Abstract
High-density lipoprotein (HDL) confers protection against cardiovascular disease partly attributable to its robust anti-oxidant activities, which is largely impaired in diabetic conditions. In this study, we analyzed the anti-oxidant activity of HDL, as represented by the arylesterase activity of paraoxonase 1 (PON1) in HDL particles, in 216 consecutive HF patients with (n = 79) or without (n = 137) type 2 diabetes, and age- and gender-matched 112 diabetic and 189 non-diabetic non-HF controls. We found arylesterase activity was significantly decreased in patients with than without HF, and was further decreased when comorbid with diabetes. After adjusting for conventional risk factors and apolipoprotein A-I levels, arylesterase activity remained correlated positively with left ventricular ejection fraction in diabetic (r = 0.325, P = 0.020) but not non-diabetic patients (r = 0.089, P = 0.415), and negatively with NT-proBNP and NYHA functional class in both subgroups. In regression analyses, a higher risk of HF was observed in diabetic than non-diabetic patients when having low arylesterase activities. In conclusion, our data demonstrate that impaired serum arylesterase activity in patients with HF is further reduced when comorbid with diabetes. The relationship of impaired arylesterase activity to HF is especially enhanced in diabetic patients.
Introduction
Patients with diabetes mellitus are at excessive risk of morbidity and mortality of congestive heart failure (HF) than the general population1–3. Besides increased coronary dysfunction and atherosclerosis in diabetic conditions, increasing evidence suggests that mechanisms independent of the vasculature, including enhanced oxidative stress, mitochondrial dysfunction, and altered substrate metabolism within the myocardium4, play a predominant role in the development of diabetic cardiomyopathy. High-density lipoprotein (HDL) is a well-established cardiovascular protective factor characterized by its reverse cholesterol transport activity as well as robust anti-oxidant and anti-inflammatory properties5. While levels of HDL cholesterol (HDL-C), a crude measure of the heterogeneous population of HDL particles, are inversely associated with the risk of cardiovascular events6,7, therapies aimed at raising HDL-C concentration failed to improve cardiovascular outcomes in randomized, controlled trails8,9. This paradox is partly explained by the findings that HDL quality rather than quantity would be a more relevant measure in association with the risk of cardiovascular events10,11, especially under pathophysiological conditions such as diabetes12,13. Arylesterase activity, predominantly conferred by HDL-associated paranoxase-1 (PON1), is a typical functional measure reflective of HDL-associated anti-oxidant activities. As one of the most abundant anti-oxidants in the body, PON1 functions to protect low-density lipoprotein (LDL) and HDL from oxidation14. Animal studies revealed that PON1 directly reduced oxidative stress in macrophages and the aortic wall15. Overexpression of PON clusters was shown to alleviate cardiac hypertrophy in response to angiotensin II16. In patients with systolic heart failure, diminished arylesterase activity of HDL was associated with higher risk of incident long-term adverse cardiac event independent of established clinical risk factors17. In diabetic conditions, the functional activity of HDL is largely impaired, which may further aggravate the imbalance between pro-oxidant and anti-oxidant substrates within the failing myocardium. Previously, we showed that decreased paraoxonase activity was associated with the presence and severity of coronary artery disease in patients with type 2 diabetes mellitus (T2DM)18. In the present study, we analyzed the relationship of seurm arylesterase activity to systolic heart failure in T2DM patients and non-diabetic subjects.
Results
Baseline clinical characteristics and biochemical measurements
Baseline characteristics were shown in Table 1. In both diabetic and non-diabetic subjects, patients with HF had lower systolic blood pressure, poorer renal function, lower levels of apolipoprotein A-I, higher incidence of previous myocardial infarction, and more frequently taking diuretics but less frequently statins than non-HF subjects. Compared with diabetic patients without HF, those with HF tended to have higher hsCRP levels. There was no difference in the proportion of patients with CAD and atherosclerosis severity as categorized by the number of diseased vessels among each group. No significant differences between diabetic and non-diabetic patients with HF were observed for left atrial diameter, left ventricular end-diastolic or end-systolic volumes, left ventricular ejection faction (LVEF), N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, and medication of ACEI/ARB, β-adrenergic receptor blockers, diuretics, statins, and antiplatelet agents.
Table 1.
Baseline characteristics.
