Abstract
Background:
It is currently unknown why people with Alzheimer’s disease (AD) receive less pain medication and report pain less frequently.
Objective:
The purpose of this study was to determine the impact of AD on thermal psychophysics and resting-state functional connectivity (RSFC) among sensory, affective, descending modulatory, and default mode structures.
Methods:
Controls (n = 23, 13 = female) and age-matched people with AD (n = 23, 13 = females) underwent psychophysical testing to rate perceptions of warmth, mild, and moderate pain and then completed resting-state fMRI. Between groups analysis in psychophysics and RSFC were conducted among pre-defined regions of interest implicated in sensory and affective dimensions of pain, descending pain modulation, and the default mode network.
Results:
People with AD displayed higher thermal thresholds for warmth and mild pain but similar moderate pain thresholds to controls. No between-group differences were found for unpleasantness at any percept. Relative to controls, people with AD demonstrated reduced RSFC between the right posterior insula and left anterior cingulate and also between right amygdala and right secondary somatosensory cortex. Moderate pain unpleasantness reports were associated with increased RSFC between right dorsolateral prefrontal cortex and left ACC in controls only.
Conclusions:
While AD had little effect on unpleasantness, people with AD had increased thermal thresholds, altered RSFC, and no association of psychophysics with RSFC in pain regions. Findings begin to elucidate that in people with AD, altered integration of pain sensation, affect, and descending modulation may, in part, contribute to decreased verbal pain reports and thus decreased analgesic administration.
Keywords: Acute pain, Alzheimer’s disease, brain mapping, dementia, experimental thermal pain, functional connectivity, neurobiology, neuroimaging, pain perception, pain threshold, psychophysics
INTRODUCTION
Untreated pain in people with Alzheimer’s disease (AD) is a serious public health problem [1]. Poorly managed pain is associated with depression [2], anxiety [3], and functional loss [4]. Thus, untreated pain in people with AD may lead to increased suffering. Results of clinical and experimental studies on pain in AD are mixed [5]. Though emerging evidence suggests that people with AD may receive significantly more non-narcotic analgesic medication relative to controls [6], a majority of studies demonstrate that when compared to controls with similar painful conditions, people with dementia generally receive less pain medication (reviewed in [7]). A likely contributor to poor pain management in people with dementia is the lack of consensus regarding behavioral pain assessment methods, particularly in people with severe dementia [8]. Additionally, most clinical and experimental studies of pain in dementia do not examine how dementia pathology (e.g., dispersion of white matter disease, tau protein burden, total amount of amyloid-β, etc.) impacts central pain processing, making interpretation of findings difficult (reviewed in [5]).
Pain is a multi-dimensional phenomenon, and it has been hypothesized that damage to brain regions responsible for encoding pain contributes to altered behavioral response and verbal reports of pain in AD [9]. A limited number of functional neuroimaging studies have begun to examine this hypothesis. Using mechanical pressure pain, Cole and colleagues [10] found that when compared to controls, people with AD demonstrated higher thresholds for just noticeable pain and verbally rated just noticeable pain as more unpleasant. They also demonstrated significantly greater and prolonged brain activation in many regions responsible for encoding pain sensitivity and pain affect. Next, Cole and colleagues examined the integrated function (resting-state functional connectivity, RSFC) in brain regions responsible for processing sensory and emotional pain using task-evoked functional connectivity during the delivery of mechanical pressure pain [11]. Increased task-evoked functional connectivity was identified in three primary nodes, namely right dorsolateral prefrontal cortex (R-dlPFC), hypothalamus (HYPO), and periaqueductal gray (PAG) [11]. It was hypothesized that dysfunction among these structures, which are thought to function as a part of a top-down pain modulatory mechanism, may provide a mechanism for altered subjective pain reports in AD. Meanwhile, Beach et al. (2016, personal communication) found that greater behavioral responsiveness to pressure pain in patients was associated with altered connectivity between limbic and ventral prefrontal structures, within the default mode network (DMN), and between the DMN and salience network. These studies provide critical insight into our understanding of central pain processing in AD. However, additional neuroimaging studies are urgently needed to further elucidate the impact of AD on pain perception.
Pain is also described as an unpleasant sensory and emotional affective experience [12]. Core sensory network regions include primary (S1), secondary (S2) somatosensory cortices [13] and posterior insula (pINS) [14], while regions responsible for encoding unpleasantness include anterior cingulate cortex (ACC) [15], dlPFC [16], and anterior insular cortex (aINS) [17]. Because of its high affinity for opiate binding coupled with high concentrations of endogenous opioids, the PAG is a key pain modulating structure [18]. Overall, the descending pain modulatory system is thought to include the PAG, HYPO, amygdala (AMY), INS, and ACC (reviewed in [19]).
In addition to the common core of recognized pain regions, recent findings suggest that the DMN is also active during experimental pain tasks and may be dependent on pain intensity [20]. For example, when compared to healthy older males, healthy older females demonstrated activation of the DMN (e.g., cuneus, precuneus, posterior cingulate cortex (PCC), hippocampus) during pain [20]. Similarly, in a sample of cognitively normal adults, when compared to ‘high’ pain, ‘low’ pain resulted in greater deactivation in DMN regions including PCC, hippocampus, precuneus, and cerebellum [21].
The DMN is generally posited to engage neural systems that are involved in the passive monitoring of the ‘external environment’ or invoking an ‘internal awareness’ (reviewed in [22]). Furthermore, the DMN has been shown to be negatively correlated with regions that increase their activity during attention demanding tasks [23–25], such as the recognition and awareness of initial pain. DMN [22] function is well established to be altered in AD [26]. Relative to controls, people with AD have decreased RSFC in PCC and hippocampus [26]. Notably, pain intensity is correlated with hippocampal volume in older adults [27], and hippocampal atrophy is a core pathologic process in AD [28]. Altered DMN function is thus another strong candidate for explaining altered pain in AD. It is logical then that Beach et al. (2016, personal communication) found associations between DMN RSFC and increased pain behaviors in people with AD, relative to controls. However, the role of altered DMN RSFC has not been established for other altered aspects of pain perception in AD, such as pain report. However, AD affects the function of multiple brain networks [29]. Thus, it is plausible that disruptions in connectivity between multiple brain regions and networks may lead to altered pain experiences and pain reports in people with AD.
