Abstract
Objective:
Maternal high fat diet (HFD) may alter the offspring intestinal immune system thereby enhancing susceptibility towards inflammatory bowel disease. The objective of current study was to investigate the impact of maternal HFD on offspring intestinal health using a mouse model of dextran sodium sulfate (DSS)-induced colitis.
Methods:
Dams were provided with either HFD (60%) or control diet. After weaning female offspring from both groups were kept on 45% HFD. At 14-weeks of age, offspring were subjected to 2.5% DSS in drinking water for 5 days, followed by 5 days of recovery.
Results:
Offspring from maternal HFD had higher body weight gain before DSS-induction, and had higher liver and fat weights with increased adipocyte size at necropsy. When subjected to DSS-treatment, HFD offspring had accelerated body weight loss and exaggerated disease activity index. HFD offspring had elevated histopathological score, interleukin (IL)-1β, IL-6, and IL-17 expression with up-regulated NF-κB signaling. Maternal HFD resulted in enhanced neutrophil infiltration associated with elevated expression of monocyte chemoattractant protein-1 (MCP-1). Furthermore, maternal HFD suppressed AMP activated protein (AMPK) activity, and decreased sirtuin 1 (SIRT1) and p53 protein contents in offspring gut.
Conclusion:
Maternal HFD consumption predisposes offspring to a higher susceptibility of inflammatory bowel disease development.
Keywords: Maternal high fat diet, offspring, adipocytes, colitis, AMPK
Introduction
According to the latest NHANES survey (2009–2010), 31.9% of non-pregnant women 20–39 years of age are with obesity, and another one third are overweight (1). Accompanying the rapidly increasing prevalence of obesity, the incidence of inflammatory bowel disease (IBD) including Crohn’s disease (CD) and ulcerative colitis (UC), has rapidly increased in Europe and North America since the last decade of 20th century. More alarming, the incidence of IBD is rising quickly in young children in the USA, which was doubled from 1991 to 2002 (2). Around 1.5 million people are suffering from IBD in the USA, and is gaining commonness in the areas of the world where people are adopting a Western lifestyle that includes less physical activity, stress, and a diet rich in fat (3). IBD has resulted in huge medical cost in the USA and around the world (4). The etiological factors of IBD mainly comprise genetics, gut microbes, and environmental factors (4). Consumption of a diet rich in saturated fat increases incidence of colitis through promoting sulphite-reducing pathobiont in interleukin (IL)-10 deficient mice, which are genetically susceptible to IBD (5). Epidemiological studies further link high fat diet exposure during infancy to the incidence of IBD in adults (6).
Maternal high fat diet (HFD) intake negatively affects fetal development, which exerts lasting effects on the health of offspring, predisposing offspring to chronic diseases such as diabetes, coronary heart diseases and hypertension (7, 8). However, knowledge regarding the impact of maternal HFD or obesity on intestinal development and health of the progeny is limited (8); and the impact of maternal HFD on the incidence and susceptibility to IBD in offspring has only been sparsely tested (9). Jacobson and colleagues demonstrated that maternal high linoleic acid (18:2n-6) intake enhanced 2,4-dinitrobenzene sulfonic acid (DNBS)-induced colitis symptoms in nursing offspring (9). In our previous studies, we found that maternal obesogenic diet induced chronic gut inflammation in offspring (10, 11) and impaired gut barrier function of offspring in non obese diabetic (NOD) mice (10). Recently, Gruber and colleagues reported that maternal HFD in combination with postnatal HFD accelerated ileitis onset in the distal ileum of offspring TNF ΔARE/WT mice, a genetically susceptible model for CD like ileitis (12). In the current study, we examined the impact of maternal HFD on the IBD susceptibility of offspring using a dextran sulfate sodium (DSS)-induced colitis mouse model.
