Abstract
Background:
Airway epithelial injury is a crucial component of acute and severe asthma pathogenesis and a promising target for treatment of refractory asthma. However, the underlying mechanism of epithelial injury remains poorly explored. Though high levels of polyamines, mainly spermine, have been found in asthma and co-morbidity, their role in airway epithelial injury and the cause of their altered levels in asthma has not been explored.
Methods:
We measured key polyamine metabolic enzymes in lung samples from normal and asthmatic subjects and in mice with OVA-induced allergic airway inflammation (AAI). Polyamine metabolism was modulated using pharmacologic/genetic modulators. Epithelial stress and apoptosis were measured by TSLP levels and TUNEL assay, respectively.
Results:
We found loss of the polyamine catabolic enzymes spermidine/spermine-N (1)-acetyltransferase-1 (SAT1) and spermine oxidase (SMOX) predominantly in bronchial epithelial cells (BECs) of human asthmatic lung samples and mice with AAI. In naïve mice, SAT1 or SMOX knockdown led to airway hyper-responsiveness, remodeling and BEC apoptosis. Conversely, in mice with AAI, overexpression of either SAT1 or SMOX alleviated asthmatic features and reduced TSLP levels and BEC apoptosis. Similarly, while pharmacological induction of SAT1 and SMOX using the polyamine analogue bis(ethyl)norspermine (BENSPM) alleviated asthmatic features with reduced TSLP levels and BEC apoptosis, pharmacological inhibition of these enzymes using BERENIL or MDL72527, respectively, worsened them. Spermine accumulation in lungs correlated with BEC apoptosis, and spermine treatment caused apoptosis of human BEAS-2B cells in vitro.
Conclusions:
Spermine induces BEC injury. Induction of polyamine catabolism may represent a novel therapeutic approach for asthma via reversing BEC stress.
Keywords: Polyamines, Catabolism, SAT1, SMOX, Spermine, Airway Epithelial Injury, Asthma
Introduction
Asthma is a complex disorder that involves an intricate interplay between structural (1) and immune cells (2). Current therapeutics available for asthma are based on the inflammatory response; however, they are ineffective for a group of patients, who develop resistance (3). Therefore, there is an imperative need to target alternative pathways of asthma pathogenesis (4). In contrast to immune cells, the airway epithelial layer is a structural boundary between the lung and the external environment, and current literature suggests that airway epithelial injury is a critical aspect of asthma pathogenesis (1,5). Stressed epithelia produce cytokines such as TSLP, IL-33 and IL-25 (2), which can cause airway inflammation and remodeling (6). Of these, TSLP has gained the most importance in asthma (7). Importantly, overexpression of TSLP in the lungs of naïve mice led to self-perpetuated asthma-like features (8). Interestingly, in a recent report, treatment with tezepelumab, a monoclonal antibody against TSLP, was found to be effective in severe asthma patients with poor steroid responsiveness (9). Further, apoptosis of bronchial epithelial cells (BECs) in the lungs of human asthmatics (10,11) and in mice with experimental asthma, reported by us (12,13) and others (14), suggests the involvement of epithelial stress in asthma. Hence, finding out the pathways involved in the survival or damage of airway epithelial cells could be a suitable approach for the discovery of alternative therapies in asthma management.
The polyamines (PAs) putrescine (PUT; H3N+(CH2)4+NH3), spermidine (SPD; H3N+ (CH2)4+NH2(CH2)3+NH3) and spermine (SPM; H3N+(CH2)3+NH2(CH2)4+NH2(CH2)3+NH3) are positively charged biological molecules at physiological pH (15). PA catabolism plays a crucial role in maintaining optimal levels of intracellular PA pools by converting longer PAs back to smaller PAs through two key catabolic enzymes, spermidine/spermine-N(1)-acetyltransferase-1 (SAT1) and spermine oxidase (SMOX) (Fig. 1, A) (16). SAT1 transfers acetyl groups onto SPM and SPD, and acetylated PAs are either exported out of the cell or oxidized by acetyl polyamine oxidase (PAOX). The rate of PAOX reaction is limited by the availability of acetylated PAs, hence SAT1 is considered a rate-limiting enzyme of this catabolic reaction (17). SMOX preferentially catabolizes SPM but not SPD, without prior acetylation, unlike PAOX (18). Various pharmacological modulators, such as 2-difluoromethylornithine (DFMO), bis(ethyl)-norspermine (BESNPM), N,N1-bis(2,3-butadienyl)-1,4-butanediamine (MDL72527) and diminazene-aceturate (BERENIL) can target key enzymes involved in the PA metabolic processes (Fig. 1, A) (19).
Figure 1.
Loss of the PA catabolic enzymes SAT1 and SMOX in human asthmatics and in mice with AAI. (A) Schematic depiction of PA metabolism. PUT is synthesized by oxidative decarboxylation of ornithine by the ornithine decarboxylase (ODC). PUT further gets converted into SPD and SPM by spermidine synthase (SRM) and spermine synthase (SMS), respectively. PA catabolism converts longer PAs back to smaller PAs through SAT1, SMOX and PAOX. Key enzymes are in filled shapes and pharmacological modulators are italicized. (B) Representative Western blots and densitometry of SAT1 and SMOX in human lung lysates (n=4). (C and D) Representative IHC images of SAT1 and SMOX in human lung sections (n=4; Original magnification: 200X). (E) Schematic presentation of the protocols for the OVA-induced AAI model without (Set-I) and with (Set II-IV) different modulators of PA catabolism, see Methods. (F) PA levels in the whole lung lysates of mice. (G) Representative Western blots for catabolic enzymes (SAT1 and SMOX) in whole lung lysates. (H) Representative IHC images of SAT1 and SMOX in lungs sections of mice (Original magnification: 200X). In images, arrows indicate expression in BECs. All data are presented as means ± s.e.m. Mice data (F-H, means ± s.e.m.) are representative from one of three independent experiments (n=5). Student’s t-test, *P<0.05; **P<0.01.