| Non-diabetic patients | Diabetic patients | |||||
|---|---|---|---|---|---|---|
| No HF (n = 189) | HF (n = 137) | P-value | No HF (n = 112) | HF (n = 79) | P-value | |
| Male, n (%) | 151 (79.9) | 110 (80.3) | 0.929 | 95 (84.8) | 69 (87.3) | 0.622 |
| Age, years | 64.36 ± 11.03 | 64.31 ± 10.52 | 0.969 | 62.45 ± 9.66 | 62.66 ± 9.77 | 0.890 |
| Body mass index, kg/m2 | 24.41 ± 3.14 | 23.83 ± 3.46 | 0.099 | 25.47 ± 2.72† | 24.61 ± 3.00 | 0.072 |
| Smoking, n (%) | 49 (25.9) | 33 (24.1) | 0.706 | 39 (34.8) | 23 (29.1) | 0.407 |
| Previous myocardial infarction, n (%) | 16 (8.5) | 24 (17.5) | 0.014 | 7 (6.3) | 18 (22.8) | 0.001 |
| Hypertension, n (%) | 129 (68.3) | 69 (50.4) | 0.001 | 81 (72.3) | 46 (58.2) | 0.042 |
| SBP, mmHg | 135.19 ± 19.73 | 123.46 ± 20.13 | <0.001 | 135.75 ± 20.06 | 126.13 ± 19.94 | 0.001 |
| DBP, mmHg | 74.88 ± 11.51 | 74.53 ± 13.20 | 0.793 | 77.54 ± 11.76 | 74.29 ± 12.22 | 0.069 |
| Fasting glucose, mmol/L | 4.87 ± 0.63 | 4.92 ± 0.71 | 0.769 | 6.64 ± 1.91† | 6.85 ± 2.04‡ | 0.283 |
| HbA1c, % | 5.70 (5.40–6.00) | 5.90 (5.60–6.10) | 0.001 | 7.00 (6.60–7.90)† | 7.30 (6.70–8.00) ‡ | 0.297 |
| HbA1c, mmol/mol | 38.80 (35.52–42.08) | 40.98 (37.71–43.17) | 0.001 | 53.01(48.63–62.84) | 56.28(49.73–63.93) | 0.297 |
| Triglyceride, mmol/L | 1.26 (0.95–1.76) | 1.18 (0.89–1.61) | 0.143 | 1.50 (1.02–2.09)* | 1.33 (0.87–1.71) | 0.053 |
| Total cholesterol, mmol/L | 3.94 ± 1.03 | 4.07 ± 1.08 | 0.326 | 3.88 ± 1.09 | 3.90 ± 1.17 | 0.891 |
| HDL cholesterol, mmol/L | 1.08 ± 0.27 | 1.05 ± 0.27 | 0.297 | 1.01 ± 0.25* | 0.98 ± 0.25 | 0.482 |
| LDL cholesterol, mmol/L | 2.35 ± 0.83 | 2.45 ± 0.85 | 0.287 | 2.25 ± 0.83 | 2.38 ± 0.91 | 0.283 |
| Apolipoprotein A-I, g/L | 1.28 ± 0.20 | 1.22 ± 0.21 | 0.007 | 1.25 ± 0.20 | 1.19 ± 0.21 | 0.049 |
| Apolipoprotein B, g/L | 0.79 ± 0.23 | 0.82 ± 0.23 | 0.296 | 0.79 ± 0.24 | 0.81 ± 0.25 | 0.521 |
| Lipoprotein (a), g/L | 0.15 (0.08–0.31) | 0.16 (0.08–0.31) | 0.396 | 0.14 (0.07–0.28) | 0.14 (0.07–0.30) | 0.874 |
| Uric acid, μmol/L | 363.87 ± 98.73 | 400.30 ± 122.40 | 0.003 | 325.44 ± 88.12† | 403.30 ± 123.35 | <0.001 |
| Blood urea nitrogen, mmol/L | 5.50 (4.60–6.53) | 6.60 (5.30–8.70) | <0.001 | 5.50 (4.70–6.68)† | 6.20 (5.30–8.53) | 0.002 |
| Serum creatinine, μmol/L | 84.00 (76.00–97.00) | 89.00 (77.00–101.00) | 0.084 | 81.5 (72.00–94.00)* | 91.50 (71.75–105.25) | 0.034 |
| eGFR, mL/min/1.73 m2 | 78.12 ± 16.15 | 74.34 ± 17.46 | 0.046 | 82.65 ± 17.21* | 77.33 ± 20.46 | 0.039 |
| hsCRP, mg/L | 1.14 (0.42–2.55) | 1.36 (0.58–7.35) | 0.057 | 0.89 (0.42–2.53) | 2.19 (0.53–6.76) | 0.008 |
| CAD, n (%) | 93 (49.2) | 63 (46.0) | 0.576 | 63 (56.3) | 42 (53.2) | 0.768 |
| 1-vessel | 46 (24.3) | 25 (18.2) | 0.222 | 23 (20.5) | 17 (21.5) | 0.859 |
| 2-vessel | 25 (13.2) | 17 (12.4) | 0.868 | 22 (19.6) | 8 (10.1) | 0.105 |
| 3-vessel | 22 (11.6) | 21 (15.3) | 0.407 | 18 (16.1) | 17 (21.5) | 0.349 |
| multi-vessel disease | 47 (24.9) | 38 (27.7) | 0.610 | 40 (35.7) | 25 (31.6) | 0.642 |
| NYHA functional class II/III/IV, n (%) | 70/59/8 | 35/35/9 | ||||
| NT-proBNP, pg/mL | 193.20 (110.95–267.55) | 2163.00 (887.80–3531.75) | <0.001 | 152.05 (84.13–227.83) | 1341 (701.03–3009.00) | <0.001 |
| Left atrial diameter, mm | 39.0 (36.0–42.0) | 47.0 (42.0–50.0) | <0.001 | 40.0 (38.0–43.0)* | 46.0 (43.0–49.0) | <0.001 |