Materials and methods
Antibodies and chemicals
Antibodies against phosphor-/total AMPK, phosphor-/total-ERK1/2, IL-6, IκBα, phosphor-/total-p38, phosphor-/total-p65, p53, and SIRT1 were from Cell Signaling Technology (Beverly, MA, USA). Anti-Ly-6B.2 monoclonal and anti-β-actin antibodies were purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA) and Sigma (Saint Louis, MO, USA), respectively. IRDye 680 goat anti-mouse and IRDye 800CW goat anti-rabbit secondary antibodies were purchased from Li-Cor Biosciences (Lincoln, NE). The Vectastain Elite ABC, and DAB peroxidase (HRP) substrate kits were purchased from Vector Laboratories Inc. (Burlingame, CA, USA). Trizol® Reagent was purchased from Sigma (Saint Louis, MO, USA). DNase I and RNeasy Mini kit were purchased from Qiagen (Valencia, CA, USA), and iScript™ cDNA synthesis kit was purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA). Colitis grade DSS (MW = 36,000–50,000) was purchased from MP Biomedicals (Santa Ana, CA, USA).
Animal care and experimental design
Maternal diet and treatment
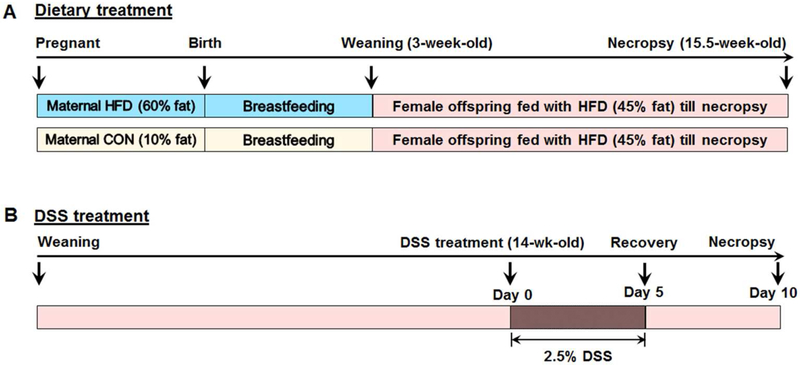
Adult (~4 months old) female C57BL/6J mice (purchased from Jackson Laboratory (Bar Harbor, ME, USA) and inbred in our facility) that had been fed regular chow diet were randomized into two groups; maternal control diet group (CON) and maternal high fat diet group (HFD). Both of the groups were fed with control diet (10% energy from fat, D12450H, Research Diets Inc., New Brunswick, NJ, USA) (Table S1&S2) up to mating with males of similar age. Mating was confirmed by the presence of vaginal plug. Then, mice were fed either CON or 60%HFD (60% energy from fat, D12492, Research Diets Inc., (Table S1&S2) diets during gestation and lactation (Fig. 1A). The 60% HFD is commonly used to induce obesity during a short duration (13). At birth, the litter sizes were balanced to 6 pups. At 3 weeks of age mice were weaned, and 9 female offspring per treatment were randomly selected for further studies. Here, we used female offspring mice because they were more vulnerable to programmed changes than males (14), and to avoid the confounding effects of sex.
Fig. 1.
Experimental design. A. Maternal and offspring dietary treatment timeframe. B. Offspring mice at age of 14-week-old were subjected to 2.5% DSS water for 5 days followed by a 5 days recovery. Offspring of both maternal HFD and CON groups were fed with a 45% HFD (45% energy from fat) from weaning to necropsy.
Offspring diets and treatment
After weaning, female offspring of both CON and HFD fed dams were fed the 45% HFD (45% energy from fat, D12451, Research Diets Inc.) (Table S1&S2) diet ad libitum for ~ 13 weeks until necropsy (Fig. 1A). The 45% HFD was used to mimic the effect of typical diet of western societies during a longer period (13). The body weights of offspring mice of both the groups were recorded weekly.
DSS treatment of offspring
At 14 weeks of age, offspring mice from both CON and HFD fed dams were subjected to 2.5% (W/V) DSS water challenge for 5 days to induce colitis, followed by five days of providing plain drinking water for recovery (Fig. 1B). Mice were monitored daily during DSS induction and the recovery stage for body weight loss, fecal consistency, and blood in the stood. Then, mice were sacrificed and tissue were collected for histological and biochemical analysis. All mice were housed in a temperature controlled room with a 12 h light and 12 h dark cycle, and had free access to diet and drinking water. Experiments were conducted per the animal procedure (BAF # 04341) approved by the Washington State University Animal Care and Use Committee.
Assessment of symptoms and colitis score
The disease activity index (DAI) score was assessed according to the combined scores of weight loss compared to initial weight, stool consistency, and bleeding in the stool using criteria detailed in Table S3 (15). The scores were recorded daily during the DSS-treatment and recovery stages. The DAI score was the summation of three individual scores (body weight loss, stool consistency, and bleeding).
