Abstract
Previous multimodal magnetic resonance imaging (MRI) studies of parkinsonian syndromes have focused primarily on motor-related basal ganglia structures. The present study investigated MRI changes in non-motor-related limbic structures in 35 Parkinson’s disease (PD), 16 multiple system atrophy parkinsonian subtype (MSA-P), 17 progressive supranuclear palsy (PSP), and 37 control subjects. Mean diffusivity (MD), fractional anisotropy (FA), transverse relaxation rate (R2*), quantitative susceptibility mapping, and volume measurements were obtained from the amygdala, hippocampus, and nucleus accumbens (NAc) to examine differences between groups, and to test for associations with clinical scores. Compared to controls, PD subjects had lower NAc volume; MSA-P subjects had higher NAc R2*; PSP subjects had higher amygdala and hippocampus MD, and lower hippocampus FA (ps ≤ 0.008). Among parkinsonian subjects, amygdala and hippocampus MD associated positively with Unified Parkinson's Disease Rating Scale (UPDRS) non-motor and activities of daily living (ADL) scores (ps ≤ 0.005). Together, these findings support the inclusion of limbic structures in future MRI studies of parkinsonian syndromes.
Keywords: Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy, magnetic resonance imaging, limbic
1. Introduction
Parkinson’s disease (PD), multiple system atrophy (MSA), and progressive supranuclear palsy (PSP) are parkinsonian syndromes with overlapping motor features. Tremors, rigidity, bradykinesia, and postural instability are signs and symptoms found in all three (McFarland, 2016). Non-motor symptoms such as mood disturbances and cognitive changes are also prevalent among PD, MSA, and PSP patients (O'Sullivan et al., 2010; Averbeck et al., 2014; Zhang et al., 2017). Magnetic resonance imaging (MRI) has been used to investigate the neural underpinnings of parkinsonian motor and non-motor symptoms (Pyatigorskaya et al., 2014). Previous multimodal MRI studies, however, have focused primarily on motor-related structures of the basal ganglia (Cochrane and Ebmeier, 2013; Wang et al., 2016). Few have used MRI to examine parkinsonian limbic structure pathologies beyond volume changes in PD (Camicioli et al., 2003; Carriere et al., 2014; Junque et al., 2005).
Post-mortem studies have demonstrated that parkinsonian syndrome neuropathologies extend beyond the basal ganglia to affect limbic structures such as the hippocampus, amygdala, and nucleus accumbens (NAc) (Bertrand et al., 2004; Kalaitzakis et al., 2009; Papp and Lantos, 1994; Piao et al., 2001; Jellinger, 2008; Yokota et al., 2010). These structures exhibit characteristic aggregates of α-synuclein in PD and MSA, (Bertrand et al., 2004; Kalaitzakis et al., 2009; Papp and Lantos, 1994; Piao et al., 2001) and neurofibrillary tangles of tau in PSP (Jellinger, 2008; Yokota et al., 2010). Diffusion tensor imaging (DTI) and susceptibility MRI have demonstrated possible sensitivities to these and other neuropathologic changes in a manner complementary to structural MRI (Cochrane and Ebmeier, 2013; Wang et al., 2016). In parkinsonian syndrome studies, the DTI measurements of mean diffusivity (MD) and fractional anisotropy (FA) have been used to reflect gray matter changes in microstructural integrity (Cochrane and Ebmeier, 2013); the apparent transverse relaxation rate (R2*) and quantitative susceptibility mapping (QSM) have revealed susceptibility differences reflective of tissue iron accumulation (Wang et al., 2016; Du et al., 2016).
Previous studies of motor structures have shown further that DTI and susceptibility MRI changes are associated with worsening motor function and increased levodopa-equivalent dosages (Barbagallo et al., 2016; Schwarz et al., 2018). It presently is unknown, however, whether such changes in limbic structures are related to changes in non-motor functions. Semi-quantitative histological assessments of PD, MSA, and PSP patients have shown that anxiety and cognitive impairment are not associated with localized cortical or subcortical proteinopathy (Prediger et al., 2012; Jellinger, 2013; Asi et al., 2014; Wakabayashi and Takahashi, 2004). Moreover, clinical studies have suggested that mood disturbances may be secondary to the stress of chronic disability (Walsh and Bennett, 2001; Hemmerle et al., 2012). This conclusion may be misleading since currently there is a lack of comprehensive in vivo assessments of limbic structure pathologies in parkinsonian patients.
Thus, to address this overall question, the primary hypothesis of the present study was that MD, FA, R2*, and QSM would reveal limbic structure changes among parkinsonian syndromes distinct from volume deviations. The secondary hypothesis was that MRI measurements demonstrating significant limbic structure changes would be associated with non-motor symptoms.
2. Methods
2.1. Study subjects
This study included 68 parkinsonian subjects and 37 age-matched controls for a total of 105 individuals. Of the parkinsonian subjects, 35 were diagnosed with PD, 16 with MSA (parkinsonian subtype; MSA-P), and 17 with PSP. Parkinsonian subjects were recruited from a tertiary movement disorder clinic, whereas controls were recruited from the spouse population and surrounding community. All subjects belonged to a longitudinal case-control cohort that was established in 2012 as part of the NINDS-sponsored Parkinson’s Disease Biomarker Program (PDBP). Parkinsonian syndrome diagnoses were made by a movement disorder specialist according to published criteria (Goetz et al., 2007; Gilman et al., 2008; Litvan et al., 1996). All PD subjects had a history of adequate response to levodopa or other dopaminergic therapies, and a history of asymmetrical symptom onset; MSA-P subjects had a history of significant autonomic and/or urinary dysfunction; and PSP subjects had a history of postural instability and vertical gaze palsy (or slowness). Both MSA-P and PSP subjects had a history of inadequate response to levodopa treatment. Diagnoses were confirmed by postmortem pathology in 13 of the 68 parkinsonian syndrome subjects (7 PD, 2 MSA-P, and 4 PSP).