| LVEDD, mm | 49.0 (47.0–53.0) | 65.0 (59.5–70.0) | <0.001 | 50.0 (47.0–53.7) | 64.00 (61.0–69.0) | <0.001 |
| LVESD, mm | 32.00 (29.0–36.0) | 54.0 (48.0–59.0) | <0.001 | 32.0 (29.0–35.0) | 53.0 (49.0–58.0) | <0.001 |
| LVEDV, mL | 117.0 (102.3–138.0) | 214.0 (177.0–257.0) | <0.001 | 118.0 (104.3–141.5) | 209.5 (184.3–263.8) | <0.001 |
| LVESV, mL | 41.0 (33.0–54.8) | 139.0 (103.5–174.0) | <0.001 | 41.0 (33.3–52.0) | 139.5 (116.0–167.5) | <0.001 |
| LVEF, % | 63.0 (60.0–67.0) | 35.0 (30.0–38.0) | <0.001 | 65.0 (60.0–69.0) | 34.0 (30.0–38.0) | <0.001 |
| Aspirin, n (%) | 134 (70.9) | 69 (50.4) | <0.001 | 72 (64.3) | 40 (50.6) | 0.059 |
| P2Y12 receptor antagonist, n (%) | 96 (50.8) | 48 (35.0) | 0.005 | 63 (56.3) | 38 (48.1) | 0.359 |
| ACE inhibitors or ARBs, n (%) | 100 (52.9) | 94 (68.6) | 0.004 | 69 (61.6) | 58 (73.4) | 0.089 |
| Beta-blockers, n (%) | 135 (71.4) | 103 (75.2) | 0.451 | 75 (67.0) | 66 (83.5) | 0.010 |
| Nitrates, n (%) | 59 (31.2) | 39 (28.5) | 0.593 | 35 (31.3) | 32 (40.5) | 0.187 |
| Statins, n (%) | 133 (70.4) | 73 (53.3) | 0.002 | 88 (78.6) | 50 (63.3) | 0.020 |
| Diuretics, n (%) | 40 (21.2) | 86 (62.8) | <0.001 | 14 (12.5) | 51 (64.6) | <0.001 |
| Oral hypoglycemic drugs, n (%) | — | — | 58 (51.8) | 33 (41.8) | 0.172 | |
| Insulin, n (%) | — | — | 16 (14.3) | 5 (6.3) | 0.083 | |
Values are given as mean ± standard deviation, median (interquartile range) or number (percentage).
HF, heart failure; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure, LDL, low-density lipoprotein; HbA1c, glycated hemoglobin A1c; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; hsCRP, high-sentivity C-reactive protein; ACE, angiotensin-converting enzyme, ARB, angiotensin II receptor blocker.
*P < 0.05, †P < 0.01, diabetic patients without HF vs. non-diabetic subjects without HF.
‡P < 0.01, diabetic patients with HF vs. non-diabetic subjects with HF.
Serum arylesterase activity in diabetic and non-diabetic HF patients
Diabetic patients tended to have lower arylesterase activity than the non-diabetic controls both in the population with (140.15 ± 45.88 vs. 166.97 ± 49.19 μmol/L/min/mL, P = 0.002) and without HF (179.44 ± 61.40 vs. 199.78 ± 70.51 μmol/L/min/mL, P = 0.005). Serum arylesterase activity were significantly decreased in both diabetic and non-diabetic patients when comorbid with HF (Fig. 1). By using two-way ANOVA, arylesterase activity was influenced both by HF (P < 0.001) and diabetes (P < 0.001), but there is no significant interaction term between heart failure and diabetes (P = 0.559, Suppl. Table 1). Among patients with HF, arylesterase activity was not influenced by aetiology of HF (ischemic vs. dilated cardiomyopathy, respectively; P = 0.881) but the presence of diabetes (P < 0.001, Suppl. Table 2).
Figure 1.
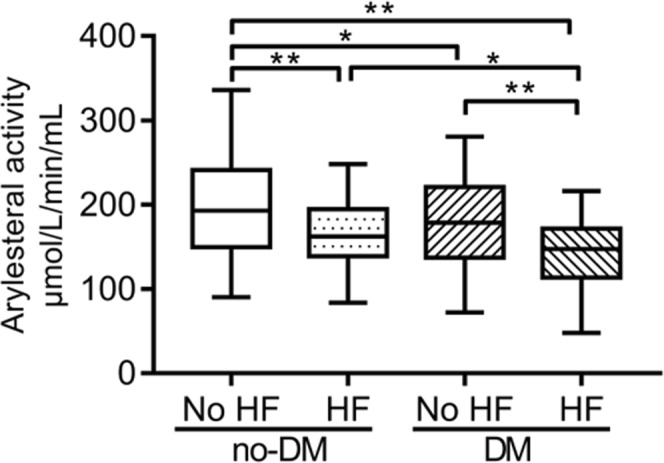
Comparison of serum arylesterase activity among diabetic and non-diabetic patients with or without HF. Diabetic patients tend to have lower arylesterase activity than the non-diabetic controls both in the population with (140.15 ± 45.88 vs. 166.97 ± 49.19 μmol/L/min/mL, P = 0.002) and without HF (179.44 ± 61.40 vs. 199.78 ± 70.51 μmol/L/min/mL, P = 0.005). Serum arylesterase activity is significantly decreased in both diabetic and non-diabetic patients when comorbid with HF. *P < 0.05; **P < 0.01.