Resting-state functional magnetic resonance imaging (fMRI) is used to describe intrinsic temporal correlations among brain regions possibly indicating connected brain [30]. Importantly, RSFC has been used to describe central alterations associated with chronic pain. Among people with newly diagnosed sub-acute back pain, RSFC was able to differentiate those who did and did not transition from sub-acute back pain to chronic low back pain [31]. Although the clinical translational relevance of RSFC in the assessment of chronic pain has yet to be definitively determined, the conceptual importance using RSFC to identify pain and monitor the efficacy of pain treatment holds great promise. When compared to standard task based fMRI, RSFC demonstrates several advantages, such as increased signal to noise ratios, decreased acquisition time, and greater ease of studying difficult patient populations [32].
Unfortunately, the vast majority of literature on RSFC and pain is from younger cohorts, with limited studies examining RSFC and pain in older populations [33, 34] and only one examining pain-related RSFC in AD (Beach et al., 2016, personal communication). Importantly, the study by Beach et al. did not examine RSFC associations with subjective pain report differences in people with AD compared to controls. Thus, the purpose of this study was to examine the impact of AD on psychophysical responses (pain report) to thermal stimuli and associated RSFC among sensory, affective, descending modulatory, and default mode structures. The current sample was drawn from a larger ongoing study examining brain activation and pain reports in response to experimental thermal pain. We recently reported psychophysical responses to pain in AD in a sample of 40 people with AD and 40 sex-age matched controls; there we found that relative to controls, people with AD required higher temperatures to detect ‘warmth’, ‘mild pain’, and ‘moderate pain’ [35]. The current study extends these findings by examining the association of psychophysical pain report with RSFC in an age- and sex-matched subsample that completed RSFC procedures from our previously published AD psychophysics paper [36]. The samples were overlapping except for one person. Here, our first hypothesis was that when compared to controls, people with AD would be less sensitive to the detection of pain and find pain less unpleasant. Because people with AD require greater stimulus intensity to detect pain [35], and they may not be immediately aware of a pain stimulus, our second hypothesis was that people with AD, when compared to controls, would have decreased RSFC between brain regions in the sensory (S1, S2, pINS) and affective (dlPFC, ACC, aINS) networks, as well as between brain regions in the sensory (S1, S2, pINS) and default mode (PAG, HYPO, AMY) networks. As Cole and colleagues [11] demonstrated increased pain modulatory connectivity in AD during evoked pain [11], our third hypothesis was that, relative to controls, people with AD would demonstrate increased RSFC between brain regions in the descending modulatory (PAG, HYPO, AMY) and sensory (S1, S2, pINS) networks, also brain regions in the descending modulatory (PAG, HYPO, AMY) and affective (dlPFC, ACC, aINS) networks. Our fourth hypothesis was that RSFC findings would correlate with psychophysical findings.
METHODS
The research reported in this study adhered to the Code of Ethics of the World Medical Association and Declaration of Helsinki and was approved by the Vanderbilt University Institutional Review Board. The current sample was drawn from a larger ongoing study examining the neurobiology of pain in dementia. The current study sample consisted of two age- and sex-balanced (each 50% female) groups of 26 AD volunteers and 26 volunteers without AD aged 65 to 92 (median age of 74).
Screening and enrollment of participants
The procedure for screening and enrollment of subjects in the parent study has been previously described [35], but in sum, subjects with a clinical diagnosis of AD were recruited from the practices of three geriatricians, two geriatric psychiatrists, and a neurologist from Vanderbilt University Medical Center. People with AD met the National Institute of Neurological and Communicative Disorders and Stroke and the Disease and Related Disorders Association (NINCDS-ADRDA) [37] criteria for probable AD. In addition to these criteria, some subjects underwent additional testing. Specifically, medical records were reviewed to confirm the presence of an AD diagnosis based on supportive documentation including the following: Mini-Mental State Exam (MMSE) [38], the Montreal Cognitive Assessment (MoCA) [39], and/or Functional Assessment Staging (FAST) Scale [40]. For the current study, the extent of global cognitive impairment in people with AD was assessed with the Folstein MMSE. Participants and caregivers were instructed to avoid drinking caffeine for four hours before MRI procedures and not to use any analgesic medications for at least 24 hours prior to data collection. Participants and their caregivers were reimbursed $100.00 each for their time.
Assessments
Participants underwent one hour of psychosocial assessments during the home visit. In people with AD who lacked capacity, legal surrogates assisted in the collection of demographic data including: a detailed list of all medications, Hollingshead Four Factor Index of Social Status (SES) [41], Brief Pain Inventory (BPI) [42], Geriatric Depression Scale (GDS) [43], MRI safety clearance, and cognitive screening with the MMSE [38]. On the day of the MRI procedures, each subject was re-assessed with the BPI and GDS, and underwent State-Trait Anxiety Inventory (STAI) [44] assessment. Tests were read to all participants with AD to facilitate increased understanding and completeness.
Thermal stimulation protocol (psychophysics)
In a room adjacent to the MRI scanner, participants underwent thermal pain psychophysics evaluation (~30min) using the Medoc Pathway Pain and Sensory Evaluation System ATS-CHEPS fMRI model [45]. Each participant was told, “There are two aspects of pain which we are interested in measuring: the intensity, how strong the pain feels, and the unpleasantness, how unpleasant or disturbing the pain is for you”. Before beginning sensory threshold testing using the Method of Limits program, practice sessions of psychophysics were conducted. The Medoc thermode (30 ×30mm) was attached to the thenar eminence of the right hand. Next, participants were shown a 0–20 pain intensity scale and a 0–20 pain unpleasantness scale [46] successfully used in prior studies of pain in people with AD [10, 11]. Each participant was read the following: “I will tell you when the metal cube that is attached to your hand will start heating up, then I will ask you to stop the heat when you feel ‘warmth’, ‘mild pain’, or ‘moderate pain.’ I will not ask you to rate any pain greater than ‘moderate pain’. After you stop the heat, I will ask you to tell me how unpleasant the previous temperature was.” A main goal of these practice sessions was to help determine if subjects could reliably understand the directions. If a subject rated ‘warmth’ less than ‘mild pain,’ which was less than “moderate pain,” that subject was included in the study. After practice sessions, each participant completed three trials determining the temperature for each percept (‘warmth’, ‘mild pain’, ‘moderate pain’) and three trials reporting unpleasantness ratings for each temperature percept identified.
After collection, each trial was averaged to find the mean warmth, mild pain, and moderate pain intensity ratings. The same procedure was repeated to determine mean affective ratings for each of the temperatures reports during the previously described intensity trials.