The involvement of PA pathway in cell survival or death has been reported (20,21). However, the effects of PAs are contextual, depending on their concentrations and cell types (22). In asthma, PA levels are increased in the blood (23) and sputum (especially SPM) (24) of patients. However, the reason for this elevation of SPM and other PAs is not known. Though there are few reports available for the effects of PAs on circulating immune cells, such as mast cells, in context of asthma (25,26), the effects of PAs on airway epithelial cells have not yet been studied. Interestingly, a recent study demonstrated survival-promoting effects of SPM on eosinophils in asthma (27). Since airway epithelium and eosinophils play protective and damaging roles in asthma, respectively, we sought to study the effect of PAs on airway epithelium.
In this study, we report for the first time that the PA catabolic enzymes SAT1 and SMOX are downregulated in asthma, predominantly in BECs. We show how reduced PA catabolism is associated with epithelial stress and the inflammatory cascade. Furthermore, pharmacological or genetic induction of PA catabolism, leading to normal PA levels, reverses asthma features.
Materials and Methods
For a detailed description, please see supplementary sections.
Animals
Male BALB/c mice (8–10 weeks old) were obtained from Central Drug Research Institute (Lucknow, India) and acclimatized for a week before the experiments. Animals were maintained following the guidelines and protocols approved by the institutional animal ethics committee.
Human samples
Human lung lysates and paraffin-embedded lung sections (from same samples) were purchased from Novus Biologicals (Colorado, USA) and BioChain Institute, Inc. (California, USA).
Cell line
Human bronchial epithelial cell line, BEAS-2B (derived from normal human bronchial epithelium) (28) was purchased (CRL-9609; ATCC, USA) and grown in serum-free LHC-9 medium (Gibco, USA).
Delivery of pharmacological compounds and plasmids, in naïve mice or mice with AAI
Pharmacological induction of SAT1 and SMOX (Fig. 1E: Set-II) was performed using the PA analogue BENSPM {20mg/kg} (29–31), a structural mimetic of SPM that exploits the regulatory feedback mechanisms of the PA pathway. For inhibition, BERENIL {20mg/kg (Sigma, USA)}, a pharmacologic inhibitor of SAT1 activity (32) and MDL72527 {40mg/kg (Sigma, USA)}, a PA analogue, which is a competitive inhibitor of SMOX (33) were used. Compounds were delivered intravenously via tail vein injections.
For genetic modulations, different plasmids were complexed with in vivo-jetPEI reagent (Polyplus, France) as per the manufacturer’s instructions, and were administered by either intratracheal or intravenous routes, as detailed in supplementary methods.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software. One-way ANOVA test was used to compare more than two groups followed by Tukey’s post-hoc analysis. Between two groups, unpaired, two-tailed Student’s t test was used.
Results
Loss of the PA catabolic enzymes SAT1 and SMOX in human asthmatic lung samples
To investigate the status of PA metabolism (Fig. 1A), expression of key metabolic enzymes was measured by Western blots in total lysates of human lung samples from both asthmatics and healthy subjects. The cellular identity of these expressions was studied by immunohistochemistry (IHC). Though the levels of ODC were unchanged (Fig. S1, A), we observed significant reductions in the levels of SAT1 and SMOX, in the lung lysates of asthmatics in comparison to healthy controls (Fig. 1B). Interestingly, IHC of the lung sections showed that these reductions in catabolic enzymes were most prominent in the BECs (Fig. 1C and D).
PA catabolism is downregulated in AAI
In order to study the role of derailed PA metabolism in epithelial injury and, thus, asthma pathogenesis, we used murine-model of AAI, which shows key features of asthma (Fig. 1E: Set-I). To confirm the suitability of the model, we checked the levels of PAs by high performance liquid chromatography (HPLC) and the expression of ODC, SAT1 and SMOX by Western blotting. To estimate the levels of PAs, lung tissues from mice with AAI (OVA/OVA) and normal mice (SHAM/PBS) were used. We found a significant increase in the levels of all mammalian PAs, PUT, SPD and SPM, in mice with AAI (Fig. 1F). However, of all PAs, the levels of SPM were increased the most (~2 fold) (Fig. 1F). Although, no differences were observed in ODC (Fig. S1, B), SAT1 and SMOX levels were significantly reduced (Fig. 1G and Fig. S1, C and D). Additionally, the activity of SAT1 was decreased (Fig. S1, E). These results were confirmed by IHC which showed that SAT1 and SMOX were downregulated predominantly in BECs (Fig. 1H and Fig. S1, F).
