Abstract
Hand preference is a prominent behavioural trait linked to human brain asymmetry. A handful of genetic variants have been reported to associate with hand preference or quantitative measures related to it. Most of these reports were on the basis of limited sample sizes, by current standards for genetic analysis of complex traits. Here we performed a genome-wide association analysis of hand preference in the large, population-based UK Biobank cohort (N = 331,037). We used gene-set enrichment analysis to investigate whether genes involved in visceral asymmetry are particularly relevant to hand preference, following one previous report. We found no evidence supporting any of the previously suggested variants or genes, nor that genes involved in visceral laterality have a role in hand preference. It remains possible that some of the previously reported genes or pathways are relevant to hand preference as assessed in other ways, or else are relevant within specific disorder populations. However, some or all of the earlier findings are likely to be false positives, and none of them appear relevant to hand preference as defined categorically in the general population. Our analysis did produce a small number of novel, significant associations, including one implicating the microtubule-associated gene MAP2 in handedness.
Introduction
Hand preference is a conspicuous behavioural trait, with about 10% of people preferring to use their left hand for many tasks, about 1% having no preference, and the large majority preferring to use the right hand1. Hand preference is probably initiated during prenatal phases, and further established in early infancy2–5. Gene expression analysis has revealed left-right differences in the human central nervous system as early as four weeks post conception6,7, which indicate that laterality is an innate and pervasive property of the central nervous system. Across different regions of the world and throughout history, the behavioural variation has persisted, albeit that cultural suppression of left-handedness has sometimes occurred8–11.
There has been much interest in possible causes of hand preference, as well as its associations with cognitive variation and disorders12–15. The existence of a genetic component for hand preference has been investigated in large twin studies, consisting of thousands of individuals. These studies found additive heritabilities of around 25% for left-hand preference16–19. In terms of individual genetic loci, monogenic models for hand preference were originally conceived, such as the dextral/chance model20. The model proposed that there are two alleles, D (dextral) and C (chance), with the homozygous DD genotype producing right-handers (directional asymmetry), the homozygous CC genotype producing random asymmetry (50% right-handers and 50% left-handers) due to a hypothesized loss of direction-setting in brain development, and the heterozygote, DC, being intermediate and producing 25% left-handers and 75% right-handers. Another related idea, conceived with respect to continuous measures of left-versus-right hand motor skill, was the ‘right-shift’ model, which proposed a major gene whose alleles cause either rightward mean asymmetry, or no directional bias21.
However, genetic linkage analyses in families with elevated numbers of left-handed members have not found convincing evidence for single major genes22–24. Genome-wide association scan (GWAS) analysis has also not found any genomic locus with a large enough effect on hand preference to be compatible with monogenic models, unless there would be extensive genetic heterogeneity (i.e. many different Mendelian effects involved in the population)25. Meanwhile, as analyses based on twins have indicated 25% heritability, there is clearly ample room for environmental influences, although these remain mostly unknown. Early life variables such as birthweight and twinning can influence handedness, but not to an extent which is remotely predictive at the individual level26,27.
Various molecular genetic studies have identified individual genes as potential contributing factors to handedness, with small effects, which would be consistent with a polygenic architecture underlying the heritable component to this trait. We review these genes briefly here:
A sib-pair analysis (N = 89 sibships with reading disability) found suggestive linkage of left versus right relative hand skill, a continuous measure based on the pegboard task28, to a broad region on chromosomes 229. A follow-up study suggested the signal came from paternal inheritance30. Further analysis suggested LRRTM1 (leucine rich repeat transmembrane neuronal 1) as the most likely gene, as it showed evidence for paternal-specific association with relative hand skill of a particular haplotype, as well as imprinted expression in cell lines31.
A CAG-repeat polymorphism in the gene AR (androgen receptor), located on chromosome X, has been studied in relation to hand preference, due to a longstanding theory that brain laterality is influenced by sex hormones32. One study found association effects which were opposite in males and females for the CAG-repeat33. A different study found that right-handedness was associated with shorter CAG repeats in both sexes34, while another, which only included males, found that mixed-handedness was associated with longer CAG repeats35.
In a set of families affected by bipolar disorder, an association was found of a variant in COMT (catechol-O-methyltransferase) with relative hand skill, although this did not survive multiple testing correction36. COMT was studied because it was considered a candidate gene for bipolar disorder37.
A study of the genes SETDB1 (SET domain bifurcated 1) and SETDB2 (SET domain bifurcated 2) found that the single nucleotide polymorphism (SNP) rs4942830 in SETDB2 was significantly associated with hand preference as measured as a quantitative trait on the Edinburgh scale38 (N = 950 healthy people), although for the binary trait of left versus right-handedness, the p-value did not survive multiple testing correction39. This result was replicated, including the stronger effect for the quantitative trait (right = 732, left = 75, healthy people)40. SETDB2 was chosen as a candidate for study as it has been shown to regulate structural left-right asymmetry in the zebrafish central nervous system41.
A GWAS study of relative hand skill, in a sample of people with reading problems (3-stage design, N = 728), found a genome-wide significant association in the gene PCSK6 (proprotein convertase subtilisin/kexin type 6)42. The same research group performed a second GWAS in a cohort of individuals from the general population (N = 2666), and found no genome-wide significant associations, and no indication of involvement of PCSK643. The GWAS results also yielded evidence that genes influencing visceral asymmetry might be involved in relative hand skill in both the dyslexic group and the general population group. For this analysis, the authors tested sets of genes for an enrichment of association signals in the GWAS data, where the sets were defined based on mutations in mice that influence the organization of the visceral organs on the left-right axis43.
In a GWAS study of 263 left vs 2092 right-handers, and 173 left vs 1412 right-handers (this was a study of healthy twins, and the GWAS analysis was divided to achieve non-relatedness of subjects), no genome-wide significant results for self-reported hand preference were found25. The most significant associations were near the neighbouring genes SCN11A/TTC21A/WDR48 (sodium voltage-gated channel alpha subunit 11/tetratricopeptide repeat domain 21 A/WD repeat domain 48), and AK3/RCL1 (adenylate kinase 3/RNA terminal phosphate cyclase like 1) with p < 1E-6, i.e. a suggestive level of association which does not survive statistical correction for multiple testing over the whole genome.
