ABSTRACT
Background
Consumption of green tea has been associated with reduced risk of breast cancer. Hormonal modulation has been suggested as one of the potential underlying mechanisms; however, it has yet to be fully elucidated in large, long-term human clinical trials.
Objective
We investigated the effects of decaffeinated green tea extract (GTE) on circulating sex hormones and insulin-like growth factor (IGF) proteins.
Methods
We conducted a placebo-controlled double-blind randomized clinical trial recruiting from 8 clinical centers in Minnesota. Participants were 538 healthy postmenopausal women randomly assigned to the GTE group (463 completed the study; mean age = 60.0 y) and 537 to the placebo group (474 completed; mean age = 59.7 y). Women in the GTE group orally took 4 decaffeinated capsules containing 1315 mg total catechins including 843 mg epigallocatechin-3-gallate daily for 1 y, whereas women in the placebo group took similar capsules containing no tea catechins. Blood sex hormones (estrone, estradiol, androstenedione, testosterone, and sex hormone-binding globulin) and IGF proteins (IGF-1 and IGF binding protein-3) were quantified at baseline and months 6 (for IGF proteins only) and 12, and were assessed as secondary outcomes of the study using a mixed-effect repeated-measures ANOVA model.
Results
Women in the GTE group had significantly higher blood total estradiol (16%; P = 0.02) and bioavailable estradiol (21%; P = 0.03) than in the placebo group at month 12. There was a statistically significant interaction between GTE supplementation and duration of treatment on estradiol and bioavailable estradiol (both Ps for interaction = 0.001). The catechol-O-methyltransferase genotype did not influence blood sex hormones before or after GTE supplementation. The circulating concentrations of IGF proteins were comparable between GTE and placebo groups at all 3 time points.
Conclusion
These results suggest that a 12-mo GTE supplementation significantly increases circulating estradiol concentrations in healthy postmenopausal women. This trial was registered at clinicaltrials.gov as NCT00917735.
Keywords: green tea extract, catechins, sex hormones, insulin-like growth factors, postmenopausal women, breast cancer
Introduction
Breast cancer mortality dropped by 39% in the United States between 1989 and 2015, yet no significant reduction in breast cancer incidence has occurred. Substantial evidence suggests that sex hormones may play a carcinogenic role in the development of breast cancer (1), particularly among postmenopausal women, by stimulating cell proliferation and mutagenesis (2, 3). Consistent with this are data showing that decreased use of postmenopausal hormone therapy results in decreased breast cancer incidence (4).
The insulin-like growth factor (IGF) system consists of several proteins including IGF-1 and its most abundant binding protein, IGF binding protein-3 (IGFBP-3) (5). IGF-1 is believed to promote carcinogenesis by stimulating mitosis, increasing DNA synthesis, and inhibiting apoptosis (6). A meta-analysis of 17 prospectively designed studies showed that higher serum concentration of IGF-1 was associated with higher risk of breast cancer (7).
Catechins, the major polyphenolic compounds in green tea, have been investigated extensively for their role in the prevention of chronic diseases including cancer (8). (-)-Epigallocatechin gallate (EGCG) is the most studied and abundant catechin, accounting for 50–80% of the total catechins in green tea (9, 10). The major metabolic pathways of catechins are methylation [via catechol-O-methyltransferase (COMT)], glucuronidation [via uridine diphosphate-glucuronosyltransferases (UGTs)], and sulfation (via sulfotransferases) in the small intestine with additional transformation in the liver, kidneys, and blood (8, 11).
Multiple in vitro and animal model studies have shown that green tea catechins can inhibit malignant transformation of mammary cells, prolong the latency period of mammary tumor development, and increase survival time of animals with mammary tumors (12–17), although epidemiological studies have produced inconsistent results (18–25). Suggested mechanisms include those mediated by hormonal pathways (26, 27). Several in vitro and in vivo studies (28–30) have shown that green tea, in particular EGCG, has modulatory effects on aromatase enzyme. Furthermore, EGCG competes with 17-β-estradiol for binding to both estrogen receptor (ER)-α and -β (31), reduces ER-α level in tumors both in vitro and in vivo (32), and downregulates the ER-α pathway in MCF-7 breast carcinoma cells (33). To date, very few human studies (34–38) have investigated the effects of green tea intake on circulating sex hormones and IGF axis proteins.
Genetic variations in COMT enzymatic activity have been studied in relation to catechin metabolism and breast cancer risk. One particular single nucleotide polymorphism (SNP), a guanine (G) to adenine (A) substitution in codon 108 of the soluble form or codon 158 of the membrane-bound form of COMT (SNP rs4680), causes a 300–400% decrease in enzymatic activity (39–41) due to increased thermolability (42). One case-control study reported that the inverse association between green tea consumption and breast cancer risk was seen primarily in Asian-American women with this SNP (43), leading to the hypothesis that these individuals receive greater benefits from green tea consumption due to slower metabolism of catechins and longer retention of the bioactive components. This polymorphism has also been reported to increase accumulation of potentially carcinogenic endogenous catechol estrogen intermediates (44, 45). However, studies assessing the effects of COMT genotype on both tea catechin metabolism (38, 46–49) and breast cancer risk (50) have yielded inconsistent results.
The objective of the present study was to investigate the effects of a daily dose of 1315 mg total catechins or 843 mg EGCG for 12 mo on circulating concentrations of sex hormones including estrone, estradiol, androstenedione, testosterone, and sex hormone-binding globulin (SHBG), and IGF-1 and IGFBP-3, in healthy postmenopausal women at high risk of breast cancer due to high breast density with different COMT genotypes. We hypothesized that green tea extract (GTE) intake would reduce blood concentrations of sex hormones and IGF-1, and increase SHBG and IGFBP-3 concentrations, in a direction consistent with prevention of breast cancer.
Methods
Study design and population
The study design and methods have been previously described in detail (51). Briefly, the Minnesota Green Tea Trial (MGTT) was a phase II randomized, double-blind, placebo-controlled trial to examine the effects of GTE supplementation on well-established biomarkers of breast cancer risk.
Study participants were recruited from 8 clinical centers in Minnesota during 2009–2013. Annual screening mammogram reports were reviewed and women aged 50–70 y at high risk of breast cancer due to “heterogeneously dense” or “extremely dense” breasts (breast tissue designated as ∼51–75% glandular or >75% glandular, respectively) as specified by the American College of Radiology (52) were considered for further screening. Eligible participants were also healthy, nonsmoking postmenopausal women who were not regular consumers of green tea (>1 cup/wk) or currently using hormone modification therapy including aromatase inhibitors, selective estrogen receptor modulators or systemic menopausal hormone therapy, or chemopreventive agents (within the last 6 mo). Additional eligibility criteria include alcohol consumption ≤7 standard drinks/wk [1 alcoholic drink size was defined as 12 ounces (355 mL) of regular beer, 5 ounces (148 mL) of wine, or 1.5 ounces (44 mL) of distilled spirits], BMI (in kg/m2) 18.5–40, stable weight for the past year (<4.5 kg change), no history of breast cancer or proliferative breast disease or breast implant, and normal liver alanine aminotransferase (ALT) concentrations at baseline (<1.5 times of the upper limit of normal, defined as 60 U/L in this study and used by Quest Diagnostics Laboratory, Wood Dale, IL).