Tissue collection and fixation
Mice were anesthetized with CO2 inhalation and followed by cervical dislocation. Organs including heart, spleen, liver, and cecum (containing lumen content) were collected and weighed. Subcutaneous fat (only lingual fat pad), visceral fat (Vis, gonadal fat collected around the ovaries in abdominal cavity), and brown adipose tissue (BAT, collected from the interscapular area) were weighed. A 5 mm segment of distal colon at a constant location, and the visceral adipose tissues were fixed in freshly prepared 4% (w/v) paraformaldehyde (pH 7.0), processed and embedded in paraffin. The remaining colon tissue containing both inflamed and non-inflamed area was rinsed in PBS, frozen in liquid nitrogen, and stored at −80 °C for biochemical analyses.
Histological evaluation of colonic ulceration and adipocyte quantification
Paraffin embedded tissues were sectioned at 5 μm thickness, deparaffinized and subjected to haematoxylin and eosin (H&E) staining. Histological examination and imaging were done under a Lecia DM2000 LED light microscope (Chicago, IL, USA). At least one image was obtained per section and 9 sections per animal at constant interval were used for microscopic examination.
The pathological score of the distal colon was evaluated and recorded blindly using previously published score criteria (15), which was the sum of the scores of crypt damage (none, basal 1/3, basal 2/3, only surface epithelium intact), severity of inflammation (none, slight, moderate, and severe), and depth of injury (none, mucosal, mucosal and submucosal, and transmural). Paraffin embedded adipose tissues were sectioned and processed as previously described (16). Image J 1.30v software (National Institute of Health, Bethesda, MD, USA) was used to measure the area and diameter of adipocytes by drawing a horizontal straight edge-to-edge line in the middle of the adipocyte. Nine sections per animal at constant interval were used and all the adipocytes per image were quantified.
Immunohistochemical analyses
Immunohistochemical analyses were carried as previously described (17). Briefly, colonic tissue sections were deparaffinized, hydrated, followed by antigen retrieval, blocking and overnight incubation with anti-Ly-6B.2 antibody (BioRad Laboratories Inc.). Signals were visualized using the Vectastain ABC and DAB kits (Vector Laboratories) and haematoxylin counterstaining. Images were taken using the Lecia DM2000 LED light microscope (Chicago, IL, USA). Neutrophil infiltration scores were assessed blindly using the criteria described previously (18). Briefly, the scores for the depth of neutrophil infiltration (scored as 0–3) and staining intensity (0–4) were recorded individually. The summation of both scores resulted in the total quantified score ranging from 0 to a maximum of 7 per distal colonic section. Nine sections per animal at constant interval were used for microscopic examination and score assessment.
Immunoblotting analyses
Immunoblotting analyses were performed as previously described (19). Band density was quantified using the Odyssey Infrared Imaging System and Image Studio™ Lite software (Li-Cor Biosciences, Lincoln, NE, USA), and normalized to the β-actin content.
Quantitative Reverse Transcriptase (qRT)-PCR analyses
Total RNA was extracted from the powdered tissue with Trizol® Reagent (Sigma). RNA was treated with DNase I (Qiagen), and then purified with RNeasy Mini kit (Qiagen). cDNA was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad). qRT PCR was performed per a published method (20). 18s was used as the reference gene. Primer sequences used in the study are listed in the Table S4.
Statistical analysis
Data were analyzed as a complete randomized design using General Linear Model of Statistical Analysis System (2000). Data were expressed as mean ± standard error of mean (SEM). Student’s T-test was used for calculating significance. A significant difference was considered as P ≤ 0.05.