A GWAS of 4268 individuals from the general population, using a questionnaire-based definition of handedness, found no association p-values below 5E-06 in the genome44.
It has become increasingly clear during the last decade that psychiatric, cognitive and behavioural traits are usually influenced by many genetic factors with very small effects of each individual locus, so that tens of thousands of subjects are needed to detect effects reliably45. None of the studies listed above approached that level of sample size or statistical power. Low power reduces the likelihood that a statistically significant result reflects a true effect46. Most of the studies summarized above made secondary use of datasets which were collected for different purposes, so that left-handedness was present at only roughly 10% within the datasets. This meant that statistical power was even less than might have been achieved in such sample sizes, for example when selection schemes balanced for handedness had been used. Given the statistical issues affecting the earlier studies, it seems possible, from a contemporary perspective on statistical genetics, that most or all of the findings were false positives.
Some of the genetic associations summarized above were identified in groups with specific disorders, including children with reading disability29,42, and families with bipolar disorder36. Therefore, even if the genetic associations were real, they may not extrapolate to the general population. Finally, a variety of different measures related to handedness were used in these studies, including binary traits based on simple questions such as ‘which hand do you write with?’, to quantitative indices based on the peg board test.
An opportunity arose recently to perform a high-powered GWAS of hand preference in a very large, adult population sample from the UK, known as the UK Biobank dataset47. This dataset comprises more than 300,000 subjects with handedness information, assessed by questionnaire (see below). SNP-based heritability analysis, based on variation over all of the autosomes (i.e. all chromosomes minus the sex chromosomes) arrived at an explained variance (SNP-heritability) for left-handedness of only 1.8% in the UK Biobank (p = 6.4e-03), which is markedly lower than previous indications from twin studies (see above), while ambidexterity had an estimated SNP-heritability of 6.8% (p = 8.7e-04)48.
Here we performed GWAS for hand preference in this large and well powered dataset, to identify novel loci associated with this trait, and to investigate whether any of the previously reported loci, summarized above, show evidence for association with binary trait handedness in the general adult population. We also applied different methods of gene-set enrichment analysis to the GWAS results, in order to test the relevance for hand preference of gene-sets that are involved in laterality of the visceral organs.
Methods
Data
We used the data collected in the UK Biobank47 and provided to Clyde Francks as part of research application 16066. Signed informed consent was obtained from all participants, and the UK Biobank has ethical approval from the UK Biobank Research Ethics Committee under number 16/NW/0274. All methods were performed in accordance with the relevant guidelines and regulations. For descriptions of all traits in the UK Biobank cohort, see http://biobank.ctsu.ox.ac.uk/crystal/search.cgi.
The phenotype 1707 ‘Handedness” was based on a touchscreen question “Are you right or left handed?”, with four answering options: ‘right-handed’, ‘left-handed’, ‘use both right and left hands equally’, and ‘prefer not to say’. We merged ‘right-handed’ and ‘use both right and left hands equally’ into the category ‘non-left-handed’ to create a dichotomous phenotype. ‘Prefer not to say’ was treated as missing data.
In a previous study of the UK Biobank dataset we modelled various early life factors with respect to left-handedness26. For the purposes of the present study, some of these variables were relevant to use as covariates when performing genetic association analysis: sex (trait 31), year of birth (trait 34), country of birth (trait 1647), and being part of a multiple birth (trait 1777). Descriptive statistics for these variables, their distributions, and their relations and effect sizes with respect to handedness, are available in that earlier reference26. The participants are older than the general population (birth years between 1934 and 1971), and females are slightly over-represented (54%)26. Although the traits ‘birthweight’ (trait 20022) and ‘being breastfed’ (trait 1677) also showed association with hand preference in our earlier study26, we did not include them in the present study as covariates, as they have significant SNP-heritabilities of their own in the UK Biobank dataset (https://nealelab.github.io/UKBB_ldsc/). In this case, some genetic loci may conceivably influence both handedness and one of these traits, while we wished to detect any genetic influences on hand preference in our GWAS without correcting for shared genetic effects.
In addition, as covariates in genetic association analysis we used the first 20 principal components which describe genome-wide diversity within the dataset, as provided by the UK Biobank49, in order to control for population stratification. We restricted the analysis to participants with British ancestry, as reported before49. Individuals from this restricted dataset were selected for the present study when they had data for the handedness phenotype and covariates used. Within this subset, we excluded randomly one individual from each pair of participants whose genetic relatedness was inferred to be 3rd degree or closer, on the basis of genome-wide genotype data, as previously calculated49, individuals were removed when there was a mismatch of their reported and genetically inferred sex, putative aneuploidy of the sex chromosomes, excessively high genome-wide heterozygosity (>0.19) or genotype missingness (missing rate >0.05). The total number of participants remaining was N = 331,037: left-handed = 31,856, non-left-handed = 299,181, the latter consisting of right-handed = 293,857 and ambidextrous = 5,324.
Imputed genotype data49 were available as dosage data for ~97 million SNPs per individual. QCtool (version: 2.0.1, revision 872b463, http://www.well.ox.ac.uk/~gav/qctool_v2/index.html) was used to assess SNP quality, and SNPs were retained if in the selected subsample the imputation ‘info’ score was >0.7, the minor allele frequency (MAF) was >0.001, and the P value for the Hardy-Weinberg equilibrium (HWE) test was >1E-07. This left ~15 million SNPs.