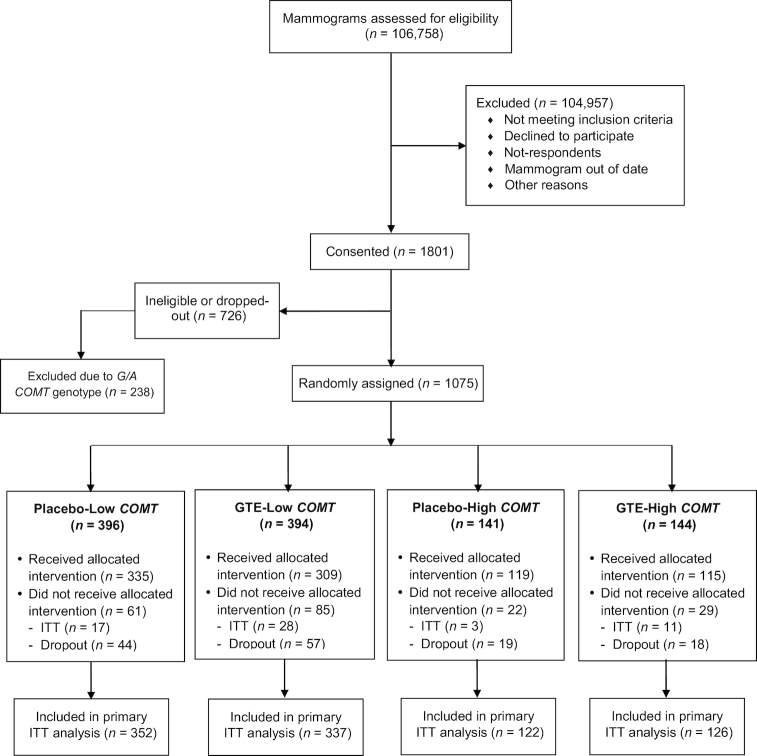
The random assignment was performed by the Investigational Drug Services pharmacy at the University of Minnesota Medical Center—Fairview using a permuted block method by treatment group and stratified by the COMT genotype [low activity (A/A or G/A) or high activity (G/G)]. We randomly assigned 1075 women into either the GTE (n = 538) or placebo (n = 537) group and further stratified by the COMT genotype into a total of 4 groups: placebo-low COMT, GTE-low COMT, placebo-high COMT, and GTE-high COMT genotype activities (Figure 1). To enroll equal number of participants with high- and low-activity COMT genotypes, we stopped randomly assigning additional participants with the COMT G/A genotype at some point toward the end of the study. As a result, we excluded 238 potential participants with the COMT G/A genotype from random assignment to allow more participants with the COMT G/G genotype to enter the study.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of postmenopausal women participants from screening to completion of the Minnesota Green Tea Trial. COMT, catechol-O-methyltransferase; GTE, green tea extract; ITT, intention-to-treat.
Participants came in monthly to the clinic for collection of biological samples and were instructed to maintain their normal diets and lifestyles. Each participant completed a health history questionnaire at the baseline including questions on demographics, lifestyle factors, and information about medical and reproductive history. In addition, each woman completed the Diet History Questionnaire I, a validated FFQ developed by the National Cancer Institute, within 1 wk of the baseline and end of the intervention. This questionnaire includes 124 food items and collects daily dietary intake with details on portion size and frequency of intake over the last year.
Written informed consent was obtained from all subjects before the screening visit, and the Institutional Review Boards of the University of Minnesota and other participating universities or institutes approved the study.
Study supplement, compliance assessment, and adverse events
Green Tea Extract Catechin Complex (Corban Complex GTB, referred to as GTE in this publication) and placebo capsules were supplied by Corban Laboratories (Eniva Nutraceutics). Mean ± SD total catechin content of each GTE capsule was 329 ± 29 mg, including 211 ± 11 mg EGCG, 27 ± 30 mg epigallocatechin (EGC), 51 ± 19 mg epicatechin gallate (ECG), and 27 ± 6 mg epicatechin (EC) (51). The GTE capsule was decaffeinated with <4 mg caffeine. The total catechin and EGCG dosage was approximately equivalent to five 8-ounce (240 mL) cups of brewed green tea per day (53). The placebo capsule appeared identical to the green tea capsule but contained 50% maltodextrin (204 mg), 49.5% microcrystalline cellulose (202 mg), 0.5% magnesium stearate (2 mg), and no caffeine.
The Investigational Drug Services pharmacy dispensed study supplements and clinic staff distributed capsules to each participant at 3-mo intervals. Participants were asked to store pills in a cool and dry place and to return all unused capsules in the original bottles to the study staff for disposal. Participants took 2 capsules in the morning with breakfast and 2 capsules in the evening with dinner. A detailed description of compliance with the GTE supplementation and a comprehensive report of the adverse events which occurred have been previously published (51, 54). Briefly, compliance to the intervention was assessed by 3 different approaches: 1) pill count, in which the compliance was calculated based on the number of returned pills and the actual number of pills that each participant should have consumed; 2) pill diary, by which each woman completed a pill log recording the date and time of consuming capsules, which was being monitored by the study staff throughout the trial; and 3) assessing the urinary concentrations of EGC and EC in a random selection of 10% of participants during the 1-y intervention (GTE: n = 43; placebo: n = 47). Participants also reported information regarding any adverse events at each clinic visit or through phone calls and emails, and all observed adverse events were recorded and graded according to the guidelines provided by the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.03. In the case of any serious adverse event (defined as fatal, life threatening, required hospital stay, or resulted in persistent or significant disability or incapacity) the study was immediately terminated for the participant.
COMT genotyping and measurement of hormones and growth factors
COMT genotyping was performed on DNA extracted from baseline buffy coats of peripheral blood samples using a DNeasy Blood & Tissue Kit column method (Qiagen) according to the manufacturer's protocol. Using the TaqMan assay, a genotyping assay was developed for the G/A transition at codon 158, rs4680 (55). The TaqMan assays were performed using a TaqMan PCR Core Reagent kit (Applied Biosystems) in accordance with the manufacturer's instructions.