In vivo knockdown of either SAT1 or SMOX increases PAs and generates asthma-like features in naïve mice
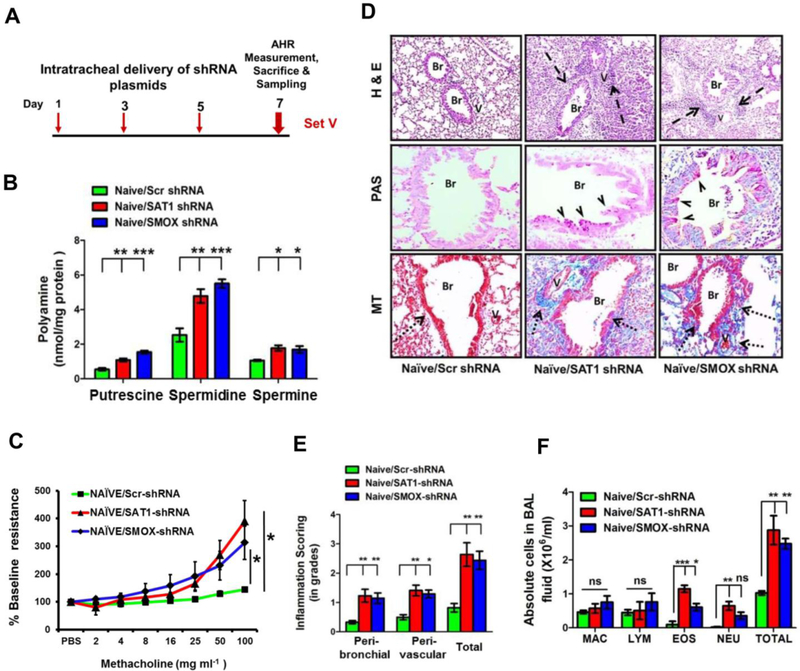
To determine whether reduced PA catabolism contributed to asthma pathophysiology, we knocked down SAT1 and SMOX in naïve mice by intratracheal delivery of respective shRNA plasmids (Fig. 2A). Knockdown of SAT1 and SMOX was confirmed by Western blot analysis (Fig. S2, A). Knockdown of SAT1 or SMOX by their respective shRNAs, but not by scrambled shRNA, elevated the levels of all PAs in the lung (Fig. 2B). Interestingly, loss of either SAT1 or SMOX led to a significant development of airway hyper-responsiveness (AHR) in the naïve mice (Fig. 2C). This AHR induction was associated with modest increase in airway inflammation (Fig. 2D and E), goblet cell metaplasia and sub-epithelial collagen deposition (Fig. 2D). Both neutrophils and eosinophils were increased in bronchoalveolar lavage (BAL) fluid (Fig. 2F). To rule out the possibility of non-specific shRNA effects, we measured the levels of interferon (IFN)-β in the lung lysates and observed no induction of interferon response (Fig. S2, B).
Figure 2.
In vivo knock- down of either SAT1 or SMOX increases PAs and generates asthma-like features in naïve mice. (A) Schematic protocol for intratracheal administration of SAT1- and SMOX-shRNA plasmids (Set-V). (B) PA levels in the whole lung lysates. (C) Airway resistance (or AHR) in response to methacholine. (D) Representative images for airway inflammation, goblet cell metaplasia and sub-epithelial collagen deposition in the lungs as measured by haematoxylin and eosin (H&E), periodic acid schiff (PAS) and Masson’s trichrome (MT) stainings, respectively. (E) Inflammation scores in the H&E stained lung sections. (F) Differential cell counts in the BAL fluid. MAC, macrophages; LYM, lymphocytes; EOS, eosinophils; NEU, neutrophils; Br, bronchus; V, vessel. Dashed arrows, arrow heads and dotted arrows indicate cellular infiltration, mucin and collagen, respectively. Original magnification: 100X for H&E and 200X for PAS and MT. Data (means ± s.e.m.) are representative from one of two independent experiments (n=5; ANOVA test followed by Tukey’s post hoc analysis or Student’s t-test, *P<0.05; **P<0.01; ***P<0.001).
Pharmacological induction of PA catabolism alleviates, while inhibition worsens asthmatic features, in mice with AAI, along with PA levels
Since both catabolic enzymes were reduced in asthma, we induced both of these enzymes together, in mice with AAI, using BENSPM, a potent pharmacological inducer of SAT1 (29,30) and SMOX (31). BENSPM competes with the natural PAs for uptake into the cell. Once inside, the analogue exploits the self-regulatory nature of the PA metabolic pathway, upregulating the catabolic pathways while downregulating biosynthesis through feedback inhibition. Western blots and IHC results confirmed the induction of SAT1 and SMOX in the lungs (Fig. 3A-B and Fig. S3, A-B). Moreover, BENSPM treatment significantly induced SAT1 activity in the lungs of mice (Fig. S3, C). Induction of PA catabolism reduced high levels of SPM and SPD in the lungs of mice with AAI (Fig. 3C). It also reduced AHR (Fig. 3D) and improved histopathological features, such as airway inflammation (Fig. 3E and F), goblet cell metaplasia, and sub-epithelial collagen deposition (Fig. 3E). Further, BENSPM treatment led to a reduction in BAL eosinophils and neutrophils to nearly undetectable levels (Fig. 3G). Interestingly, these mice also showed a marked reduction in TSLP levels (Fig. 3H), indicating alleviation of BEC stress. This reversal of asthma features and epithelial stress was associated with a striking reduction of ova-specific IgE (Fig. 3I) and pro-inflammatory cytokines, IL-4, IL-5 and IL-13 (Fig. 3J–L). Since a greater level of epithelial stress has been reported in severe asthmatics than in intermittent asthma patients (34), we sought to further increase the levels of SPM and SPD in the lungs of mice with AAI by using inhibitors of SAT1 and SMOX, BERENIL (32) and MDL72527 (33) (Fig. 1E: Set-III), respectively. As expected, we observed higher levels of PAs in the lungs of mice with AAI after either BERENIL or MDL72527 treatment (Fig. S4, A). Interestingly, this further increase in PAs was associated with aggravation of asthmatic features in mice with AAI (Fig. S4, B-E), including epithelial stress (Fig. S5, A), ova-specific IgE (Fig. S5, B) and pro-inflammatory cytokines levels (Fig. S5, C–E).
Figure 3.