Results
Maternal HFD affects organ weight and visceral adipocyte size in offspring
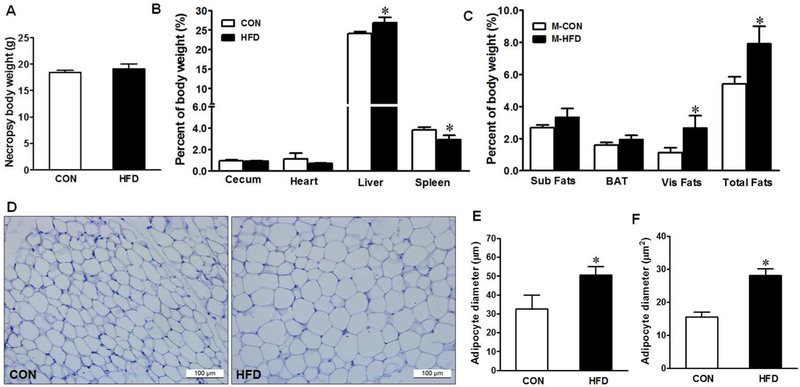
The offspring from maternal HFD group had a significantly higher liver and lower spleen weights per percentage of body weight than those from maternal CON group (Fig. 2B), though their body weights were not different at necropsy (Fig. 2A). Similarly, the maternal HFD enhanced fat mass, including subcutaneous, BAT, and visceral fats (Fig. 2C), and visceral adipocyte diameter and area (Fig. 2D–F).
Fig. 2.
Body and organ weight in offspring of CON (□) or HFD (■) fed dams at necropsy. (A) Body weight, (B) Organ weight, (C) Fats weight, (D) Representative images of hematoxylin and eosin (H&E) staining of visceral adipose tissue section, (E) Adipocyte diameter (μm), and (F) Adipocyte area (μm2). Sub = Subcutaneous fats, BAT = Brown adipose tissue, and Vis = Visceral fats. Means ± SEM, n = 9, *: P ≤ 0.05.
Maternal HFD affects body weight loss and disease activity index in DSS-induced colitis
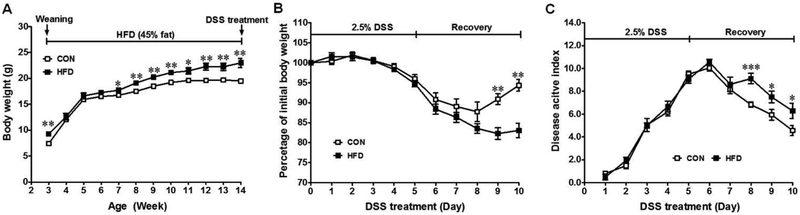
Although body weight in offspring from maternal HFD group was significantly higher than that of maternal CON group before DSS induction (Fig. 3A), DSS-treatment resulted in a greater loss of body weight in maternal HFD offspring at the recovery stage (Fig. 3B). Colitis induction was further confirmed by diarrhea and blood in the stool in addition to body weight loss as shown by the DAI scores (Fig. 3C). The DAI score increased during DSS-treatment for both groups, which was significantly greater in maternal HFD offspring during the recovery stage (Fig. 3C). Further the colonic length was shorter (4.33 ± 0.14 cm) in maternal HFD fed offspring than maternal CON group (4.64 ± 0.17 cm), though it was not significant.
Fig. 3.
Symptoms of DSS-induced colitis in offspring of CON (□) or HFD (■) fed dams. (A) Weekly body weight of offspring after weaning and before DSS treatment, (B) Body weight loss and (C) Disease activity index during DSS-treatment and recovery process, a higher score correlates with severer symptoms. Means ± SEM, n = 9, *: P ≤ 0.05, **: P ≤ 0.01, and ***: P ≤ 0.001.
Maternal HFD enhances histologic distortion and inflammation in the colon of offspring mice subjected to DSS induction
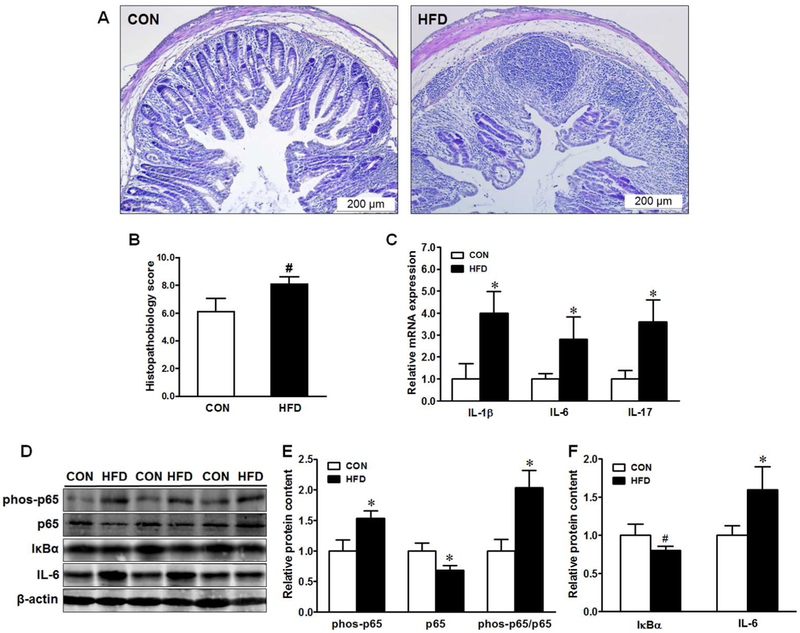
Histological evaluation revealed that DSS-treatment caused injuries to the distal colonic tissues, which resulted in the loss of crypt architecture (Fig. 4A). The colon in maternal HFD offspring was more susceptible to DSS challenge with a trend of higher histopathological score (P ≤ 0.10) than those from CON offspring (Fig. 4A&B), indicating aggravated ulceration in the distal colon.
