g and 0.5130 ± 0.1763 mmol/g, respectively, which are significantly higher than those of 3 and 5. It is different from the case of 3 and 5; the BODIPY loading amount herein is size dependent, and smaller-sized 4 (9 Å) was more uploaded than its diiodio-substituted analog of 2 (11 Å), which is a typical phenomenon in the selective host-guest adsorption. In addition, the release kinetics of 3, 3′, 5, and 5′ were investigated in boiling ethanol. As expected, the encapsulated BODIPY in 3′ and 5′ was readily extracted with EtOH with a rapid BODIPY release. In contrast, no BODIPY leaching occurred in 3 and 5 under the same conditions because they were firmly anchored by the covalent imine bond. By BDF in this way, the nanocrystallization of BODIPY via COF platform has been successfully achieved, which laid a solid foundation for their applications in biomedical treatment.
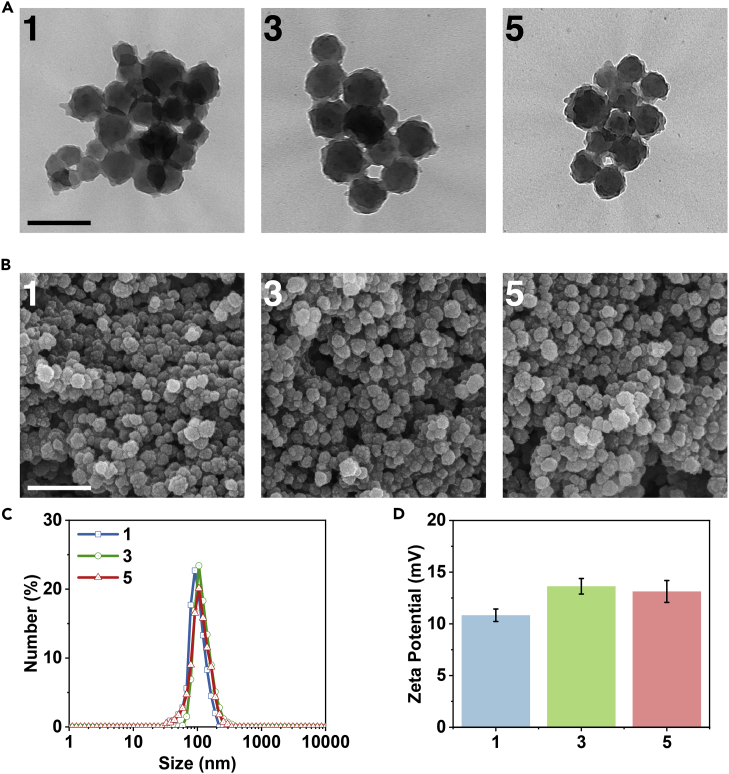
Besides structural characterization, the morphology of 1, 3, and 5 was investigated by scanning electron microscopy and transmission electron microscope. As indicated in Figures 3A and 3B, the obtained nanoparticles (NPs) of 1, 3, and 5 were uniformly distributed and their diameter was ca. 110 nm, which is further supported by the dynamic light scattering (DLS) measurement in PBS (Figure 3C). The slight difference in size might be caused by the solvation effect depending on the different measurements (Röder et al., 2017). It was worth noting that NBPDA played an important role in the synthesis of nanosized 1 (Zhao et al., 2017). With the aid of CF3COOH, the –Boc group could in situ hydrolyze and gradually release p-phenylenediamine under the given conditions. In this way, the Schiff condensation rate was significantly decreased, which was favorable for the formation of highly crystalline 1. On the other hand, if p-phenylenediamine was used instead of NBPDA, only micron-sized LZU-1 was obtained (Figure S6). The surface chemistries of 1, 3, and 5 were also studied. As shown in Figure 3D, 1, 3, and 5 displayed positive zeta potentials in PBS (pH = 6.5), which might be caused by the protonation of N atoms in COFs. NPs with electropositive zeta potential should be of great benefit to the tumor cell uptake owing to their high affinity for the negatively charged tumor cell membranes (Chakraborty et al., 2018, Intlekofer and Finley, 2019).
Figure 3.
NP Microtopography Characterization
(A) Transmission electron microscopic images of 1, 3, and 5. Scale bar, 200 nm.
(B) Scanning electron microscopic images of 1, 3, and 5. Scale bar, 500 nm.
(C) DLS size profiles of 1, 3, and 5 in PBS (pH = 6.5) at 25°C.
(D) Zeta potentials of 1, 3, and 5 in PBS (pH = 6.5). Data are presented as mean ± SD (n = 3).
Chemical, Light, and Colloidal Stability
The stabilities of 3 and 5 were evaluated from three aspects: chemical stability in PBS, anti-bleaching ability under light irradiation, and colloidal stability of their PBS dispersions (see Chemical Stability, Light Stability, and Colloidal Stability in Transparent Methods for experimental details). After soaking in PBS (pH = 6.5) for 24 h, no change in their PXRD patterns was observed, indicating 3 and 5 featured excellent chemical stability in tumor cell microenvironment (Figure S7). In addition, there were no detectable changes in their UV-vis spectra under green laser (1 W/cm2) illumination for 30 min, demonstrating that 3 and 5 were not affected by external light sources, even with the high-intensity lasers (Figure S8). Moreover, when the PBS solutions of 3 and 5 were allowed to stand at room temperature after 24 h, no coagulation was observed, and the measured particle size by DLS and zeta potentials remained unchanged (Figure S9).
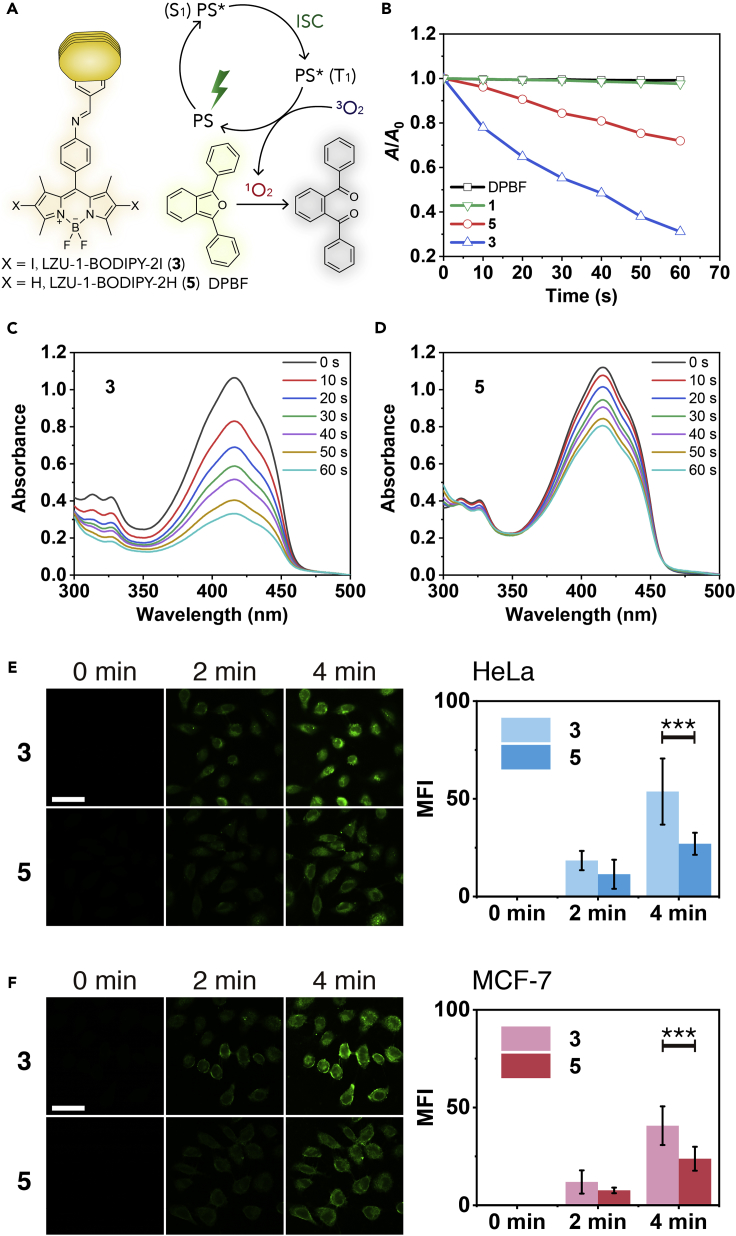
Singlet Oxygen Generation
For PDT treatment, the 1O2 generation of 3 and 5 in PBS upon green light-emitting diode (LED) irradiation was measured by using 1,3-diphenylisobenzofuran (DPBF) (Zhang et al., 2018c) as a 1O2 probe (see Singlet Oxygen Generation in PBS in Transparent Methods for experimental details). As shown in Figures 4A–4D, the DPBF absorbance at 414 nm in the presence of 3 and 5 under green LED irradiation (40 mW/cm2) remarkably decreased within 1 min, implying 1O2 generation. In contrast, negligible changes were observed in control experiments (1 + DPBF + light, and DPBF only) under the same conditions, which indicated that 3 and 5 herein were highly efficient COF-based PSs. Because intersystem crossing was significantly promoted by heavy-atom effect (Wu et al., 2011, Zou et al., 2017), 3 exhibited much higher 1O2 generation efficiency than of 5.
Figure 4.
Singlet Oxygen Generation Induced by 3 and 5
(A) DPBF as a chemical probe for detecting 1O2.
(B) Comparison of the decay rate of DPBF in PBS (pH = 6.5) induced by 1, 3, and 5 under green LED irradiation (40 mW/cm2).
(C and D) UV-vis spectra of DPBF induced by the PBS (pH = 6.5) dispersions of (C) 3 and (D) 5 (2 mL, 10 μM, BODIPY equiv.) under green LED irradiation (40 mW/cm2).