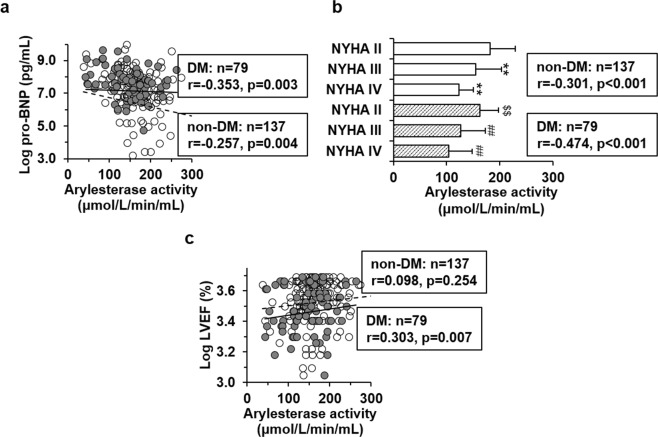
In non-diabetic patients with HF, arylesterase activity was associated inversely with ages (r = −0.371, P < 0.001), and positively with levels of HDL cholesterol (r = 0.270, P = 0.002) and apoA-I (r = 0.257, P = 0.004), all of which were attenuated in diabetic subjects (Table 2). Serum arylesterase activity was inversely correlated with log-transformed NT-proBNP levels (non-diabetes: r = −0.257, P = 0.004; diabetes: r = −0.353, P = 0.003; Fig. 2a) and NYHA functional class (non-diabetes: r = −0.301, P < 0.001; diabetes: r = −0.474, P < 0.001; Fig. 2b) both in non-diabetic and diabetic subgroups, whereas a positive correlation between arylesterase activity and log-transformed LVEF was only present in diabetic patients (r = 0.303, P = 0.007; Fig. 2c). After adjustment for confounding risk factors and apoA-I levels, these correlations remained significant (Table 2).
Table 2.
Correlation of clinical and laboratory variables with arylesterase activity in heart failure patients
| Variables correlated to serum arylesterase activity | Non-diabetic patients with HF | Diabetic patients with HF | |||
|---|---|---|---|---|---|
| Correlation coefficient | P-value | Correlation coefficient | P-value | ||
| Unadjusted | Age | −0.371 | <0.001 | −0.216 | 0.056 |
| Log HbA1c | 0.035 | 0.699 | 0.068 | 0.554 | |
| Total cholesterol | 0.171 | 0.055 | 0.212 | 0.068 | |
| HDL cholesterol | 0.270 | 0.002 | 0.111 | 0.344 | |
| ApoA-I | 0.257 | 0.004 | 0.255 | 0.028 | |
| eGFR | 0.142 | 0.100 | 0.190 | 0.096 | |
| Log NT-proBNP | −0.257 | 0.004 | −0.353 | 0.003 | |
| Log LVEF | 0.098 | 0.254 | 0.303 | 0.007 | |
| NYHA class | −0.301 | <0.001 | −0.474 | <0.001 | |
| Adjusted* | Log NT-proBNP | −0.227 | 0.035 | −0.313 | 0.025 |
| Log LVEF | 0.089 | 0.415 | 0.325 | 0.020 | |
| NYHA class | −0.451 | <0.001 | −0.554 | <0.001 | |
HF, heart failure; HbA1c, glycated hemoglobin A1c; eGFR, estimated glomerular filtration rate; hsCRP, high-sentivity C-reactive protein; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVEF, left ventricular ejection fraction.
*After adjustment for ages, sex, log transformed hsCRP, eGFR, apo A-I, history of smoking, hypertension and myocardial infarction.
Figure 2.
Correlation of serum arylesterase activity with pro-BNP, NYHA functional classes and left ventricular ejection fraction (LVEF) in patients with heart failure. Pro-BNP (a) and LVEF (c) were logarithmically transformed before plotting. Open dots and dashed line, non-diabetic subjects (n = 137); closed dots and solid line, patients with type 2 diabetes (n = 79). (b) Shown is arylesterase activity in non-diabetic (open box) and diabetic (box with diagonal lines) patients grouped by different NYHA functional classes. **p < 0.01 vs. non-diabetic patients with NYHA II functional class; ##p < 0.01 vs. diabetic patients with NYHA II functional class; $$p < 0.01 vs. non-diabetic patients with the same NYHA functional class.
In addition, ROC analysis showed potential superiority of the serum arylesterase activity in evaluating the presence and severity of CHF (NYHA class III-IV) in diabetic (AUC: 0.697, 95% CI: 0.623~0.770, P < 0.001 and AUC: 0.763, 95% CI: 0.659~0.867, P < 0.001, respectively) over non-diabetic patients (AUC: 0.643, 95% CI: 0.584~0.702, P < 0.001 and AUC: 0.652, 95% CI: 0.561~0.743, P = 0.002, respectively).