Brain imaging acquisition
Immediately following the collection of psychophysics as described above, subjects were assisted to a comfortable position on the MRI table. After acquiring a survey and structural scan, participants completed a 16-min functional task paradigm. Then, after approximately 1min, a 5-min resting state sequence was completed (echo time (TE) = 35ms; time to report (TR) = 2000ms; field of view (FOV) = 240×240×131.6; 30 slices; 150 dynamics; voxel size=3mm×3 mm, 4mm slice thickness). Thus, the psychophysics used in the current analysis were collected approximately 30min prior to the resting state scan. To limit the possibility of subjects falling asleep, subjects were instructed to remain awake with eyes open during the resting state scan. Research assistants and the MRI technicians spoke with each subject immediately before the scan to ensure the subject’s level of comfort with continuing the scanning procedures. Immediately after the 5-min RSFC scan, study personnel spoke to the subject to ensure a timely response.
Analysis of head motion
Because head motion may impact correlation analyses [47], head motion analyses using Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect/toolbox) was used to detect motion outliers. This approach was comprised of three steps. The first step was an evaluation of the motion outliers for each volume across the full blood oxygen level dependent (BOLD) fMRI time course (300s, TR = 2s, 150 volumes). Normalized mean motion was computed from the x, y, z, pitch, roll, and yaw axes by which in-scanner head motion was measured as displacement from a central point along some axis. This normalized mean motion was represented by a single motion vector, and volumes whose normalized mean motion exceeded the threshold (2mm in the current analysis) were marked as outliers. For each outlier volume, an additional delta function regressor was added to the confound model. The subject’s entire fMRI time course was removed if more than half of the volumes were identified as outliers. The second component of this approach was the comparison of the individual subject motion relative to the normalized mean motion of the sample. If motion for a single subject exceeded three standard deviations from the mean, or if the normalized mean motion for a subject exceeded the threshold (1mm for this approach) across all volumes, then the resting state time course for the subject was removed from the sample. The third step used the Mann-Whitney U test to determine if the difference in total head motion between the AD and control groups was statistically significantly different.
Regions of interest (ROIs) seed selection
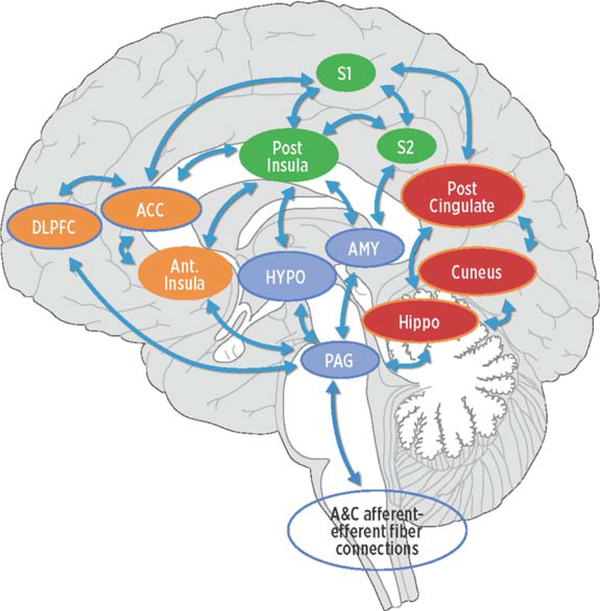
We used an ROI-to-ROI approach focusing on four distinct pain-related networks, each consisting of three ROIs. Specific networks included lateral/sensory, medial/affective, descending modulatory, and DMN (see conceptual model, Fig. 1). Lateral or sensory pain ROIs included S1, S2, and pINS [10]. Medial or affective pain pathway ROIs included ACC, dlPFC, and aINS [10]. Descending pain modulation ROIs included PAG, HYPO, and AMY [19]. DMN ROIs included PCC, hippocampus, and precuneus. While the dlPFC is part of the top-down modulation system [11], the literature frequently describes the dlPFC in the affective network (reviewed in [5]). For the purposes of this study, we placed the dlPFC in the affective network. We defined bilateral regions of interest using the TD Brodmann Area (BA) maps and Automated Anatomical Labeling (AAL) regions [48] provided in the Wake Forest U (WFU) PickAtlas Toolbox [49, 50] extension in SPM12 as the following: AMY, HYPO, PCC, precuneus, and hippocampus, ACC=BA 24, 32, & 33; dlPFC =BA 9 & 46 [10]; S1 =BA 1,2,3 [10]; and S2 =BA 40,44 [10]. We defined the PAG as a single 6mm sphere centered on MNI coordinates x=6, y = −30, z = 14 [51]. We subdivided the insula into left (L-)aINS with a 6mm sphere centered on MNI coordinates x = −38, y=6, z = 2; R-aINS with a 6mm sphere centered on MNI coordinates x = 35, y=7, z = 3; L-pINS with a 6mm sphere centered on MNI coordinate x = −38, y = −6, z = 5; and R-pINS=6mm sphere centered on MNI coordinate x = 35, y = −11, z = 6 [52]. See Table 1 for each ROI and corresponding Atlas.
Fig. 1.
Conceptual model of four networks believed to be involved in pain processing and pain interpretation. Green areas represent common structures in the sensory/discriminative (lateral) pain pathway (pINS, S1, and S2). Orange areas represent common structures identified in the affective/motivational (medial) pain pathway (ACC, AINS, dlPFC). The blue regions represent common structures in the descending modulatory system (AMY, HYPO, PAG). Red areas represent core structures in the default mode network (posterior cingulate gyrus, cuneus, hippocampus). The arrows represent multiple cortical connections between regions and systems indicating the complex interconnectedness of brain regions involved with pain. pINS, posterior insula; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; ACC, anterior cingulate cortex; aINS, anterior insula; dlPFC, dorsolateral prefrontal cortex; AMY, amygdala; HYPO, hypothalamus; PAG, periaqueductal gray.
Table 1.