Genome-wide association analysis
GWAS analysis was run using linear regression assuming an additive genetic model, with BGENIE v1.249. We applied the widely-used threshold P value of 5E-08 to assign significance in the context of genome-wide multiple testing, which accounts for the number of SNPs tested in a modern GWAS study, and the correlation structure between SNPs in European ancestry populations50,51. The GWAS for left-handed versus non-left-handed was our primary analysis, the results of which we used for downstream analyses at the whole gene level and gene-set level (below). However, in addition, we ran GWAS for the trait right-handed vs non-right-handed and ambidextrous vs non-ambidextrous, for look-ups of previously reported individual SNPs (see Introduction).
When we ran GWAS for ‘ambidextrous’ versus ‘non-ambidextrous’, due to the much smaller size of the ambidextrous group than the other handedness groups, we raised the MAF threshold to 0.01.
In addition, we applied LDSC52 version 1.0.0 to the results of the GWAS of left-handed versus non-left-handed participants, to investigate the evidence for a polygenic component to the trait. This analysis was based on 1,171,358 SNPs which remained after pruning for LD following default settings, based on the reference panel eur_w_ld_chr, as provided with the software.
Previously implicated SNPs and genes
We checked the GWAS catalog (http://www.ebi.ac.uk/gwas/search, 3 July 2018) for the terms ‘handedness’ and ‘hand’ to find SNPs and genes previously implicated in traits related to hand preference. In addition, we checked the literature through Pubmed (July 2018) for ((‘hand preference’ OR ‘hand skill’ OR ‘handedness’) AND genetic) to identify SNPs or genes found in non-GWAS studies (such as candidate gene studies). We also checked references in the papers we found. The results of this search are summarized in the Introduction.
In the results of our GWAS for hand preference in the UK Biobank, we first checked the p-values for association, for each of the specific SNPs reported in the previous papers (Table 1). We also generated association scores for each individual gene implicated in the previous papers, by use of the Magma program as implemented in the FUMA package v1.3.3c (see Supp. Table 1 for specific settings)53 (November 2018). Each gene-based score summarizes the overall level of association at all SNPs spanning a given gene, into one single score and generates a corresponding P value for that entire gene.
Table 1.
Significance of association with hand preference measures in the UK Biobank, for SNPs and genes previously implicated in the literature.
| Gene | CHR | Start | End | SNP | p-genEa | P-SNP lefta | p-SNP rightb | P-SNP ambic | Ref |
|---|---|---|---|---|---|---|---|---|---|
| LRRTM1 | 2 | 80515483 | 80531874 | rs1007371 | 0.42 | 0.81 | 0.74 | 0.80 | 31 |
| PCSK6 | 15 | 101840818 | 102065405 | rs7182874 | 0.22 | 0.97 | 0.90 | 0.69 | 43 |
| COMT | 22 | 19929130 | 19957498 | rs4680 | 0.78 | 0.95 | 0.89 | 0.64 | 36 |
| SETDB2 | 13 | 50018429 | 50069138 | rs4942830 | 0.52 | 0.45 | 0.94 | 0.11 | 39 |
| SCN11A | 3 | 38887260 | 38992052 | rs883565 | 0.80 | 0.14 | 0.09 | 0.47 | 25 |
| AK3 | 9 | 4711155 | 4742043 | rs296859 | 0.77 | 0.98 | 0.92 | 0.76 | 25 |
| AR | X | 66764465 | 66950461 | micro-satellitesd | 0.76 | — | — | — | 33 |
Notes: Gene-level association is shown for the left-handed versus non-left-handed phenotype, and SNP level association for all three hand preference contrasts. START and END are according to the reference genome build 37 (see methods). P values are not adjusted for multiple testing, as they are all anyway greater than 0.05. aUsing GWAS of left-handed vs non-left-handed. bUsing GWAS of right-handed vs non-right-handed. cUsing GWAS of ambidextrous vs non-ambidextrous. dThe original study made use of a microsatellite repeat polymorphism which was not genotyped or imputed in the UK Biobank dataset.
Gene-sets involved in visceral laterality
To test for the involvement of genes associated with visceral asymmetry in relative hand skill, Brandler et al.43 had selected a number of mouse phenotypes related to visceral asymmetry (see Introduction), such as ‘dextrocardia’ and ‘situs inversus’, from all those available in the Mouse Genome Informatics (MGI) resource (http://www.informatics.jax.org/). In this database, mutations in specific genes have been linked to resulting phenotypes, such that each phenotype has a set of individual genes annotated to it. The exact criteria by which Brandler et al. selected their specific phenotypes for analysis were not described. In addition, new relevant mouse laterality phenotypes, and new associated genes, may have been added since 2013. We therefore made an up-to-date selection of phenotypes related to visceral asymmetry in the MGI, in the following way: In the hierarchical tree for mammalian phenotypes (http://www.informatics.jax.org/vocab/mp_ontology, MGI 6.12, last updated 06/19/2018), we searched for ‘heterotaxy’, ‘situs inversus’, ‘isomerism’ and ‘dextrocardia’, and also included all child terms if they also related to asymmetry phenotypes. We only included terms with at least 15 genes. This approach resulted in a list of 11 mouse phenotypes (See Results). The mouse genes were then mapped to corresponding human genes, using homologene.data (v68, May 2014) from ftp://ftp.ncbi.nih.gov/pub/HomoloGene/current, by matching mouse genes with human genes that had the same HID (HomoloGene group identifier). The list of phenotypes and the associated human genes can be found in Supplementary Table 2.
In the Online Mendelian Inheritance in Man database OMIM (https://omim.org/, last updated on 26 Jun 2018), we also identified all genes in the human phenotypic series ‘Primary Ciliary Dyskinesia’ (MIM #244400), because half of the patients with this disorder present with situs inversus. These genes were used to form one additional gene-set, thus in this study we tested a total of twelve sets, eleven from the mouse database and one based directly on human genetics (See Results).
Seven of our mouse phenotypes were among those previously analysed by Brandler et al. (Supp. Table 3). We also repeated our analyses using the exact same list of mouse phenotypes used by Brandler et al. (Supp. Table 3). However, the gene lists associated with each mouse phenotype may have changed since Brandler et al. performed their study five years ago, as new information on genes affecting visceral asymmetry can have been incorporated.