Pharmacological induction of PA catabolism using BENSPM reduces PAs and alleviates asthmatic features in mice with AAI. (A and B) Representative Western blot and IHC images for the confirmation of induction of SAT1 and SMOX in mouse lungs with AAI (OVA/VEHICLE). (C) PA levels in whole lung lysates. (D) AHR to methacholine. (E) Representative images for airway inflammation, goblet cell metaplasia and sub-epithelial collagen deposition in the lungs. (F) Inflammation scores in the H&E-stained lung sections. (G) Differential cell counts in the BAL fluid. MAC, macrophages; LYM, lymphocytes; EOS, eosinophils; NEU, neutrophils. (H-L) Levels of TSLP, OVA-specific IgE and pro-inflammatory cytokines in the BAL fluid, serum and total lung lysates, respectively as measured by ELISA. Br, bronchus; V, vessel. Cellular infiltration, mucin and collagen in lungs is indicated by dashed arrows, arrow heads and dotted arrows, respectively. Original magnification in images: 100X for H&E and MT and 200X for PAS and IHC. Data (means ± s.e.m.) are representative from one of two independent experiments (n=6; ANOVA test followed by Tukey’s post hoc analysis or Student’s t-test, *P<0.05; **P<0.01; ***P<0.001).
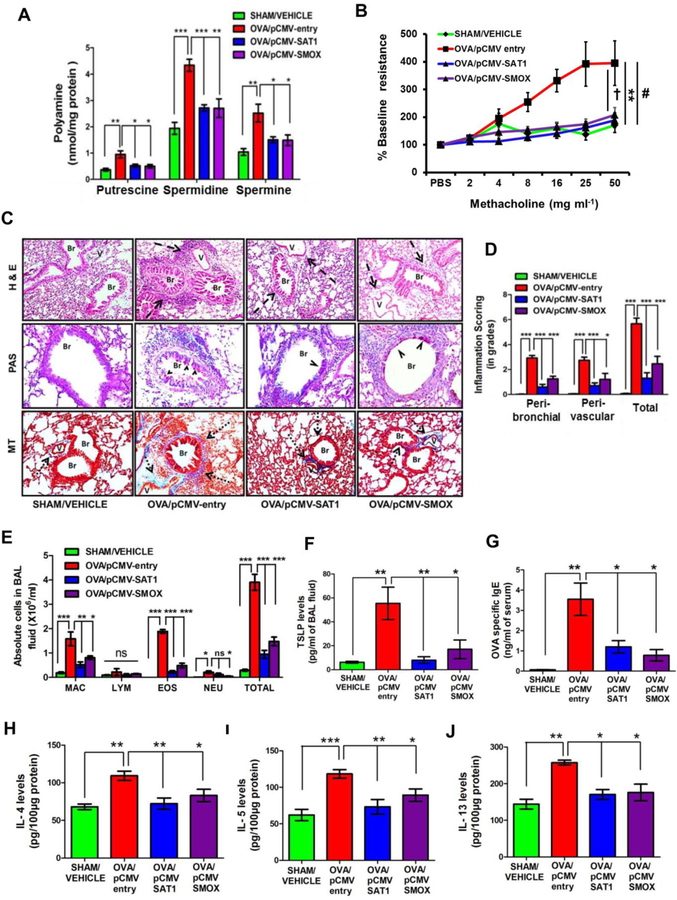
Overexpression of either SAT1 or SMOX reduces PAs and alleviates asthmatic features in mice with AAI
To further dissect the therapeutic effects of inducing PA catabolism in asthma, we tested the individual contribution of SAT1 and SMOX in reversing asthmatic features. For this, we intravenously administered SAT1- or SMOX-overexpressing plasmids in mice with AAI (Fig.1E: Set-IV). Western blot analysis and IHC results (Fig. S6, A–B) confirmed the overexpression of SAT1 and SMOX in the lungs. Importantly, overexpression of either SAT1 or SMOX resulted in a significant decrease in PAs (Fig. 4A) and AHR (Fig. 4B) that was associated with reductions in airway inflammation, airway remodeling, airway eosinophilia, and in the levels of TSLP, ova-specific IgE, IL-4, IL-5 and IL-13 (Fig. 4C–J). Absence of IFN-β induction in these mouse lungs excluded the possibility of non-specific effects by plasmid administration (Fig. S6, C).
Figure 4.
Overexpression of either SAT1 or SMOX reduces PAs and alleviates asthmatic features in mice with AAI. (A and B) Polyamine levels in the whole lung lysates and AHR of lungs to methacholine in mice with AAI (OVA/pCMV-entry) and SAT1 or SMOX overexpression plasmids. (C) Representative images of airway inflammation, goblet cell metaplasia and sub-epithelial collagen deposition in the lungs. (D) Inflammation scores in the H&E-stained lung sections. (E) Differential cell counts in the BAL fluid. MAC, macrophages; LYM, lymphocytes; EOS, eosinophils; NEU, neutrophils. (F-J) Levels of TSLP, OVA-specific IgE and pro-inflammatory cytokines in the BAL fluid, serum and total lung lysates, respectively. Br, bronchus; V, vessel. Dashed arrows, arrow heads and dotted arrows indicate cellular infiltration, mucin and collagen, respectively. Original magnification in images: 100X for H&E, MT and 200X for PAS. Data (means ± s.e.m.) are representative from one of two independent experiments (n=6; ANOVA test followed by Tukey’s post hoc analysis or Student’s t-test, *P<0.05; **P<0.01; ***P<0.001), (In panel B, for AHR data, **P<0.01 for SHAM/VEHICLE versus OVA/pCMV-entry, #P <0.05 for OVA/pCMV-entry versus OVA/pCMV-SAT1 and †P <0.05 for OVA/pCMV-entry versus OVA/pCMV-SMOX group).