All controls demonstrated a Mini-Mental State Examination score ≥24, were screened for neurological and neuropsychiatric disorders through a study questionnaire, and were cleared of neuropathologic findings upon later review of their MRIs. All subjects or their legal guardians were willing and able to provide written informed consent. Both control and parkinsonian subjects were excluded if they had any condition that would preclude an MRI examination, a history of cerebrovascular disease, and/or renal or liver failure. History or treatment for mood disorders and/or cognitive impairment were not exclusion criteria since these were features of interest in the present study. In all, 12 subjects (7 PD, 3 MSA-P, and 3 PSP) had present or past history of antidepressant use; 7 subjects (5 PD, 1 MSA-P, and 1 PSP) were on medications for cognitive impairment.
For parkinsonian subjects, disease duration was defined as the time from the date of first diagnosis to study visit date. Disease severity was assessed using Hoehn and Yahr staging (Hoehn and Yahr, 1967). Levodopa equivalent daily dosage (LEDD) was calculated using previously published criteria (Tomlinson et al., 2010). In all subjects, the Montreal Cognitive Assessment (MoCA) was used to evaluate global cognitive status, the Hamilton Anxiety Rating Scale (HAM-A) to assess anxiety levels, and the Hamilton Depression Rating Scale (HAM-D) to evaluate depression levels. All subjects also were administered the Unified Parkinson’s Disease Rating Scale Parts I, II and III (UPDRS-I, -II, and -III). UPDRS-I assesses non-motor symptoms experienced by patients, whereas UPDRS-II and -III evaluate motor symptoms experienced by patients in activities of daily living (ADL) and motor signs observed by trained personnel, respectively. Neurobehavioral assessments and MRI scans were performed while parkinsonian subjects were in a medication ‘on’ state since only 26 of the 68 parkinsonian subjects were able to undergo examination in a medication ‘off’ state. Collection of all clinical and imaging measurements was approved by the institutional review board at the Pennsylvania State Milton S. Hershey Medical Center.
2.2. MRI image acquisition and post-processing
Brain MRIs from all study subjects were obtained using a 3T MR imaging system (Magnetom Trio; Siemens, Erlangen, Germany). T1-weighted images were acquired using an MP-RAGE sequence with the following parameters: TR/TE = 1540/2.34 ms, FoV = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm (no gap), slice number = 176. T2-weighted images were acquired using a fast spin-echo sequence with TR/TE=2500/316 ms and the same resolution parameters as the T1-weighted images. DTI parameters were as follows: TR/TE = 8300/82 ms, b-value = 1000 s/mm2, diffusion gradient directions = 42, 7 b=0 scans, FoV = 256 × 256 mm, matrix = 128 × 128, slice thickness = 2 mm (no gap), slice number = 65. T2*-weighted images (for QSM and R2* maps) were acquired using a multiple-gradient-echo sequence: six echoes with TEs ranging from 7 to 47 ms at an equal interval of 8 ms, TR = 54 ms, flip angle = 20°, FoV = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1.5 mm (no gap), slice number = 64.
DTI images were processed using DTIPrep (NIRAL, UNC-Chapel Hill, North Carolina), where intersection and intervolume correlation analysis, eddy currents, and motion artifact correction were performed for quality control before estimation of mean diffusivity (MD) and fractional anisotropy (FA) maps. For R2* and QSM, six magnitude images taken from the multiple-gradient-echo were aligned by affine registration and then averaged to generate a mean magnitude image to correct for potential head motion. R2* maps were generated from a voxel-wise, nonlinear, Levenberg-Marquardt algorithm to fit the monoexponential function s = s0e−TE × R2* using an in-house MATLAB (MathWorks, Natick, Massachusetts) tool. QSM maps were generated using morphology-enabled dipole inversion (MEDI) with a nonlinear formulation (Liu et al., 2013; Liu et al., 2012).
2.3. ROI Segmentation
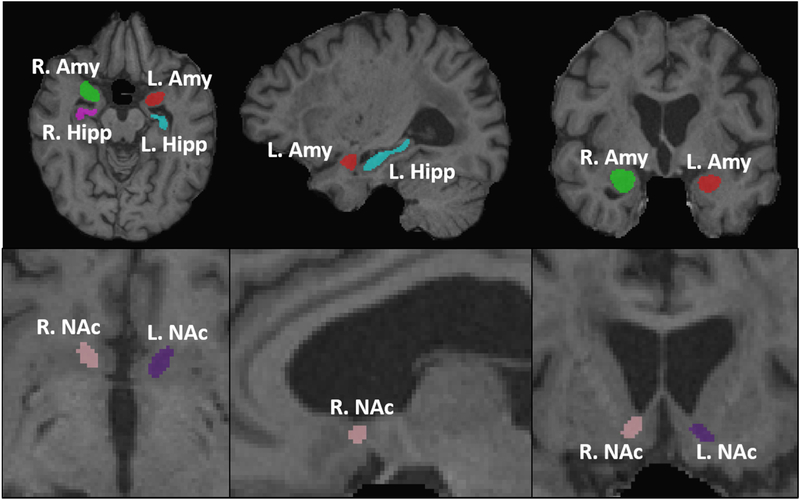
ROIs for the hippocampus, amygdala, and nucleus accumbens (NAc) were defined on a study-specific T1-weighted template. To reduce partial volume effects after automatic segmentation in the lower resolution DTI and susceptibility MRI images, template ROIs were reduced by 1 voxel along structure boundaries. ROIs for each subject were defined from template ROIs using AutoSeg (http://www.nitrc.org/projects/autoseg/) with a warping option from Advanced Normalization Tools software (ANTs; http://stnava.github.io/ANTs) (Figure 1). DTI and susceptibility MRI images then were registered to individual T2-weighted images using ANTs. The resultant transformations were applied to MD, FA, R2*, and QSM maps using B-spline interpolation to bring maps and segmented ROIs into the same space (Figure 1). Segmentation results were inspected visually at each slice for misalignments and manually adjusted by a blinded investigator. MD, FA, R2*, and QSM values were calculated from mean intensities across each ROI. Volume measurements were normalized by dividing by total intracranial volume (TIV; sum of gray matter, white matter, and CSF) to yield a percentage of TIV measurement. For each modality, right- and left-side values were compared using two-sided t-tests to test for possible hemispheric asymmetries. Since there were no hemisphere differences in any ROI (amygdala, hippocampus, or NAc) using any MRI modality (MD, FA, R2*, QSM, or volume), subsequent group comparisons used measurements that were the average of the right- and left-side values.