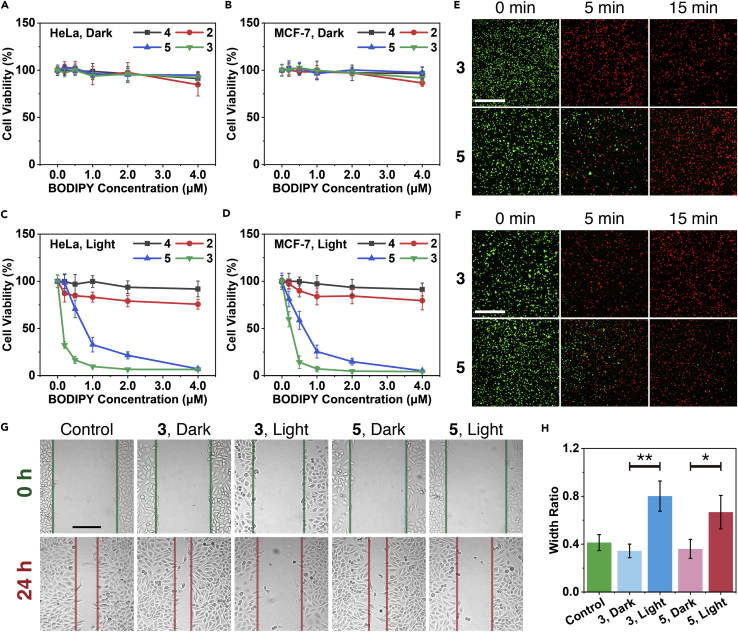
(E and F) HeLa (E) and MCF-7 (F) cells laser scanning confocal images of intracellular 1O2. Scale bar, 50 μm. Cells were incubated with DPBS dispersion of 3 or 5 (200 μL, 0.2 μM, BODIPY equiv.) in CO2 incubator for 30 min, and further incubated with SOSG (5 μM, 200 μL) for 15 min. The cells were exposed to green LED (40 mW/cm2) for different times and imaged with a laser scanning confocal microscope. The concentration of 1O2 in cells was reflected by mean fluorescence intensity (MFI) of green fluorescence. Data were presented as mean ± SD (n = 5, ***p < 0.001).
As shown above, 3 and 5 exhibited excellent ability to induce 1O2 generation in PBS. To evaluate their intracellular 1O2 production ability, Singlet Oxygen Sensor Green (SOSG), which is a specific green fluorescent probe for 1O2 in cells (Jia et al., 2018), was used as a fluorescent probe to detect intracellular 1O2 generation triggered by 3 and 5 (see Intracellular Singlet Oxygen Generation in Transparent Methods for experimental details). As the irradiation time prolonged, the green fluorescence in HeLa and MCF-7 cancer cells gradually increased, suggesting that 3 and 5 could effectively induce 1O2 generation in cells. As shown in Figures 4E and 4F, the induced fluorescence intensity caused by 3 was 1.99 times higher than that caused by 5 for HeLa cells, and 1.71 times for MCF-7 cells under irradiation with green LED (40 mW/cm2) for 4 min. This meant that 3 containing heavy iodine atoms was more efficient in intracellular 1O2 production.
In Vitro PDT Experiment
The dark toxicities of 2, 3, 4, and 5 were evaluated before intracellular PDT (see In Vitro PDT Experiment in Transparent Methods for experimental details). As shown in Figures 5A and 5B, the standard 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (van Meerloo et al., 2011) showed that the cell viability for the HeLa and MCF-7 cancer cells induced by 2, 3, 4, and 5 was more than 80%, even with a high BODIPY concentration up to 4.0 μM, indicating that their dark toxicity was negligible. Detailed study by the standard MTT assay indicated that 2, 3, 4, and 5 exhibited very different phototoxicity for the cells under the given conditions (green LED, 40 mW/cm2, 15 min). As shown in Figures 5C and 5D, the free BODIPY molecule of 2 with heavy iodine atoms exhibited very weak phototoxicity to HeLa and MCF-7 cells. The cell viability of HeLa and MCF-7 was up to 75.7% ± 5.2% and 79.6% ± 9.8% even with 4.0 μM of 2 under the light irradiation. Meanwhile, the free BODIPY molecule of 4 without iodine atoms showed even lower phototoxicity under the same conditions. The corresponding cell viability of HeLa and MCF-7 was more than 90%. This poor phototoxicity of 2 and 4 was clearly attributed to their poor water solubility and cell membrane permeability. In contrast to 2 and 4, 3 and 5 exhibited excellent phototoxicity. The HeLa cell viability was sharply down to 32.4% ± 3.5% even with concentration of 3 as low as 0.2 μM (BODIPY equiv.). For MCF-7 cells, a low cell viability of 14.3% ± 6.7% was observed with concentration of 3 at 0.5 μM (BODIPY equiv.). Compared with 3, 5 without heavy atoms exhibited a lower phototoxicity. For example, the cell viability of HeLa and MCF-7 was 32.8% ± 7.7% and 25.6% ± 6.9% with 1.0 μM 5 (BODIPY equiv.). Thus the nanocrystallization of 2 and 4 via COF 1 significantly enhanced their cell membrane permeability and intracellular phototoxicity, and consequently, made their practical PDT application available.
Figure 5.
In Vitro PDT Induced by 3 and 5
(A–D) MTT assays of HeLa (A and C) and MCF-7 (B and D) cancer cells incubated with 2, 3, 4, and 5. Cells were incubated with DPBS dispersion of 2, 3, 4, or 5 (100 μL, 0, 0.2, 0.5, 1.0, 2.0, 4.0 μM, BODIPY equiv.) for 30 min. Then the cells were exposed to green LED (40 mW/cm2) for 0 min (A and B) and 15 min (C and D), respectively. After additional 24-h incubation, the relative cell viabilities were detected by the standard MTT assay. Data were presented as mean ± SD (n = 5).
(E and F) Laser scanning confocal images of HeLa (E) or MCF-7 (F) cells co-stained with calcein-AM (green, live cells) and propidium iodide (red, dead cells) after being incubated with 3 or 5 (2.0 μM, BODIPY equiv.) with green LED irradiation (40 mW/cm2) for 0, 5, 15 min, respectively. Scale bar, 500 μm.
(G and H) In vitro scratch assays. MCF-7 cell monolayer with scratches was incubated with 3 or 5 (500 μL, 0.5 μM, BODIPY equiv.) for 30 min. Then cells were exposed to green LED (40 mW/cm2) for 0 or 5 min, respectively. The cells that were not incubated with 3 and 5 were used as controls. Representative images (G) and scratch width ratio of 0 h and 24 h (H) are shown. Scale bar, 200 μm. Data were presented as mean ± SD (n = 3, **p < 0.01, *p < 0.05).
The PDT efficacity of 3 and 5 was further confirmed by calcein-AM and propidium iodide (PI) double staining (Guo et al., 2017b) (see Calcein-AM/PI Double Stain in Transparent Methods for experimental details). As shown in Figures 5E and 5F, after incubation with 3 or 5 (2.0 μM BODIPY equiv.) for 30 min, the proportion of the dead HeLa and MCF-7 cells (red ones) significantly increased with the prolongation of irradiation time. This result again demonstrated that 3 and 5 possessed excellent PDT efficacy, but with ignorable dark toxicity.
As we know, invasiveness was one of the important features of cancer (Altorki et al., 2019, Stuelten et al., 2018). MCF-7 cells' migrating ability damage caused by PDT was assessed using cell scratch assays (see In Vitro Scratch Assay in Transparent Methods for experimental details), which could to some extent mimic the in vivo cell migration (Liang et al., 2007). As shown in Figures 5G and 5H, after incubation with 3 and 5 and subsequent light irradiation, only few MCF-7 cells migrated, whereas the invasive ability of the unilluminated MCF-7 cells was not affected by 3 and 5. Therefore, we could conclude that the PDT induced by 3 and 5 can significantly decrease the MCF-7 cell migration in vitro.
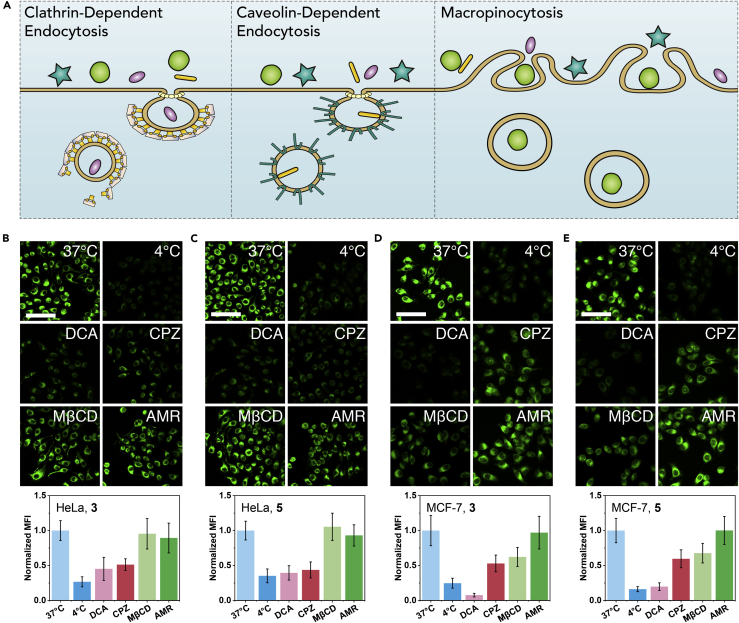
Cellular Uptake Mechanism
The excellent PDT efficacity of 3 and 5 inspired us to explore the intrinsic mechanism of cell death (Figure 6, see Cellular Uptake Mechanism in Transparent Methods for experimental details). As a result, low temperature (4°C) and dichloroacetate (DCA, inhibiting aerobic glycolysis through inhibiting pyruvate dehydrogenase kinase; Kato et al., 2007, Nayak et al., 2018) inhibited cell uptake of 3 and 5. This suggested that cellular uptake of 3 and 5 was an energy-dependent process and was closely related to the energy provided by aerobic glycolysis metabolism in cancer cells. Considering the energy-dependent pathway of cellular uptake by NPs (Mayor and Pagano, 2007, Shi and Tian, 2019), cells were pre-treated with chlorpromazine (CPZ, clathrin-dependent endocytosis inhibitor), amiloride (AMR, micropinocytosis inhibitor), and methyl-β-cyclodextrin (MβCD, caveolin-dependent endocytosis inhibitor), and then incubated with 3 or 5. We found that HeLa and MCF-7 cells showed different cell uptake inhibition. For HeLa, CPZ significantly inhibited cellular uptake, demonstrating HeLa uptake of 3 and 5 by clathrin-dependent endocytosis. For MCF-7, CPZ and MβCD significantly inhibited cellular uptake, implying MCF-7 uptake of 3 and 5 by both clathrin-dependent endocytosis and caveolin-dependent endocytosis. AMR had no significant effect on the cell uptake, indicating that micropinocytosis has a very limited contribution to the cell uptake herein.