SPM accumulation in the lungs positively correlates with the apoptosis of BECs
To determine the effect of accumulated PAs on cell stress, we used Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays in our murine models of AAI and in naïve mice with different modulators of PA catabolism. Interestingly, we observed predominant apoptosis of the BECs in the lungs, where the PAs were found to be accumulated, i.e., after knockdown of SAT1 and SMOX (Fig. 5A). However, reductions in the levels of apoptosis were observed where the levels of PAs were reduced after BENSPM treatment (Fig. 5B) or overexpression of SAT1 or SMOX (Fig. 5C). Additionally, more BEC apoptosis was observed in MDL72527- and BERENIL-treated AAI mice than in vehicle-treated AAI mice (Fig. S7, A). Quantification of apoptosis and its association with PA levels indicated a pro-apoptotic role of accumulated PAs in BECs. We correlated the levels of the individual PAs with the total number of apoptotic BECs in the lungs. Interestingly, of all of the PAs altered, SPM showed the strongest correlation with BEC apoptosis (Pearson’s correlation r value = 0.8148, r2 = 0.6639 and p<0.0001); conversely, SPD and PUT were not well correlated with apoptosis (correlation r values < 0.5) (Fig. 5D). As previous studies showed an active role of mitochondrial dysfunction in epithelial injury (35,36), we measured the levels of cytochrome c (cyto c) and cleaved-caspase-3 (c-csp-3) in lung cytosols. Interestingly, the trends in cyto c and c-csp-3 levels were similar to that observed with apoptosis of BECs (Fig. 5E-G and Fig. S7, B–E).
Figure 5.
SPM accumulation in the lungs positively correlates with the apoptosis of BECs. (A-C) Representative images of TUNEL assay and quantification of TUNEL-positive BECs after SAT1 and SMOX modulations in mice, i.e., after (A) knock down by shRNA, (B) induction using BENSPM and (C) overexpression by their respective plasmids. (D) Linear regression plots and Pearson’s correlation coefficients for the percentage of apoptotic BECs with respect to SPM, SPD and PUT levels in the lungs for different murine models used in this study. (E-G) Representative Western blots of cyto c and c-csp-3 in the lung cytosols after SAT1 and SMOX modulations, i.e., after (E) knock down by shRNA, (F) induction using BENSPM and (G) overexpression by plasmids. Br, bronchus. Arrows indicate TUNEL-positive BEC nuclei in brown (original magnification, 1000X). Data (means ± s.e.m.) are representative from one of two independent experiments (n=5–6; ANOVA test followed by Tukey’s post hoc analysis, *P<0.05; **P<0.01; ***P<0.001).
SPM is the most active PA in inducing apoptosis of human BECs
After finding a strong correlation between accumulation of SPM and apoptosis of BECs in the lungs, we tested the causal role of each PA in inducing apoptosis of BEAS-2B cells by culturing them with different concentrations of PUT, SPD and SPM. The extent of apoptosis was quantified using AnnexinV/7-AAD assay. Of the PAs, SPM was found to be the most active PA in inducing the apoptosis of BEAS-2B, followed by SPD, whereas, PUT was ineffective (Fig. 6A and B). To determine if the apoptosis of BEAS-2B was due to the dysfunction of mitochondria, we measured cyto c, in the cytosols of BEAS-2B. Cyto c levels were increased with increasing concentrations of SPM (Fig. 6C). Moreover, the cleavage of csp-3 showed a concentration-dependent increase (Fig. 6D).
Figure 6.
SPM is the most active PA in inducing apoptosis of human BECs. (A) Fold change in the apoptosis of BEAS-2B cells that were treated with different concentrations of PUT, SPD and SPM for 24 hours, with respect to non-treated control cells. (B) Representative dot plots for the most active PA, SPM, quantified in panel A; Quadrants Q1 and Q2 together show the total apoptotic cells. (C and D) Levels of cyto c in the cytosols and flow cytometry analysis of c-csp-3 in BEAS-2B cells after treatment with different concentrations of SPM. Data (means ± s.e.m.) are representative from one of at least three independent experiments (ANOVA test followed by Tukey’s post hoc analysis, *P<0.05; **P<0.01; ***P<0.001).
Discussion
Normally, PAs are tightly maintained at levels optimal for cellular functions. Alterations in PA levels, either high (21,23,24,30,37,38) or low (21,39) have been associated with various diseases. In asthma, despite the evidence for increased PAs, a mechanistic understanding has remained elusive.
In this study, we report for the first time that PA catabolism is downregulated in asthma (predominantly in the BECs) and resulting high levels of SPM induce BEC stress and apoptosis. In the normal lungs of mouse and human, we observed higher expressions of SAT1 and SMOX in the BECs, in comparison to other structural cells. Though inflammatory cells in the lung sections of asthmatic patients and mice with AAI have shown similar expression of SAT1; SMOX expression was moderate in the inflammatory cells of human lungs in comparison to mouse. Hence downregulation of SAT1 and SMOX in BECs may lead to the accumulation of PAs, as was seen in lungs of mice with AAI. Additionally, another PA catabolic enzyme diamine oxidase (DAO) utilizes substrate PUT but not SPD or SPM. Moreover, the activity of DAO is very low in the lungs (almost negligible) (40). Therefore, the contribution of SAT1 and SMOX is most important in context of asthma. Further, significant apoptosis of BECs after SAT1 or SMOX knockdown in naïve mice lungs indicates a crucial role of PA catabolism in the maintenance of lung epithelial homeostasis. Moreover, SAT1 was reported to be highly expressed in the normal epithelial cells from nasal brushings (41).
Interestingly, SAT1 or SMOX knockdown in naïve mice lungs increased PA levels and induced asthma-like features, supporting a causal role of SAT1 and SMOX in asthma pathophysiology. Similarly, SPM nebulization in naïve mice was reported to induce AHR (24). In airway smooth muscle cells (ASMCs), interaction of SAT1 with α9β1 integrin was shown to reduce AHR by catabolizing PAs locally (42). However, in the absence of integrin, this effect was not observed even after induction of SAT1. Moreover, SAT1 expression in ASMCs was not tested in asthma. In contrast to ASMCs, we found predominant reduction of SAT1 in BECs. Hence, the induction of asthma-like features after knockdown is likely due to BEC stress (Fig. 5A) (8,12). In contrast to knockdown experiments, overexpression of either SAT1 or SMOX alleviated AAI with BEC stress; this further strengthens the importance of both enzymes in asthma. Further, we found worsening of asthmatic features in AAI after SAT1 and SMOX inhibition using BERENIL and MDL72527, respectively, that was associated with higher levels of SPM, BEC stress and apoptosis. This aggravated response could be implicated in severe asthmatic conditions, where airway epithelial shedding is greater than in mild intermittent asthma (34).