Gene-set enrichment analysis
We ran three different gene-set enrichment algorithms that have been developed for application to GWAS results. Brandler et al., in their investigation of gene-sets involved in visceral laterality, had used MAGENTA54. For consistency we also used MAGENTA (v2, 21 July 2011), plus two newer algorithms called Magma (55, v1.06) and Pascal (56, no version number). The three enrichment algorithms work in different ways, but essentially they first assign a single association score to each gene based on the GWAS data, and then test whether the genes in a given set show, on average, more evidence for association with the trait in question than the rest of the genes in the genome for which scores could be calculated (hence these are sometimes called ‘competitive’ analyses)57, while accounting for non-independence of SNPs due to linkage disequilibrium. For Magma and Pascal, we used default settings, while for MAGENTA we chose the settings as used by Brandler et al. (parameter settings for all analyses can be found in Supp. Table 1). Pascal and Magma offered the option to calculate gene-scores based on the single most associated SNP within a gene (the ‘max’ or ‘top’ option), or on a combination of SNPs within the gene (the ‘sum’ option), and we ran both approaches with both softwares, for a robust assessment of whether the previously implicated gene-sets in the literature might affect handedness in the UK Biobank.
Results
Candidate SNPs, genes and pathways from the previous literature
None of the SNPs previously reported in the literature (see Introduction) had even a nominally significant effect on left-handedness in the UK Biobank dataset (Table 1), i.e. no P values were less than 0.05. Nor was there any effect at the whole-gene-level, for any of the previously reported genes (Table 1). Also for the trait definitions of ‘ambidextrous vs non-ambidextrous and ‘right-handed vs non-right-handed’, none of the previously claimed SNPs showed significant association in the UK Biobank dataset (Table 1).
The gene-set enrichment analyses did not convincingly support the proposition that sets related to visceral asymmetry are particularly associated with left-handedness (Table 2, Supp. Table 3). There was one potential enrichment involving the gene-set ‘right-sided isomerism’ in the analysis with MAGENTA, but this was not supported by either of the other methods.
Table 2.
Gene-set enrichment results for visceral asymmetry-related gene-sets, based on the UK Biobank GWAS for left-handedness versus non-left-handedness
| mouse ID | Description | Pascal sum P | Pascal max P | Magma sum P | Magma max P | Magenta P |
|---|---|---|---|---|---|---|
| MP:0000508 | right-sided isomerism | 0.65 | 0.83 | 0.98 | 0.46 | 0.006a |
| MP:0000531 | right pulmonary isomerism | 0.64 | 0.68 | 0.97 | 0.76 | 0.06 |
| MP:0000542 | left-sided isomerism | 0.77 | 0.59 | 0.52 | 0.73 | 0.10 |
| MP:0000644 | Dextrocardia | 0.68 | 0.87 | 0.85 | 0.84 | 0.23 |
| MP:0001706 | abnormal left-right axis patterning | 0.93 | 0.84 | 0.67 | 0.93 | 0.41 |
| MP:0002766 | situs inversus | 0.40 | 0.51 | 0.70 | 1.00 | 0.49 |
| MP:0003178 | left pulmonary isomerism | 0.70 | 0.52 | 0.55 | 0.90 | 0.13 |
| MP:0004133 | Heterotaxia | 0.80 | 0.89 | 0.16 | 0.99 | 0.35 |
| MP:0010808 | right-sided stomach | 0.34 | 0.72 | 0.86 | 0.95 | 0.17 |
| MP:0011250 | abdominal situs ambiguus | 0.08 | 0.62 | 0.70 | 0.94 | 0.31 |
| MP:0011252 | situs inversus totalis | 0.23 | 0.67 | 0.71 | 0.99 | 0.44 |
| PCD | human primary ciliary dyskinesia | 0.33 | 0.31 | 0.84 | 0.70 | 0.30 |
Notes: Different softwares and settings were used. See Supplementary Table 1 for the settings. The uncorrected P values for each gene-set and enrichment analysis are shown. aFDR = 0.035.
Novel findings arising from the GWAS
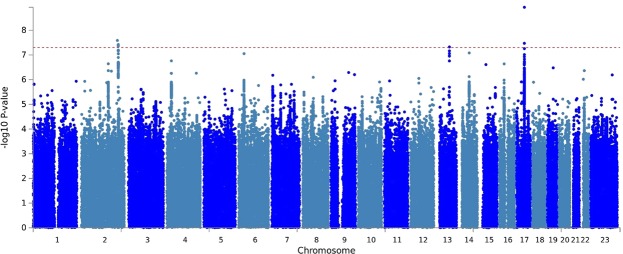
In the GWAS for left-handedness vs non-left-handedness (Fig. 1), we found the following significant associations, with P values below the threshold 5E-08 for correcting for multiple testing over the whole genome: on chromosome 2q34 the SNP rs142367408 (p = 3.8E-08), located 28 kilobases upstream of the gene MAP2 (Microtubule associated protein 2); on chromosome 17q21 the SNP rs144216645 (p = 1.2E-09) within a large inversion haploblock spanning the genes STH, KANSL1, ARL17B, ARL17A, LRRC37A, LRRC37A2 and NSF; on chromosome 13q22 the SNP rs11454570 (p = 4.7E-08) within an intron of the non-coding RNA-gene LINC00381.
Figure 1.
Manhattan plot of GWAS results for left-handed vs non-left-handed people in the UK Biobank sample. The dashed line marks the genome-wide significance threshold of p = 5 × 10−8.