Figure 6.
Cellular Uptake Mechanism of 3 and 5
(A) Energy-dependent pathways of cellular internalization of NPs.
(B–E) Cellular uptake mechanism of 3 (B and D) and 5 (C and E) in HeLa (B and C) and MCF-7 (D and E) cells. The cells were treated with 3 or 5 (5 μg/mL) for 30 min, at 37°C, 4°C, and 37°C while pre-treating with DCA (15 mM), CPZ (10 μg/mL), MβCD (10 mg/mL), and AMR (75 μg/mL) for 1 h. The cellular uptake was reflected by MFI of green fluorescence. Data were presented as mean ± SD (n = 5). Scale bar, 100 μm.
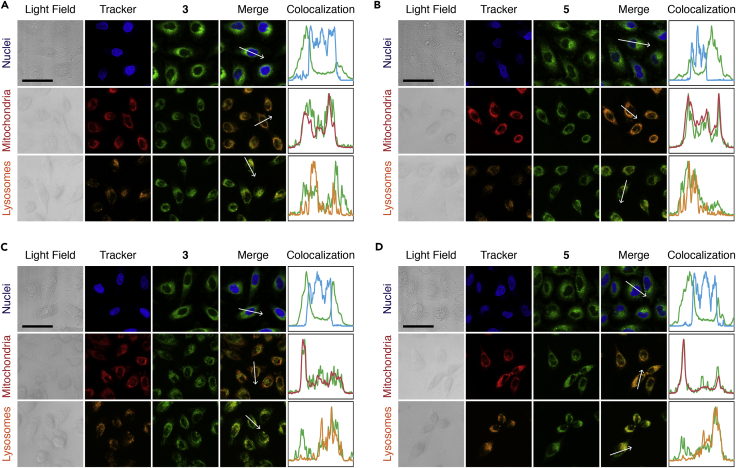
Subcellular Localization
Cell death caused by PDT was directly related to the 1O2-generated location (Gomes-da-Silva et al., 2018, Liu et al., 2011). Owing to the high local concentration of the in situ-formed 1O2, cellular structures with high PS concentrations were preferentially damaged by PDT, which induced subsequent cellular events. In view of this consideration, the distribution of 3 and 5 in cells was examined (Figure 7). After incubation with 3 and 5, the nuclei, mitochondria, and lysosomes of the cancer cells were labeled with Hoechst 33258, MitoTracker Deep Red FM, and LysoTracker Red DND-99, respectively (see Subcellular Localization of Cell Nucleus, Subcellular Localization of Mitochondria, and Subcellular Localization of Lysosomes in Transparent Methods for experimental details). Laser confocal imaging showed that 3 and 5 were basically not localized in the nucleus, but mainly colocalized with lysosomes and mitochondria. We therefore assumed that 3 and 5 might induce cell death through the lysosome- (Kroemer and Jäättelä, 2005) and mitochondrion-associated pathways (Fulda et al., 2010) instead of the nucleus-associated pathway. Lysosomes are the main organelles that decompose exogenous NPs, and destruction of lysosomes can prevent NPs from being degraded (Dong et al., 2018, Gyparaki and Papavassiliou, 2014). Mitochondria are major contributors to endogenous reactive oxygen species and are closely related to oxidative stress and apoptosis in tumor cells (Vyas et al., 2016a, Wong et al., 2017). Therefore targeting mitochondria and lysosomes may be beneficial for efficient PDT.
Figure 7.
Subcellular Localization Studies of 3 and 5
(A–D) Subcellular localization of 3 (A and C) and 5 (B and D) in HeLa (A and B) and MCF-7 (C and D) cancer cells. HeLa and MCF-7 cancer cells were pretreated with 3 or 5 (5 μg/mL, 30 min), and subsequently co-incubated with Hoechst 33258 (5 μg/mL, 15 min), MitoTracker Deep Red FM (25 nM, 15 min), and LysoTracker red DND-99 (50 nM, 15 min). The fluorescence intensity profiles along the line segments in merged images showed that 3 and 5 colocalized with mitochondria and lysosomes, instead of nucleus. Scale bar, 50 μm.
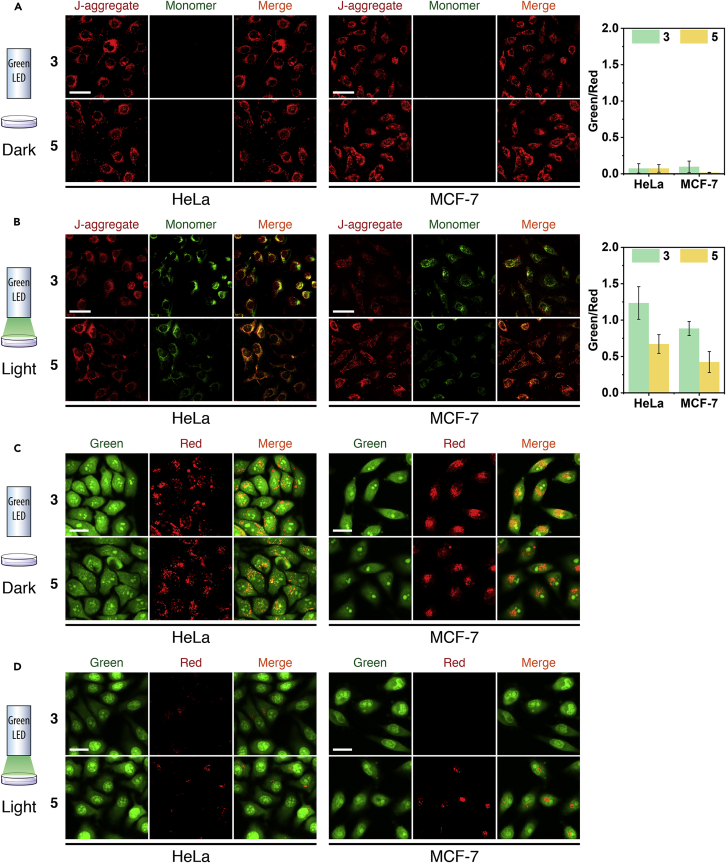
Cell Death Mechanism
Mitochondrial membrane potential (MMP, ΔΨ) was a hallmark event related to cell death by the mitochondrion-associated pathway (Burke, 2017, Weinberg and Chandel, 2015). The ΔΨ detection can be easily achieved by JC-1 (see Mitochondrial Membrane Potential (MMP, ΔΨ) in Transparent Methods for experimental details), which was a reliable fluorescent probe for ΔΨ (Lv et al., 2016, Tan et al., 2018). As shown in Figures 8A and 8B, after incubation with 3 and 5 (0.2 μM, BODIPY equiv.), only red fluorescence of JC-1 was observed without light irradiation owing to the formation of JC-1 J-aggregate, indicating that the ΔΨ was normal and 3 or 5 was not dark toxic. When irradiated with green LED, the JC-1 fluorescence changed from red to green due to the JC-1 monomer formation, demonstrating that a significant ΔΨ decrease occurred and the cell death caused by 3 and 5 was through the mitochondrion-associated pathway. It was worth noting that 3-induced fluorescence intensity was significantly stronger than that of 5, further indicating the heavy-atom effect on photosensitization.
Figure 8.
Characterization of Mitochondrial Membrane Potential (ΔΨ) Reduction and Lysosomal Membrane Permeabilization (LMP) Induced by 3 and 5
(A and B) Laser scanning confocal images of cells stained with JC-1 (10 μg/mL, 10 min) after being incubated with 3 or 5 (0.2 μM, BODIPY equiv.) with green LED irradiation (40 mW/cm2) for 0 min (A) and 4 min (B), respectively. ΔΨ reduction was reflected by the MFI ratios of green and red fluorescence. Data are presented as mean ± SD (n = 5). Scale bar, 50 μm.
(C and D) Laser scanning confocal images of cells stained with AO (5 μg/mL, 10 min) after being incubated with 3 or 5 (0.2 μM, BODIPY equiv.) with green LED irradiation (40 mW/cm2) for 0 min (C) and 4 min (D), respectively. Scale bar, 25 μm.
In addition, the lysosome-associated-pathway-induced cell death was also examined based on lysosomal membrane permeabilization (LMP) (Marques et al., 2013). For this, acridine orange (AO) was selected as the fluorescent dye to perform the experiment (see Lysosomal Membrane Permeabilization (LMP) in Transparent Methods for experimental details). As shown in Figures 8C and 8D, a significant intracellular red dot fluorescence was observed due to the formation of protonated AO in the intact lysosomes (pH 4–5), implying that 3 and 5 did not damage the lysosomes in dark. However, the intracellular red fluorescence was weakened or even disappeared under light irradiation, which demonstrated that AO was released from the lysosome into the cytoplasm (pH ∼7.2), and moreover, deprotonated in the weak alkaline environment to emit diffuse green fluorescence (Al-Eisawi et al., 2016, Boya and Kroemer, 2008). This observation suggested that 3- and 5-triggered 1O2 can induce LMP, and effectively promoted cell death via lysosome-associated pathway. Again, 3 exhibited more effective LMP because of the heavy-atom effect.