Logistic regression analyses
Next, we analyzed the association between arylesterase activity and HF by different regression models. In the univariate analysis, there was a stepwise increase in the odds ratio for HF with decreasing tertiles of arylesterase activity both in diabetic and non-diabetic subjects (p for trend both <0.001). Compared with the highest tertile, the lowest and intermediate tertiles of arylesterase activity correspond to a higher risk of HF in diabetic (7.167- and 5.584-fold, respectively) than non-diabetic patients (3.482- and 2.615-fold, respectively). After adjusting for conventional risk factors including age, sex, history of smoking, hypertension, and previous myocardial infarction (model 2), or with further adjustment for renal function and apoA-I levels (model 3) and medications (model 4), these associations persisted significant in both subgroups. Consistently, the odds ratio for HF in lower tertiles of arylesterase activity tended to be associated with higher risk of HF in diabetic than non-diabetic patients (Table 3).
Table 3.
Logistic regression analysis of association between arylesterase activity and heart failure.
| Arylesterase activity | Non-diabetic patients | Diabetic patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p for trend | Tertile 1 | Tertile 2 | Tertile 3 | p for trend | |
| Model 1a | 3.482 (1.964–6.174) | 2.615 (1.509–4.533) | 1 | <0.001 | 7.167 (2.872–17.884) | 5.584 (2.183–14.288) | 1 | <0.001 |
| Model 2b | 4.063 (2.188–7.547) | 2.960 (1.648–5.315) | 1 | <0.001 | 7.053 (2.709–18.361) | 4.876 (1.835–12.960) | 1 | <0.001 |
| Model 3c | 3.727 (1.919–7.240) | 2.939 (1.558–5.546) | 1 | <0.001 | 5.365 (1.967–14.631) | 4.236 (1.566–11.461) | 1 | 0.004 |
| Model 4d | 4.455 (2.178–9.115) | 3.637 (1.827–7.242) | 1 | <0.001 | 4.554 (1.613–12.860) | 3.846 (1.373–10.770) | 1 | 0.013 |
aUnadjusted.
bAdjusted for age, sex, history of smoking, hypertension, and previous myocardial infarction.
cModel 2 and additional adjustment for eGFR and apoA-I.
dModel 3 and additional adjustment for intake of beta-adrenergic blockers, RAAS inhibitors, and statin.
Discussion
The major findings of the present study are that impaired serum arylesterase activity in patients with HF is further reduced when comorbid with diabetes. There are no significant interaction effects between heart failure and diabetes on arylesterase activity. The relationship of impaired arylesterase activity to HF is enhanced in diabetic than non-diabetic patients.
Impaired vascular perfusion and low-grade chronic inflammation are pivotal mechanisms contributing to the progression of heart failure. In diabetic patients, the ischemic and inflammatory disorders are usually aggravated due to the presence of metabolic disturbance, diffuse vascular injury, and imbalance between pro-oxidants and anti-oxidants in the myocardium19. As a well-established pluripotent atheroprotective factor, the biological function of HDL was shown to be inversely correlated to the risk of cardiovascular disease. However, given the specificity of diabetic cardiomyopathy and markedly impaired functions of HDL in diabetes, it is noteworthy that HDL-associated proteins are subjected to substantial oxidation and glycation under diabetic conditions, which leads to protein dysfunction and the discrepancy between quantity and quality of apoA-I and its associated enzymes. Hence, the beneficial effect of HDL and its role in the risk assessment of heart failure in diabetic patients needs better characterization. In line with previous reports20, we showed that the anti-oxidant activity of HDL, as represented by PON1-associated arylesterase activity, was substantially decreased in patients with HF. Especially, the impaired arylesterase activity in diabetic population was further reduced when comorbid with HF. After adjusting for conventional risk factors including apoA-I, arylesterase activity remained significantly correlated to LVEF in diabetic but not non-diabetic patients. ROC analysis further showed that areas under curve of arylesterase activity in evaluating the presence and severity of HF tended to be larger in diabetic than non-diabetic subjects. Finally, the logistic regression analyses demonstrated that the odds ratio for HF was increased with reduced arylesterase levels both in diabetic and non-diabetic patients. Of importance, lower tertiles of arylesterase activity corresponded to a higher risk of HF in diabetic than non-diabetic patients.
Irrespective of the specific aetiology, heightened oxidative stress in diabetes aggravates perturbations of the milieu within the failing myocardium. Treatments targeting at reducing myocardial oxidative damage have provided prospective potential in attenuating cardiomyopathy in diabetic animal studies21. Transfer of human ApoA-I to increase HDL levels has effectively reduced the development of streptozotocin-induced diabetic cardiomyopathy22. Consistently, our findings suggest that impaired anti-oxidant activity of HDL is associated with, and may also contribute to the development of HF especially in diabetic settings. Paradoxically, the anti-oxidant capability of HDL, as represented by the PON1-associated arylesterase activity, is markedly impaired in diabetic conditions, which may further exacerbate the imbalance between anti-oxidant and pro-oxidant responses in the myocardium and thereby leading to HF progression.