The results for right-handed vs non-right-handed people were similar to those for left-handed versus non-left-handed (Supp. Fig. S1), which was expected as only the relatively small group of ambidextrous people had moved between groups. For right-handed versus non-right-handed, the region around MAP2 on chromosome 2 did not reach genome-wide significance. Instead, the major histocompatibility complex region on chromosome 6p21 showed significant associations. The most associated SNP was rs9366770 (p = 2.2E-09), located in an exon of the non-coding RNA gene HCG27. The inversion region on chromosome 17 was also associated with the right-handed versus non-right-handed phenotype, again most significantly with the SNP rs144216645 (p = 2.3E-08). An additional association on chromosome Xq25 was detected with the SNP rs767669906 (p = 1.0E-08), located 44 kilobases downstream of the gene DCAF12L1. However, with a minor allele frequency of 0.0035, this signal may not be reliable.
Zoomed in figures showing the significantly associated regions can be found in Figs S2–S5.
The GWAS for ‘ambidextrous’ vs ‘non-ambidextrous’ showed no significant associations.
QQ plots from the GWAS analyses are shown in Figs S6–S8. Analysis with LDSC indicated a SNP-based heritability for left-handedness h2 = 0.0251 (s.e = 0.0033; liability scale). This analysis also indicated that 72% (95CI 0.59–0.84) of the inflation which is observable in the QQ plot for the left-handed versus non-left-handed GWAS (Fig. S6) was due to a polygenic component, including the top associations, rather than due to unaccounted population structure (λGC = 1.10, mean Χ2 = 1.114, intercept = 1.0324).
Discussion
In a very large dataset comprising hundreds of thousands of adult subjects from the general population, we found no evidence for an involvement in hand preference of any previously claimed genetic variants, genes, or gene-sets from the literature. We consider the most likely explanation to be that the earlier findings were false positives, as the study sample sizes were orders of magnitude smaller than the present analysis (see Introduction). However, as the earlier studies used various different trait measures related to handedness, and some made use of selected disorder populations, then it remains possible that some of the earlier findings were indeed real, but not detectable in the present analysis (see Introduction).
There was no evidence for an involvement, in hand preference, of common variants in genes related to visceral left-right patterning. Note that Brandler et al.43 performed analysis of a quantitative handedness index based on peg moving, and included a sample with reading disability, when they originally reported such a relationship. Our present analysis was not an attempt to directly replicate the study of Brandler et al., but rather it was a related investigation, of genes involved in visceral asymmetry with respect to questionnaire-defined, binary-trait hand preference, in a large dataset from the general population. In the rare genetic disorder Primary Ciliary Dyskinesia (PCD)(MIM # 244400), recessive mutations in specific genes involved in ciliary biology result in a 50% chance of mirror-reversed left-right visceral asymmetry, a condition known as situs inversus totalis (SIT)58. When PCD and SIT co-occur, it appears that the rate of left-handedness is unchanged from the general population59. However, there may be an increase of left-handedness in people with SIT when they do not have PCD60,61, which would suggest the existence of some developmental mechanisms which link visceral asymmetry with brain asymmetry. Nonetheless, a recent genome-wide mutation screening study did not identify any individual genes as likely candidates to cause non-PCD SIT with left-handedness62, and it is possible that the aetiology is multifactorial or even non-genetic62,63. At present, the study by Brandler et al. remains the only one to report tentative evidence for a molecular genetic link between visceral laterality and handedness.
Our GWAS analysis in the UK Biobank dataset produced three novel loci associated with left-hand preference at a genome-wide significant level, on 2q34, 17q21, and 13q22. The most likely causative gene within the large chromosome 17q21 inversion cannot be determined on the basis of these data, as the region spans at least twelve genes showing similar levels of association with the trait (Supp. Fig. S2). This region shows association with numerous other human traits (see e.g. http://big.stats.ox.ac.uk/variant/17-43659975-T-C). The variant on chromosome 13 resides in an intron of a non-coding RNA-gene of unknown function, LINC00381 (Supp Fig. S3). This SNP is part of a short A-repeat, but is in LD with other SNPs (www.ensembl.org). For the locus on chromosome 2q34, the most probable gene appears to be MAP2, which encodes microtubule-associated protein 2: the association signal extends from upstream into this gene’s coding region, and the single most associated SNP is associated with expression differences of MAP2 in oesophageal tissue, according to the GTeX database (https://www.gtexportal.org/home/ Release V7 (dbGaP Accession phs000424.v7.p2)), and not with any other gene’s expression level (Supp. Fig. S4). MAP2 protein is a well-known marker for neuronal cells, and specifically the perikarya and dendrites of these cells64. MAP2 is involved in neurogenesis65, and almost exclusively expressed in the brain66. The protein plays a role during the development of various brain structures (e.g.)67,68, and in mice seems to play a role in motor skills69. One report suggested that MAP2 is more highly expressed in right hippocampus than left during development in the rat70. How exactly this gene may act in affecting motor laterality in human development remains unknown. Regardless, as this genetic association is based on hundreds of thousands of study participants, MAP2 must be considered the single most reliably implicated protein-coding gene in left-handedness yet identified, although replication will be desirable.
When pooling ambidextrous people together with left-handers, to create a ‘right-handed’ versus ‘non-right-handed’ phenotype, an additional locus within the MHC region of chromosome 6p was detected. Again this is a very broad region of linkage disequilibrium spanning many genes. We found that the most associated SNP is an eQTL for the non-coding RNA gene HCG27 (HLA complex group 27) in the cerebellar hemisphere and frontal cortex, according to the GTeX portal. Other associated SNPs within the region, rs3130976 (p = 2.0E-08) and rs2854008 (p = 2.4E-08), are eQTLs for MICB (MHC class I polypeptide-related sequence B) and C4A (complement C4A) in the cerebellar hemisphere, but are also eQTLs for other genes in other tissues. We cannot conclude which may be the most likely causative gene or genes in the region, on the basis of this information.