Regions of interest used and various atlases used from the WFU-PickAtlas toolbox extension in SPM 12
| ROI | Atlas/Coordinates [REF] |
|---|---|
| Left Anterior Insula | MNI −38, 6, 2 6mm Sphere [52] |
| Left Anterior Cingulate Cortex | TD Brodmann Areas 24, 32, 33 [50, 78, 79] |
| Left Amygdala | AAL [80] |
| Left Dorsolateral Prefrontal Cortex | AAL [80] |
| Left Hippocampus | AAL [80] |
| Left Hypothalamus | AAL [80] |
| Left Posterior Insula | MNI −38, −6, 5 6mm Sphere [52] |
| Left Posterior Cingulate Cortex | AAL [80] |
| Left Precuneus | AAL [80] |
| Left Primary Somatosensory Cortex | TD Brodmann Areas 1, 2, 3 [50, 78, 79] |
| Left Secondary Somatosensory Cortex | TD Brodmann Areas 40, 44 [50, 78, 79] |
| Periaqueductal Gray | MNI 6, −30, 14 6mm Sphere [51] |
| Right Anterior Insula | MNI 35, 7, 3 6mm Sphere [52] |
| Right Anterior Cingulate Cortex | TD Brodmann Areas 24, 32, 33 [50,78, 79] |
| Right Amygdala | AAL [80] |
| Right Dorsolateral Prefrontal Cortex | AAL [80] |
| Right Hippocampus | AAL [80] |
| Right Hypothalamus | AAL [80] |
| Right Posterior Insula | MNI 35, −11, 6 6mm Sphere [52] |
| Right Posterior Cingulate Cortex | AAL [80] |
| Right Precuneus | AAL [80] |
| Right Primary Somatosensory Cortex | TD Brodmann Areas 1, 2, 3 [50, 78, 79] |
| Right Secondary Somatosensory Cortex | TD Brodmann Areas 40, 44 [50, 78, 79] |
ROI, region of interest; WFU, Wake Forest University, MNI, Montreal Neuroimaging Institute; TD, Talairach Daemon; AAL, Automated Anatomical Labeling.
Calculation of RSFC
Standard preprocessing was accomplished with SPM12 and ART including motion correction (z = 9; threshold = 2 mm), slice timing correction, band-pass filtering of 0.01–0.1Hz, co-registration to structural images, spatial normalization to MNI space, and spatial smoothing with an 8mm Gaussian kernel. Individual subject’s mean white matter, cerebrospinal fluid, and motion parameters were included in the first level model as covariates of no interest. The CONN-fMRI Functional Connectivity toolbox v15.g (http://www.nitrc.org/projects/conn/) as cited in [53] and CompCor [54] were used to perform the latter nuisance regressor analyses.
Next, RSFC analysis was performed with the CONN toolbox [53], which performs time series preprocessing, volume exclusion, and connectivity calculations. Connectivity matrices for the ROIs were compared between people with AD and healthy older adults. To examine overall connectivity in a network, the median connectivity value within each ROI was calculated for each subject and then the correlation between ROIs were compared between groups. Positive and negative correlation thresholds were at T > 2.34, corresponding to p < 0.01 uncorrected [55]. Analyses were adjusted for regional gray matter volume (GMV). To compensate for between-subject differences in GMV with respect to total intracranial volume (TIV), the residual difference between TIV and GMV was entered as a second level covariate in the between-groups comparisons.
General and psychophysical analyses
These analyses were conducted using SPSS (Version 23). Nominal data were summarized using frequency distributions and the AD groups compared using Chi-Square Tests of Independence. Due to skewed distributions, the continuous data were summarized by median and inter-quartile range (IQR) and compared using Mann-Whitney Tests. Data were rank-transformed for use in subsequent analyses. Linear regressions within each group were used to generate the associations of each of the pain temperature/sensation self-reports with the extracted RSFC findings for each group. Due to the known and observed confounding associations of depressive symptoms with pain, the GDS scores were entered as covariates in those regression models. The resulting beta coefficients were compared using the z-statistic. An uncorrected alpha of 0.05 (p < 0.05) was used for determining statistical significance of psychophysical findings.
RESULTS
Analysis of head motion
Three AD subjects (two female) were identified as having excessive normalized mean motion > 1 mm. In order to maintain age and sex balanced groups, age-matched controls (two female) were also removed. The final sample consisted of 46 people, (22 female, 11 with AD; 24 male, 12 with AD). The groups did not differ on normalized mean head motion (p = 0.307).
Demographics
No statistically significant differences were observed between the groups in terms of SES, BPI current pain, BPI average pain, or anxiety scores (p > 0.05). The participants with an AD diagnosis did have statistically significantly higher levels of depressive symptoms as measured by the GDS than did those in the controls (p = 0.001; Table 2).
Table 2.
Demographic and clinical summaries by Alzheimer’s disease diagnosis
| Total | AD | Control | p-value | |
|---|---|---|---|---|
| (N = 46) | (N = 23) | (N=23) | ||
| Median [IQR] | Median [IQR] | Median [IQR] | ||
| Age | 71.0 [68–79] | 74.0 [68–79] | 69.0 [68–78] | 0.086 |
| Standardized measures | ||||
| Total SES score1 | 54.0 [43–58] | 52.0 [42–57] | 55.5 [43–64] | 0.786 |
| MMSE score2 | 27.0 [21–30] | 21.0 [14–24] | 30.0 [29–30] | <0.001 |
| BPI-SF average pain3 | 0.0 [0–2] | 0.0 [0–3] | 1.0 [0–2] | 0.838 |
| BPI-SF pain right now3 | 0.0 [0–0] | 0.0 [0–0] | 0.0 [0–0] | 0.268 |
| GDS-SF score4 | 1.5 [0–4] | 3.0 [1–5] | 0.0 [0–2] | 0.001 |
| STAI state score5 | 47.5 [45–51] | 47.0 [44–50] | 48.0 [45–51] | 0.360 |
| STAI trait score5 | 47.0 [45–50] | 47.0 [44–50] | 47.0 [45–50] | 0.907 |
SES (Hollingshead Four Factor Index of Social Status; range=8 lowest to 66=highest).
MMSE (Folstein Mini Mental State Examination; range = 0completelycognitivelyimpairedto30completelycognitivelyintact).
BPI-SF (Brief Pain Inventory Short Form; range = 0 no pain to10 = most pain).
GDS-SF (Geriatric Depression Scale Short Form; range 0 = no indication of depression to 15 = high possibility of depression).
STAI (Spielberger State or Trait Anxiety Inventory; range=20 increased anxiety to 80 least amount of anxiety).
Psychophysics
Summaries of self-report psychophysics data are shown in Table 3. Relative to controls, people with AD demonstrated less sensitivity to warmth (p = 0.030) and mild pain (p = 0.039) detection levels. No statistically significant differences between the groups were observed in terms of unpleasantness or affective responses to those perceived pain stimulus intensities (p > 0.05).
Table 3.