Induction of PA catabolism appears to be a viable therapeutic approach in asthma. BENSPM treatment reduced PAs and potently alleviated AAI along with epithelial stress. The striking reduction in OVA-specific IgE levels after BENSPM treatment could be due to reduced TSLP levels, as TSLP stimulates T cells to express IL-4 (43) that is known to induce IgE synthesis from B cells in asthma (44). While it is not possible to definitively separate the interlinked inflammatory response and epithelial stress in AAI, the overall data is consistent with BENSPM alleviating BEC injury by reducing PA accumulation, thereby attenuating the release of TSLP and damping the well-known feed-forward loop between AAI and epithelial injury. Recently, an antibody against TSLP, markedly inhibited exacerbations in severe asthmatics and reduced IgE levels (9). Importantly, this effect was independent of asthma subtypes such as eosinophilic or neutrophilic. This suggests that therapeutic interventions targeting BEC stress and TSLP release may be most relevant for severe asthmatics. Restoring normal PA catabolism seems to be one such approach. Moreover, the effects of BENSPM treatment are consistent with observations in Parkinson’s disease model, where BENSPM induced SAT1 reduced PAs and alleviated disease pathology; while BERENIL worsened it (30). In contrast, in tumour lines, BENSPM induced PA catabolism from hundreds to thousands-fold and resulted in cytotoxicity (45,46). Whereas, in our study BENSPM did not induce SAT-1 activity to such an extent and thus reduced epithelial stress.
In a study by North et al., an attempt was made to inhibit PA biosynthesis using DFMO. However, that strategy could not reduce high levels of SPM and targeting ODC worsened AAI and peri-bronchial collagen deposition in mice, although it attenuated AHR (24). The reason for these unexpected results could be, (a) the observed unchanged levels of ODC in AAI; (b) inability of DFMO to consistently reduce SPM levels, due to additional compensatory mechanisms and incomplete inhibition of ODC (47,48). Thus, there was no effect on SPM levels in their model, and the observed changes in AHR could be because of SPD levels. SPM was the most effective PA in inducing AHR in their study and in inducing BEC injury in our study. In contrast, our strategy of using BENSPM did bring down the elevated levels of SPM and alleviated asthmatic features.
BECs are damaged and sensitive to apoptosis in the lungs of asthmatics (10,11) and in mice with OVA-induced AAI, as reported by us and others (12–14,49). Thus, we speculated that these high levels of PAs could lead to apoptosis of BECs. In our in vivo models with modulated PA catabolism, we found high levels of SPM to be well correlated with BECs apoptosis. Our studies with human BECs in culture demonstrated that SPM was the most active PA inducing apoptosis. The concentrations of SPM (10–100 µmol/L) inducing BEC apoptosis are physiologically relevant, as in asthmatic patients during attacks, upto 30 µmol/L SPM in the blood (23), and upto 316 nmol/g [~µmol/L] SPM in mouse lungs were reported (50).
The SPM induced apoptosis was associated with cyto c release from mitochondria and caspase-3 activation, and is in agreement with earlier studies, where SPM treatment to cells or cell-free preparations of mitochondria induced cyto c release (51) and activated caspase-3 (52). Although, we have demonstrated an association of mitochondrial dysfunction with SPM, the causation and mechanism for this effect remains to be elucidated.
Interestingly, in the context of growth and repair of gastrointestinal mucosa, depletion of PAs protected intestinal epithelial cells from apoptosis (53). Similarly, our results showed the diminished wound-healing, after SPM addition to BEAS-2B (Fig. S8), indicating its possible non-apoptotic role in controlling cell proliferation in addition to its apoptotic effects (Fig. 5). Such dual effects of SPM are likely to be context dependent, ranging from finer regulatory control in physiological processes such as healing, to injury and apoptosis in pathological conditions. Thus, moderately increased SPM levels may compromise epithelial wound repair, a known feature of asthma, while striking elevations may cause the epithelial injury. Thus, changes in polyamine metabolism could lead to asthma risk or perpetuate asthma-like changes, depending on context. This warrants further attention and investigation. In contrast, a recent study demonstrated that SPM prolongs eosinophil survival by inhibiting normal apoptosis (27). Therefore, the effects of PAs on cellular apoptosis may not be true for all cell types. Thus, it is conceivable that increased SPM levels can have direct and indirect effects, such as eosinophil survival, AHR induction and BECs damage, resulting in severe asthmatic conditions.
Importantly, elevated SPM levels were well-correlated with oxidative/nitrosative stress during obesity (54), suggesting that PA metabolism could be important in obese-asthma. The increasing prevalence of obese-asthma and its poor response to standard anti-inflammatory therapy provides an impetus for further exploration of this area. PA metabolism is known to be a valid drug target, as DFMO is approved for the treatment of Human African Trypanosomiasis and is currently in colorectal cancer chemoprevention trials (55,56). Thus, the reduction of PAs, through the induction of PA catabolism, represents a novel therapeutic target for asthma that holds considerable promise for further investigation.
Supplementary Material
Acknowledgments
VJ received research fellowship from DBT, Delhi, India. Authors acknowledge Dr. Shantanu Sengupta, Ajay Bhat for providing HPLC facility (IGIB) and Dr. K.M. Manjaiah, Dr. Kapil Chobhe for liquid scintillation counting facility at IARI, Delhi. The help by Sarita Mishra, Puneet Gupta, Sulagna Bhattacharya and Dr. Vijay Pal Singh, IGIB, are highly acknowledged. This work was supported by projects MLP5502 and BSC0016 (CSIR); GAP-84 (DST); GAP-0124 (Wellcome Trust); CA204345 (NIH).