Sex hormones including estradiol, estrone, androstenedione, and testosterone were quantified at the Quest Diagnostics Nichols Institute (San Juan Capistrano, CA) by LC/tandem MS (LC/MS/MS) on a Finnigan TSQ Quantum Ultra LC/MS/MS instrument (ThermoFisher) after extraction and separation procedures (56). Free and bioavailable concentrations of estradiol and testosterone were calculated from total estradiol and testosterone, and SHBG using a validated algorithm and absolute individual serum albumin concentrations (57, 58), but only data for bioavailable fractions are reported here. Assay sensitivities were 2 pg/mL for estradiol, 10 pg/mL for estrone, 5 ng/dL for androstenedione, and 1 ng/dL for testosterone. For hormone values below the detection limit, we assigned the value to half the minimum detectable limit. In each batch, 3–4 blinded quality control serum samples were inserted to evaluate laboratory assay variability. The average intra-assay CVs across all batches were 11.0% for estrone, 20.5% for estradiol, 11.6% for androstenedione, and 13.1% for testosterone. Interassay variabilities were 14.4%, 26.1%, 13.7%, and 15.9% for estrone, estradiol, androstenedione, and testosterone, respectively.
Concentration of SHBG was quantified via the Human SHBG Quantikine ELISA Kit (R&D Systems). The final colorimetric analysis was done on a Synergy H1 Plate Reader (Biotek). Plates were read at 450 nm with a background wavelength of 570 nm and results were calculated using linear regression analysis after log/log transformation. Each sample was analyzed in duplicate and the mean value of 2 measures was used in the final statistical analysis. In addition, a quality control sample was analyzed with each batch to determine reproducibility of the method. Intra- and interassay CVs were 6.9% and 10.8%, respectively.
IGF-1 and IGFBP-3 were quantified using Human Quantikine ELISA kits (R&D Systems) in duplicates with a quality control sample in each batch. The intra- and interassay CVs were 1.7% and 6.5% for IGF-1, and 1.6% and 4.4% for IGFBP-3, respectively.
Sex steroid hormones and SHBG were assayed on fasting morning serum samples collected at baseline and month 12 from all participants and also on a subset of 371 participants (184 in GTE and 187 in placebo group) at month 6. IGF-1 and IGFBP-3 were measured on fasting plasma at baseline and months 6 and 12 from all participants. All samples from each participant and an equal number of samples from the GTE and placebo groups were measured in the same analysis batch with laboratory technicians blinded to the study treatment status. ALT concentrations were measured on nonfasting serum samples using spectrophotometry methodology by Quest Diagnostics Laboratory (Wood Dale, IL).
Statistical analysis
Statistical analysis was conducted on logarithmically transformed values and data are presented as geometric means because hormones were nonnormally distributed. Departure from a Hardy-Weinberg equilibrium was tested using the chi-square test. Pearson partial correlation coefficients adjusted for age and BMI were used to evaluate the correlations between baseline circulating concentrations of sex hormones and IGF proteins.
We used a mixed-effects repeated-measures ANOVA model with adjustment for age and BMI at baseline and taking baseline value as the covariate (59) to assess the overall effect of GTE treatment on circulating concentrations of sex hormones and IGFs over the 12-mo treatment period with simultaneous comparisons of endpoint measures at baseline and month 12 (and month 6 where appropriate for IGF proteins). The same mixed-effect models allowed for the test of a potential interaction effect between GTE treatment and time point on the hormonal measurements. Besides age and BMI, the mixed-effect ANOVA models included COMT genotype, past tea drinking status, and years since last menstrual period at baseline. The modifying effects of COMT genotype on sex hormone concentrations were similarly examined using ANOVA for measurements of hormones at baseline alone or mixed-effect models for those during GTE treatment. One-factor ANOVA was used to compare geometric means of sex hormone concentrations across different COMT genotypes and age groups. P values for interaction of GTE treatment and age on the hormonal changes were calculated by a generalized linear model while adjusting for BMI and hormone concentrations at baseline.
The MGTT was originally designed to have 81% power to detect a 3.4% reduction in percentage mammographic density as the study primary endpoint with a total sample size of 800 (400 per arm) assuming a 2-sided significance level of 0.05. However, in the post hoc power calculations based on parameter estimates from the results of our previous studies (60, 61), the present study with a total sample size of 936 (468 per treatment group), and assuming a 2-tailed α of 0.05, had an 80% statistical power to detect a 10.9% change in the circulating concentrations of estrone and a 4.5% change of IGF-1 by GTE as the secondary outcomes of the MGTT. We had limited statistical power to detect an interaction between GTE supplementation and COMT genotype on sex hormones because of a relatively small number of subjects with the high-activity COMT genotype.
Data analysis was conducted according to the intention-to-treat principle. All statistical analyses including sample size calculation were performed using the SAS version 9.4 (SAS Institute Inc.) statistical software package. All reported P values are 2-sided. P values <0.05 were considered statistically significant.
Results
Figure 1 details the recruitment, random assignment scheme, and participant flow through the trial. More than 100,000 women's mammograms were screened for eligibility and 1075 were finally randomly assigned into the study by treatment group and stratified by the COMT genotype.
In total, 937 women completed the clinical trial, whereas 138 (12.8%) women dropped out from the trial. Two women did not provide blood samples at month 6. Therefore, the present analysis included 935 women for IGF protein evaluations: 462 in the GTE and 473 in the placebo group. Sex hormone results are available for all 937 women.
Overall, participants tolerated the GTE supplementation well without significant differences in the percentage of women with adverse events between the study groups (54). More than 98% of the reported adverse events were mild (grade I or II according to the Common Terminology Criteria for Adverse Events guidelines) and the most common categories of adverse events were infections/infestations and gastrointestinal disorders. When adverse events were further examined by the gastrointestinal and elevated liver enzyme concentration cases, it was shown that significantly higher numbers of participants in the GTE group experienced nausea (P = 0.001) and elevated concentrations of ALT (P < 0.001) than in the placebo group. In contrast, the number of women reporting diarrhea was greater in the placebo group than in the GTE group (P = 0.02).
As previously reported (51), baseline demographics, lifestyle characteristics, and energy and nutrient intakes did not differ between the GTE and placebo groups, although participants in the GTE group had a higher intake of vitamin supplement per day than those in the placebo group.
BMI at baseline was positively associated with estrone (r = 0.24) and estradiol (r = 0.26) but negatively associated with SHBG (r = −0.37), IGF-1 (r = −0.10), and IGFBP-3 (r = −0.14) (all Ps ≤ 0.0001) (data not shown). Table 1 shows age- and BMI-adjusted partial correlation coefficients between measurements of circulating sex steroid hormones and IGF proteins. Estrone and estradiol were highly correlated (r = 0.95), whereas androstenedione and testosterone (r = 0.43) and IGF-1 and IGFBP-3 (r = 0.46) were moderately correlated (all Ps ≤ 0.005).