Psychophysical summaries by Alzheimer’s disease diagnosis
| Total | AD | Control | p-value |
|---|---|---|---|
| (N=46) | (N = 23) | (N = 23) | |
| Median [IQR] | Median [IQR] | Median [IQR] | |
| Just Noticeable Warmth | |||
| Temp 33.0 [32–34] | 33.0 [32–34] | 32.0 [32–33] | 0.030 |
| Affect 0.0 [0–1] | 0.0 [0–1] | 0.0 [0–1] | 0.877 |
| Mild Pain | |||
| Temp 35.2 [34–40] | 37.0 [35–41] | 35.0 [34–38] | 0.039 |
| Affect 4.0 [2–5] | 5.0 [3–6] | 4.0 [1–5] | 0.333 |
| Moderate Pain | |||
| Temp 40.0 [37–44] | 42.0 [37–44] | 38.0 [37–43] | 0.533 |
| Affect 7.0 [5–9] | 8.0 [5–9] | 6.0 [5–9] | 0.925 |
Temp: Temperature in degrees Celsius, range = 30 to 55. Affect: Verbal unpleasantness rating, range 0 = neutral to 20 = extremely distressing.
RSFC
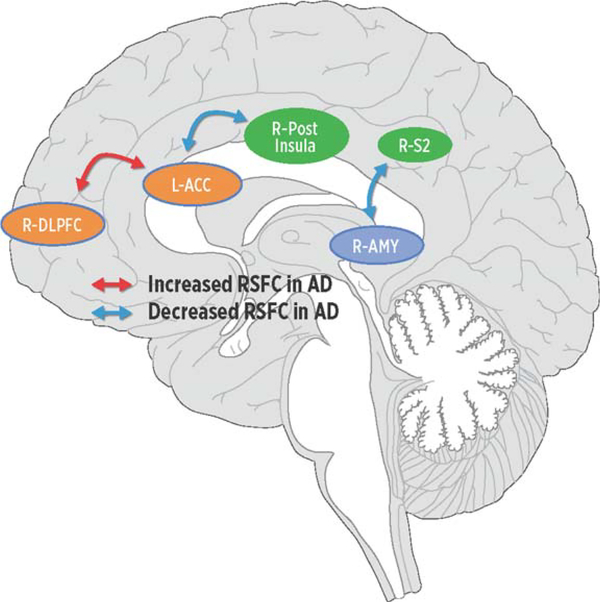
Relative to controls, and after controlling for depressive symptoms (GDS scores) and total GMV, people with AD demonstrated decreased RSCF between sensory (R-pINS) and affective regions (bilateral ACC) as well as sensory (R-S2) and descending modulatory (R-AMY) regions. In contrast, and compared to controls, people with AD demonstrated increased RSFC between regions modulating pain affect (R-dlPFC) and (L-ACC); see Fig. 2 and Table 4). The Supplementary Table 1 shows all ROI-to-ROI connections surviving p < 0.05, with corresponding T statistic.
Fig. 2.
Regions in networks demonstrating increased (red arrows) or decreased (blue arrows) resting-state functional connectivity (RSFC) in Alzheimer’s disease (AD). Relative to controls, people with AD demonstrated increased RSFC between the cognitive (dlPFC) and affective (ACC) regions in the medial pain system while conversely people with AD displayed decreased RSFC (ACC) regions in the medial network and sensory (pINS) network and between the sensory (S2) network and the descending modulatory (AMY) network. dlPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; pINS, posterior insula; S2, secondary somatosensory cortex; AMY, amygdala.
Table 4.
ROI-to-ROI connections for correlations greater and less in healthy controls than in subjects with a diagnosis of Alzheimer’s disease
| Source | Seed | T | 1p-corrected |
|---|---|---|---|
| ROI-to-ROI Connectivity for Healthy>AD | |||
| Right Posterior Insula | Left ACC | 3.22 | 0.0025 |
| Right ACC | 2.71 | 0.0096 | |
| Right Amygdala | Right S2 | 2.76 | 0.0085 |
| ROI-to-ROI Connectivity for Healthy<AD | |||
| Right DLPFC | Left ACC | −3.30 | 0.0020 |
Threshold corrected T > 2.35, p < 0.01.
Associations between psychophysics and RSFC
Each of the thermal stimuli and unpleasantness reports across all percepts (warmth, mild pain, moderate pain) were correlated with extracted overall RSFC beta weights in each ROI for each participant. This unadjusted analysis resulted in a single statistically significant between-group difference in strength and direction of the association between unpleasantness ratings of moderate intensity pain and the RSFC between the R-dlPFC and L-ACC (z test of differences = 2.17, p = 0.030). This finding was the result of a moderate correlation between affective response to moderate pain and RSFC in controls (0.50, p = 0.015) that was absent in those with AD (−0.09, p = 0.704).
DISCUSSION
The current study examined the impact of AD on pain-related psychophysics and RSFC of brain regions involved in pain processing. Specifically, we examined how AD and control subjects’ psychophysical responses to thermal stimuli were related to RSFC among pain-related sensory, affective, descending modulatory ROIs as well as the DMN. Our first hypothesis was that when compared to controls, people with AD would be less sensitive to the detection of pain and find pain less unpleasant; this hypothesis was partially supported. When compared to controls, people with AD displayed decreased thermal sensitivity to the detection of both warmth and mild pain; no differences were found for unpleasantness for any thermal percept. Thus, while lower level thermal pain thresholds were increased in people with AD, pain-induced affect for perceptually matched pain levels was virtually equal to those of controls. The current study’s psychophysical findings were similar to the results from the larger one, from which this sample was drawn [35].
Our psychophysical findings here are generally in agreement with prior studies by Cole et al. [10] and Gibson et al. [56], who both found increased perceptual detection thresholds for pressure and CO2 laser pain modalities, respectively. Nevertheless, data are rather mixed with respect to whether, and in which direction, AD may alter pain thresholds and/or unpleasantness. Other than aforementioned studies (including the current one) showing mild increases in pain-related thresholds, multiple studies have found equal pain thresholds between AD and controls [57–59]. Further, despite early reports of increased pain tolerance in AD (thought to reflect reduced pain affect [58]), more recent studies find evidence of reduced tolerance [10, 59] as well as equal [59–61] or slightly higher subjective pain ratings [62] in patients versus controls. Likely reasons for mixed findings with respect to effects of AD on subjective aspects of pain include: varied scales for reporting pain levels; various pain induction modalities; perceptual matching and quantitative sensory testing versus fixed intensity paradigms. Regardless, results of thermal psychophysical testing here suggest that, while thermal pain-related affect is unchanged by AD (at least for up to moderate pain), thermal thresholds to reach those percepts are altered by the disease. The clinical result of these altered perceptual sensitivities in AD may thus be reduced pain report and under-treatment of pain in patients [36].