In Vivo PDT Experiment
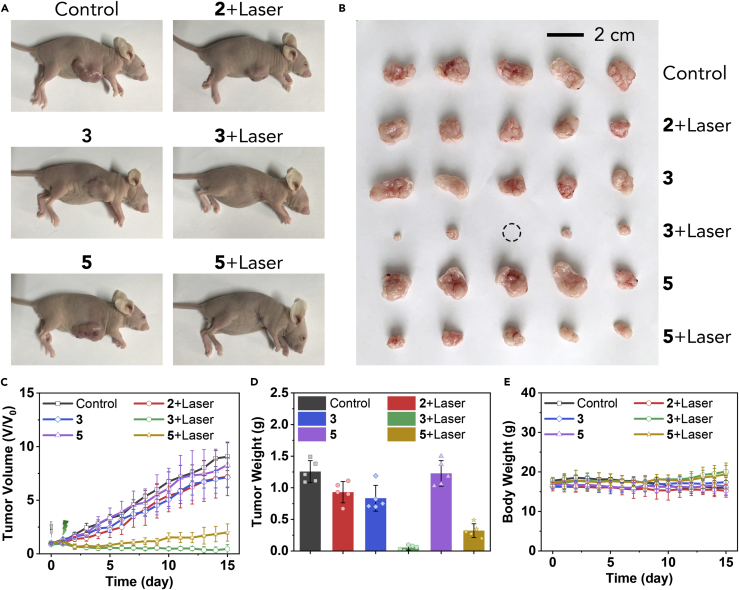
All the aforementioned results proved that 3 and 5 were excellent COF-based nano-PSs. This encouraged us to continue to examine their in vivo anti-tumor ability (see MCF-7 Xenograft Model and In Vivo PDT Experiment in Transparent Methods for experimental details). As shown in Figures 9A–9D, tumors in nude mice treated with Dulbecco's phosphate-buffered saline (DPBS) increased rapidly. For the groups injected with 3 and 5 without light irradiation, a similar tumor growth trend was observed, but there was no necrosis of any tissue. This indicated that 3 and 5 were negligibly toxic to the biological tissues. For the group injected with 2 with light irradiation (green LED, 1.0 W/cm2), some slight signs of tumor inhibition effect were observed at the early treatment stages but then quickly recurred, and eventually there was no significant difference from the control group. The results of the 2 + laser group ruled out the individual role of the small molecular BODIPY and green laser irradiation in tumor inhibition. As expected, 3- and 5-induced PDT effectively inhibited tumor growth under green laser irradiation (1.0 W/cm2), and no recrudescent signs were observed during the whole experimental period. Compared with the 5 + laser group, the results from 3 + laser group strongly suggested that the heavy-atom effect can effectively enhance the in vivo PDT efficacy, which is entirely consistent with their in vitro PDT examination.
Figure 9.
In Vivo PDT Experiment
Animals were used in PDT experiments when the tumor size reached ∼150 mm3. Day 0, intratumoral injection; day 1, for the treatment group, light treatment was performed on the tumor site. The mice continued to be fed for 14 days. See MCF-7 Xenograft Model and In Vivo PDT Experiment in Transparent Methods for experimental details.
(A) Representative photographs of MCF-7 tumor-bearing nude mice at the end of the treatment.
(B) Photographs of tumor tissue obtained after treatment. Scale bar, 2 cm.
(C) Tumor volume of the nude mice in various groups during the treatment. Data are presented as mean ± SD (n = 5).
(D) Tumor weight obtained after treatment. Data are presented as mean ± SD (n = 5).
(E) Body weight of the mice in various groups during the treatment. Data are presented as mean ± SD (n = 5).
Besides, Figure 9E showed that all nude mice with intratumor injection presented a steady growth or no change in their body weight, whereas the body weight of the control group was slightly decreased. Histopathological examination revealed that there was no substantial damage or distinct inflammation lesions in organs, including the heart, liver, spleen, lung, and kidney (Figure S10, see Histopathological Examination in Transparent Methods for experimental details). All these results confirmed that 3 and 5 had negligible adverse impact and favorable biocompatibility on organisms and were indeed suitable for in vivo anti-tumor application.
Discussion
COFs have picked up intense attention of researchers worldwide due to their promising applications in heterogeneous catalysis (Ding and Wang, 2013, Tu et al., 2018, Yan et al., 2018), energy storage (Cao et al., 2019, Xiao and Xu, 2018, Zhang et al., 2017b, Zheng et al., 2019, Zhou and Wang, 2017), analytical chemistry (Qian et al., 2018b, Zhang et al., 2018d, Zhang et al., 2019), and light conversion (Banerjee et al., 2018). As an emerging class of organic crystalline materials, COFs offered some specific advantages not found in other types of materials: modularity, porosity, stability, and metal free (Chandra et al., 2013, Kandambeth et al., 2012). The application of COFs in biomedical field, especially cancer therapeutics, however, is still in its infancy owing to the difficulty in their size- and structure-controlled synthesis and poor aqueous dispersibility (Li and Yang, 2017) (see Table S1 for details). On the other hand, the bulky and active organic PSs, such as BODIPY herein, were sometimes hard to be safely anchored onto the COF framework by the bottom-up approach especially under the rigorous solvothermal conditions. In addition, the covalent decoration of PSs on the established COF framework by the existing PSM approach (Ma and Scott, 2018) might be limited by the stability and activity of the pre-embedded active precursors under the COF synthetic conditions (Xu et al., 2016), and also the incoming bulky functional moiety of PSs might lead to crystallinity decrease, structural transformation, and even structure collapse of the COF pristine, although some very impressive PSM examples have been reported very recently (Bunck and Dichtel, 2013, Chen et al., 2014, Daugherty et al., 2019, Dong et al., 2016, Guo et al., 2017a, Haase et al., 2018, Han et al., 2018, Huang et al., 2015a, Huang et al., 2015b, Li et al., 2018c, Li et al., 2019, Lohse et al., 2016, Lu et al., 2018, Mitra et al., 2017, Nagai et al., 2011, Qian et al., 2017, Qian et al., 2018a, Rager et al., 2017, Sun et al., 2017, Sun et al., 2018a, Sun et al., 2018b, Vardhan et al., 2019, Waller et al., 2016, Waller et al., 2018, Xu et al., 2014, Xu et al., 2015a, Xu et al., 2015b) (see Table S2 for details).
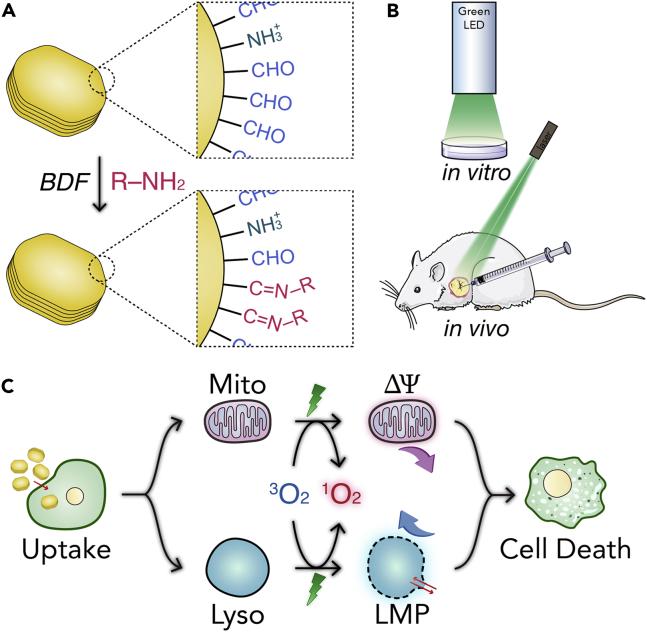
On the other hand, COFs could be considered as a type of porous crystalline organic “reactive end-group polymers.” In principle, these intrinsic bonding defects in COF matrix can be further modified and tailored to the desired application via BDF approach. As shown in Figure 10A, by reaction of the free end –CHO group on 1 with –NH2 moiety on 2 or 4, the organic PSs of BODIPY were covalently attached to the COF NPs via Schiff-base condensation under the given conditions to generate the NCOF-based PSs. As a consequence, the organic BODIPY components have been successfully nanocrystallized without altering the pristine COF structure and the inherent property of BODIPY; moreover, the possible BODIPY leaching was also effectively avoided due to the covalent decoration. Indeed, both 3 and 5 featured a clear chemical composition and excellent chemical, aqueous, and photostability. In addition, their nanometer size (ca. 110 nm), low dark toxicity, and extremely high phototoxicity made them become attractive NCOF-based PSs for PDT, which was well evidenced by the in vitro and in vivo experiments (Figure 10B).
Figure 10.
Bonding Defects Functionalization Strategy of Nanoscale COFs for PDT
(A) Diagram of bonding defect functionalization (BDF) of NCOF LZU-1 (1).
(B) Diagram of in vitro and in vivo PDT.
(C) Diagram of 3- and 5-induced cell death mechanism.
In in vitro experiments, we took the HeLa and MCF-7 cancer cell lines to examine the PDT of 3 and 5 in detail. Intracellular 1O2 imaging, cytostatic, and calcein-AM/PI double staining tests all supported that 3 and 5 are excellent nanomedical agents for PDT, especially 3 with heavy atoms of iodine. Furthermore, the spread of cancer cells was effectively inhibited by 3- and 5-involved PDT, as revealed by in vitro scratch assays. Notably, the in vitro PDT performance exhibited by 3 was better than those of reported BODIPY-based PDT systems (see Table S3 for detailed comparative analysis). Their high-efficacy PDT, especially 3, was further confirmed by the in vivo experiments, and they are able to effectively inhibit the MCF-7 xenograft growth without detectable systemic toxicity. More detailed mechanism study (Figure 10C) showed that 3 and 5 were taken up by cancer cells via energy-dependent endocytosis, and that they were mainly localized to lysosomes and mitochondria, and consequently led to cell death through mitochondrion- and lysosome-associated pathways.