TABLE 1.
Pearson partial correlation coefficients (r) between baseline circulating sex steroid hormones and IGF proteins in postmenopausal women1
| Sex hormone or IGF protein | Estrone | Estradiol | Bioavailable estradiol | Androstenedione | Testosterone | Bioavailable testosterone | SHBG | IGF-1 | IGFBP-3 |
|---|---|---|---|---|---|---|---|---|---|
| Estrone | 1.00 | 0.95* | 0.96* | 0.09* | 0.07** | 0.05 | 0.01 | −0.01 | −0.01 |
| Estradiol | — | 1.00 | 0.99* | 0.06 | 0.03 | 0.01 | 0.02 | −0.01 | −0.02 |
| Bioavailable estradiol | — | — | 1.00 | 0.06 | 0.02 | 0.03 | −0.02 | −0.003 | −0.02 |
| Androstenedione | — | — | — | 1.00 | 0.43* | 0.42* | −0.07** | 0.10* | 0.004 |
| Testosterone | — | — | — | — | 1.00 | 0.80* | 0.10 * | 0.06 | 0.003 |
| Bioavailable testosterone | — | — | — | — | — | 1.00 | −0.37* | 0.12* | 0.09** |
| SHBG | — | — | — | — | — | — | 1.00 | −0.18* | −0.14* |
| IGF-1 | — | — | — | — | — | — | — | 1.00 | 0.46* |
| IGFBP-3 | — | — | — | — | — | — | — | — | 1.00 |
1 n = 937. Values are adjusted for age and BMI at baseline. *,**Significant correlation: *P ≤ 0.005, **P < 0.05. IGF, insulin-like growth factor; IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein 3; SHBG, sex hormone-binding globulin.
Table 2 presents results for the effects of GTE on circulating concentrations of sex steroid hormones. Both total and bioavailable estradiol were significantly higher in the GTE group than in the placebo group (P = 0.02 and P = 0.03, respectively) at month 12. There was a statistically significant interaction effect between treatment and time on total and bioavailable estradiol (both Ps ≤ 0.001). Furthermore, there was a statistically significant interaction effect between GTE treatment and the duration of treatment on total and bioavailable testosterone (both Ps ≤ 0.01). The concentrations of estrone and androstenedione in both the placebo and GTE groups were reduced over the 12-mo study period. GTE supplementation had no overall or modifying effect on circulating concentrations of estrone, androstenedione, or SHBG (Table 2). Similar results were obtained when the analyses included the measurements at month 6 on a subset of study participants (184 in GTE and 187 in placebo) (data not shown).
TABLE 2.
Circulating concentrations of sex steroid hormones at baseline and month 12 by treatment group in postmenopausal women randomly assigned to the GTE or placebo group1
| Sex hormone and time point | GTE (n = 463) | Placebo (n = 474) | P 2 | P 3 | P 4 |
|---|---|---|---|---|---|
| Estrone, pg/mL | — | — | 0.32 | 0.31 | |
| Baseline | 22.7 (21.7, 23.7) | 23.8 (22.7, 24.8) | 0.12 | ||
| Month 12 | 22.3 (21.2, 23.5) | 22.6 (21.5, 23.7) | 0.86 | ||
| Estradiol, pg/mL | — | — | 0.63 | 0.001 | |
| Baseline | 3.3 (3.1, 3.7) | 3.7 (3.4, 4.1) | 0.10 | ||
| Month 12 | 3.7 (3.4, 4.1) | 3.2 (2.9, 3.5) | 0.02 | ||
| Bioavailable estradiol, pg/mL | — | — | 0.78 | 0.001 | |
| Baseline | 1.5 (1.4, 1.7) | 1.7 (1.5, 1.9) | 0.07 | ||
| Month 12 | 1.7 (1.5, 1.9) | 1.4 (1.3, 1.6) | 0.03 | ||
| Androstenedione, pg/mL | — | — | 0.69 | 0.19 | |
| Baseline | 488 (480, 496) | 499 (491, 507) | 0.08 | ||
| Month 12 | 477 (461, 493) | 472 (456, 488) | 0.91 | ||
| Testosterone, pg/mL | — | — | 0.63 | 0.01 | |
| Baseline | 154 (150, 158) | 159 (155, 163) | 0.09 | ||
| Month 12 | 161 (155, 167) | 153 (147, 159) | 0.10 | ||
| Bioavailable testosterone, pg/mL | — | — | 0.69 | 0.01 | |
| Baseline | 39.3 (38.1, 40.6) | 40.8 (39.6, 42.1) | 0.12 | ||
| Month 12 | 41.6 (39.8, 43.5) | 39.4 (37.7, 41.2) | 0.13 | ||
| SHBG, nmol/L | 0.42 | 0.46 | |||
| Baseline | 67.9 (66.9, 69.0) | 69.2 (68.2, 70.3) | 0.08 | ||
| Month 12 | 68.5 (66.5, 70.6) | 68.8 (66.8, 70.9) | 0.88 |
1 n = 937. Values are geometric means (95% CIs). GTE, green tea extract; SHBG, sex hormone-binding globulin.
2 P values for difference between GTE and placebo groups at baseline and month 12 were derived from a single linear mixed-effect model with adjustment for age, BMI, and hormone concentration at baseline.
3 P values for the overall treatment effect between GTE and placebo groups were derived from a mixed-effects repeated-measures ANOVA with adjustment for age, BMI, and hormone concentration at baseline.
4 P values for interaction between treatment and time were derived from a mixed-effects repeated-measures ANOVA with adjustment for age, BMI, and hormone concentration at baseline.
The frequencies of the COMT G/G, G/A, and A/A genotypes among all study participants who were genotyped including those not randomly assigned (n = 1445) were 22.5%, 51.1%, and 26.4%, respectively. Genotype frequencies for the COMT gene (SNP rs4680) were in Hardy-Weinberg equilibrium (P = 0.35). The concentrations of circulating sex steroid hormones measured at baseline were comparable among women possessing the G/G, A/A, and G/A COMT genotypes (Supplemental Table 1). Furthermore, the COMT genotype did not have a significant modifying effect in interaction with GTE supplementation on the circulating concentration of sex hormones (all Ps for interaction ≥0.51) (Table 3).
TABLE 3.