Fig. 4.
Histopathological score and inflammatory mediators in offspring of CON (□) or HFD (■) fed dams. (A) Representative images of H&E staining of distal colonic sections, (B) Histopathological score, (C) mRNA expression of pro-inflammatory cytokines, (D-F) Representative immunoblotting bands and statistical data of p65, IκB-α and IL-6. Means ± SEM, n = 9, *: P ≤ 0.05, #: P ≤ 0.10.
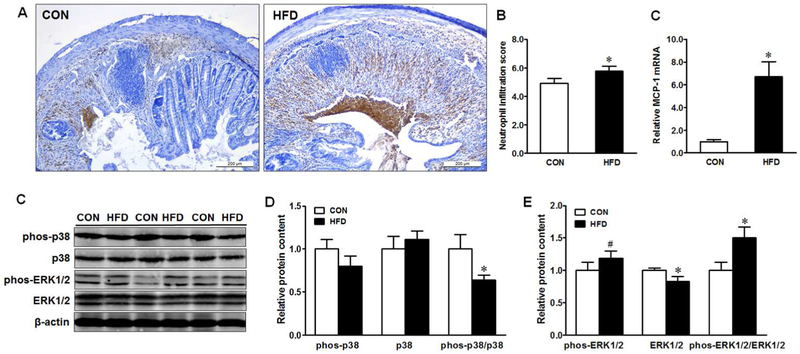
To gain more insights on the altered histopathology, we further analyzed the expression of pro-inflammatory cytokines and associated inflammatory signaling. Maternal HFD elevated interleukin (IL)-1β, IL-6, and IL-17 gene expression (Fig. 4C) and increased IL-6 protein in offspring gut (Fig. 4F). NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling was exaggerated in the colonic tissues as indicated by enhanced phosphorylation of p65 (Fig. 4D&E) and decreased IκBα (inhibitor κB alpha, which sequesters and inactivates NF-κB Rel/p65 in cytoplasm) content (Fig. 4D&F). In addition, maternal HFD resulted in more extensive and severe neutrophil infiltration in the colonic tissue of offspring mice (Fig. 5A&B), associated with elevated expression of monocyte chemoattractant protein-1 (MCP-1) (Fig. 5C). Accordingly, we observed decreased p38 phosphorylation (Fig. 5C&D) and enhanced ERK1/2 phosphorylation (Fig. 5C&E) in maternal HFD offspring. These findings collectively suggest that maternal HFD rendered offspring mice to be more susceptible to inflammation and colitis induced by DSS.
Fig. 5.
Neutrophil immunohistochemical staining in distal colonic tissues of offspring of CON (□) or HFD (■) fed dams. (A) Representative images of neutrophil staining, (B) Neutrophil infiltration score, (C) mRNA expression of MCP-1, (D-F) Representative immunoblotting bands and statistical data of p38 and ERK1/2. Means ± SEM, n = 9, *: P ≤ 0.05, #: P ≤ 0.10.