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nature Medicine 2012;18:684–692. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012;18:673–683. [DOI] [PubMed] [Google Scholar]
- 3.Dunican EM, Fahy JV. Asthma and corticosteroids: time for a more precise approach to treatment. European Respiratory Journal 2017;49:1701167. [DOI] [PubMed] [Google Scholar]
- 4.Trevor JL, Deshane JS. Refractory asthma: Mechanisms, targets, and therapy. Allergy: European Journal of Allergy and Clinical Immunology 2014;69:817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirose K, Iwata A, Tamachi T, Nakajima H. Allergic airway inflammation: key players beyond the Th2 cell pathway. Immunological Reviews 2017;278:145–161. [DOI] [PubMed] [Google Scholar]
- 6.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clinical immunology (Orlando, Fla) 2012;143:222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell PD, O’Byme PM. Epithelial-Derived Cytokines in Asthma. Chest 2017;151:1338–1344. [DOI] [PubMed] [Google Scholar]
- 8.Zhou B, Comeau MR, Smedt T De, Liggitt HD, Dahl ME, Lewis DB et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nature Immunology 2005;6:1047–1053. [DOI] [PubMed] [Google Scholar]
- 9.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM et al. Tezepelumab in Adults with Uncontrolled Asthma. The New England journal of medicine 2017;377:936–946. [DOI] [PubMed] [Google Scholar]
- 10.NAYLOR B The shedding of the mucosa of the bronchial tree in asthma. Thorax 1962;17:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou C, Yin G, Liu J, Liu X, Zhao S. Epithelial apoptosis and loss in airways of children with asthma. The Journal of asthma: official journal of the Association for the Care of Asthma 2011;48:358–365. [DOI] [PubMed] [Google Scholar]
- 12.Mabalirajan U, Rehman R, Ahmad T, Kumar S, Singh S, Leishangthem GD et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Scientific reports 2013;3:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabalirajan U, Rehman R, Ahmad T, Kumar S, Leishangthem GD, Singh S et al. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Scientific reports 2013;3:1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorscheid DR, Low E, Conforti A, Shifrin S, Sperling AI, White SR. Corticosteroid-induced apoptosis in mouse airway epithelium: effect in normal airways and after allergen-induced airway inflammation. The Journal of allergy and clinical immunology 2003;111:360–366. [DOI] [PubMed] [Google Scholar]
- 15.Wallace HM, Fraser AV, Hughes A. REVIEW ARTICLE A perspective of polyamine metabolism. Biochemical Journal 2003;376:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiler N Catabolism of polyamines. Amino Acids 2004;26:217–233. [DOI] [PubMed] [Google Scholar]
- 17.Casero RA, Pegg AE. Spermidine/spermine N1-acetyltransferase--the turning point in polyamine metabolism. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 1993;7:653–661.0 [PubMed] [Google Scholar]
- 18.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM et al. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochemical and biophysical research communications 2003. ;304:605–611. [DOI] [PubMed] [Google Scholar]
- 19.Wallace HM, Fraser AV. Inhibitors of polyamine metabolism: Review article. Amino Acids 2004;26:353–365. [DOI] [PubMed] [Google Scholar]
- 20.Pegg AE. Functions of Polyamines in Mammals. Journal of Biological Chemistry 2016;291:14904–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging 2011;3:716–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seiler N, Raul F. Polyamines and apoptosis. Journal of cellular and molecular medicine 2005;9:623–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurosawa M, Shimizu Y, Tsukagoshi H, Ueki M. Elevated levels of peripheral-blood, naturally occurring aliphatic polyamines in bronchial asthmatic patients with active symptoms. Allergy 1992;47:638–643. [DOI] [PubMed] [Google Scholar]
- 24.North ML, Grasemann H, Khanna N, Inman MD, Gauvreau GM, Scott JA. Increased ornithine-derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. American journal of respiratory cell and molecular biology 2013;48:694–702. [DOI] [PubMed] [Google Scholar]
- 25.Kurosawa M, Uno D, Kobayashi S. Naturally occurring aliphatic polyamines-induced histamine release from rat peritoneal mast cells. Allergy 1991;46:349–354. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi T, Harada Y, Moriyama S, Furuta K, Tanaka S, Miyaji T et al. Vesicular Polyamine Transporter Mediates Vesicular Storage and Release of Polyamine from Mast Cells. The Journal of biological chemistry 2017;292:3909–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilmarinen P, Moilanen E, Erjefält JS, Kankaanranta H. The polyamine spermine promotes survival and activation of human eosinophils. The Journal of allergy and clinical immunology 2015;136:482–4.e11. [DOI] [PubMed] [Google Scholar]
- 28.Kinnula VL, Yankaskas JR, Chang L, Virtanen I, Linnala A, Kang BH et al. Primary and immortalized (BEAS 2B) human bronchial epithelial cells have significant antioxidative capacity in vitro. American Journal of Respiratory Cell and Molecular Biology 1994;11:568–576. [DOI] [PubMed] [Google Scholar]
- 29.Fogel-Petrovic M, Kramer DL, Vujcic S, Miller J, McManis JS, Bergeron RJ et al. Structural basis for differential induction of spermidine/spermine N1-acetyltransferase activity by novel spermine analogs. Molecular pharmacology 1997;52:69–74. [DOI] [PubMed] [Google Scholar]