Our second hypothesis was that, when compared to controls, people with AD would have decreased RSFC between brain regions in the sensory (S1, S2, pINS) and affective (dlPFC, ACC, aINS) networks, and between brain regions in the sensory (S1, S2, pINS) and default mode (PAG, HYPO, AMY) networks; this hypothesis was partially supported. Relative to controls, people with AD demonstrated decreased RSFC between R-pINS and bilateral ACC and between R-AMY and R-S2. Our third hypothesis was that, when compared to controls, people with AD would demonstrate increased RSFC between descending modulatory and sensory ROIs, and between descending modulatory and affective ROIs; this hypothesis was not supported. In fact, increased connectivity was found between R-dlPFC and L-ACC in people with AD when compared to controls. None of the selected brain regions in the DMN showed significantly different RSFC between controls and people with AD.
Our third hypothesis was that relative to controls, people with AD would demonstrate increased RSFC between brain regions in the descending modulatory (PAG, HYPO, AMY) and sensory (S1, S2, pINS) networks, and brain regions in the descending modulatory (PAG, HYPO, AMY) and affective (dlPFC, ACC, aINS) networks; this hypothesis was partially supported. We found that people with AD demonstrated decreased RSCF between sensory (R-pINS) and affective regions (bilateral ACC) as well as sensory (R-S2) and descending modulatory (R-AMY) regions. In contrast, and compared to controls, people with AD demonstrated increased RSFC between in regions modulating pain affect (R-dlPFC) and (L-ACC).
Our fourth hypothesis that RSFC findings would correlate with psychophysical findings was partially supported. We found a single significant between-group difference in the association between unpleasantness ratings of moderate intensity pain and the RSFC between the R-dlPFC and L-ACC. Though reduced sensitivity to pain detection in AD versus controls coincided with decreased sensory pain RSFC in people with AD, a specific statistical relationship between these results was not found. This may pertain to the limited spatial scope of our a priori defined ROIs; we simply may not have had the necessary spatial coverage to capture related RSFC correlates of altered pain detection in people with AD.
The current results extend and build on the paucity of studies examining the relationship between pain report and brain function in AD. Our findings partially agree with those of Cole and colleagues’ studies [10, 11] examining pressure pain-induced activation and functional connectivity in people with AD and healthy older adults. Using both ROI-to-ROI and whole brain seed analyses they found that, relative to controls, people with AD demonstrated increased activation and functional connectivity across several medial pain/pain modulatory regions, namely involving the dlPFC, HYPO, and PAG. Here, no associations could be found between thermal pain detection or unpleasantness with the PAG or HYPO, possibly because of the resting-state nature of the current study. Nevertheless, it is intriguing that increased R-dlPFC to ACC connectivity in AD, compared to healthy older adults consistently, occurred in both a prior pain induction study [11] and in the current study. It further remained consistently associated with relatively equal moderate pain ratings between groups.
While the dlPFC is involved in pain-related cognition [63] and subsequent modulation [16, 19, 64], the ACC functions as part of the affective-motivational dimension of central pain processing [15]. Together the two work in concert to bring about affective judgments and subsequent goal-directed behaviors [65, 66]. Increased RSFC between dlPFC and ACC found here, as well as by Cole et al., may thus reflect a baseline compensatory means of preserving cognitive evaluation, top-down modulation, and goal-directed behaviors related to affective states such as pain [11, 67, 68]. However, the downstream effects of this aberrant baseline RSFC on pain processing remain only partly elaborated [11]. Further derangement of pain processing not captured here or by Cole et al. is also possible, for example between ventromedial prefrontal and temporal-limbic structures (Beach et al., 2016, personal communication). The end result may thus be heightened emotional appraisal in AD, compared to healthy older adults, despite equal or slightly reduced perceptual pain thresholds [35], as suggested by multiple recent studies finding reduced pain tolerance [59] and increased pain behaviors such as facial expressions [60, 61, 69] in patients. Further, coupled with memory decline and impaired pain-related semantic fluency, one may be left with paradoxical findings of increased pain behaviors [62] and decreased verbal reports of pain by people with AD [70].
There are some limitations to consider when interpreting the results from the current study. ROI definition required use of a combination of atlases and specific coordinate generated spheres from specific examples in the pain literature. This method may result in less spatial and functional specificity in regions such as the ACC, which is divided into multiple functional and anatomic subregions [71], but does allow for a basic comparison between studies defining the same ROI. Atrophy is a common phenomenon in AD and the relationship between atrophy and decreased cognition is well documented [72, 73]. Since we used a combination of anatomical and spherical ROI masks, it is possible that regional atrophy secondary to AD or general aging resulted in ROI timeseries that inadvertently included voxels from white matter or CSF [74]. To help account for regional atrophy and improve tissue segmentation, we co-registered functional timeseries to structural images before normalizing to MNI space. We further calculated an adjusted GMV and used the residual difference as a second level covariate in our group level analyses. While we excluded participants who met DSM-IV criteria for major depression, continuous measures of depression scale scores (as measured by the GDS) were higher in the AD participants than in the control participants. Therefore, we controlled for depression score to control for potential effects of depression on outcome measures. Work in other populations has suggested the depression can influence pain processing. While our study cannot address this relationship, future studies comparing people with dementia and depression to those with dementia without depression would be able to examine specifically the effects of depression on pain processing. Consideration should also be given to treating sub-syndromal depression in people with AD, potentially with pain medications that have been shown to have antidepressant properties (e.g., tramadol) or with antidepressants that treat pain (e.g., duloxetine).
Conclusions
Though resting-state MRI provides a feasible, non-invasive method for examining intrinsic neural network activity without an in-scanner pain stimulus, making assumptions about the pain system integrity in AD is a challenge. Despite this limitation, the potential use of RSFC to guide management of pain in difficult patient populations such as AD holds great promise. When compared to task-evoked studies, RSFC offers better signal to noise ratios [75] with the ability to examine multiple regions and networks simultaneously. Prior work has demonstrated the influence of RSFC on somatosensation and pain perception [76, 77]. Furthermore, RSFC has been used to measure the transition from acute to chronic back pain [31]. Therefore, it is logical to use RSFC to measure pain-related brain function in people with AD, particularly in more advanced patients who may no longer be able to reliably self-report pain. Regardless, more research is needed to demonstrate the feasibility of using RSFC in this capacity.