Figure 1. Representative images of segmentation results.
Representative auto-segmentation results of the amygdala (Amy), hippocampus (Hipp), and nucleus accumbens (NAc). Axial (left), sagittal (center), and coronal (right) slices illustrating ROIs of the amygdala and hippocampus (top row), and NAc (bottom row).
2.3. Statistical Analysis
2.3.1. Group comparisons
Differences in sex frequency were assessed by the Chi-square test. Age and disease duration were compared by one-way analysis of variance. Clinical scores (MoCA, HAM-A, HAM-D, and UPDRS) were compared by one-way analyses of covariance (ANCOVA) with adjustments for age and sex. Group differences in MRI measurements (MD, FA, R2*, QSM, volume) for each ROI were assessed by pairwise, one-way ANCOVAs with adjustments for age and sex. Statistical significance was defined by the Bonferroni method as P < 0.0083 (0.05/6) to adjust for six pairwise comparisons.
2.3.2. MRI associations with clinical scores
We also explored whether MRI measurements showing group differences from controls were associated with MoCA, HAM-A, HAM-D, and UPDRS scores among parkinsonian subjects. In linear models, MoCA, HAM-A, HAM-D, and UPDRS-I, -II, and -III were explained separately by MRI measurements, age, sex, and disease duration. The strength of the association was determined by the coefficient estimate for each MRI measurement after individually introducing them into an age-, sex-, and disease duration-controlled model. Statistical significance was defined by the Bonferroni method as P < 0.0021 (0.05/24) to adjust for the number of tested associations. All statistical analyses were performed using R version 3.4.2 (R Core Team, Vienna, Austria).
3. Results
3.1. Demographics and clinical data
Table 1 shows the demographic and clinical data for study subjects. As expected, there was no difference in age among groups since this was part of our overall design. There also was no difference in sex among groups, although there were more females than males in the MSA-P group. Therefore, we adjusted for sex in later group comparisons. Disease duration did not differ among the parkinsonian groups. MoCA scores were similar among control, PD, and MSA-P subjects but were significantly lower in PSP subjects. UPDRS-II scores in PD subjects were significantly lower than in PSP subjects. Control subjects had an average UPDRS-III score of 7.9. This is below a recent population estimate of the average UPDRS-III score for aged adults without parkinsonian syndrome diagnoses (12.5 ± 9.8) (Keezer et al., 2016).
Table 1.
Demographics and clinical scores
| Controls | PD | MSA-P | PSP | P | |
|---|---|---|---|---|---|
| No. of subjects | 37 | 35 | 16 | 17 | - |
| Sex, female/malea | 14/23 | 11/24 | 10/6 | 4/13 | 0.100 |
| Ageb | 70.4 ± 7.8 | 71.0 ± 7.4 | 66.4 ± 8.2 | 72.5 ± 9.7 | 0.152 |
| Disease durationb | - | 3.4 ± 3.6 | 4.1 ± 3.3 | 3.3 ± 2.9 | 0.745 |
| LEDD (mg/dy)c | - | 706.5 ± 426.0 | 752.3 ± 279.4 | 558.8 ± 282.1 | 0.264 |
| H & Yc | 0.4 ± 1.0 | 2.5 ± 1.2 | 3.4 ± 1.1 | 3.2 ± 1.0 | <0.001 |
| MoCAc | 25.0 ± 2.4 | 22.5 ± 4.2 | 23.9 ± 2.6 | 19.8 ± 4.7 | <0.001 |
| HAM-Ac | 4.5 ± 4.6 | 9.0 ± 6.5 | 11.1 ± 8.9 | 8.4 ± 5.7 | 0.001 |
| HAM-Dc | 2.6 ± 3.1 | 6.7 ± 5.0 | 8.1 ± 7.5 | 5.2 ± 5.0 | <0.001 |
| UPDRS-Ic | 4.1 ± 4.5 | 11.8 ± 8.2 | 11.9 ± 8.4 | 10.8 ± 6.7 | <0.001 |
| UPDRS-IIc | 1.4 ± 4.3 | 14.9 ± 13.3 | 22.6 ± 13.5 | 26.0 ± 12.7 | <0.001 |
| UPDRS-IIIc | 7.9 ± 13.2 | 37.3 ± 27.3 | 50.5 ± 19.1 | 41.8 ± 26.5 | <0.001 |
Data represent sums or mean ± standard deviation.
Group differences using the Chi-square test.
Group differences using one-way analysis of variance.
Group differences using one-way analysis of covariance with adjustments for age and sex.