Circulating concentrations of sex steroid hormones by COMT genotype in postmenopausal women randomly assigned to the GTE or placebo group1
| COMT genotype | |||||||
|---|---|---|---|---|---|---|---|
| Sex hormone | G/G (n = 248) | A/A (n = 300) | G/A (n = 389) | ||||
| Time point | GTE (n = 126) | PLC (n = 122) | GTE (n = 156) | PLC (n = 144) | GTE (n = 181) | PLC (n = 208) | P 2 |
| Estrone, pg/mL | — | — | — | — | — | — | 0.71 |
| Baseline | 23.5 (21.4, 25.7) | 24.1 (22.0, 26.5) | 22.2 (20.3, 24.3) | 22.3 (20.3, 24.5) | 22.7 (20.9, 24.7) | 24.4 (22.6, 26.3) | |
| Month 12 | 24.1 (21.8, 26.8) | 23.5 (21.2, 26.1) | 22.4 (20.6, 24.3) | 21.9 (20.1, 23.9) | 21.3 (19.6, 23.1) | 22.3 (20.7, 24.0) | |
| Estradiol, pg/mL | — | — | — | — | — | — | 0.53 |
| Baseline | 3.3 (2.7, 4.0) | 3.2 (2.6, 3.9) | 3.5 (3.0, 4.2) | 3.7 (3.1, 4.4) | 3.3 (2.8, 3.9) | 4.0 (3.4, 4.7) | |
| Month 12 | 4.1 (3.3, 5.0) | 3.0 (2.4, 3.7) | 3.4 (2.9, 4.0) | 3.3 (2.8, 3.8) | 3.8 (3.3, 4.4) | 3.2 (2.7, 3.6) | |
| Bioavailable estradiol, pg/mL | — | — | — | — | — | — | 0.54 |
| Baseline | 1.5 (1.2, 1.8) | 1.4 (1.2, 1.8) | 1.6 (1.3, 1.9) | 1.6 (1.4, 2.0) | 1.5 (1.2, 1.8) | 1.8 (1.6, 2.2) | |
| Month 12 | 1.9 (1.5, 2.3) | 1.3 (1.1, 1.7) | 1.5 (1.3, 1.8) | 1.5 (1.2, 1.7) | 1.7 (1.5, 2.0) | 1.5 (1.3, 1.7) | |
| Androstenedione, pg/mL | — | — | — | — | — | — | 0.51 |
| Baseline | 480 (440, 523) | 490 (449, 534) | 484 (448, 522) | 501 (462, 542) | 478 (447, 511) | 521 (490, 555) | |
| Month 12 | 494 (461, 530) | 479 (446, 514) | 453 (420, 489) | 468 (432, 506) | 467 (439, 496) | 487 (460, 516) | |
| Testosterone, pg/mL | — | — | — | — | — | — | 0.95 |
| Baseline | 158 (143, 174) | 173 (157, 190) | 147 (133, 162) | 155 (140, 172) | 149 (135, 164) | 161 (147, 176) | |
| Month 12 | 165 (150, 181) | 159 (145, 174) | 152 (138, 168) | 151 (136, 167) | 158 (145, 172) | 157 (145, 170) | |
| Bioavailable testosterone, pg/mL | — | — | — | — | — | — | 0.84 |
| Baseline | 40.1 (36.0, 44.6) | 44.4 (39.8, 49.4) | 37.2 (33.3, 41.5) | 39.0 (34.8, 43.8) | 38.2 (34.5, 42.3) | 42.4 (38.6, 46.7) | |
| Month 12 | 43.0 (39.0, 47.5) | 40.4 (36.6, 44.7) | 38.3 (34.4, 42.7) | 38.3 (34.2, 42.8) | 40.9 (37.2, 45.1) | 41.9 (38.3, 45.9) | |
| SHBG, nmol/L | — | — | — | — | — | — | 0.97 |
| Baseline | 70.2 (64.5, 76.3) | 69.4 (63.7, 75.5) | 68.8 (64.3, 73.6) | 71.6 (66.7, 76.8) | 66.8 (62.2, 71.7) | 66.5 (62.3, 71.1) | |
| Month 12 | 67.9 (62.5, 73.9) | 70.5 (64.8, 76.7) | 71.4 (66.6, 76.5) | 70.8 (65.8, 76.1) | 67.7 (62.7, 73.0) | 65.5 (61.1, 70.3) | |
1 n = 937. Values are geometric means (95% CIs). COMT, catechol-O-methyltransferase; GTE, green tea extract; PLC, placebo; SHBG, sex hormone-binding globulin.
2 P values for the interaction between treatment and COMT genotype derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI at baseline.
Baseline plasma concentrations of IGF-1 and IGFBP-3, and the IGF-1:IGFBP-3 ratio were comparable between both the GTE and placebo groups (Table 4). The median (IQR) of IGF-1 was 88.5 ng/mL (73.3– 109 ng/mL) in the GTE group and 88.6 ng/mL (74.8–105 ng/mL) in the placebo group. The median (IQR) of IGFBP-3 was 2069 ng/mL (1804–2378 ng/mL) in the GTE group and 2076 ng/mL (1822–2340 ng/mL) in the placebo group. Corresponding P values for the overall time effect for IGF-1 were 0.02 and 0.0004 in the GTE and placebo groups, respectively; and 0.02 in the GTE group and 0.0009 in the placebo group for the IGF-1:IGFBP-3 ratio. Circulating concentration of IGFBP-3 remained unchanged over the 12-mo treatment period in the GTE group. The supplementation of GTE did not have any effect on the circulating concentrations of IGF-1 and IGFBP-3 or the IGF-1:IGFPB-3 ratio at month 6 or month 12 (all Ps for overall treatment ≥0.12). There was no significant interaction between the GTE treatment and the duration of treatment on any IGF measures (all Ps for interaction ≥0.19).
TABLE 4.
Circulating concentrations of IGF-1 and IGFBP-3, and IGF-I:IGFBP-3 ratio at baseline and months 6 and 12 by treatment group among postmenopausal women randomly assigned to the GTE or placebo group1
| IGF protein | GTE (n = 462) | Placebo (n = 473) | P 2 | P 3 | P 4 |
|---|---|---|---|---|---|
| IGF-1, ng/mL | — | — | 0.66 | 0.31 | |
| Baseline | 87.6 (87.2, 88.1) | 88.2 (87.7, 88.6) | 0.10 | ||
| Month 6 | 83.7 (82.5, 85.0) | 83.3 (82.1, 84.5)5 | 0.59 | ||
| Month 12 | 83.8 (82.4, 85.2) | 83.0 (81.6, 84.3) | 0.35 | ||
| IGFBP-3, ng/mL | — | — | 0.60 | 0.51 | |
| Baseline | 2046 (2039, 2053) | 2050 (2043, 2057) | 0.38 | ||
| Month 6 | 2019 (1999, 2039) | 2032 (2012, 2052)5 | 0.41 | ||
| Month 12 | 2046 (2025, 2067) | 2043 (2023, 2064) | 0.81 | ||
| IGF-1:IGFBP-3 ratio | — | — | 0.12 | 0.19 | |
| Baseline | 0.0429 (0.0427, 0.0432) | 0.0429 (0.0427, 0.0431) | 0.96 | ||
| Month 6 | 0.0416 (0.0410, 0.0421) | 0.0409 (0.0404, 0.0414)5 | 0.11 | ||
| Month 12 | 0.0410 (0.0405, 0.0416) | 0.0405 (0.0400, 0.0411) | 0.23 |
1 n = 935. Values are geometric means (95% CIs). GTE, green tea extract; IGF, insulin-like growth factor; IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein 3.