The risk of suffering some type of pain and developing AD (or related dementias) concurrently rises with age. As the population of older adults continues to increase, so will the numbers of persons with dementia who have pain. Here we found that older adults with AD of mild to moderate cognitive impairment, in comparison to cognitively healthy older adults, displayed increased warmth detection and thermal pain thresholds (up to mild pain) and relatively equal degrees of affect. As summarized in Fig. 2, psychophysical results occurred in the context of people with AD having: 1) reduced RSFC between pain sensory and affective structures (R-pINS to ACC) and between sensory and pain modulatory structures (R-S2 to R-AMY); 2) increased RSFC between two cognitive evaluative and affective regions (R-dlPFC to L-ACC). The latter finding was specifically associated with pain affect ratings of controls yet not identified in AD. Our findings, combined with our recently reported psychophysical analysis [35], begin to describe a possible neurobiological mechanism that may help explain decreased pain reports in AD. Increasing our understanding of the neurobiology of pain in AD is a critical step in the development of interventions and the refinement of tools to help decrease the risk of suffering in this highly vulnerable population. Future imaging studies should include task-evoked analysis to pain as well as task-evoked functional connectivity. A larger scale study across a range AD severity would provide additional information on the time course of these relationships. Because sex can impact the experience of pain, future studies should explore whether there are sex-specific differences in pain processing in the AD population.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the John A. Hartford Foundation, Mayday Fund, Vanderbilt Office of Clinical and Translational Scientist Development, Vanderbilt Clinical and Translational Research Scholars Program, and the National Institutes of Health National Institute on Aging [grants number K23 AG046379-01A1 and number R21 AG045735-01A1]. The contents are solely the responsibility of the authors and do not necessarily represent the official views of these institutions. Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University. REDCap is maintained by Vanderbilt Institute for Clinical and Translational Research which is supported by the National Institutes of Health National Center for Advancing Translational Sciences [grant number UL1 TR000011].
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-1187r1).
SUPPLEMENTARYMATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-161187.
REFERENCES
- [1].Herr K, Zwakhalen S, Swafford K (2016) Observation of pain in dementia. Curr Alzheimer Res, doi: 10.2174-1567205013666160602234526 [DOI] [PubMed] [Google Scholar]
- [2].Cohen-Mansfield J, Marx MS (1993) Pain and depression in the nursing home: Corroborating results. J Gerontol 48, P96–P97. [DOI] [PubMed] [Google Scholar]
- [3].Thibodeau MA, Welch PG, Katz J, Asmundson GJG (2013) Pain-related anxiety influences pain perception differently in men and women: A quantitative sensory test across thermal pain modalities. Pain 154, 419–426. [DOI] [PubMed] [Google Scholar]
- [4].Creamer P, Lethbridge-Cejku M, Hochberg MC(2000)Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxford) 39, 490–496. [DOI] [PubMed] [Google Scholar]
- [5].Monroe TB, Gore JC, Chen LM, Mion LC, Cowan RL (2012) Pain in people with Alzheimer’s disease: Potential applications for psychophysical and neurophysiological research. J Geriatr Psychiatry Neurol 25, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haasum Y, Fastbom J, Fratiglioni L, Kåreholt I, Johnell K (2011) Pain treatment in elderly persons with and without dementia. Drugs Aging 28, 283–293. [DOI] [PubMed] [Google Scholar]
- [7].Scherder E, Oosterman J, Swaab D, Herr K, Ooms M, Ribbe M, Sergeant J, Pickering G, Benedetti F (2005) Recent developments in pain in dementia. BMJ 330, 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Monroe TB, Mion LC (2012) Patients with advanced dementia: How do we know if they are in pain? Geriatr Nurs 33, 226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scherder E, Sergeant J, Swaab D (2003) Pain processing in dementia and its relation to neuropathology. Lancet Neurol 2, 677–686. [DOI] [PubMed] [Google Scholar]
- [10].Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, Gibson SJ (2006) Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain 129, 2957–2965. [DOI] [PubMed] [Google Scholar]
- [11].Cole LJ, Gavrilescu M, Johnston LA, Gibson SJ, Farrell MJ, Egan GF (2011) The impact of Alzheimer’s disease on the functional connectivity between brain regions underlying pain perception. Eur J Pain 15, 568. e561–511. [DOI] [PubMed] [Google Scholar]
- [12].International Association for the Study of Pain, IASP Taxonomy, http://www.iasp-pain.org/Taxonomy, Accessed March 12, 2012.
- [13].Treede RD, Kenshalo DR, Gracely RH, Jones AK (1999) The cortical representation of pain. Pain 79, 105–111. [DOI] [PubMed] [Google Scholar]
- [14].Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F (2002) Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb Cortex 12, 376–385. [DOI] [PubMed] [Google Scholar]
- [15].Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971. [DOI] [PubMed] [Google Scholar]
- [16].Lorenz J, Minoshima S, Casey KL (2003) Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain 126, 1079–1091. [DOI] [PubMed] [Google Scholar]
- [17].Rainville P (2002) Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol 12, 195–204. [DOI] [PubMed] [Google Scholar]
- [18].Basbaum AI, Fields HL (1984) Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7, 309–338. [DOI] [PubMed] [Google Scholar]
- [19].Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55, 377–391. [DOI] [PubMed] [Google Scholar]
- [20].Monroe TB, Gore JC, Bruehl SP, Benningfield MM, Dietrich MS, Chen LM, Newhouse P, Fillingim R, Chodkowski B, Atalla S (2015) Sex differences in psychophysical and neurophysiological responses to pain in older adults: A cross-sectional study. Biol Sex Differ 6, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL (2010) Exploring the brain in pain: Activations, deactivations and their relation. Pain 148, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- [23].Chang C, Glover GH (2010) Time–frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50, 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fransson P (2005) Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Greicius MD, Menon V (2004) Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. J Cogn Neurosci 16, 1484–1492. [DOI] [PubMed] [Google Scholar]
- [27].Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB (2009) Hippocampal correlates of pain in healthy elderly adults: A pilot study. Neurology 73, 1567–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K (2006) Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage 31, 496–504. [DOI] [PubMed] [Google Scholar]
- [29].Damoiseaux JS, Prater KE, Miller BL, Greicius MD (2012) Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging 33, 828. e819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Horwitz B (2003) The elusive concept of brain connectivity. Neuroimage 19, 466–470. [DOI] [PubMed] [Google Scholar]