Conclusion
We can reasonably assume that the BDF approach herein is flexible and versatile, and that it can be used to construct new types of NCOF-based nanomedicines by covalently grafting various functional species such as bioactive, chemotherapeutic, and targeting agents, and so on via bonding defects decoration. In doing so, the functionality of the COF-based materials could be significantly enriched, and their application scope would be effectively expanded. To the best of our knowledge, this is the first example of NCOF-based materials for PDT. In the past two decades, nanotherapeutics, as an emerging medical treatment approach, has provided a promising way for clinical cancer treatment (Chen et al., 2017, Hartshorn et al., 2018, Wang et al., 2018b). A wide variety of nanomaterials (Croissant et al., 2018, Yang et al., 2018, Zhang et al., 2018a) have been developed for cancer therapeutics. PDT as a minimally invasive method has received much more attention due to its unique tissue selectivity (Agostinis et al., 2011, Li et al., 2018b). We believe that the NCOF-based PDT materials via BDF herein with both nano- and organic PS merits not only expanded the type of nanomaterials for PDT but also might open up new avenues for the design and fabrication of many more new nanomedicines, especially for cancer therapy.
Limitations of Study
This study focused on the free end –CHO on LZU-1 by BDF, and no chemical modification on the free –NH2 of LZU-1 NP was performed. If so, other types of functional molecules such as antineoplastic drugs might be further grafted on the NCOF by BDF. In doing so, more advanced and effective NCOF-based medicines would be achieved.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are grateful for financial support from NSFC, China (grant nos. 21671122, 21475078, and 21805172), the Taishan Scholar's Construction Project and Changjiang Scholar Project at SDNU, and the Doctoral Fund of Shandong Province (Grant No. ZR2017BB067).
Author Contributions
Y.-B.D., Q.G. and Y.-A.L. conceived this work. Y.-B.D., Y.-A.L., and S.-J.Z. directed the research. Q.G., D.-D.F., Y.-A.L., X.-M.K., Z.-Y.W., and W.-Y.L. carried out the experiments and characterizations. Q.G. analyzed the data. Y.-B.D. and Q.G. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: April 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.03.028.
Contributor Information
Yan-An Li, Email: yananli@sdnu.edu.cn.
Shao-Jun Zhang, Email: vickie5454@163.com.
Yu-Bin Dong, Email: yubindong@sdnu.edu.cn.
Supplemental Information
References
- Abánades Lázaro I., Forgan R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019;380:230–259. [Google Scholar]
- Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Eisawi Z., Stefani C., Jansson P.J., Arvind A., Sharpe P.C., Basha M.T., Iskander G.M., Kumar N., Kovacevic Z., Lane D.J.R. Novel mechanism of cytotoxicity for the selective selenosemicarbazone, 2-acetylpyridine 4,4-Dimethyl-3-selenosemicarbazone (Ap44mSe): lysosomal membrane permeabilization. J. Med. Chem. 2016;59:294–312. doi: 10.1021/acs.jmedchem.5b01399. [DOI] [PubMed] [Google Scholar]
- Altorki N.K., Markowitz G.J., Gao D., Port J.L., Saxena A., Stiles B., McGraw T., Mittal V. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer. 2019;19:9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Phua S.Z.F., Lim W.Q., Jana A., Luo Z., Tham H.P., Zhao L., Gao Q., Zhao Y. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 2016;52:4128–4131. doi: 10.1039/c6cc00853d. [DOI] [PubMed] [Google Scholar]
- Banerjee T., Gottschling K., Savasci G., Ochsenfeld C., Lotsch B.V. H2 evolution with covalent organic framework photocatalysts. ACS Energy Lett. 2018;3:400–409. doi: 10.1021/acsenergylett.7b01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand B., Passador K., Goze C., Denat F., Bodio E., Salmain M. Metal-based BODIPY derivatives as multimodal tools for life sciences. Coord. Chem. Rev. 2018;358:108–124. [Google Scholar]
- Bhanja P., Mishra S., Manna K., Mallick A., Das Saha K., Bhaumik A. Covalent organic framework material bearing phloroglucinol building units as a potent anticancer agent. ACS Appl. Mater. Interfaces. 2017;9:31411–31423. doi: 10.1021/acsami.7b07343. [DOI] [PubMed] [Google Scholar]
- Bisbey R.P., Dichtel W.R. Covalent organic frameworks as a platform for multidimensional polymerization. ACS Cent. Sci. 2017;3:533–543. doi: 10.1021/acscentsci.7b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolze F., Jenni S., Sour A., Heitz V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. (Camb.) 2017;53:12857–12877. doi: 10.1039/c7cc06133a. [DOI] [PubMed] [Google Scholar]
- Boya P., Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- Bunck D.N., Dichtel W.R. Postsynthetic functionalization of 3D covalent organic frameworks. Chem. Commun. (Camb.) 2013;49:2457–2459. doi: 10.1039/c3cc40358k. [DOI] [PubMed] [Google Scholar]
- Burke P.J. Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer. 2017;3:857–870. doi: 10.1016/j.trecan.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Li B., Zhu R., Pang H. Design and synthesis of covalent organic frameworks towards energy and environment fields. Chem. Eng. J. 2019;355:602–623. [Google Scholar]
- Chakraborty A., Dalal C., Jana N.R. Colloidal nanobioconjugate with complementary surface chemistry for cellular and subcellular targeting. Langmuir. 2018;34:13461–13471. doi: 10.1021/acs.langmuir.8b00376. [DOI] [PubMed] [Google Scholar]
- Chandra S., Kandambeth S., Biswal B.P., Lukose B., Kunjir S.M., Chaudhary M., Babarao R., Heine T., Banerjee R. Chemically stable multilayered covalent organic nanosheets from covalent organic frameworks via mechanical delamination. J. Am. Chem. Soc. 2013;135:17853–17861. doi: 10.1021/ja408121p. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang W., Zhu G., Xie J., Chen X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017;2:17024. doi: 10.1038/natrevmats.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Furukawa K., Gao J., Nagai A., Nakamura T., Dong Y., Jiang D. Photoelectric covalent organic frameworks: converting open lattices into ordered donor–acceptor heterojunctions. J. Am. Chem. Soc. 2014;136:9806–9809. doi: 10.1021/ja502692w. [DOI] [PubMed] [Google Scholar]
- Croissant J.G., Zink J.I., Raehm L., Durand J.-O. Two-Photon-excited silica and organosilica nanoparticles for spatiotemporal cancer treatment. Adv. Healthc. Mater. 2018;7:1701248. doi: 10.1002/adhm.201701248. [DOI] [PubMed] [Google Scholar]
- Das G., Benyettou F., Sharama S.K., Prakasam T., Gándara F., de la Peña-O’Shea V.A., Saleh N.i., Pasricha R., Jagannathan R., Olson M.A. Covalent organic nanosheets for bioimaging. Chem. Sci. 2018;9:8382–8387. doi: 10.1039/c8sc02842g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty M.C., Vitaku E., Li R.L., Evans A.M., Chavez A., Dichtel W. Improved synthesis of β-ketoenamine-linked covalent organic frameworks via monomer exchange reactions. Chem. Commun. (Camb.) 2019;55:2680–2683. doi: 10.1039/c8cc08957d. [DOI] [PubMed] [Google Scholar]
- Ding S.-Y., Gao J., Wang Q., Zhang Y., Song W.-G., Su C.-Y., Wang W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 2011;133:19816–19822. doi: 10.1021/ja206846p. [DOI] [PubMed] [Google Scholar]
- Ding S.-Y., Wang W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 2013;42:548–568. doi: 10.1039/c2cs35072f. [DOI] [PubMed] [Google Scholar]
- Dong B., Wang L., Zhao S., Ge R., Song X., Wang Y., Gao Y. Immobilization of ionic liquids to covalent organic frameworks for catalyzing the formylation of amines with CO2 and phenylsilane. Chem. Commun. (Camb.) 2016;52:7082–7085. doi: 10.1039/c6cc03058k. [DOI] [PubMed] [Google Scholar]
- Dong K., Wang Z., Zhang Y., Ren J., Qu X. Metal–organic framework-based nanoplatform for intracellular environment-responsive endo/lysosomal escape and enhanced cancer therapy. ACS Appl. Mater. Interfaces. 2018;10:31998–32005. doi: 10.1021/acsami.8b11972. [DOI] [PubMed] [Google Scholar]
- Durantini A.M., Heredia D.A., Durantini J.E., Durantini E.N. BODIPYs to the rescue: potential applications in photodynamic inactivation. Eur. J. Med. Chem. 2018;144:651–661. doi: 10.1016/j.ejmech.2017.12.068. [DOI] [PubMed] [Google Scholar]
- Fang Q., Wang J., Gu S., Kaspar R.B., Zhuang Z., Zheng J., Guo H., Qiu S., Yan Y. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 2015;137:8352–8355. doi: 10.1021/jacs.5b04147. [DOI] [PubMed] [Google Scholar]