Maternal HFD adversely affects AMP-activated protein kinase activity in offspring mice with DSS-induced colitis
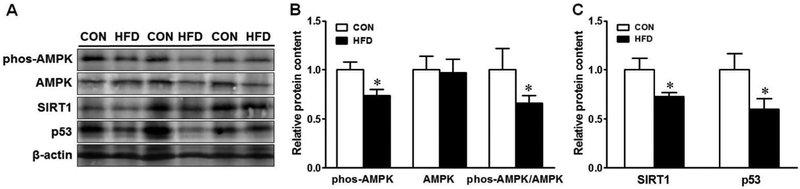
Maternal HFD reduced AMPK phosphorylation (Fig. 6A&B) in offspring colonic tissues. Consistently, both AMPK interacting proteins, SIRT1 and p53, were decreased (Fig. 6C) in offspring mice from HFD fed dams.
Fig. 6.
AMP-activated protein kinase (AMPK) signaling in the colon of offspring of CON (□) or HFD (■) fed dams. (A) Representative immunoblotting bands, (B) Relative protein expression of AMPK, (C) Relative protein expression of SIRT1 and p53. Means ± SEM, n = 9, *: P ≤ 0.05.
Discussion
The high fat diet is a part of the Western lifestyle and has been proposed to increase the risk of IBD (3). The rapid increase of IBD incidence in young children (2) suggests the potential role of maternal obesogenic diet in the development of this disease, but direct evidence remains missing. In the current study, we assessed impact of maternal HFD on the incidence of IBD in offspring using a DSS-induced colitis mouse model. We found that maternal HFD significantly aggravated the symptoms of acute colitis in the female offspring.
In this study, we initiated HFD (60%) dietary treatments after mating and maintained during gestation and lactation in order to avoid maternal obesity that could induce gestational diabetes and other physiological changes in addition to the effect of high fat diet (21). All offspring were fed obesogenic diet after weaning to mimic the typical diet of Western societies. The maternal HFD offspring were heavier at weaning, body weight was higher in offspring of maternal HFD compared to CON group from 7 weeks till DSS induction. The higher weight at weaning in the HFD fed group offspring was likely due to the maternal HFD (60%) diet consumption during gestation and lactation. Our findings are consistent with previous investigations that maternal high linoleic acid (18:2n-6) intake resulted a higher body weight in the nursing rats (9), and maternal HFD consumption enhanced body weight gain and adiposity in the offspring mice (22, 23). During the recovery period (post DSS induction), however, the body weight loss was higher in offspring of maternal HFD, suggesting severer colitis in these mice compared to offspring of maternal CON group. Consistently, during the course of recovery, a higher DAI score was detected in the maternal HFD fed group, so for the histopathological score, showing severer colitis and slower recovery of offspring of HFD fed dams. DSS, administered to mice in the drinking water, triggers intestinal inflammation by binding to medium chain length fatty acids in the mouse colon, resulting in the disruption of colonic epithelial barrier (24), and eliciting inflammation (25). Activated immune system produces pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α that further amplify the NF-κB inflammatory cascade (26). IL-17 in return mediates neutrophil infiltration and upregulation of TNF-α and IL-6, which are the key mediators in chronic inflammation of UC (26, 27). Consistent with previous reports and the observed severer histopathological damage (9), maternal HFD enhanced IL-1β, IL-6, and IL-17 production with amplified NF-κB signaling in the offspring. These findings are aligned with earlier observations that maternal obesity predisposes offspring gut to inflammation in sheep (11) and NOD mice (10), as well as in young rats (9).
In IBD, excessive activation and infiltration of neutrophils in colonic lesions result in epithelial cell necrosis, mucosal injury and exaggerated colitis symptoms (28). High density neutrophil flux across epithelial monolayers from basolateral to apical surface disrupts epithelium (29), and weakens the epithelial barrier, which worsens inflammation and IBD symptoms (28). Maternal HFD exacerbated mucosal neutrophil infiltration in offspring subjected to DSS-treatment, which could contribute to the enhanced IL-17, and severer tissue damage and elevated histopathological score in DSS-induced colitis. Consistent with enhanced neutrophil infiltration, MCP-1, the main chemokine regulating migration and infiltration of neutrophils (30), was increased in offspring gut from HFD fed dams. These data were supported by a recent publication where maternal HFD promotes an early onset of ileitis in a genetically-driven CD mouse model, with enhanced neutrophil infiltration (12). Rapid apoptosis and removal of neutrophils are vital in the resolution of inflammation to avoid undesirable tissue damage. In human neutrophils, lipopolysaccharide or TNFα-stimulated ERK-activation contributes to the delayed apoptosis (31), while activation of p38 MAPK induced neutrophil apoptosis in vitro (32). Accordingly, the enhanced ERK1/2 phosphorylation and decreased p38 activation were detected in the offspring gut from HFD fed dams, indicating that maternal HFD might delay neutrophil apoptosis in the inflamed colonic tissues.