Abbreviations: PD = Parkinson’s disease; MSA-P = multiple system atrophy (parkinsonian type); PSP = progressive supranuclear palsy; LEDD = levodopa equivalent daily dose; H & Y = Hoehn and Yahr Stage; MoCA = Montreal Cognitive Assessment; HAM-A = Hamilton Anxiety Rating Scale; HAM-D = Hamilton Depression Rating Scale; UPDRS-I, -II, -III = Unified Parkinson’s Disease Rating Scale Parts I, II, and III
3.2. Group differences in MRI measurements
3.2.1. Comparisons with controls
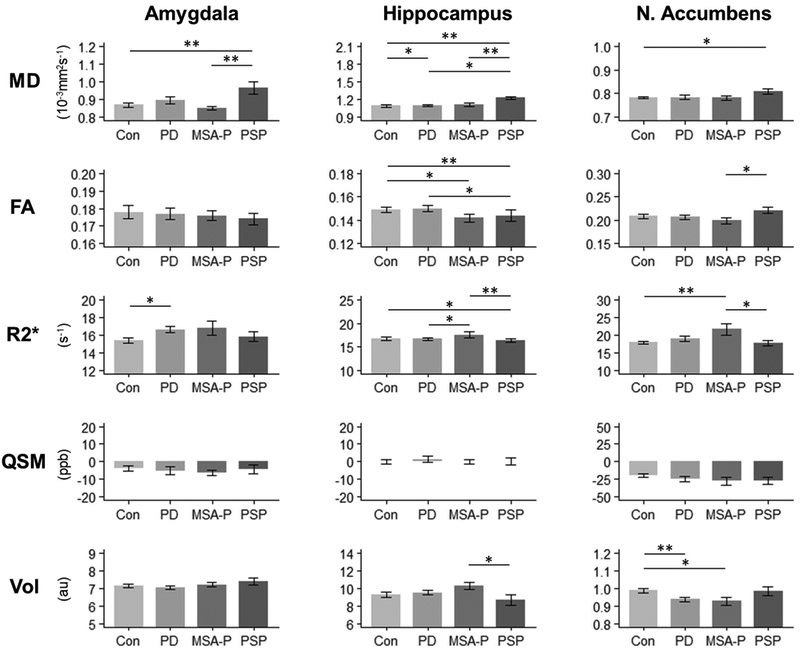
Mean MRI measurements and group differences are presented in Figure 2 and Supplemental Table 1. Compared to controls, PD subjects had lower NAc volume (p = 0.006); MSA-P subjects had higher NAc R2* values (p = 0.003); and PSP subjects had higher amygdala (p = 0.001) and hippocampus (p < 0.001) MD and lower hippocampus FA (p = 0.008) values. Differences from controls that did not survive correction for multiple comparisons were: higher amygdala R2* (p = 0.011) and hippocampus MD (p = 0.040) values in PD subjects; lower NAc volume (p = 0.009) and hippocampus FA (p = 0.019) values in MSA-P subjects; and higher NAc MD (p = 0.027) and hippocampus R2* (p = 0.035) values in PSP subjects. QSM measurements were not significantly different in the amygdala, hippocampus, or NAc in parkinsonian subjects compared to controls (p > 0.050).
Figure 2. MRI measurements by limbic structure.
Group comparisons of MD, FA, R2*, QSM, and volume in the amygdala, hippocampus, and NAc. Bars represent mean ± standard deviation. * Indicates P < 0.05. ** Indicates significance after Bonferroni correction at P < 0.0083.
Abbreviations: MD = mean diffusivity; FA = fractional anisotropy; R2* = transverse relaxation rate; QSM = quantitative susceptibility mapping; Vol = volume; Amy = amygdala; Hipp = hippocampus; NAc = nucleus accumbens; Con = Controls; PD = Parkinson's disease; MSA-P = multiple system atrophy (parkinsonian type); PSP = progressive supranuclear palsy; ppb = parts per billion; au = arbitrary unit
3.2.2. Comparisons among parkinsonian syndromes
Amygdala (p = 0.004) and hippocampus (p < 0.001) MD values were higher in PSP subjects compared to MSA-P subjects. Hippocampus R2* values were higher in MSA-P subjects than in PSP subjects (p = 0.003). Group differences that did not survive correction for multiple comparisons were: higher hippocampus MD (p = 0.014) and lower hippocampus FA (p = 0.036) values in PSP subjects than in PD subjects; higher hippocampus R2* values in MSA-P subjects than in PD subjects (p = 0.018); higher hippocampus volume (p = 0.037), higher NAc R2* (p = 0.040), and lower NAc FA (p = 0.033) values in MSA-P subjects than in PSP subjects. Among parkinsonian syndrome subjects, there were no differences in QSM measurements in any limbic structure.
3.3. MRI associations with clinical scores
Associations between the four MRI measurements demonstrating group differences from controls (amygdala MD, hippocampus MD and FA, nucleus accumbens R2*) and MoCA, HAM-A, HAM-D, and UPDRS scores among parkinsonian syndrome subjects are shown in Table 2. Notably, amygdala MD values were associated positively with UPDRS-I (β = 26.91, p = 0.003) and UPDRS-II (β = 47.63, p = 0.002) scores. Hippocampus MD values also were associated positively with UPDRS-I (β = 11.56, p = 0.005) and UPDRS-II (β = 19.83, p = 0.003) scores. Of these, only the association between amygdala MD and UPDRS-II survived the correction for multiple comparisons. Hippocampus FA and NAc R2* values did not demonstrate associations approaching significance with any clinical score.
Table 2.
Associations between MRI measurements and clinical scores among all subjects
| Associations | Amy MD |
Hipp MD |
Hipp FA |
NAc R2* |
||||
|---|---|---|---|---|---|---|---|---|
| βa | Pb | β | P | β × 10 | P | β | P | |
| MoCA | −6.30 | 0.182 | −2.41 | 0.254 | 6.22 | 0.086 | 0.15 | 0.147 |
| HAM-A | 8.41 | 0.277 | 1.83 | 0.597 | −6.71 | 0.261 | −0.22 | 0.203 |
| HAM-D | 7.47 | 0.231 | 1.56 | 0.577 | −6.37 | 0.185 | −0.13 | 0.362 |
| UPDRS-I | 26.91 | 0.003 | 11.56 | 0.005 | −12.26 | 0.094 | −0.43 | 0.039 |
| UPDRS-II | 47.63 | 0.002 | 19.83 | 0.003 | −30.32 | 0.012 | −0.26 | 0.468 |
| UPDRS-III | 61.98 | 0.034 | 29.78 | 0.022 | −39.48 | 0.083 | −0.05 | 0.941 |
MRI measurement coefficient estimates in age- and sex-controlled linear models.