2 P value for difference between the GTE and placebo groups at baseline, month 6, and month 12 from a single linear mixed-effects model adjusted for age, BMI, and IGF protein concentration at baseline.
3 P values for overall treatment effect were derived from a mixed-effects repeated-measures ANOVA with adjustment for age, BMI, and IGF protein concentration at baseline.
4 P values for interaction between treatment and time were derived from a mixed-effects repeated-measures ANOVA with adjustment for age, BMI, and IGF protein concentration at baseline.
5Two subjects with missing data on month 6 were excluded from this analysis.
Age was significantly inversely associated with baseline circulating concentrations of estradiol, bioavailable estradiol (both Ps = 0.0005), androstenedione (P = 0.002), and IGF-1 (P < 0.0001), but positively associated with testosterone (P = 0.01) among all women who completed the study (Supplemental Table 2). There was a significant interaction between GTE consumption and age on circulating concentration of estrone (P = 0.009), but no effect on other sex steroid hormones (Supplemental Table 3). We also examined but did not detect any evidence of effect modification by BMI, past tea drinking status, or years of menopause with the GTE treatment on the circulating concentrations of sex steroid hormones (data not shown).
Discussion
This study investigated the potential effects of daily supplementation with 1315 mg total catechins (843 mg as EGCG) for 1 y on circulating concentrations of sex steroid hormones and IGF axis proteins in 937 healthy postmenopausal women. The study showed that GTE supplementation did not result in reduction of sex steroid hormone and IGF protein concentrations in blood. The concentrations of total and bioavailable estradiol were significantly higher in the GTE than in the placebo group at the end of the 12-mo treatment. COMT genotype did not have any significant impact on blood concentrations of hormones before or after GTE treatment.
Our results are inconsistent with the findings from previous observational studies. Previous observational studies, mostly in Asian populations, have shown that green tea intake may reduce circulating sex hormones. Nagata et al. (34) reported that green tea intake was inversely correlated with estradiol on day 11 of the menstrual cycle in 50 premenopausal Japanese women after adjustment for age and BMI. In addition, plasma estrone concentrations were 13% lower among healthy postmenopausal Chinese women (n = 130) who drank green tea regularly than among non–green tea drinkers (35). In the same study, circulating estradiol and androstenedione concentrations were also lower among regular green tea drinkers although the differences were not statistically significant. All these observational studies were limited by modest sample sizes (<140 in total) and the potential of unmeasured confounding factors. Importantly, the previous studies assessed the effect of brewed infusions with relatively low doses, whereas we used GTEs as a capsule containing a high dose of tea catechins. The catechin contents in brewed tea infusions vary greatly and depend on the original source of tea leaves, brewing time, and water temperature. Further studies should be performed to increase our understanding of differences due to ethnicity, green tea compared with catechins, and dose.
Our null findings on green tea intake and circulating IGF proteins are similar to the results observed among Asian-American women (36). However, our results differed from the observation findings reported by Maruyama et al. (62), who found higher serum concentrations of IGF-1 in association with intake of ≥5 servings of green tea per week but no association with IGFBP-3 in Japanese women.
Results from our clinical trial are in accordance with 2 shorter and smaller intervention studies (37, 38) that did not observe significant reductions in sex hormones and IGF axis proteins after consumption of green tea supplement compared with placebo. Wu et al. (37) showed that supplementation with 400 mg and 800 mg EGCG for 2 mo in the form of Polyphenon E capsules among 103 healthy postmenopausal women did not lower serum concentrations of estrone, estradiol, testosterone, androstenedione, and SHBG compared with those in the placebo group. The same pattern of null results was observed for both IGF-1 and IGFBP-3. Similarly, another intervention study which administered daily Polyphenon E intake of 800 mg, 1200 mg, and 1600 mg EGCG for 6 mo did not result in reduction of sex hormones or IGF axis proteins in women with a history of breast cancer (38). Participants in this study (n = 40) were mostly white, overweight postmenopausal women, who were 10–170 mo postdiagnosis of an estrogen and progesterone receptor negative breast carcinoma.
We observed a significant interaction between GTE treatment and age on circulating concentrations of estrone. Compared with the baseline estrone concentrations, at the end of the 12-mo treatment women ages 50–54 y in the GTE group showed an 11% increase in estrone, whereas their counterparts in the placebo group had a 15% decrease in estrone concentrations. GTE supplementation did not have a significant impact on other sex hormones studied in women 50 y or older.
Although unexpected and of unknown etiology, the GTE supplementation effect on counteracting the decrease in estradiol from aging is interesting. Future studies are warranted on understanding the biological mechanism of GTE in altering both estradiol and estrone, especially in younger menopausal women.
The present study did not show any effect of COMT genotype on circulating concentrations of sex hormones. This null finding is consistent with the results from Dunning et al. (63) and Tworoger et al. (64) among healthy postmenopausal women. To the best of our knowledge, no other GTE intervention trial has examined the modifying effect of COMT genotype on sex hormones.
The primary strengths of this study include the randomized, double-blind, placebo-controlled design with the largest sample size and the longest treatment duration of its kind. Analysis of circulating hormones was performed by the gold-standard method of LC/MS/MS. The study had an excellent retention rate and participants showed great compliance with treatment. Study participants’ compliance rate was 96.5% on average as determined by pill count, and in an objective measure of compliance assessment, women in the GTE group had >10 times increase in urinary EGC concentrations and 16 times higher urinary concentrations of EC compared with their placebo counterparts. This study has several limitations as well. Participants were postmenopausal women and predominantly white, which make the extrapolation of our results to a more heterogeneous population difficult. Second, we had limited data on the past habitual tea intake. Also, we had low statistical power to test the effects of COMT genotype on circulating sex hormones owing to low prevalence of the high-activity COMT genotype (G/G) among our study participants. Finally, this study was based on a GTE (not green tea drinking), and the single dose of the present study precluded evaluating the effect of GTE at different doses on the sex hormones or IGFs.
In summary, findings of this study suggest that daily GTE supplementation with 843 mg EGCG for 12 mo did not reduce sex steroid hormones but it significantly increased estradiol concentration. Genetic polymorphisms in the COMT gene did not have any effect on circulating concentrations of sex steroid hormones. The findings of epidemiological studies on the effect of green tea intake alone or in combination with COMT genetic polymorphisms, if true, would be caused through pathways other than the sex steroid hormones. Further studies are warranted to better understand the complexity of GTE effects on the circulating concentrations of sex steroid hormones.