- Feng X., Ding X., Chen L., Wu Y., Liu L., Addicoat M., Irle S., Dong Y., Jiang D. Two-dimensional artificial light-harvesting antennae with predesigned high-order structure and robust photosensitising activity. Sci. Rep. 2016;6:32944. doi: 10.1038/srep32944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice C., Akinyemiju T.F., Al Lami F.H., Alam T., Alizadeh-Navaei R., Allen C., Alsharif U., Alvis-Guzman N., Amini E., Anderson B.O. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S., Galluzzi L., Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- Gomes-da-Silva L.C., Zhao L., Bezu L., Zhou H., Sauvat A., Liu P., Durand S., Leduc M., Souquere S., Loos F. Photodynamic therapy with redaporfin targets the endoplasmic reticulum and Golgi apparatus. EMBO J. 2018;37:e98354. doi: 10.15252/embj.201798354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q., Li Y.-A., Li W.-Y., Dong Y.-B. Photodynamic therapy based on nanoscale metal–organic frameworks: from material design to cancer nanotherapeutics. Chem. Asian J. 2018;13:3122–3149. doi: 10.1002/asia.201801221. [DOI] [PubMed] [Google Scholar]
- Guan Q., Zhou L.-L., Li Y.-A., Dong Y.-B. Diiodo-bodipy-encapsulated nanoscale metal–organic framework for pH-driven selective and mitochondria targeted photodynamic therapy. Inorg. Chem. 2018;57:10137–10145. doi: 10.1021/acs.inorgchem.8b01316. [DOI] [PubMed] [Google Scholar]
- Guo H., Wang J., Fang Q., Zhao Y., Gu S., Zheng J., Yan Y. A quaternary-ammonium-functionalized covalent organic framework for anion conduction. CrystEngComm. 2017;19:4905–4910. [Google Scholar]
- Guo L., Niu G., Zheng X., Ge J., Liu W., Jia Q., Zhang P., Zhang H., Wang P. Single near-infrared emissive polymer nanoparticles as versatile phototheranostics. Adv. Sci. 2017;4:1700085. doi: 10.1002/advs.201700085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyparaki M.-T., Papavassiliou A.G. Lysosome: the cell's ‘suicidal bag’ as a promising cancer target. Trends Mol. Med. 2014;20:239–241. doi: 10.1016/j.molmed.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Haase F., Troschke E., Savasci G., Banerjee T., Duppel V., Dörfler S., Grundei M.M.J., Burow A.M., Ochsenfeld C., Kaskel S. Topochemical conversion of an imine- into a thiazole-linked covalent organic framework enabling real structure analysis. Nat. Commun. 2018;9:2600. doi: 10.1038/s41467-018-04979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Huang J., Yuan C., Liu Y., Cui Y. Chiral 3D covalent organic frameworks for high performance liquid chromatographic enantioseparation. J. Am. Chem. Soc. 2018;140:892–895. doi: 10.1021/jacs.7b12110. [DOI] [PubMed] [Google Scholar]
- Hartshorn C.M., Bradbury M.S., Lanza G.M., Nel A.E., Rao J., Wang A.Z., Wiesner U.B., Yang L., Grodzinski P. Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano. 2018;12:24–43. doi: 10.1021/acsnano.7b05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Chen X., Krishna R., Jiang D. Two-Dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization. Angew. Chem. Int. Ed. 2015;54:2986–2990. doi: 10.1002/anie.201411262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Krishna R., Jiang D. Tailor-made pore surface engineering in covalent organic frameworks: systematic functionalization for performance screening. J. Am. Chem. Soc. 2015;137:7079–7082. doi: 10.1021/jacs.5b04300. [DOI] [PubMed] [Google Scholar]
- Hynek J., Zelenka J., Rathouský J., Kubát P., Ruml T., Demel J., Lang K. Designing porphyrinic covalent organic frameworks for the photodynamic inactivation of bacteria. ACS Appl. Mater. Interfaces. 2018;10:8527–8535. doi: 10.1021/acsami.7b19835. [DOI] [PubMed] [Google Scholar]
- Intlekofer A.M., Finley L.W.S. Metabolic signatures of cancer cells and stem cells. Nat. Metab. 2019;1:177–188. doi: 10.1038/s42255-019-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q., Ge J., Liu W., Zheng X., Chen S., Wen Y., Zhang H., Wang P. A magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv. Mater. 2018;30:1706090. doi: 10.1002/adma.201706090. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Wang Y., Sun L., Yuan B., Tian Y., Xiang L., Li Y., Li Y., Li J., Wu A. Dual ATP and pH responsive ZIF-90 nanosystem with favorable biocompatibility and facile post-modification improves therapeutic outcomes of triple negative breast cancer in vivo. Biomaterials. 2019;197:41–50. doi: 10.1016/j.biomaterials.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Josefsen L.B., Boyle R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics. 2012;2:916–966. doi: 10.7150/thno.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan J.-L., Jiang Y., Xue A., Yu Y.-H., Wang Q., Zhou Y., Dong Y.-B. Surface decorated porphyrinic nanoscale metal–organic framework for photodynamic therapy. Inorg. Chem. 2018;57:5420–5428. doi: 10.1021/acs.inorgchem.8b00384. [DOI] [PubMed] [Google Scholar]
- Kandambeth S., Mallick A., Lukose B., Mane M.V., Heine T., Banerjee R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (Acid/Base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012;134:19524–19527. doi: 10.1021/ja308278w. [DOI] [PubMed] [Google Scholar]
- Kandambeth S., Venkatesh V., Shinde D.B., Kumari S., Halder A., Verma S., Banerjee R. Self-templated chemically stable hollow spherical covalent organic framework. Nat. Commun. 2015;6:6786. doi: 10.1038/ncomms7786. [DOI] [PubMed] [Google Scholar]
- Kato M., Li J., Chuang J.L., Chuang D.T. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure. 2007;15:992–1004. doi: 10.1016/j.str.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Jäättelä M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Li M., Long S., Kang Y., Guo L., Wang J., Fan J., Du J., Peng X. De novo design of phototheranostic sensitizers based on structure-inherent targeting for enhanced cancer ablation. J. Am. Chem. Soc. 2018;140:15820–15826. doi: 10.1021/jacs.8b09117. [DOI] [PubMed] [Google Scholar]
- Li W., Yang C.-X., Yan X.-P. A versatile covalent organic framework-based platform for sensing biomolecules. Chem. Commun. 2017;53:11469–11471. doi: 10.1039/c7cc06244c. [DOI] [PubMed] [Google Scholar]
- Li X., Lee S., Yoon J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018;47:1174–1188. doi: 10.1039/c7cs00594f. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang C., Cai S., Lei X., Altoe V., Hong F., Urban J.J., Ciston J., Chan E.M., Liu Y. Facile transformation of imine covalent organic frameworks into ultrastable crystalline porous aromatic frameworks. Nat. Commun. 2018;9:2998. doi: 10.1038/s41467-018-05462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Hu X., Zheng X., Liu Y., Liu S., Yue Y., Xie Z. Self-assembled organic nanorods for dual chemo-photodynamic therapies. RSC Adv. 2018;8:5493–5499. doi: 10.1039/c8ra00067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Ding X., Feng Y., Feng W., Han B.-H. Structural and dimensional transformations between covalent organic frameworks via linker exchange. Macromolecules. 2019;52:1257–1265. [Google Scholar]
- Li Z., Yang Y.-W. Creation and bioapplications of porous organic polymer materials. J. Mater. Chem. B. 2017;5:9278–9290. doi: 10.1039/c7tb02647a. [DOI] [PubMed] [Google Scholar]
- Liang C.-C., Park A.Y., Guan J.-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Lin G., Ding H., Chen R., Peng Z., Wang B., Wang C. 3D porphyrin-based covalent organic frameworks. J. Am. Chem. Soc. 2017;139:8705–8709. doi: 10.1021/jacs.7b04141. [DOI] [PubMed] [Google Scholar]
- Lismont M., Dreesen L., Wuttke S. Metal-organic framework nanoparticles in photodynamic therapy: current status and perspectives. Adv. Funct. Mater. 2017;27:1606314. [Google Scholar]
- Liu L., Zhang Z., Xing D. Cell death via mitochondrial apoptotic pathway due to activation of Bax by lysosomal photodamage. Free Radic. Biol. Med. 2011;51:53–68. doi: 10.1016/j.freeradbiomed.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Liu S., Hu C., Liu Y., Zhao X., Pang M., Lin J. One-pot synthesis of DOX@covalent organic framework with enhanced chemotherapeutic efficacy. Chem. Eur. J. 2019;25:4315–4319. doi: 10.1002/chem.201806242. [DOI] [PubMed] [Google Scholar]
- Liu W., Cao Y., Wang W., Gong D., Cao T., Qian J., Iqbal K., Qin W., Guo H. Mechanochromic luminescent covalent organic frameworks for highly selective hydroxyl radical detection. Chem. Commun. (Camb.) 2019;55:167–170. doi: 10.1039/c8cc07783e. [DOI] [PubMed] [Google Scholar]
- Liu T., Hu X., Wang Y., Meng L., Zhou Y., Zhang J., Chen M., Zhang X. Triazine-based covalent organic frameworks for photodynamic inactivation of bacteria as type-II photosensitizers. J Photochem. Photobiol. B. 2017;175:156–162. doi: 10.1016/j.jphotobiol.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Liu X., Hu M., Wang M., Song Y., Zhou N., He L., Zhang Z. Novel nanoarchitecture of Co-MOF-on-TPN-COF hybrid: ultralowly sensitive bioplatform of electrochemical aptasensor toward ampicillin. Biosens. Bioelectron. 2019;123:59–68. doi: 10.1016/j.bios.2018.09.089. [DOI] [PubMed] [Google Scholar]
- Liu Y., Song N., Li Z., Chen L., Xie Z. Near-infrared nanoparticles based on aza-BDP for photodynamic and photothermal therapy. Dyes Pigm. 2019;160:71–78. [Google Scholar]