Significance of the coefficient in age- and sex-controlled linear models.
Bold indicates significance after Bonferroni correction at P < 0.0021.
Abbreviations: MoCA = Montreal Cognitive Assessment; HAM-A = Hamilton Anxiety Rating Scale; HAM-D = Hamilton Depression Rating Scale; UPDRS-I, -II, -III = Unified Parkinson’s Disease Rating Scale Parts I, II, and III; MD = mean diffusivity; FA = fractional anisotropy; R2* = transverse relaxation rate; Amy = amygdala; Hipp = hippocampus; NAc = nucleus accumbens
4. Discussion
This is the first study to focus on limbic structure pathologies in parkinsonian syndrome subjects using multimodal MRI. The findings reveal that PD, MSA-P, and PSP subjects have distinct patterns of DTI and R2* changes in the hippocampus, amygdala, and NAc that may reflect underlying differences in disease neuropathology that are not apparent in volume estimations. The associations between amygdala and hippocampus DTI measurements and UPDRS I and II scores suggest that these measurements in particular are relevant to parkinsonian symptoms. Together, these results support the inclusion of limbic structures in future MRI-based research on parkinsonian syndrome pathologies.
4.1. Parkinsonian limbic structure MRI findings
4.1.1. PD MRI findings
Previous studies of hippocampal volume in PD have yielded mixed findings. Camicioli et al. (2003) reported lower volumes in PD patients that were associated with cognitive impairment, whereas Tanner et al. (2017) found volume loss to be associated with disease duration. On the other hand, in combined volume and DTI assessments of the hippocampus in PD, both Carlesimo et al. (2012) and Yao et al. (2016) found no volume changes, but higher MD. The results from the current study are in agreement with the latter findings, suggesting that microstructural alterations are discernable by MD when macrostructural differences are not present.
The lower NAc volume we found in PD subjects matches the previous finding of NAc atrophy by Carriere et al. (2014). It is known that PD patients exhibit higher frequencies of impulse control disorders (ICDs) (Averbeck et al., 2014). It is unclear, however, whether ICD behaviors in PD, such as compulsive eating, compulsive shopping, hypersexuality, and pathologic gambling, are attributable entirely to dopaminergic medication effects (to which they are strongly associated), or perhaps due to pathologic involvement of limbic structures such as the NAc (Weintraub et al., 2010). The trend for higher amygdala R2* values in PD is interesting for the same reason, as both the NAc and amygdala are known to mediate functions related to ICDs such as motivation processing, emotion processing, and reward-seeking behaviors (Baxter and Murray, 2002; Stuber et al., 2011; Ikemoto and Panksepp, 1999). The present study, however, did not incorporate an adequate measure of ICDs to suggest that lower NAc volume and higher amygdala R2* values were reflective of underlying neuropathologic substrates for Parkinson’s ICDs. In follow-up analyses, NAc volume and amygdala R2* were not associated with dopamine dysregulation syndrome (DDS) subscores of the UPDRS-III after controlling for sex, age, and disease duration. DDS subscores also were not associated with LEDD, or with NAc volume and amygdala R2* after controlling for LEDD. Future histologic and MRI studies may examine NAc and amygdala changes in relation to more comprehensive measures of ICDs to test whether associations between pathology and behavior exist.
4.1.2. MSA-P MRI findings
MSA-P subjects exhibited both higher R2* values and lower volumes in the NAc. Beyond the post-mortem findings by Spokes et al. (1979) of NAc dopamine depletion, pathologic involvement of the NAc in MSA has not been reported previously. Indeed, Papp and Lantos (1994) found the NAc to be spared of the oligodendroglial inclusions characteristic of MSA in a comprehensive assessment of brain structures. The results here are intriguing since MSA patients are not known to exhibit the impulsive-compulsive behaviors seen in PD and PSP (O'Sullivan et al., 2010; Averbeck et al., 2014). Whereas ICDs may not be prevalent in MSA, symptoms such as depression, anxiety, and apathy are frequent (Ceponiene et al., 2016). It has not been determined yet whether NAc changes in MSA are related to such neuropsychiatric presentations.
Amygdala R2* values also were higher in MSA-P patients, although the difference was non-significant. Nevertheless, the finding may reflect actual pathology since a recent post-mortem study of 35 MSA brains revealed that neuronal inclusions were present in all sampled amygdalae (Cykowski et al., 2015). Moreover, higher amygdala R2* values may be related to autonomic dysfunction in MSA (Gilman et al., 2008). The central nucleus of the amygdala (CeA) is known to modulate the autonomic nervous system through the hypothalamus (Ressler, 2010). Whether pathology in the amygdala contributes to autonomic dysfunction in MSA is unknown and needs to be investigated further.
4.1.3. PSP MRI findings
Prior DTI studies of gray matter structure changes in PSP have reported higher MD and lower FA values in the striatum compared to controls (Piattella et al., 2015). In the present study, we demonstrated that similar changes also occur in the amygdala and hippocampus. The hippocampal MD and FA findings in particular may be related to memory impairments known to occur in PSP (Litvan et al., 1989). Consistent with this hypothesis, PSP subjects in our cohort demonstrated significantly lower MoCA scores compared to PD and MSA subjects despite comparable disease durations.