There may have been some particular sources of noise in the UK Biobank definitions of hand preference. The UK Biobank participants are older than the general population (birth years between 1934 and 1971), so that some effects in this cohort may be quantitatively or qualitatively different in younger cohorts. An earlier analysis of the UK biobank dataset26 suggested a reduction in left-hand preference of roughly 20% among the oldest participants (born around 1940), relative to younger participants (born around 1970), which suggests that some of the older left-handed people were forced to switch to right-handedness during childhood. Our GWAS analysis did adjust for year of birth as a covariate effect, but hand switching may have reduced power. In addition, the location of birth within the UK was found to influence the probability of being left-handed26, likely primarily for cultural rather than biological reasons. Country of birth was therefore included as a covariate in our GWAS. Furthermore, the ambidextrous group was previously found to give a high rate of inconsistent answers between different assessment visits26. However, ambidextrous people comprised only a relatively small subset.
It is striking that common genetic variants in the UK Biobank dataset have little overall effect on hand preference, as measured by a very low SNP-based heritability (only 2–3% as measured here, and alsewhere)48,71, while previous twin-based studies have indicated a heritability of roughly 25% for left-hand preference (see Introduction). Nonetheless, our analysis with LDSC52 did detect evidence for an overall polygenic component to this trait. It has been noted before that behavioural traits can show a particularly large difference between twin and SNP-based heritability estimates72. A commonly given explanation is that much of the heritability may be due to rare SNPs and mutations, which are not well captured by genotyping arrays and imputation protocols in GWAS studies. Rare mutations can be relatively recent in origin, and under negative selection. Whether these possibilities apply to hand preference will need to be investigated in future large-scale genome sequencing studies.
Conclusion
In conclusion, previously reported genetic associations with measures related to handedness, found in datasets other than the UK biobank, were not supported by analysis of this very large cohort. We also found no supportive evidence that common variants within genes involved in visceral left-right patterning contribute to hand preference. We consider the most likely explanation for the discrepancy to be that the previous findings were noise, which arose from limited sample sizes. Differences of trait measurement and population selection might also be involved. Regardless, what is known of the molecular genetic basis of hand preference remains tentative and extremely limited, with the gene MAP2 providing the most robust lead so far.
Supplementary information
Acknowledgements
This research was conducted using the UK Biobank Resource under Application Number 16066. We thank all participants of the UK Biobank for their contribution. CGFdeK was supported by an Open Programme grant (824.14.005) to C.F. from the Netherlands Organization for Scientific Research (NWO). C.F. was supported by the Max Planck Society (Germany).
Author Contributions
C.F. initiated the study and edited the manuscript. C.G.F.d.K. carried out and interpreted the data analysis, and wrote the manuscript.
Data Availability
Data were obtained from the UK Biobank cohort, as part of research application 16066, with Clyde Francks as the principal applicant. Summary descriptions of these data can be found at http://biobank.ctsu.ox.ac.uk/crystal/. For use of these data, application must be made to http://www.ukbiobank.ac.uk/register-apply/. The gene-sets used in this analysis are added to the manuscript as Supplementary Table 2. GWAS summary statistics are available from the NHGRI-EBI GWAS Catalog https://www.ebi.ac.uk/gwas/downloads/summary-statistics.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42515-0.
References
- 1.Gilbert AN, Wysocki CJ. Hand preference and age in the United States. Neuropsychologia. 1992;30:601–608. doi: 10.1016/0028-3932(92)90065-T. [DOI] [PubMed] [Google Scholar]
- 2.Hepper PG, Wells DL, Lynch C. Prenatal thumb sucking is related to postnatal handedness. Neuropsychologia. 2005;43:313–315. doi: 10.1016/j.neuropsychologia.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Hepper PG. The developmental origins of laterality: Fetal handedness. Developmental Psychobiology. 2013;55:588–595. doi: 10.1002/dev.21119. [DOI] [PubMed] [Google Scholar]
- 4.Parma V, Brasselet R, Zoia S, Bulgheroni M, Castiello U. The origin of human handedness and its role in pre-birth motor control. Sci Rep. 2017;7:16804. doi: 10.1038/s41598-017-16827-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharoun SM, Bryden PJ. Hand preference, performance abilities, and hand selection in children. Front Psychol. 2014;5:82. doi: 10.3389/fpsyg.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Kovel CGF, et al. Left-Right Asymmetry of Maturation Rates in Human Embryonic Neural Development. Biol Psychiatry. 2017;82:204–212. doi: 10.1016/j.biopsych.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 7.de Kovel CGF, Lisgo SN, Fisher SE, Francks C. Subtle left-right asymmetry of gene expression profiles in embryonic and foetal human brains. Sci Rep-Uk. 2018;8:12606. doi: 10.1038/s41598-018-29496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faurie C, Raymond M. Handedness frequency over more than ten thousand years. Proc Biol Sci. 2004;271(Suppl 3):S43–45. doi: 10.1098/rsbl.2003.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coren S, Porac C. Fifty centuries of right-handedness: the historical record. Science. 1977;198:631–632. doi: 10.1126/science.335510. [DOI] [PubMed] [Google Scholar]
- 10.Medland SE, Perelle I, Monte VD, Ehrman L. Effects of culture, sex, and age on the distribution of handedness: An evaluation of the sensitivity of three measures of handedness. Laterality. 2004;9:287–297. doi: 10.1080/13576500342000040. [DOI] [PubMed] [Google Scholar]
- 11.Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- 12.Francks C. Exploring human brain lateralization with molecular genetics and genomics. Annals of the New York Academy of Sciences. 2015;1359:1–13. doi: 10.1111/nyas.12770. [DOI] [PubMed] [Google Scholar]
- 13.Duboc V, Dufourcq P, Blader P, Roussigne M. Asymmetry of the Brain: Development and Implications. Annu Rev Genet. 2015;49:647–672. doi: 10.1146/annurev-genet-112414-055322. [DOI] [PubMed] [Google Scholar]