AMP-activated protein kinase (AMPK) is the central regulator of cellular energy balance (22). AMPK mediates the inflammatory responses such as IL-6 expression (33), and reduced AMPK protein level results in impaired epithelial barrier function (34). The reduced AMPK level due to maternal HFD in offspring with DSS-induced colitis suggested that AMPK signaling might be important in the pathophysiological effects of maternal HFD on inflammation and colitis in offspring gut. AMPK inhibits inflammation through inducing the expression or activation of a number of mediators such as SIRT1, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), p53, and Forkhead box O factors (35, 36). AMPK activates SIRT1 deacetylase via increasing cellular NAD+ levels (35) while SIRT1 stimulates LKB1 activity, which subsequently activates AMPK (37). SIRT1 deacetylates the RelA/p65 and inhibits NF-κB signaling (36). Thus, the reduction in AMPK and SIRT1 might contribute to the enhanced activation of NF-κB signaling in HFD offspring gut. In this sense, AICAR-induced AMPK activation down-regulates the innate and adaptive immune responses of TNBS induced colitis (38). AMPK also activates p53, a tumor suppressor that mediates growth arrest or induces apoptosis (39). Mice deficient in p53 developed tumors upon DSS-treatment even without azoxymethane (AOM) injection (40), suggesting a vital protective role of p53 in IBD pathogenesis. Consistent with decreased AMPK activation, p53 protein was decreased in offspring colon of HFD fed dams, which likely contributes to the colitis induction in the maternal HFD offspring.
In conclusion, maternal HFD exposure accelerated offspring body weight gain, but enhanced DSS-induced body weight loss, and colitis symptoms by activating NF-κB signaling and stimulating IL-1β, IL-6, and IL-17 expression, as well as neutrophil induction in colonic tissues. Furthermore, maternal HFD exposure suppressed AMPK activity and down-regulated SIRT1 and p53 that further aggravated DSS-colitis in the offspring. Collectively, this study suggests that maternal HFD consumption predisposes offspring mice to IBD and related inflammatory gut diseases.
Supplementary Material
What is already known about this subject?
High fat diet (HFD) consumption promotes inflammation
HFD consumption can enhance susceptibility to IBD
Maternal HFD is related to the increased risk for IBD in offspring
What does your study add?
Maternal high fat diet HFD aggravates colitis in the offspring by activating NF-κB signaling
Maternal HFD enhances colonic inflammation and distortion in the offspring induced by DSS
Maternal HFD suppresses AMPK activity and SIRT1content in offspring gut
Acknowledgements
We thank Bo Wang, Xiaofei Sun, Dr. Hanying Zhang and Dr. Shuming Zhang for their assistance in mice operation and tissue collection.
FUNDING: This work was financially supported by NIH R15HD073864.