4.1.4. Possible proteinopathy-related sensitivities of DTI and R2*
Few studies have examined possible associations between protein pathology, and tissue and cellular changes in limbic structures of parkinsonian subjects. Using MRI, our study detected proteinopathy-related differences that future neuropathology comparisons may examine. DTI measurements were more sensitive to changes in PSP, a tauopathy, whereas R2* was more sensitive to changes in PD and MSA-P, both synucleinopathies. This pattern suggests that DTI measurements, particularly MD, may better reflect microstructural changes in tauopathies, whereas R2* may better reflect changes in α-synucleinopathies.
It is also noteworthy that limbic structure differences found in R2* were not seen in QSM. We believe that this is not due to a lack of QSM sensitivity, as significant differences in the substantia nigra were found between PD and control subjects using a similar cohort (Du et al., 2018). As a cleaner measurement of susceptibility, QSM is a more selective measure of iron (Wang and Liu, 2014). Therefore, the R2* changes observed here may reflect microstructural changes more so than differences in iron deposition. We observed a similar result in our recent MRI-neuropathology correlation study of the substantia nigra, where R2* was associated strongly with α- synuclein burden but not with Perls’ stain for iron (Lewis et al., 2018). Since R2* captures both susceptibility and the transverse relaxation rate, it is possible that measurements unrelated to iron content are influenced strongly by local cell structure properties (Wang and Liu, 2014).
4.2. Clinical relevance of MRI findings in limbic structures
In the present study, the respective positive and negative associations of hippocampus MD values with non-motor scores (UPDRS-I) were expected since it is known that the hippocampus is involved in non-motor cognitive functions such as memory and learning. As Albouy et al. (2013) previously reviewed, studies also have shown that the hippocampus may interact with the striatum in the acquisition and consolidation of motor-related memory. The positive association of hippocampus MD values with ADL (UPDRS-II) scores is consistent with a possible hippocampal role in motor function.
The positive association of amygdala MD values with non-motor scores fits the current understanding of the non-motor functions of the amygdala in mediating the processing of emotions (Baxter and Murray, 2002). The association of amygdala MD values with ADL scores suggest that the amygdala also may have an effect on movement. A possible pathway by which this may occur is through regulation of mood. Anxiety and depression are known to influence ADL mobility, motor symptoms, and quality of life among parkinsonian syndromes (Kuhn et al., 1996).
Alternatively, these associations may be explained by overall disease progression. The relationships between MRI measurements and UPDRS-I, -II, and -III scores may be due to parallel processes of non-motor and motor deterioration, and not clinical manifestations of limbic pathologies. Nonetheless, the current study suggests that limbic contributions to overall disease phenotype are worth exploring. Future studies may employ a more comprehensive battery of neuropsychiatric tests in order to align limbic pathologies with specific behavioral deficits.
4.3. Limitations and future directions
There were limitations in the present study that present opportunities for future investigation. First, of the 68 parkinsonian subjects included in the study, 13 had pathology-confirmed diagnoses. Future studies of limbic structures from this study cohort will update analyses with additional post-mortem confirmations. Second, groups were imbalanced in both number and gender proportions, a limitation that was addressed by adjusting for age and gender in our analyses. Third, segmentation of small structures, such as the NAc, continue to be challenged by technological limitations. Accurate delineation of the hypothalamus, which we ideally would have included here, is presently limited in 3T MRI (Makris et al., 2013). Future developments in image acquisition and processing capabilities will improve upon analyses of these small structures. Lastly, future studies would benefit from additional neuropsychiatric measurements, such as those assessed by the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease–Rating Scale (QUIP-RS) (Weintraub et al., 2012). QUIP-RS scores would allow future studies to test directly whether NAc R2* and volume changes are reflective of impulsive-compulsive behaviors.
4.4. Conclusions
This study of PD, MSA-P, and PSP subjects demonstrates that DTI and R2* reveal distinct pathologies in the amygdala, hippocampus, and NAc. These findings support the inclusion of limbic structures in future MRI-based research efforts. As DTI and R2* are not capable of describing exact microstructural changes, future neuropathology studies may examine the MRI differences observed here to enhance our understanding of parkinsonian disease pathology and progression.
Supplementary Material
Highlights.
Multimodal MRI reveals limbic structure changes among parkinsonian syndromes.
Susceptibility MRI reflects limbic changes in Parkinson’s disease and multiple system atrophy.
Diffusion tensor imaging reflects limbic changes in progressive supranuclear palsy.
Limbic changes are associated with non-motor symptoms and activities of daily living.
5. Acknowledgements
We express gratitude to all of the study subjects who volunteered for this study and to the study personnel who contributed to its success.
Funding: This study was funded by the following sources: National Institute of Neurological Disorders and Stroke (NS060722 and NS082151 to XH), the Hershey Medical Center Clinical Research Center (National Center for Research Resources, Grant UL1 RR033184 that is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127), National Center for Advancing Translational Sciences (TL1 TR002016), the PA Department of Health Tobacco CURE Funds, the Translational Brain Research Center, the Michael J. Fox Foundation for Parkinson’s Research, Alzheimer’s Association, Alzheimer’s Research UK, and the Weston Brain Institute.
6. Disclosure Statements
Ernest W. Wang: Received funding from the National Center for Advancing Translational Sciences.
Guangwei Du: Received funding from the National Institute of Environmental Health Sciences (NIEHS), the Michael J. Fox Foundation for Parkinson’s Research, the Alzheimer’s Association, Alzheimer’s Research UK, the Weston Brain Institute, and the Department of Defense.
Mechelle M. Lewis: Received funding from the NIEHS, the Michael J. Fox Foundation for Parkinson’s Research, the Alzheimer’s Association, Alzheimer’s Research UK, the Weston Brain Institute, Bristol Myers Squibb, Biogen, Pfizer, and the Department of Defense.