Supplementary Material
Acknowledgments
We thank William Smith and Sarah Bedell for their assistance in sample analyses. The authors’ contributions were as follows—AHW, GU, MCY, DY, MSK, and J-MY: contributed to the conception and design of the study; MSK and HS: led the acquisition of data and conducted the research; HS and RW: performed the statistical analyses; HS: drafted the manuscript; HS, MSK, and J-MY: had primary responsibility for the final content; and all authors: contributed to interpreting the results and reviewed and approved the final manuscript.
Notes
Supported by NIH/National Cancer Institute grant R01 CA127236 (to MSK, Principal Investigator), Department of Defense/US Army Medical Research and Materiel Command grant W81XWH-11-1-0013 (to HS), NIH/National Cancer Institute training grant in cancer epidemiology T32CA186873, the University of Minnesota Graduate School Doctoral Dissertation Fellowship (to HS), and the National Center for Advancing Translational Sciences of the NIH, award number UL1TR000114.
Author disclosures: HS, AHW, GU, CJT, RW, MCY, DY, MSK, and J-MY, no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ALT, alanine aminotransferase; COMT, catechol-O-methyltransferase; EC, epicatechin; EGC, epigallocatechin; EGCG, epigallocatechin gallate; GTE, green tea extract; IGF, insulin-like growth factor; IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein 3; MGTT, Minnesota Green Tea Trial; SHBG, sex hormone-binding globulin; SNP, single nucleotide polymorphism; UGT, uridine diphosphate-glucuronosyltransferase.
References
- 1. Key T, Appleby P, Barnes I, Reeves G, The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–16. [DOI] [PubMed] [Google Scholar]
- 2. Russo J, Russo IH. Genotoxicity of steroidal estrogens. Trends Endocrinol Metab. 2004;15(5):211–14. [DOI] [PubMed] [Google Scholar]
- 3. Samavat H, Kurzer MS. Estrogen metabolism and breast cancer. Cancer Lett. 2015;356(2 Pt A):231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–4. [DOI] [PubMed] [Google Scholar]
- 5. Annunziata M, Granata R, Ghigo E. The IGF system. Acta Diabetol. 2011;48(1):1–9. [DOI] [PubMed] [Google Scholar]
- 6. Belfiore A, Frasca F. IGF and insulin receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(4):381–406. [DOI] [PubMed] [Google Scholar]
- 7. Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm. 2007;4(6):819–25. [DOI] [PubMed] [Google Scholar]
- 9. Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 2006;7(7):755–809. [DOI] [PubMed] [Google Scholar]
- 10. Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37(8):693–704. [DOI] [PubMed] [Google Scholar]
- 11. Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (–)-epigallocatechin gallate. Drug Metab Dispos. 2003;31(5):572–9. [DOI] [PubMed] [Google Scholar]
- 12. Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132(8):2307–11. [DOI] [PubMed] [Google Scholar]
- 13. Leong H, Mathur PS, Greene GL. Inhibition of mammary tumorigenesis in the C3(1)/SV40 mouse model by green tea. Breast Cancer Res Treat. 2008;107(3):359–69. [DOI] [PubMed] [Google Scholar]
- 14. Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clin Cancer Res. 2005;11(5):1918–27. [DOI] [PubMed] [Google Scholar]
- 15. Hirose M, Mizoguchi Y, Yaono M, Tanaka H, Yamaguchi T, Shirai T. Effects of green tea catechins on the progression or late promotion stage of mammary gland carcinogenesis in female Sprague-Dawley rats pretreated with 7,12-dimethylbenz(a)anthracene. Cancer Lett. 1997;112(2):141–7. [DOI] [PubMed] [Google Scholar]
- 16. Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96(2):239–43. [DOI] [PubMed] [Google Scholar]
- 17. Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001;82(3):387–98. [DOI] [PubMed] [Google Scholar]
- 18. Shrubsole MJ, Lu W, Chen Z, Shu XO, Zheng Y, Dai Q, Cai Q, Gu K, Ruan ZX, Gao YT et al.. Drinking green tea modestly reduces breast cancer risk. J Nutr. 2009;139(2):310–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003;106(4):574–9. [DOI] [PubMed] [Google Scholar]
- 20. Zhang M, Holman CD, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in southeast China. Carcinogenesis. 2007;28(5):1074–8. [DOI] [PubMed] [Google Scholar]
- 21. Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, Willett WC, Tsugane S, Japan Public Health Center-Based Prospective Study Group. Green tea drinking and subsequent risk of breast cancer in a population-based cohort of Japanese women. Breast Cancer Res. 2010;12(5):R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagano J, Kono S, Preston DL, Mabuchi K. A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan). Cancer Causes Control. 2001;12(6):501–8. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki Y, Tsubono Y, Nakaya N, Koizumi Y, Tsuji I. Green tea and the risk of breast cancer: pooled analysis of two prospective studies in Japan. Br J Cancer. 2004;90(7):1361–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inoue M, Robien K, Wang R, Van Den Berg DJ, Koh WP, Yu MC. Green tea intake, MTHFR/TYMS genotype and breast cancer risk: the Singapore Chinese Health Study. Carcinogenesis. 2008;29(10):1967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dai Q, Shu XO, Li H, Yang G, Shrubsole MJ, Cai H, Ji B, Wen W, Franke A, Gao YT et al.. Is green tea drinking associated with a later onset of breast cancer?. Ann Epidemiol. 2010;20(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141(3):980–7. [DOI] [PubMed] [Google Scholar]
- 27. Zhou JR, Yu L, Zhong Y, Blackburn GL. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133(2):516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monteiro R, Azevedo I, Calhau C. Modulation of aromatase activity by diet polyphenolic compounds. J Agric Food Chem. 2006;54(10):3535–40. [DOI] [PubMed] [Google Scholar]
- 29. Goodin MG, Rosengren RJ. Epigallocatechin gallate modulates CYP450 isoforms in the female Swiss-Webster mouse. Toxicol Sci. 2003;76(2):262–70. [DOI] [PubMed] [Google Scholar]
- 30. Satoh K, Sakamoto Y, Ogata A, Nagai F, Mikuriya H, Numazawa M, Yamada K, Aoki N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. 2002;40(7):925–33. [DOI] [PubMed] [Google Scholar]
- 31. Goodin MG, Fertuck KC, Zacharewski TR, Rosengren RJ. Estrogen receptor-mediated actions of polyphenolic catechins in vivo and in vitro. Toxicol Sci. 2002;69(2):354–61. [DOI] [PubMed] [Google Scholar]