Footnotes
DISCLOSURE: The authors declare no conflict of interest
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307: 491–497. [DOI] [PubMed] [Google Scholar]
- 2.Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr 2010;50: 27–31. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126: 1504–1517. [DOI] [PubMed] [Google Scholar]
- 4.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142: 46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 5.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012;487: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12: 205–217. [DOI] [PubMed] [Google Scholar]
- 7.Williams CB, Mackenzie KC, Gahagan S. The effect of maternal obesity on the offspring. Clin Obstet Gynecol 2014;57: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu MJ, Du M, Ford SP. CELL BIOLOGY SYMPOSIUM: Impacts of maternal obesity on placental and gut inflammation and health. J Anim Sci 2014;92: 1840–1849. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson K, Mundra H, Innis SM. Intestinal responsiveness to experimental colitis in young rats is altered by maternal diet. Am J Physiol Gastrointest Liver Physiol 2005;289: G13–20. [DOI] [PubMed] [Google Scholar]
- 10.Xue Y, Wang H, Du M, Zhu MJ. Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in nonobese diabetic mice. J Nutr Biochem 2014;25: 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan X, Huang Y, Wang H, Du M, Hess BW, Ford SP, et al. Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm Bowel Dis 2011;17: 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber L, Hemmerling J, Schuppel V, Muller M, Boekschoten MV, Haller D. Maternal High-fat Diet Accelerates Development of Crohn’s Disease-like Ileitis in TNFDeltaARE/WT Offspring. Inflamm Bowel Dis 2015;21: 2016–2025. [DOI] [PubMed] [Google Scholar]
- 13.Wang C-Y, Liao JK. A Mouse Model of Diet-Induced Obesity and Insulin Resistance. Methods Mol Biol 2012;821: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol 2008;1: 170–178. [PMC free article] [PubMed] [Google Scholar]
- 15.Hamamoto N, Maemura K, Hirata I, Murano M, Sasaki S, Katsu K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)). Clin Exp Immunol 1999;117: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, et al. Up-Regulation of Toll-Like Receptor 4/Nuclear Factor-κB Signaling Is Associated with Enhanced Adipogenesis and Insulin Resistance in Fetal Skeletal Muscle of Obese Sheep at Late Gestation. Endocrinology 2010;151: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu MJ, Du M, Hess BW, Nathanielsz PW, Ford SP. Periconceptional nutrient restriction in the ewe alters MAPK/ERK1/2 and PI3K/Akt growth signaling pathways and vascularity in the placentome. Placenta 2007;28: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 18.Kang Y, Xue Y, Du M, Zhu MJ. Preventive effects of Goji berry on dextran sulfate-sodium-induced colitis in mice. J Nutr Biochem 2016;40: 70–76. [DOI] [PubMed] [Google Scholar]
- 19.Bibi S, Kang Y, Yang G, Zhu M. Grape seed extract improves small intestinal health through suppressing inflammation and regulating alkaline phosphatase in IL-10-deficient mice. J Funct Foods 2016;20: 245–252. [Google Scholar]
- 20.Wang H, Xue Y, Zhang H, Huang Y, Yang G, Du M, et al. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice. Mol Nutr Food Res 2013;57: 2253–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010;140: 387–398. [DOI] [PubMed] [Google Scholar]
- 22.Tong JF, Yan X, Zhao JX, Zhu MJ, Nathanielsz PW, Du M. Metformin mitigates the impaired development of skeletal muscle in the offspring of obese mice. Nutr Diabetes 2011;1: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang X, Yang Q, Fu X, Rogers CJ, Wang B, Pan H, et al. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J Physiol 2016;594: 4453–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, et al. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One 2012;7: e32084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gkouskou KK, Deligianni C, Tsatsanis C, Eliopoulos AG. The gut microbiota in mouse models of inflammatory bowel disease. Front Cell Infect Microbiol 2014;4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng SC, Benjamin JL, McCarthy NE, Hedin CR, Koutsoumpas A, Plamondon S, et al. Relationship between human intestinal dendritic cells, gut microbiota, and disease activity in Crohn’s disease. Inflamm Bowel Dis 2011;17: 2027–2037. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 2004;126: 989–996; discussion 947. [DOI] [PubMed] [Google Scholar]
- 28.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol 2001;159: 2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest 1991;88: 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol 1999;162: 1685–1691. [PubMed] [Google Scholar]
- 31.Simard FA, Cloutier A, Ear T, Vardhan H, McDonald PP. MEK-independent ERK activation in human neutrophils and its impact on functional responses. J Leukoc Biol 2015;98: 565–573. [DOI] [PubMed] [Google Scholar]
- 32.Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. Role of p38-mitogen-activated protein kinase in spontaneous apoptosis of human neutrophils. J Immunol 1999;162: 1692–1700. [PubMed] [Google Scholar]
- 33.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 2008;181: 8633–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharl M, Paul G, Barrett KE, McCole DF. AMP-activated protein kinase mediates the interferon-gamma-induced decrease in intestinal epithelial barrier function. J Biol Chem 2009;284: 27952–27963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458: 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004;23: 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 2008;283: 27628–27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan Q, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol 2010;80: 1708–1717. [DOI] [PubMed] [Google Scholar]
- 39.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 2005;18: 283–293. [DOI] [PubMed] [Google Scholar]
- 40.Chang WC, Coudry RA, Clapper ML, Zhang X, Williams KL, Spittle CS, et al. Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis 2007;28: 2375–2381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.