- 30.Lewandowski NM, Ju S, Verbitsky M, Ross B, Geddie ML, Rockenstein E et al. Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proceedings of the National Academy of Sciences 2010;107:16970–16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaturvedi R, de Sablet T, Peek RM, Wilson KT, Wilson KT. Spermine oxidase, a polyamine catabolic enzyme that links Helicobacter pylori CagA and gastric cancer risk. Gut microbes 2012;3:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby PR, Porter CW. Inhibition of enzymes of polyamine back-conversion by pentamidine and berenil. Biochemical pharmacology 1992;44:830–832. [DOI] [PubMed] [Google Scholar]
- 33.Bellelli A, Cavallo S, Nicolini L, Cervelli M, Bianchi M, Mariottini P et al. Mouse spermine oxidase: a model of the catalytic cycle and its inhibition by N,N1-bis(2,3-butadienyl)-1,4-butanediamine. Biochemical and Biophysical Research Communications 2004;322:1–8. [DOI] [PubMed] [Google Scholar]
- 34.VIGNOLA AM, CHANEZ P, CAMPBELL AM, SOUQUES F, LEBEL B, ENANDER I et al. Airway Inflammation in Mild Intermittent and in Persistent Asthma. American Journal of Respiratory and Critical Care Medicine 1998;157:403–409. [DOI] [PubMed] [Google Scholar]
- 35.Mabalirajan U, Dinda AK, Kumar S, Roshan R, Gupta P, Sharma SK et al. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. Journal of immunology (Baltimore, Md: 1950) 2008;181:3540–3548. [DOI] [PubMed] [Google Scholar]
- 36.Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. Journal of immunology (Baltimore, Md: 1950) 2009;183:5379–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grasemann H, Shehnaz D, Enomoto M, Leadley M, Belik J, Ratjen F. L-Ornithine Derived Polyamines in Cystic Fibrosis Airways. PLoS ONE 2012;7:e46618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babbar N, Gerner EW. Targeting polyamines and inflammation for cancer prevention. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer 2011;188:49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soda K, Kano Y, Chiba F. Food polyamine and cardiovascular disease--an epidemiological study. Global journal of health science 2012;4:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuguchi H, Imamura I, Takemura M, Fukui H. Purification and characterization of diamine oxidase (histaminase) from rat small intestine. Journal of biochemistry 1994;116:631–635. [DOI] [PubMed] [Google Scholar]
- 41.Chu C-Y, Qiu X, Wang L, Bhattacharya S, Lofthus G, Corbett A et al. The Healthy Infant Nasal Transcriptome: A Benchmark Study. Scientific reports 2016;6:33994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C, Kudo M, Rutaganira F, Takano H, Lee C, Atakilit A et al. Integrin α9β1 in airway smooth muscle suppresses exaggerated airway narrowing. Journal of Clinical Investigation 2012;122:2916–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. Journal of immunology (Baltimore, Md: 1950) 2007;178:1396–1404. [DOI] [PubMed] [Google Scholar]
- 44.Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. The Journal of allergy and clinical immunology 2000;105:S547–58. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Kramer DL, Diegelman P, Vujcic S, Porter CW. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer research 2001;61:6437–6444. [PubMed] [Google Scholar]
- 46.Shappell NW, Miller JT, Bergeron RJ, Porter CW. Differential effects of the spermine analog, N1, N12-bis(ethyl)-spermine, on polyamine metabolism and cell growth in human melanoma cell lines and melanocytes. Anticancer research;12:1083–1089. [PubMed] [Google Scholar]
- 47.Pegg AE, McCann PP. Polyamine metabolism and function. The American journal of physiology 1982;243:C212–21. [DOI] [PubMed] [Google Scholar]
- 48.Muth A, Madan M, Archer JJ, Ocampo N, Rodriguez L, Phanstiel O. Polyamine transport inhibitors: design, synthesis, and combination therapies with difluoromethylornithine. Journal of medicinal chemistry 2014;57:348–363. [DOI] [PubMed] [Google Scholar]
- 49.Mabalirajan U, Ahmad T, Rehman R, Leishangthem GD, Dinda AK, Agrawal A et al. Baicalein reduces airway injury in allergen and IL-13 induced airway inflammation. PloS one 2013;8:e62916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teixeira D, Santaolaria ML, Meneu V, Alonso E. Dietary arginine slightly and variably affects tissue polyamine levels in male swiss albino mice. The Journal of nutrition 2002;132:3715–3720. [DOI] [PubMed] [Google Scholar]
- 51.Stefanelli C, Stanic ‘ I, Zini M, Bonavita F, Flamigni F, Zambonin L et al. Polyamines directly induce release of cytochrome c from heart mitochondria. The Biochemical journal 2000;347 Pt 3:875–880. [PMC free article] [PubMed] [Google Scholar]
- 52.Stefanelli C, Bonavita F, Stanic’ I, Pignatti C, Flamigni F, Guarnieri C et al. Spermine triggers the activation of caspase-3 in a cell-free model of apoptosis. FEBS letters 1999;451:95–98. [DOI] [PubMed] [Google Scholar]
- 53.Yuan Q, Ray RM, Johnson LR. Polyamine depletion prevents camptothecin-induced apoptosis by inhibiting the release of cytochrome c. American journal of physiology Cell physiology 2002;282:C1290–7. [DOI] [PubMed] [Google Scholar]
- 54.Codoner-Franch P, Tavarez-Alonso S, Murria-Estal R, Herrera-Martin G, Alonso-Iglesias E. Polyamines Are Increased in Obese Children and Are Related to Markers of Oxidative/Nitrosative Stress and Angiogenesis. The Journal of Clinical Endocrinology & Metabolism 2011;96:2821–2825. [DOI] [PubMed] [Google Scholar]
- 55.Burchmore RJS, Ogbunude POJ, Enanga B, Barrett MP. Chemotherapy of human African trypanosomiasis. Current pharmaceutical design 2002;8:256–267. [DOI] [PubMed] [Google Scholar]
- 56.Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer prevention research (Philadelphia, Pa) 2008;1:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.