- 32. Sartippour MR, Pietras R, Marquez-Garban DC, Chen HW, Heber D, Henning SM, Sartippour G, Zhang L, Lu M, Weinberg O et al.. The combination of green tea and tamoxifen is effective against breast cancer. Carcinogenesis. 2006;27(12):2424–33. [DOI] [PubMed] [Google Scholar]
- 33. Farabegoli F, Barbi C, Lambertini E, Piva R. (-)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect Prev. 2007;31(6):499–504. [DOI] [PubMed] [Google Scholar]
- 34. Nagata C, Kabuto M, Shimizu H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr Cancer. 1998;30(1):21–4. [DOI] [PubMed] [Google Scholar]
- 35. Wu AH, Arakawa K, Stanczyk FZ, Van Den Berg D, Koh WP, Yu MC. Tea and circulating estrogen levels in postmenopausal Chinese women in Singapore. Carcinogenesis. 2005;26(5):976–80. [DOI] [PubMed] [Google Scholar]
- 36. Wu AH, Butler LM. Green tea and breast cancer. Mol Nutr Food Res. 2011;55(6):921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res (Phila). 2012;5(3):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crew KD, Brown P, Greenlee H, Bevers TB, Arun B, Hudis C, McArthur HL, Chang J, Rimawi M, Vornik L et al.. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev Res (Phila). 2012;5(9):1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61(18):6716–22. [PubMed] [Google Scholar]
- 40. Syvanen AC, Tilgmann C, Rinne J, Ulmanen I. Genetic polymorphism of catechol-O-methyltransferase (COMT): correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and Parkinsonian patients in Finland. Pharmacogenetics. 1997;7(1):65–71. [DOI] [PubMed] [Google Scholar]
- 41. Yager JD. Catechol-O-methyltransferase: characteristics, polymorphisms and role in breast cancer. Drug Discov Today Dis Mech. 2012;9(1–2):e41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weinshilboum RM, Raymond FA. Inheritance of low erythrocyte catechol-o-methyltransferase activity in man. Am J Hum Genet. 1977;29(2):125–35. [PMC free article] [PubMed] [Google Scholar]
- 43. Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63(21):7526–9. [PubMed] [Google Scholar]
- 44. Liehr JG, Ricci MJ. 4-Hydroxylation of estrogens as marker of human mammary tumors. Proc Natl Acad Sci U S A. 1996;93(8):3294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–82. [DOI] [PubMed] [Google Scholar]
- 46. Inoue-Choi M, Yuan JM, Yang CS, Van Den Berg DJ, Lee MJ, Gao YT, Yu MC. Genetic association between the COMT genotype and urinary levels of tea polyphenols and their metabolites among daily green tea drinkers. Int J Mol Epidemiol Genet. 2010;1(2):114–23. [PMC free article] [PubMed] [Google Scholar]
- 47. Miller RJ, Jackson KG, Dadd T, Mayes AE, Brown AL, Lovegrove JA, Minihane AM. The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol Nutr Food Res. 2012;56(6):966–75. [DOI] [PubMed] [Google Scholar]
- 48. Brown AL, Lane J, Holyoak C, Nicol B, Mayes AE, Dadd T. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr. 2011;106(12):1880–9. [DOI] [PubMed] [Google Scholar]
- 49. Miller RJ, Jackson KG, Dadd T, Nicol B, Dick JL, Mayes AE, Brown AL, Minihane AM. A preliminary investigation of the impact of catechol-O-methyltransferase genotype on the absorption and metabolism of green tea catechins. Eur J Nutr. 2012;51(1):47–55. [DOI] [PubMed] [Google Scholar]
- 50. Sak K. The Val158Met polymorphism in COMT gene and cancer risk: role of endogenous and exogenous catechols. Drug Metab Rev. 2017;49(1):56–83. [DOI] [PubMed] [Google Scholar]
- 51. Samavat H, Dostal AM, Wang R, Bedell S, Emory TH, Ursin G, Torkelson CJ, Gross MD, Le CT, Yu MC et al.. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: study rationale, design, methods, and participant characteristics. Cancer Causes Control. 2015;26(10):1405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. ACR. Breast Imaging Reporting and Data System Atlas (BI-RADS® Atlas)—Mammography. 4th ed. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 53. Dueñas M, González-Manzano S, González-Paramás A, Santos-Buelga C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J Pharm Biomed Anal. 2010;51(2):443–9. [DOI] [PubMed] [Google Scholar]
- 54. Dostal AM, Samavat H, Bedell S, Torkelson C, Wang R, Swenson K, Le C, Wu AH, Ursin G, Yuan JM et al.. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem Toxicol. 2015;83:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee LG, Connell CR, Bloch W. Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucleic Acids Res. 1993;21(16):3761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75(2):169–75. [DOI] [PubMed] [Google Scholar]
- 57. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–72. [DOI] [PubMed] [Google Scholar]
- 58. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512–19. [DOI] [PubMed] [Google Scholar]
- 59. Bland JM, Altman DG. Best (but oft forgotten) practices: testing for treatment effects in randomized trials by separate analyses of changes from baseline in each group is a misleading approach. Am J Clin Nutr. 2015;102(5):991–4. [DOI] [PubMed] [Google Scholar]
- 60. Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Short-term soy and probiotic supplementation does not markedly affect concentrations of reproductive hormones in postmenopausal women with and without histories of breast cancer. J Altern Complement Med. 2005;11(6):1067–74. [DOI] [PubMed] [Google Scholar]
- 61. Wangen KE, Duncan AM, Merz-Demlow BE, Xu X, Marcus R, Phipps WR, Kurzer MS. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85(9):3043–8. [DOI] [PubMed] [Google Scholar]
- 62. Maruyama K, Iso H, Ito Y, Watanabe Y, Inaba Y, Tajima K, Nakachi K, Tamakoshi A. Associations of food and nutrient intakes with serum IGF-I, IGF-II, IGFBP-3, TGF-b1, total SOD activity and sFas levels among middle-aged Japanese: the Japan Collaborative Cohort study. Asian Pac J Cancer Prev. 2009;10(Suppl):7–22. [PubMed] [Google Scholar]
- 63. Dunning AM, Dowsett M, Healey CS, Tee L, Luben RN, Folkerd E, Novik KL, Kelemen L, Ogata S, Pharoah PD et al.. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004;96(12):936–45. [DOI] [PubMed] [Google Scholar]
- 64. Tworoger SS, Chubak J, Aiello EJ, Ulrich CM, Atkinson C, Potter JD, Yasui Y, Stapleton PL, Lampe JW, Farin FM et al.. Association of CYP17, CYP19, CYP1B1, and COMT polymorphisms with serum and urinary sex hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13(1):94–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.