Interestingly, our two-way ANOVA showed no significant interaction term between HF and diabetes on HDL-associated arylesterase activity, suggesting that different mechanisms may additively contribute to the markedly decreased arylesterase activity in diabetic patients with HF. HF is characterized by hemodynamic abnormalities as well as systemic and local inflammation20,23. Previously, Kim et al. found that serum amyloid A (SAA), a sensitive maker of systemic inflammation, was markedly elevated in HF patients versus controls20. Especially, a significant inverse correlation was detected between PON1 activity and SAA levels20. Increased SAA associates with HDL, thereby resulting in impairment of its anti-inflammatory properties and cholesterol efflux capacity20,24. Additionally, oxidation of HDL was shown to compromise its cholesterol efflux capacity and the stability/activity of its related enzymes25,26. Therefore, oxidative stress and inflammatory disorders are likely to be key factors underlying the compromised HDL functional capacity in the setting of HF.
On the other hand, non-enzymatic glycation of HDL plays an essential role for the altered functionality in diabetic conditions27,28. In vivo studies showed that non-enzymatic glycation of apoA-I impairs its anti-inflammatory properties and the activity of lecithin: cholesterol acyltransferase (LCAT) in mediating cholesterol esterification29,30. Previously, we reported that increased apoA-I glycation is associated with the severity of coronary artery disease and plaque progression18. Our recent study revealed that apoA-IV, another apolipoprotein within HDL particles, is also subjected to substantial glycation in diabetic patients as assessed by LC-MS analysis31. In vitro glycation of HDL appears to result in changes of apolipoprotein conformation and thereby decreased functional activity of paraoxonase. Noteworthy, Mastorikou et al. reported that glycation of PON1 is increased in patients with diabetes, and in vitro glycation of PON1 dramatically reduces its ability to metabolize membrane hydroperoxides32. In line with these reports, we showed that PON1-associated arylesterase activity was significantly reduced in diabetic patients regardless of the presence of HF. However, no association was observed between arylesterase activity and glucose or HbA1C levels in diabetic patients with HF. Thus, it is likely that hyperglycemia contributes, but not the solo determinant factor, to the impaired functional activities of PON1 in diabetic patients. Determining the influence of intensive glycemic control and other key factors on the HDL-associated anti-oxidant activities in diabetic patients is important to optimize strategies to restore HDL physiological functions in diabetic conditions.
We recognize limitations in our findings. First, the study was cross-sectional, thereby only allowing us to detect association. Due to the study design, predictions and causal inferences are impossible. Second, the overall sample size was modest and therefore the ability to definitely evaluate the association between HDL function with HF was limited. Third, pre-clinical diastolic cardiac dysfunction is common in patients with diabetes. Further studies focusing on evaluating of HDL function in diabetic patients with ejection fraction-preserved heart failure patients is warranted for the risk stratification at early stage. Fourth, the association of HDL-associated arylesterase activity and adverse cardiovascular outcomes in diabetic patients with HF awaits further investigation. Finally, PON1 possesses paraoxonase activity in addition to the arylesterase activity as measured in the present study. Unlike arlyesterase activity, no association was observed between paraoxonase activity and adverse HF outcomes33. However, the potential involvement of paraoxonase activity of PON1 in the pathogenesis of HF, especially in diabetic conditions, cannot be excluded and awaits further characterization.
Conclusions
This study provides evidence that serum arylesterase activity is reduced both in diabetic and non-diabetic patients with HF. The relationship of arylesterase activity to the presence and severity of HF is enhanced in diabetic than non-diabetic patients. Therefore, diabetic patients with HF is more likely to develop severe HF when having low serum arylesterase activity due to, at least partly, insufficient anti-oxidant capacity. Strategies aimed at restoring the serum arylesterase activity might be useful to counter the over-activated oxidative stress within the myocardium in diabetic patients.
Methods
This study was approved by the Institutional Review Board of Rui Jin Hospital, Shanghai Jiaotong University School of Medicine, and was registered (NCT02089360). Informed consent was obtained in written form from all patients and clinical investigation was conducted according to the principle of the Declaration of Helsinki.
Study population
Subjects met the inclusion criteria as follows, (1) symptomatic HF (New York Heart Association class II to IV) for at least 3 months, (2) left ventricular ejection fraction (LVEF) <40%, as documented by echocardiography, within the past 6 months as performed as part of routine care, and (3) age >18 years were enrolled consecutively from the Department of Cardiology, Rui Jin Hospital, Shanghai Jiao-Tong University School of Medicine between February 2014 and June 2016. At last a total of 216 stable systolic HF patients with (n = 79) or without (n = 137) T2DM were enrolled. Another gender- and age-matched 189 non-diabetic and 112 patients with T2DM without HF underwent angiography not in the setting of acute coronary syndrome in Rui Jin hospital during the same period were recruited as respective controls. T2DM was defined according to the American Diabetes Association criteria34 as: a fasting plasma glucose levels ≥7.0 mmol/L, or 2-h postprandial plasma glucose readings ≥11.1 mmol/L by multiple determinations, or taking oral hypoglycemic drugs or parenteral insulin. Patients with acute coronary syndrome, primary valvular heart disease, pulmonary heart disease, a history of viral myocarditis, type 1 diabetes, chronic viral or bacterial infections, tumors or immune disorders were excluded. Detailed information of all the patients was obtained on demographics, clinical manifestation, medications, and LVEF determined by echocardiography.