- Lohse M.S., Bein T. Covalent organic frameworks: structures, synthesis, and applications. Adv. Funct. Mater. 2018;28:1705553. [Google Scholar]
- Lohse M.S., Stassin T., Naudin G., Wuttke S., Ameloot R., De Vos D., Medina D.D., Bein T. Sequential pore wall modification in a covalent organic framework for application in lactic acid adsorption. Chem. Mater. 2016;28:626–631. [Google Scholar]
- Lu K., He C., Lin W. Nanoscale metal–organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J. Am. Chem. Soc. 2014;136:16712–16715. doi: 10.1021/ja508679h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Ma Y., Li H., Guan X., Yusran Y., Xue M., Fang Q., Yan Y., Qiu S., Valtchev V. Postsynthetic functionalization of three-dimensional covalent organic frameworks for selective extraction of lanthanide ions. Angew. Chem. Int. Ed. 2018;57:6042–6048. doi: 10.1002/anie.201712246. [DOI] [PubMed] [Google Scholar]
- Lv W., Zhang Z., Zhang K.Y., Yang H., Liu S., Xu A., Guo S., Zhao Q., Huang W. A mitochondria-targeted photosensitizer showing improved photodynamic therapy effects under hypoxia. Angew. Chem. Int. Ed. 2016;55:9947–9951. doi: 10.1002/anie.201604130. [DOI] [PubMed] [Google Scholar]
- Ma X., Scott T.F. Approaches and challenges in the synthesis of three-dimensional covalent-organic frameworks. Commun. Chem. 2018;1:98. [Google Scholar]
- Mallidi S., Anbil S., Bulin A.-L., Obaid G., Ichikawa M., Hasan T. Beyond the barriers of light penetration: strategies, perspectives and possibilities for photodynamic therapy. Theranostics. 2016;6:2458–2487. doi: 10.7150/thno.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques C., Oliveira C.S.F., Alves S., Chaves S.R., Coutinho O.P., Côrte-Real M., Preto A. Acetate-induced apoptosis in colorectal carcinoma cells involves lysosomal membrane permeabilization and cathepsin D release. Cell Death Dis. 2013;4:e507. doi: 10.1038/cddis.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S., Pagano R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Kandambeth S., Biswal B.P., Khayum M.A., Choudhury C.K., Mehta M., Kaur G., Banerjee S., Prabhune A., Verma S. Self-exfoliated guanidinium-based ionic covalent organic nanosheets (iCONs) J. Am. Chem. Soc. 2016;138:2823–2828. doi: 10.1021/jacs.5b13533. [DOI] [PubMed] [Google Scholar]
- Mitra S., Sasmal H.S., Kundu T., Kandambeth S., Illath K., Díaz Díaz D., Banerjee R. Targeted drug delivery in covalent organic nanosheets (CONs) via sequential postsynthetic modification. J. Am. Chem. Soc. 2017;139:4513–4520. doi: 10.1021/jacs.7b00925. [DOI] [PubMed] [Google Scholar]
- Mosquera J., García I., Liz-Marzán L.M. Cellular uptake of nanoparticles versus small molecules: a matter of size. Acc. Chem. Res. 2018;51:2305–2313. doi: 10.1021/acs.accounts.8b00292. [DOI] [PubMed] [Google Scholar]
- Nagai A., Chen X., Feng X., Ding X., Guo Z., Jiang D. A squaraine-linked mesoporous covalent organic framework. Angew. Chem. Int. Ed. 2013;52:3770–3774. doi: 10.1002/anie.201300256. [DOI] [PubMed] [Google Scholar]
- Nagai A., Guo Z., Feng X., Jin S., Chen X., Ding X., Jiang D. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2011;2:536. doi: 10.1038/ncomms1542. [DOI] [PubMed] [Google Scholar]
- Nayak M.K., Dhanesha N., Doddapattar P., Rodriguez O., Sonkar V.K., Dayal S., Chauhan A.K. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv. 2018;2:2029–2038. doi: 10.1182/bloodadvances.2018022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V., Grünwald M. Microscopic origins of poor crystallinity in the synthesis of covalent organic framework COF-5. J. Am. Chem. Soc. 2018;140:3306–3311. doi: 10.1021/jacs.7b12529. [DOI] [PubMed] [Google Scholar]
- Nian F., Huang Y., Song M., Chen J.-J., Xue J. A novel fabricated material with divergent chemical handles based on UiO-66 and used for targeted photodynamic therapy. J. Mater. Chem. B. 2017;5:6227–6232. doi: 10.1039/c7tb01295k. [DOI] [PubMed] [Google Scholar]
- Peng Y., Wong W.K., Hu Z., Cheng Y., Yuan D., Khan S.A., Zhao D. Room temperature batch and continuous flow synthesis of water-stable covalent organic frameworks (COFs) Chem. Mater. 2016;28:5095–5101. [Google Scholar]
- Qian C., Qi Q.-Y., Jiang G.-F., Cui F.-Z., Tian Y., Zhao X. Toward covalent organic frameworks bearing three different kinds of pores: the strategy for construction and COF-to-COF transformation via heterogeneous linker exchange. J. Am. Chem. Soc. 2017;139:6736–6743. doi: 10.1021/jacs.7b02303. [DOI] [PubMed] [Google Scholar]
- Qian H.-L., Li Y., Yan X.-P. A building block exchange strategy for the rational fabrication of de novo unreachable amino-functionalized imine-linked covalent organic frameworks. J. Mater. Chem. A. 2018;6:17307–17311. [Google Scholar]
- Qian H.-L., Yang C.-X., Wang W.-L., Yang C., Yan X.-P. Advances in covalent organic frameworks in separation science. J. Chromatogr. A. 2018;1542:1–18. doi: 10.1016/j.chroma.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Qin J.-S., Yuan S., Zhang L., Li B., Du D.-Y., Huang N., Guan W., Drake H.F., Pang J., Lan Y.-Q. Creating well-defined hexabenzocoronene in zirconium metal–organic framework by postsynthetic annulation. J. Am. Chem. Soc. 2019;141:2054–2060. doi: 10.1021/jacs.8b11042. [DOI] [PubMed] [Google Scholar]
- Rager S., Dogru M., Werner V., Gavryushin A., Gotz M., Engelke H., Medina D.D., Knochel P., Bein T. Pore wall fluorescence labeling of covalent organic frameworks. CrystEngComm. 2017;19:4886–4891. [Google Scholar]
- Rajora M.A., Lou J.W.H., Zheng G. Advancing porphyrin's biomedical utility via supramolecular chemistry. Chem. Soc. Rev. 2017;46:6433–6469. doi: 10.1039/c7cs00525c. [DOI] [PubMed] [Google Scholar]
- Röder R., Preiß T., Hirschle P., Steinborn B., Zimpel A., Höhn M., Rädler J.O., Bein T., Wagner E., Wuttke S. Multifunctional nanoparticles by coordinative self-assembly of his-tagged units with metal–organic frameworks. J. Am. Chem. Soc. 2017;139:2359–2368. doi: 10.1021/jacs.6b11934. [DOI] [PubMed] [Google Scholar]
- Ruyra À., Yazdi A., Espín J., Carné-Sánchez A., Roher N., Lorenzo J., Imaz I., Maspoch D. Synthesis, culture medium stability, and in vitro and in vivo Zebrafish embryo toxicity of metal–organic framework nanoparticles. Chem. Eur. J. 2015;21:2508–2518. doi: 10.1002/chem.201405380. [DOI] [PubMed] [Google Scholar]
- Shi X., Tian F. Multiscale modeling and simulation of nano-carriers delivery through biological barriers—a review. Adv. Theory Simul. 2019;2:1800105. [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Stuelten C.H., Parent C.A., Montell D.J. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat. Rev. Cancer. 2018;18:296–312. doi: 10.1038/nrc.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Aguila B., Perman J., Earl L.D., Abney C.W., Cheng Y., Wei H., Nguyen N., Wojtas L., Ma S. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal. J. Am. Chem. Soc. 2017;139:2786–2793. doi: 10.1021/jacs.6b12885. [DOI] [PubMed] [Google Scholar]
- Sun Q., Aguila B., Earl L.D., Abney C.W., Wojtas L., Thallapally P.K., Ma S. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv. Mater. 2018;30:1705479. doi: 10.1002/adma.201705479. [DOI] [PubMed] [Google Scholar]
- Sun Q., Aguila B., Perman J.A., Butts T., Xiao F.-S., Ma S. Integrating superwettability within covalent organic frameworks for functional coating. Chem. 2018;4:1726–1739. [Google Scholar]
- Sun Q., Fu C.-W., Aguila B., Perman J., Wang S., Huang H.-Y., Xiao F.-S., Ma S. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J. Am. Chem. Soc. 2018;140:984–992. doi: 10.1021/jacs.7b10642. [DOI] [PubMed] [Google Scholar]
- Tamames-Tabar C., Cunha D., Imbuluzqueta E., Ragon F., Serre C., Blanco-Prieto M.J., Horcajada P. Cytotoxicity of nanoscaled metal–organic frameworks. J. Mater. Chem. B. 2014;2:262–271. doi: 10.1039/c3tb20832j. [DOI] [PubMed] [Google Scholar]
- Tan J., Namuangruk S., Kong W., Kungwan N., Guo J., Wang C. Manipulation of amorphous-to-crystalline transformation: towards the construction of covalent organic framework hybrid microspheres with NIR photothermal conversion ability. Angew. Chem. Int. Ed. 2016;55:13979–13984. doi: 10.1002/anie.201606155. [DOI] [PubMed] [Google Scholar]
- Tan Y., Zhu Y., Zhao Y., Wen L., Meng T., Liu X., Yang X., Dai S., Yuan H., Hu F. Mitochondrial alkaline pH-responsive drug release mediated by Celastrol loaded glycolipid-like micelles for cancer therapy. Biomaterials. 2018;154:169–181. doi: 10.1016/j.biomaterials.2017.07.036. [DOI] [PubMed] [Google Scholar]
- Tu W., Xu Y., Yin S., Xu R. Rational design of catalytic centers in crystalline frameworks. Adv. Mater. 2018;30:1707582. doi: 10.1002/adma.201707582. [DOI] [PubMed] [Google Scholar]
- van Meerloo J., Kaspers G.J.L., Cloos J. Cell sensitivity assays: the MTT assay. In: Cree I.A., editor. Cancer Cell Culture: Methods and Protocols. Humana Press; 2011. pp. 237–245. [DOI] [PubMed] [Google Scholar]