- 14.Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nature Reviews Neuroscience. 2006;7:655. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- 15.Corballis MC. Left brain, right brain: facts and fantasies. PLoS Biol. 2014;12:e1001767. doi: 10.1371/journal.pbio.1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuoksimaa E, Koskenvuo M, Rose RJ, Kaprio J. Origins of handedness: A nationwide study of 30161 adults. Neuropsychologia. 2009;47:1294–1301. doi: 10.1016/j.neuropsychologia.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medland SE, et al. Genetic influences on handedness: data from 25,732 Australian and Dutch twin families. Neuropsychologia. 2009;47:330–337. doi: 10.1016/j.neuropsychologia.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medland SE, Duffy DL, Wright MJ, Geffen GM, Martin NG. Handedness in twins: joint analysis of data from 35 samples. Twin Res Hum Genet. 2006;9:46–53. doi: 10.1375/183242706776402885. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Ando J. Genetic and environmental structure of individual differences in hand, foot, and ear preferences: A twin study. Laterality: Asymmetries of Body. Brain and Cognition. 2014;19:113–128. doi: 10.1080/1357650X.2013.790396. [DOI] [PubMed] [Google Scholar]
- 20.McManus IC. Handedness, language dominance and aphasia: a genetic model. Psychological medicine. Monograph supplement. 1985;8:1–40. doi: 10.1017/S0264180100001879. [DOI] [PubMed] [Google Scholar]
- 21.Annett M. Handedness and Cerebral Dominance. The Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10:459–469. doi: 10.1176/jnp.10.4.459. [DOI] [PubMed] [Google Scholar]
- 22.Kavaklioglu T, Ajmal M, Hameed A, Francks C. Whole exome sequencing for handedness in a large and highly consanguineous family. Neuropsychologia. 2016;93:342–349. doi: 10.1016/j.neuropsychologia.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Somers M, et al. Linkage analysis in a Dutch population isolate shows no major gene for left-handedness or atypical language lateralization. J Neurosci. 2015;35:8730–8736. doi: 10.1523/JNEUROSCI.3287-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren DM, Stern M, Duggirala R, Dyer TD, Almasy L. Heritability and linkage analysis of hand, foot, and eye preference in Mexican Americans. Laterality. 2006;11:508–524. doi: 10.1080/13576500600761056. [DOI] [PubMed] [Google Scholar]
- 25.Armour JA, Davison A, McManus IC. Genome-wide association study of handedness excludes simple genetic models. Heredity (Edinb) 2014;112:221–225. doi: 10.1038/hdy.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Kovel CGF, Carrion-Castillo A, Francks C. A large-scale population study of early life factors influencing left-handedness. Sci Rep. 2019;9:584. doi: 10.1038/s41598-018-37423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston, D. W., Nicholls, M. E. R., Shah, M. A. & Shields, M. A. Handedness, Health and Cognitive Development: Evidence from Children in the NLSY. (Forschungsinstitut zur Zukunft der Arbeit, Bonn, Germany, 2010).
- 28.Annett M, Turner A. Laterality and the growth of intellectual abilities. British Journal of Educational Psychology. 1974;44:37–46. doi: 10.1111/j.2044-8279.1974.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 29.Francks C, et al. A Genomewide Linkage Screen for Relative Hand Skill in Sibling Pairs. The American Journal of Human Genetics. 2002;70:800–805. doi: 10.1086/339249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francks C, et al. Parent-of-origin effects on handedness and schizophrenia susceptibility on chromosome 2p12–q11. Hum. Mol. Genet. 2003;12:3225–3230. doi: 10.1093/hmg/ddg362. [DOI] [PubMed] [Google Scholar]
- 31.Francks C, et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Molecular Psychiatry. 2007;12:1129. doi: 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- 33.Medland SE, et al. Opposite effects of androgen receptor CAG repeat length on increased risk of left-handedness in males and females. Behav Genet. 2005;35:735–744. doi: 10.1007/s10519-005-6187-3. [DOI] [PubMed] [Google Scholar]
- 34.Arning L, et al. Handedness and the X chromosome: the role of androgen receptor CAG-repeat length. Sci Rep. 2015;5:8325. doi: 10.1038/srep08325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hampson E, Sankar JS. Hand preference in humans is associated with testosterone levels and androgen receptor gene polymorphism. Neuropsychologia. 2012;50:2018–2025. doi: 10.1016/j.neuropsychologia.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Savitz J, van der Merwe L, Solms M, Ramesar R. Lateralization of hand skill in bipolar affective disorder. Genes Brain Behav. 2007;6:698–705. doi: 10.1111/j.1601-183X.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 37.Maier W, Hofgen B, Zobel A, Rietschel M. Genetic models of schizophrenia and bipolar disorder: overlapping inheritance or discrete genotypes? European archives of psychiatry and clinical neuroscience. 2005;255:159–166. doi: 10.1007/s00406-005-0583-9. [DOI] [PubMed] [Google Scholar]
- 38.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 39.Ocklenburg S, et al. Left-Right Axis Differentiation and Functional Lateralization: a Haplotype in the Methyltransferase Encoding Gene SETDB2 Might Mediate Handedness in Healthy Adults. Mol Neurobiol. 2016;53:6355–6361. doi: 10.1007/s12035-015-9534-2. [DOI] [PubMed] [Google Scholar]
- 40.Crespi B, Read S, Hurd P. The SETDB2 locus: evidence for a genetic link between handedness and atopic disease. Heredity. 2018;120:77–82. doi: 10.1038/s41437-017-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu PF, et al. Setdb2 restricts dorsal organizer territory and regulates left-right asymmetry through suppressing fgf8 activity. Proc Natl Acad Sci USA. 2010;107:2521–2526. doi: 10.1073/pnas.0914396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scerri TS, et al. PCSK6 is associated with handedness in individuals with dyslexia. Hum. Mol. Genet. 2011;20:608–614. doi: 10.1093/hmg/ddq475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandler WM, et al. Common variants in left/right asymmetry genes and pathways are associated with relative hand skill. PLoS Genet. 2013;9:e1003751. doi: 10.1371/journal.pgen.1003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eriksson N, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6:e1000993. doi: 10.1371/journal.pgen.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku CS, Loy EY, Pawitan Y, Chia KS. The pursuit of genome-wide association studies: where are we now? J Hum Genet. 2010;55:195–206. doi: 10.1038/jhg.2010.19. [DOI] [PubMed] [Google Scholar]
- 46.Button KS, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature reviews. Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 47.Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neale, B. Heritability of >2,000 traits and disorders in the UK Biobank, http://www.nealelab.is/blog/2017/9/15/heritability-of-2000-traits-and-disorders-in-the-uk-biobank (2017).