Biochemical measurements
Peripheral venous blood samples of the patients were collected after an overnight fast after admission to the hospital. Serum glucose, blood urea nitrogen, creatinine, uric acid, triglyceride, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, lipoprotein (a), apolipoprotein (apo) A-I, and apo B were measured using standard laboratory techniques on a Hitachi 912 Analyzer (Roche Diagnostics, Mannheim, Germany). The estimated glomerular filtration rate (eGFR) was computed using the Chronic Kidney Disease Epidemiology Collaboration equation35. Blood HbA1c concentration was measured using ion-exchange high performance liquid chromatography with Bio-rad Variant Hemoglobin Testing System (Bio-Rad Laboratories, USA). Serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) was determined using a commercially available electrochemiluminescence immunoassay kit (Roche Diagnostics). Serum levels of high-sentivity C-reactive protein (hsCRP) were determined by ELISA (Biocheck Laboratories, Toledo, OH, USA).
Measurement of serum PON1 arylesterase activity
PON1 arylesterase activity was analyzed from peripheral venous blood samples collected after an overnight fast after admission to the hospital using a photometric assay with phenyl acetate (cat# 108723, Sigma-Aldrich, St Louis, Mo) as the substrate, as previously described18. Briefly, initial hydrolysis rates were determined at 270 nm in 100-fold diluted serum (final) in reaction mixtures composed of 10 mmol/L phenyl acetate, 100 mmol/L Tris hydrochloride, pH = 8, and 2 mmol/L calcium chloride at 37 °C. An extinction coefficient (at 270 nm) of 1310/M/cm was used for calculating units of arylesterase activity. PON1 arylesterase activity was monitored in triplicates and the results are presented as μmol/L/min per mL.
Statistical analyses
Data are expressed as mean ± standard deviation (SD) or median (inter-quartile range) for continuous variables, and frequencies (percentages) for categorical ones. For continuous variables, the existence of normal distribution was ascertained by the Kolmogorov–Smirnov test. Data that were not normally distributed were logarithmically transformed for HbA1c, NT-proBNP, LVEF, hsCRP before analyses were made. Differences among groups were analyzed by one-way analysis of variance (ANOVA) or the Kruskal-Wallis analysis followed by post hoc test with Fisher’s Least Significant Difference (LSD) correction. The Chi-square test was used to compare nominal scale variables. Pearson’s and Spearman’s correlation tests were used to assess the relation between variables. Receiver-operating characteristic (ROC) analyses were used to determine the power of arylesterase activity for detecting the presence or severity of HF and the areas under the curve were compared using the DeLong method36.
Different models were established in the regression analysis to evaluate the association between different tertiles of serum arylesterase activity and HF in non-diabetic and diabetic subjects. In model 1, univariate analysis was performed. In model 2, the association was adjusted for conventional risk factors including age, sex, history of smoking, hypertension, and previous myocardial infarction. In model 3, a further adjustment was made for eGFR and apoA-I levels. In model 4, medication status was further adjusted.
All statistical analyses were performed using the SPSS 23.0 for Windows (SPSS, Inc., Chicago, IL, USA). A P value < 0.05 was considered statistically significant for all tests including multiple comparisons after LSD correction.
Supplementary information
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant No. 81670451, 81470469, 81770430, 81870179), Shanghai Rising-Star Program (Grant No. 17QA1403000), Shanghai Municipal Commission of Health and Family Planning (Grant No. 2018YQ17).
Author Contributions
C.L., X.Q.W. and L.L. contributed to all stages including conception and design of the study, as well as acquisition, analysis and interpretation of the data. C.L. contributed to drafting the article and X.Q.W. and L.L. participating in revising and final approval of the version to be submitted. J.W.C., Z.H.L. and F.W. collected data and contributed reagents, materials and analysis tools. F.H.D., S.Y., R.Y.Z. and W.F.S. contributed to the data acquisition, analysis, writing of the manuscript and revised it critically for important intellectual content.
Data Availability
The study protocol, datasets and statistical code for the current study are available from the corresponding authors on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chang Li and Jia Wei Chen contributed equally.
Contributor Information
Lin Lu, Email: rjlulin1965@163.com.
Xiao Qun Wang, Email: Xiaoqun_Wang@hotmail.com.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42518-x.