- Vardhan H., Verma G., Ramani S., Nafady A., Al-Enizi A.M., Pan Y., Yang Z., Yang H., Ma S. Covalent organic framework decorated with vanadium as a new platform for Prins reaction and sulfide oxidation. ACS Appl. Mater. Interfaces. 2019;11:3070–3079. doi: 10.1021/acsami.8b19352. [DOI] [PubMed] [Google Scholar]
- Vyas S., Zaganjor E., Haigis M.C. Mitochondria and cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas V.S., Vishwakarma M., Moudrakovski I., Haase F., Savasci G., Ochsenfeld C., Spatz J.P., Lotsch B.V. Exploiting noncovalent interactions in an imine-based covalent organic framework for quercetin delivery. Adv. Mater. 2016;28:8749–8754. doi: 10.1002/adma.201603006. [DOI] [PubMed] [Google Scholar]
- Waller P.J., AlFaraj Y.S., Diercks C.S., Jarenwattananon N.N., Yaghi O.M. Conversion of imine to oxazole and thiazole linkages in covalent organic frameworks. J. Am. Chem. Soc. 2018;140:9099–9103. doi: 10.1021/jacs.8b05830. [DOI] [PubMed] [Google Scholar]
- Waller P.J., Lyle S.J., Osborn Popp T.M., Diercks C.S., Reimer J.A., Yaghi O.M. Chemical conversion of linkages in covalent organic frameworks. J. Am. Chem. Soc. 2016;138:15519–15522. doi: 10.1021/jacs.6b08377. [DOI] [PubMed] [Google Scholar]
- Wang P., Zhou F., Zhang C., Yin S.-Y., Teng L., Chen L., Hu X.-X., Liu H.-W., Yin X., Zhang X.-B. Ultrathin two-dimensional covalent organic framework nanoprobe for interference-resistant two-photon fluorescence bioimaging. Chem. Sci. 2018;9:8402–8408. doi: 10.1039/c8sc03393e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang L., Li Z., Xie Z. BODIPY-containing nanoscale metal-organic frameworks for photodynamic therapy. Chem. Commun. 2016;52:5402–5405. doi: 10.1039/c6cc01048b. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sun S., Zhang Z., Shi D. Nanomaterials for cancer precision medicine. Adv. Mater. 2018;30:1705660. doi: 10.1002/adma.201705660. [DOI] [PubMed] [Google Scholar]
- Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.-S., Dighe P.A., Mezera V., Monternier P.-A., Brand M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017;292:16804–16809. doi: 10.1074/jbc.R117.789271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Guo H., Wu W., Ji S., Zhao J. Organic triplet sensitizer library derived from a single chromophore (BODIPY) with long-lived triplet excited state for triplet–triplet annihilation based upconversion. J. Org. Chem. 2011;76:7056–7064. doi: 10.1021/jo200990y. [DOI] [PubMed] [Google Scholar]
- Xiao P., Xu Y. Recent progress in two-dimensional polymers for energy storage and conversion: design, synthesis, and applications. J. Mater. Chem. A. 2018;6:21676–21695. [Google Scholar]
- Xu F., Xu H., Chen X., Wu D., Wu Y., Liu H., Gu C., Fu R., Jiang D. Radical covalent organic frameworks: a general strategy to immobilize open-accessible polyradicals for high-performance capacitive energy storage. Angew. Chem. Int. Ed. 2015;54:6814–6818. doi: 10.1002/anie.201501706. [DOI] [PubMed] [Google Scholar]
- Xu H.-S., Ding S.-Y., An W.-K., Wu H., Wang W. Constructing crystalline covalent organic frameworks from chiral building blocks. J. Am. Chem. Soc. 2016;138:11489–11492. doi: 10.1021/jacs.6b07516. [DOI] [PubMed] [Google Scholar]
- Xu H., Chen X., Gao J., Lin J., Addicoat M., Irle S., Jiang D. Catalytic covalent organic frameworks via pore surface engineering. Chem. Commun. 2014;50:1292–1294. doi: 10.1039/c3cc48813f. [DOI] [PubMed] [Google Scholar]
- Xu H., Gao J., Jiang D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015;7:905–912. doi: 10.1038/nchem.2352. [DOI] [PubMed] [Google Scholar]
- Yan X., Song Y., Liu J., Zhou N., Zhang C., He L., Zhang Z., Liu Z. Two-dimensional porphyrin-based covalent organic framework: a novel platform for sensitive epidermal growth factor receptor and living cancer cell detection. Biosens. Bioelectron. 2019;126:734–742. doi: 10.1016/j.bios.2018.11.047. [DOI] [PubMed] [Google Scholar]
- Yan Y., He T., Zhao B., Qi K., Liu H., Xia B.Y. Metal/covalent–organic frameworks-based electrocatalysts for water splitting. J. Mater. Chem. A. 2018;6:15905–15926. [Google Scholar]
- Yang B., Chen Y., Shi J. Material chemistry of two-dimensional inorganic nanosheets in cancer theranostics. Chem. 2018;4:1284–1313. [Google Scholar]
- Yoon S., Rossi J.J. Aptamers: uptake mechanisms and intracellular applications. Adv. Drug Deliv. Rev. 2018;134:22–35. doi: 10.1016/j.addr.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.-C., Sun B., Cao A.-M., Wang D., Wan L.-J. Heterogeneous nucleation and growth of highly crystalline imine-linked covalent organic frameworks. Chem. Commun. 2018;54:5976–5979. doi: 10.1039/c8cc02381f. [DOI] [PubMed] [Google Scholar]
- Zhang W., Lin W., Pei Q., Hu X., Xie Z., Jing X. Redox-hypersensitive organic nanoparticles for selective treatment of cancer cells. Chem. Mater. 2016;28:4440–4446. [Google Scholar]
- Zhang C., Zhang S., Yan Y., Xia F., Huang A., Xian Y. Highly fluorescent polyimide covalent organic nanosheets as sensing probes for the detection of 2,4,6-trinitrophenol. ACS Appl. Mater. Interfaces. 2017;9:13415–13421. doi: 10.1021/acsami.6b16423. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Riduan S.N., Wang J. Redox active metal– and covalent organic frameworks for energy storage: balancing porosity and electrical conductivity. Chem. Eur. J. 2017;23:16419–16431. doi: 10.1002/chem.201702919. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wu W., Li R.-Q., Qiu W.-X., Zhuang Z.-N., Cheng S.-X., Zhang X.-Z. Peptide-based multifunctional nanomaterials for tumor imaging and therapy. Adv. Funct. Mater. 2018;28:1804492. [Google Scholar]
- Zhang G., Li X., Liao Q., Liu Y., Xi K., Huang W., Jia X. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018;9:2785. doi: 10.1038/s41467-018-04910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Yang Z., Meng X., Cao Y., Zhang Y., Dai W., Lu H., Yu Z., Dong H., Zhang X. Peroxidase-like Fe3O4 nanocomposite for activatable reactive oxygen species generation and cancer theranostics. Mater. Chem. Front. 2018;2:1184–1194. [Google Scholar]
- Zhang S., Yang Q., Wang C., Luo X., Kim J., Wang Z., Yamauchi Y. Porous organic frameworks: advanced materials in analytical chemistry. Adv. Sci. 2018;5:1801116. doi: 10.1002/advs.201801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Chen Y., Huang W., Wang Y., Hu X. A novel AuNPs-doped COFs composite as electrochemical probe for chlorogenic acid detection with enhanced sensitivity and stability. Sens. Actuators B Chem. 2018;276:362–369. [Google Scholar]
- Zhang T., Ma N., Ali A., Wei Q., Wu D., Ren X. Electrochemical ultrasensitive detection of cardiac troponin I using covalent organic frameworks for signal amplification. Biosens. Bioelectron. 2018;119:176–181. doi: 10.1016/j.bios.2018.08.020. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen J., Peng S., Peng S., Zhang Z., Tong Y., Miller P.W., Yan X.-P. Emerging porous materials in confined spaces: from chromatographic applications to flow chemistry. Chem. Soc. Rev. 2019 doi: 10.1039/c8cs00657a. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Guo L., Gándara F., Ma Y., Liu Z., Zhu C., Lyu H., Trickett C.A., Kapustin E.A., Terasaki O. A synthetic route for crystals of woven structures, uniform nanocrystals, and thin films of imine covalent organic frameworks. J. Am. Chem. Soc. 2017;139:13166–13172. doi: 10.1021/jacs.7b07457. [DOI] [PubMed] [Google Scholar]
- Zheng W., Tsang C.-S., Lee L.Y.S., Wong K.-Y. Two-dimensional metal-organic framework and covalent-organic framework: synthesis and their energy-related applications. Mater. Today Chem. 2019;12:34–60. [Google Scholar]
- Zhou J., Wang B. Emerging crystalline porous materials as a multifunctional platform for electrochemical energy storage. Chem. Soc. Rev. 2017;46:6927–6945. doi: 10.1039/c7cs00283a. [DOI] [PubMed] [Google Scholar]
- Zhou L.-L., Guan Q., Li Y.-A., Zhou Y., Xin Y.-B., Dong Y.-B. One-pot synthetic approach toward porphyrinatozinc and heavy-atom involved Zr-NMOF and its application in photodynamic therapy. Inorg. Chem. 2018;57:3169–3176. doi: 10.1021/acs.inorgchem.7b03204. [DOI] [PubMed] [Google Scholar]
- Zhou N., Ma Y., Hu B., He L., Wang S., Zhang Z., Lu S. Construction of Ce-MOF@COF hybrid nanostructure: label-free aptasensor for the ultrasensitive detection of oxytetracycline residues in aqueous solution environments. Biosens. Bioelectron. 2019;127:92–100. doi: 10.1016/j.bios.2018.12.024. [DOI] [PubMed] [Google Scholar]
- Zou J., Yin Z., Ding K., Tang Q., Li J., Si W., Shao J., Zhang Q., Huang W., Dong X. BODIPY derivatives for photodynamic therapy: influence of configuration versus heavy atom effect. ACS Appl. Mater. Interfaces. 2017;9:32475–32481. doi: 10.1021/acsami.7b07569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.