- 49.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoggart CJ, Clark TG, De Iorio M, Whittaker JC, Balding DJ. Genome-wide significance for dense SNP and resequencing data. Genet Epidemiol. 2008;32:179–185. doi: 10.1002/gepi.20292. [DOI] [PubMed] [Google Scholar]
- 51.Genome-Wide Significance Project, Panagiotou, O. A. & Ioannidis, J. P. A What should the genome-wide significance threshold be? Empirical replication of borderline genetic associations. International Journal of Epidemiology. 2011;41:273–286. doi: 10.1093/ije/dyr178. [DOI] [PubMed] [Google Scholar]
- 52.Ni G, et al. Estimation of Genetic Correlation via Linkage Disequilibrium Score Regression and Genomic Restricted Maximum Likelihood. The American Journal of Human Genetics. 2018;102:1185–1194. doi: 10.1016/j.ajhg.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nature communications. 2017;8:1826. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segrè AV, et al. Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits. PLOS Genetics. 2010;6:e1001058. doi: 10.1371/journal.pgen.1001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLOS Computational Biology. 2015;11:e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lamparter D, Marbach D, Rueedi R, Kutalik Z, Bergmann S. Fast and Rigorous Computation of Gene and Pathway Scores from SNP-Based Summary Statistics. PLoS Comput Biol. 2016;12:e1004714. doi: 10.1371/journal.pcbi.1004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evangelou M, Rendon A, Ouwehand WH, Wernisch L, Dudbridge F. Comparison of methods for competitive tests of pathway analysis. PLoS One. 2012;7:e41018. doi: 10.1371/journal.pone.0041018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng H, Xia H, Deng S. Genetic basis of human left-right asymmetry disorders. Expert Rev Mol Med. 2015;16:e19. doi: 10.1017/erm.2014.22. [DOI] [PubMed] [Google Scholar]
- 59.McManus IC, Martin N, Stubbings GF, Chung EM, Mitchison HM. Handedness and situs inversus in primary ciliary dyskinesia. Proc Biol Sci. 2004;271:2579–2582. doi: 10.1098/rspb.2004.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vingerhoets G, et al. Brain structural and functional asymmetry in human situs inversus totalis. Brain Struct Funct. 2018;223:1937–1952. doi: 10.1007/s00429-017-1598-5. [DOI] [PubMed] [Google Scholar]
- 61.Vingerhoets G, Gerrits R, Bogaert S. Atypical brain functional segregation is more frequent in situs inversus totalis. Cortex. 2018;106:12–25. doi: 10.1016/j.cortex.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Postema, M. C., Carrion-Castillo, A., Fisher, S. E., Vingerhoets, G. & Francks, C. The genetics of situs inversus totalis without primary ciliary dyskinesia. bioRxiv, 10.1101/422964 (2018). [DOI] [PMC free article] [PubMed]
- 63.Aylsworth AS. Clinical aspects of defects in the determination of laterality. Am. J. Med. Gen. 2001;101:345–355. doi: 10.1002/ajmg.1219. [DOI] [PubMed] [Google Scholar]
- 64.Caceres A, et al. Differential subcellular localization of tubulin and the microtubule-associated protein MAP2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. J Neurosci. 1984;4:394–410. doi: 10.1523/JNEUROSCI.04-02-00394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. The Journal of Cell Biology. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fagerberg L, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Molecular & cellular proteomics: MCP. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin-Lopez Eduardo, Meller Sarah J., Greer Charles A. Development of piriform cortex interhemispheric connections via the anterior commissure: progressive and regressive strategies. Brain Structure and Function. 2018;223(9):4067–4085. doi: 10.1007/s00429-018-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blumcke I, et al. Distinct expression pattern of microtubule-associated protein-2 in human oligodendrogliomas and glial precursor cells. J Neuropathol Exp Neurol. 2001;60:984–993. doi: 10.1093/jnen/60.10.984. [DOI] [PubMed] [Google Scholar]
- 69.Tamakoshi K, Kawanaka K, Onishi H, Takamatsu Y, Ishida K. Motor Skills Training Improves Sensorimotor Dysfunction and Increases Microtubule-Associated Protein 2 mRNA Expression in Rats with Intracerebral Hemorrhage. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2016;25:2071–2077. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Moskal JR, Kroes RA, Otto NJ, Rahimi O, Claiborne BJ. Distinct patterns of gene expression in the left and right hippocampal formation of developing rats. Hippocampus. 2006;16:629–634. doi: 10.1002/hipo.20198. [DOI] [PubMed] [Google Scholar]
- 71.Ge T, Chen CY, Neale BM, Sabuncu MR, Smoller JW. Phenome-wide heritability analysis of the UK Biobank. PLoS Genet. 2017;13:e1006711. doi: 10.1371/journal.pgen.1006711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheesman R, et al. Childhood behaviour problems show the greatest gap between DNA-based and twin heritability. Translational psychiatry. 2017;7:1284. doi: 10.1038/s41398-017-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were obtained from the UK Biobank cohort, as part of research application 16066, with Clyde Francks as the principal applicant. Summary descriptions of these data can be found at http://biobank.ctsu.ox.ac.uk/crystal/. For use of these data, application must be made to http://www.ukbiobank.ac.uk/register-apply/. The gene-sets used in this analysis are added to the manuscript as Supplementary Table 2. GWAS summary statistics are available from the NHGRI-EBI GWAS Catalog https://www.ebi.ac.uk/gwas/downloads/summary-statistics.