References
- 1.Iribarren C, et al. Glycemic Control and Heart Failure Among Adult Patients With Diabetes. Circulation. 2001;103:2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 2.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. Journal of the American College of Cardiology. 2010;55:300–305. doi: 10.1016/j.jacc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/circulationaha.106.679597. [DOI] [PubMed] [Google Scholar]
- 5.Stone NJ, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 6.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. The American journal of medicine. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 7.Boekholdt SM, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128:1504–1512. doi: 10.1161/circulationaha.113.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barter P, et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GG, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. The New England journal of medicine. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 10.Woudberg NJ, et al. Pharmacological Intervention to Modulate HDL: What Do We Target? Frontiers in pharmacology. 2017;8:989. doi: 10.3389/fphar.2017.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaefer EJ, Anthanont P, Asztalos BF. High-density lipoprotein metabolism, composition, function, and deficiency. Current opinion in lipidology. 2014;25:194–199. doi: 10.1097/mol.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Femlak M, Gluba-Brzozka A, Cialkowska-Rysz A, Rysz J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids in health and disease. 2017;16:207. doi: 10.1186/s12944-017-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava RAK. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Molecular and cellular biochemistry. 2017 doi: 10.1007/s11010-017-3165-z. [DOI] [PubMed] [Google Scholar]
- 14.Watson AD, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. The Journal of clinical investigation. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozenberg O, Shih DM, Aviram M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis. 2005;181:9–18. doi: 10.1016/j.atherosclerosis.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Pei JF, et al. Human paraoxonase gene cluster overexpression alleviates angiotensin II-induced cardiac hypertrophy in mice. Science China. Life sciences. 2016;59:1115–1122. doi: 10.1007/s11427-016-0131-4. [DOI] [PubMed] [Google Scholar]
- 17.Tang WH, et al. Diminished antioxidant activity of high-density lipoprotein-associated proteins in systolic heart failure. Circulation. Heart failure. 2011;4:59–64. doi: 10.1161/circheartfailure.110.958348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y, et al. Association of elevated apoA-I glycation and reduced HDL-associated paraoxonase1, 3 activity, and their interaction with angiographic severity of coronary artery disease in patients with type 2 diabetes mellitus. Cardiovascular diabetology. 2015;14:52. doi: 10.1186/s12933-015-0221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Rosa S, et al. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic. Links. Frontiers in endocrinology. 2018;9:2. doi: 10.3389/fendo.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JB, et al. Heart failure is associated with impaired anti-inflammatory and antioxidant properties of high-density lipoproteins. The American journal of cardiology. 2013;112:1770–1777. doi: 10.1016/j.amjcard.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142:375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Van Linthout S, et al. Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation. 2008;117:1563–1573. doi: 10.1161/circulationaha.107.710830. [DOI] [PubMed] [Google Scholar]
- 23.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. The New England journal of medicine. 1999;341:577–585. doi: 10.1056/nejm199908193410806. [DOI] [PubMed] [Google Scholar]
- 24.Han CY, et al. Serum amyloid A impairs the antiinflammatory properties of HDL. The Journal of clinical investigation. 2016;126:266–281. doi: 10.1172/JCI83475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rifici VA, Khachadurian AK. Oxidation of high density lipoproteins: characterization and effects on cholesterol efflux from J774 macrophages. Biochimica et biophysica acta. 1996;1299:87–94. doi: 10.1016/0005-2760(95)00198-0. [DOI] [PubMed] [Google Scholar]
- 26.Deakin S, Moren X, James RW. HDL oxidation compromises its influence on paraoxonase-1 secretion and its capacity to modulate enzyme activity. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1146–1152. doi: 10.1161/ATVBAHA.107.141747. [DOI] [PubMed] [Google Scholar]
- 27.Ferretti G, Bacchetti T, Marchionni C, Caldarelli L, Curatola G. Effect of glycation of high density lipoproteins on their physicochemical properties and on paraoxonase activity. Acta diabetologica. 2001;38:163–169. doi: 10.1007/s592-001-8074-z. [DOI] [PubMed] [Google Scholar]
- 28.Hedrick CC, et al. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43:312–320. doi: 10.1007/s001250050049. [DOI] [PubMed] [Google Scholar]
- 29.Nobecourt E, et al. Nonenzymatic glycation impairs the antiinflammatory properties of apolipoprotein A-I. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:766–772. doi: 10.1161/atvbaha.109.201715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calvo C, Ulloa N, Del Pozo R, Verdugo C. Decreased activation of lecithin:cholesterol acyltransferase by glycated apolipoprotein A-I. European journal of clinical chemistry and clinical biochemistry: journal of the Forum of European Clinical Chemistry Societies. 1993;31:217–220. doi: 10.1515/cclm.1993.31.4.217. [DOI] [PubMed] [Google Scholar]
- 31.Dai Y, et al. Glycated Apolipoprotein A-IV Induces Atherogenesis in Patients With CAD in Type 2 Diabetes. Journal of the American College of Cardiology. 2017;70:2006–2019. doi: 10.1016/j.jacc.2017.08.053. [DOI] [PubMed] [Google Scholar]
- 32.Mastorikou M, Mackness B, Liu Y, Mackness M. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolize membrane lipid hydroperoxides. Diabetic medicine: a journal of the British Diabetic Association. 2008;25:1049–1055. doi: 10.1111/j.1464-5491.2008.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammadah M, et al. High-density lipoprotein-associated paraoxonase-1 activity for prediction of adverse outcomes in outpatients with chronic heart failure. European journal of heart failure. 2017;19:748–755. doi: 10.1002/ejhf.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Diabetes, A. Standards of medical care in diabetes–2012. Diabetes care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol, datasets and statistical code for the current study are available from the corresponding authors on reasonable request.