Eun-Young Lee: Received funding from the NIEHS, Pfizer, and the Department of Defense.
Sol De Jesus: Received funding from Bristol Myers Squibb and Pfizer.
Sangam Kanekar: Nothing to disclose.
Lan Kong: Received funding from the NIEHS, the Michael J. Fox Foundation for Parkinson’s Research, the Alzheimer’s Association, Alzheimer’s Research UK, and the Weston Brain Institute.
Xuemei Huang: Received funding from the NIEHS, the Michael J. Fox Foundation for Parkinson’s Research, the Alzheimer’s Association, Alzheimer’s Research UK, the Weston Brain Institute, Bristol Myers Squibb, Biogen, Pfizer, and the Department of Defense. She has received consultant fees and honorarium from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The data contained in this manuscript has not been previously published, has not been submitted elsewhere, and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
All study subjects provided written informed consent, and collection of all clinical and imaging measurements was approved by the institutional review board at the Pennsylvania State Milton S. Hershey Medical Center.
All authors have reviewed the contents of this manuscript and approve of its contents and validate the accuracy of the data.
References
- McFarland NR. Diagnostic Approach to Atypical Parkinsonian Syndromes. Continuum (Minneap Minn), 22 (2016), pp. 1117–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SS, Djamshidian A, Ahmed Z, Evans AH, Lawrence AD, Holton JL, Revesz T and Lees AJ. Impulsive-compulsive spectrum behaviors in pathologically confirmed progressive supranuclear palsy. Mov Disord, 25 (2010), pp. 638–642. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, O'Sullivan SS and Djamshidian A. Impulsive and compulsive behaviors in Parkinson's disease. Annu Rev Clin Psychol, 10 (2014), pp. 553–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cao B, Ou R, Wei QQ, Zhao B, Yang J, Wu Y and Shang H. Non-motor symptoms and the quality of life in multiple system atrophy with different subtypes. Parkinsonism Relat Disord, 35 (2017), pp. 63–68. [DOI] [PubMed] [Google Scholar]
- Pyatigorskaya N, Gallea C, Garcia-Lorenzo D, Vidailhet M and Lehericy S. A review of the use of magnetic resonance imaging in Parkinson's disease. Ther Adv Neurol Disord, 7 (2014), pp. 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane BJ and Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology, 80 (2013), pp. 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Luo XG and Gao C. Utility of susceptibility-weighted imaging in Parkinson's disease and atypical Parkinsonian disorders. Transl Neurodegener, 5 (2016), pp. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K and Kaye JA. Parkinson's disease is associated with hippocampal atrophy. Mov Disord, 18 (2003), pp. 784–790. [DOI] [PubMed] [Google Scholar]
- Carriere N, Besson P, Dujardin K, Duhamel A, Defebvre L, Delmaire C and Devos D. Apathy in Parkinson's disease is associated with nucleus accumbens atrophy: A magnetic resonance imaging shape analysis. Mov Disord, 29 (2014), pp. 897–903. [DOI] [PubMed] [Google Scholar]
- Junque C, Ramirez-Ruiz B, Tolosa E, Summerfield C, Marti MJ, Pastor P, Gomez-Anson B and Mercader JM. Amygdalar and hippocampal MRI volumetric reductions in Parkinson's disease with dementia. Mov Disord, 20 (2005), pp. 540–544. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Lechowicz W, Szpak GM, Lewandowska E, Dymecki J and Wierzba-Bobrowicz T. Limbic neuropathology in idiopathic Parkinson's disease with concomitant dementia. Folia Neuropathol, 42 (2004), pp. 141–150. [PubMed] [Google Scholar]
- Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RK and Gentleman SM. Dementia and visual hallucinations associated with limbic pathology in Parkinson's disease. Parkinsonism Relat Disord, 15 (2009), pp. 196–204. [DOI] [PubMed] [Google Scholar]
- Papp MI and Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain, 117 ( Pt 2) (1994), pp. 235–243. [DOI] [PubMed] [Google Scholar]
- Piao YS, Hayashi S, Hasegawa M, Wakabayashi K, Yamada M, Yoshimoto M, Ishikawa A, Iwatsubo T and Takahashi H. Co-localization of alpha-synuclein and phosphorylated tau in neuronal and glial cytoplasmic inclusions in a patient with multiple system atrophy of long duration. Acta Neuropathol, 101 (2001), pp. 285–293. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Different tau pathology pattern in two clinical phenotypes of progressive supranuclear palsy. Neurodegener Dis, 5 (2008), pp. 339–346. [DOI] [PubMed] [Google Scholar]
- Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S and Mann DM. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol, 120 (2010), pp. 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Liu T, Lewis MM, Kong L, Wang Y, Connor J, Mailman RB and Huang X. Quantitative susceptibility mapping of the midbrain in Parkinson's disease. Mov Disord, 31 (2016), pp. 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo G, Sierra-Pena M, Nemmi F, Traon AP, Meissner WG, Rascol O and Peran P. Multimodal MRI assessment of nigro-striatal pathway in multiple system atrophy and Parkinson disease. Mov Disord, 31 (2016), pp. 325–334. [DOI] [PubMed] [Google Scholar]
- Schwarz ST, Mougin O, Xing Y, Blazejewska A, Bajaj N, Auer DP and Gowland P. Parkinson's disease related signal change in the nigrosomes 1-5 and the substantia nigra using T2* weighted 7T MRI. Neuroimage Clin, 19 (2018), pp. 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger RD, Matheus FC, Schwarzbold ML, Lima MM and Vital MA. Anxiety in Parkinson's disease: a critical review of experimental and clinical studies. Neuropharmacology, 62 (2012), pp. 115–124. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Mild cognitive impairment in Parkinson disease: heterogenous mechanisms. J Neural Transm (Vienna), 120 (2013), pp. 157–167. [DOI] [PubMed] [Google Scholar]
- Asi YT, Ling H, Ahmed Z, Lees AJ, Revesz T and Holton JL. Neuropathological features of multiple system atrophy with cognitive impairment. Mov Disord, 29 (2014), pp. 884–888. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K and Takahashi H. Pathological heterogeneity in progressive supranuclear palsy and corticobasal degeneration. Neuropathology, 24 (2004), pp. 79–86. [DOI] [PubMed] [Google Scholar]
- Walsh K and Bennett G. Parkinson's disease and anxiety. Postgrad Med J, 77 (2001), pp. 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerle AM, Herman JP and Seroogy KB. Stress, depression and Parkinson's disease. Exp Neurol, 233 (2012), pp. 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley AC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ and LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord, 22 (2007), pp. 41–47. [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo A Durr CJ Fowler H Kaufmann T Klockgether A Lees W Poewe N Quinn T Revesz D Robertson P Sandroni K Seppi and Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology, 71 (2008), pp. 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E and Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology, 47 (1996), pp. 1–9. [DOI] [PubMed] [Google Scholar]
- Hoehn MM and Yahr MD. Parkinsonism: onset, progression and mortality. Neurology, 17 (1967), pp. 427–442. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R and Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord, 25 (2010), pp. 2649–2653. [DOI] [PubMed] [Google Scholar]
- Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P and Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med, 69 (2013), pp. 467–476. [DOI] [PubMed] [Google Scholar]
- Liu T, Xu W, Spincemaille P, Avestimehr AS and Wang Y. Accuracy of the morphology enabled dipole inversion (MEDI) algorithm for quantitative susceptibility mapping in MRI. IEEE Trans. Med. Imaging, 31 (2012), pp. 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keezer MR, Wolfson C and Postuma RB. Age, Gender, Comorbidity, and the MDS-UPDRS: Results from a Population-Based Study. Neuroepidemiology, 46 (2016), pp. 222–227. [DOI] [PubMed] [Google Scholar]
- Tanner JJ, McFarland NR and Price CC. Striatal and Hippocampal Atrophy in Idiopathic Parkinson's Disease Patients without Dementia: A Morphometric Analysis. Front Neurol, 8 (2017), pp. 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C and Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology, 78 (2012), pp. 1939–1945. [DOI] [PubMed] [Google Scholar]
- Yao N, Cheung C, Pang S, Shek-kwan Chang R, Lau KK, Suckling J, Yu K, Ka-Fung Mak H, Chua SE, Ho SL and McAlonan GM. Multimodal MRI of the hippocampus in Parkinson's disease with visual hallucinations. Brain Struct Funct, 221 (2016), pp. 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub J Koester MN Potenza AD Siderowf M Stacy V Voon J Whetteckey GR Wunderlich and Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol, 67 (2010), pp. 589–595. [DOI] [PubMed] [Google Scholar]
- Baxter MG and Murray EA. The amygdala and reward. Nat Rev Neurosci, 3 (2002), pp. 563–573. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K and Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature, 475 (2011), pp. 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S and Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev, 31 (1999), pp. 6–41. [DOI] [PubMed] [Google Scholar]
- Spokes EG, Bannister R and Oppenheimer DR. Multiple system atrophy with autonomic failure: clinical, histological and neurochemical observations on four cases. J Neurol Sci, 43 (1979), pp. 59–82. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Edland SD, Reid TN, Al Rizaiza A and Litvan I. Neuropsychiatric symptoms and their impact on quality of life in multiple system atrophy. Cogent Psychology, 3 (2016), pp. [Google Scholar]
- Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, Schmeichel AM and Parisi JE. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain, 138 (2015), pp. 2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ. Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry, 67 (2010), pp. 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piattella MC, Upadhyay N, Bologna M, Sbardella E, Tona F, Formica A, Petsas N, Berardelli A and Pantano P. Neuroimaging evidence of gray and white matter damage and clinical correlates in progressive supranuclear palsy. J Neurol, 262 (2015), pp. 1850–1858. [DOI] [PubMed] [Google Scholar]
- Litvan I, Grafman J, Gomez C and Chase TN. Memory impairment in patients with progressive supranuclear palsy. Arch Neurol, 46 (1989), pp. 765–767. [DOI] [PubMed] [Google Scholar]
- Du G, Lewis MM, Sica C, He L, Connor JR, Kong L, Mailman RB and Huang X. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson's patients. Mov Disord, 2018. May 14. doi: 10.1002/mds.27318. [Epub ahead of print] (2018), pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y and Liu T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson. Med, 73 (2014), pp. 82–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MM, Du G, Baccon J, Snyder AM, Murie B, Cooper F, Stetter C, Kong L, Sica C, Mailman RB, Connor JR and Huang X. Susceptibility MRI captures nigral pathology in patients with parkinsonian syndromes. Mov Disord, (2018), pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, King BR, Maquet P and Doyon J. Hippocampus and striatum: dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus, 23 (2013), pp. 985–1004. [DOI] [PubMed] [Google Scholar]
- Kuhn W, Heye N, Muller T, Kraus P, Klotz P, Friedrich B, Welter FL and Przuntek H. The motor performance test series in Parkinson's disease is influenced by depression. J Neural Transm (Vienna), 103 (1996), pp. 349–354. [DOI] [PubMed] [Google Scholar]
- Makris N, Swaab DF, van der Kouwe A, Abbs B, Boriel D, Handa RJ, Tobet S and Goldstein JM. Volumetric parcellation methodology of the human hypothalamus in neuroimaging: normative data and sex differences. Neuroimage, 69 (2013), pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Mamikonyan E, Papay K, Shea JA, Xie SX and Siderowf A. Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Rating Scale. Mov Disord, 27 (2012), pp. 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.