- [31].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV (2012) Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15, 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fox MD, Greicius M (2010) Clinical applications of resting state functional connectivity. Front Syst Neurosci 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Duke Han S, Buchman AS, Arfanakis K, Fleischman DA, Bennett DA (2013) Functional connectivity networks associated with chronic musculoskeletal pain in old age. Int J Geriatr Psychiatry 28, 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Song JJ, DeRidder D, Schlee W, Van de Heyning P, Vanneste S (2013) Distressed aging: The differences in brain activity between early- and late-onset tinnitus. Neurobiol Aging 34, 1853–1863. [DOI] [PubMed] [Google Scholar]
- [35].Monroe TB, Gibson SJ, Bruehl SP, Gore JC, Dietrich MS, Newhouse P, Atalla S, Cowan RL (2016) Contact heat sensitivity and reports of unpleasantness in communicative people with mild to moderate cognitive impairment in Alzheimer’s disease: A cross-sectional study. BMC Med 14, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Monroe TB, Misra SK, Habermann RC, Dietrich MS, Cowan RL, Simmons SF (2013) Pain reports and pain medication treatment in nursing home residents with and without dementia. Geriatr Gerontol Int 14, 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol 6, 734–746. [DOI] [PubMed] [Google Scholar]
- [38].Folstein MF, Folstein SE, McHugh PR (1975) Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [39].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [40].Reisberg B (1988) Functional assessment staging (FAST). Psychopharmacol Bull 24, 653–659. [PubMed] [Google Scholar]
- [41].Hollingshead AB (1975) Four factor index of social status, Yale University, New Haven. [Google Scholar]
- [42].Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS (2004) Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 20, 309–318. [DOI] [PubMed] [Google Scholar]
- [43].Kurlowicz L (1999) The Geriatric Depression Scale (GDS). Geriatr Nurs (Lond) 20, 212–213. [PubMed] [Google Scholar]
- [44].Spielberger R, Gorsuch R, Lushene R (1970) State-Trait Anxiety Inventory. Consulting Psychologists, Palo Alto, CA. [Google Scholar]
- [45].Medoc Advanced Medical, Systems (2006) Medoc Ltd. Advanced Medical Systems, Durham, NC, p. 3. [Google Scholar]
- [46].Petzke F, Harris RE, Williams DA, Clauw DJ, Gracely RH (2005) Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. Eur J Pain 9, 325–335. [DOI] [PubMed] [Google Scholar]
- [47].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012)Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lancaster J, Rainey L, Summerlin J, Freitas C, Fox P, Evans A, Toga A, Mazziotta J (1997) Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp 5, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Maldjian JA, Laurienti PJ, Burdette JH (2004) Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21, 450–455. [DOI] [PubMed] [Google Scholar]
- [50].Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- [51].Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I (2013) The importance of context: When relative relief renders pain pleasant. Pain 154, 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Deen B, Pitskel NB, Pelphrey KA (2011) Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex 21, 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2, 125–141. [DOI] [PubMed] [Google Scholar]
- [54].Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chang C, Glover GH (2009) Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage 47, 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gibson SJ, Voukelatos X, Ames D, Flicker L, Helme RD (2001) An examination of pain perception and cerebral event-related potentials following carbon dioxide laser stimulation in patients with Alzheimer’s disease and age-matched control volunteers. Pain Res Manag 6, 126–132. [DOI] [PubMed] [Google Scholar]
- [57].Jensen-Dahm C, Madsen CS, Waldemar G, Ballegaard M, Hejl AM, Johnsen B, Jensen TS (2016) Contact heat evoked potentials (CHEPs) in patients with mild-moderate Alzheimer’s disease and matched control–a pilot study.Pain Med 17, 675–684. [DOI] [PubMed] [Google Scholar]
- [58].Benedetti F, Vighetti S, Ricco C, Lagna E, Bergamasco B, Pinessi L, Rainero I (1999) Pain threshold and tolerance in Alzheimer’s disease. Pain 80, 377–382. [DOI] [PubMed] [Google Scholar]
- [59].Jensen-Dahm C, Werner MU, Dahl JB, Jensen TS, Ballegaard M, Hejl A-M, Waldemar G (2014) Quantitative sensory testing and pain tolerance in patients with mild to moderate Alzheimer disease compared to healthy control subjects. Pain 155, 1439–1445. [DOI] [PubMed] [Google Scholar]
- [60].Kunz M, Mylius V, Scharmann S, Schepelman K, Lautenbacher S (2009) Influence of dementia on multiple components of pain. Eur J Pain 13, 317–325. [DOI] [PubMed] [Google Scholar]
- [61].Kunz M, Scharmann S, Hemmeter U, Schepelmann K, Lautenbacher S (2007) The facial expression of pain in patients with dementia. Pain 133, 221–228. [DOI] [PubMed] [Google Scholar]
- [62].Beach PA, Huck JT, Miranda MM, Bozoki AC (2015) Autonomic, behavioral, and subjective pain responses in Alzheimer’s disease. Pain Med 16, 1930–1942. [DOI] [PubMed] [Google Scholar]
- [63].Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, Gee J (2004) What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain 127, 628–649. [DOI] [PubMed] [Google Scholar]
- [64].Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL (2002) A unique representation of heat allodynia in the human brain. Neuron 35, 383–393. [DOI] [PubMed] [Google Scholar]
- [65].Amemori K-i, Amemori S, Graybiel AM (2015) Motivation and affective judgments differentially recruit neurons in the primate dorsolateral prefrontal and anterior cingulate cortex. J Neurosci 35, 1939–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288, 1835–1838. [DOI] [PubMed] [Google Scholar]
- [67].Benedetti F, Ardunio C, Costa S, Vighetti S, Tarenzi L, Rainero I, Asteggiano G (2006) Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less efective. Pain 121, 133–144. [DOI] [PubMed] [Google Scholar]
- [68].Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE (2003) Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci 23, 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Beach PA, Huck JT, Miranda MM, Foley KT, Bozoki AC (2016) Effects of Alzheimer’s disease on the facial expression of pain. Clin J Pain 32, 478–487. [DOI] [PubMed] [Google Scholar]
- [70].Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S (2009) Pain in dementia. Pain 145, 276–278. [DOI] [PubMed] [Google Scholar]
- [71].Vogt B (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Neurosci 6, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner M, Jagust W, Reed B, Norman D, Schuff N, Kusdra L (2000) Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology 55, 1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Karas G, Burton E, Rombouts S, Van Schijndel R, O’Brien J, Scheltens P, McKeith I, Williams D, Ballard C, Barkhof F (2003) A comprehensive study of gray matter loss in patients with Alzheimer’s disease using optimized voxel-based morphometry. Neuroimage 18, 895–907. [DOI] [PubMed] [Google Scholar]
- [74].Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, Rombouts SA, Maris E, Barkhof F, Scheltens P, Stam CJ (2010) Loss of ‘small-world’networks in Alzheimer’s disease: Graph analysis of FMRI resting-state functional connectivity. PLoS One 5, e13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fox MD, Snyder AZ, Zacks JM, Raichle ME (2006) Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci 9, 23–25. [DOI] [PubMed] [Google Scholar]
- [76].Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S (2007) Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104, 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ploner M, Lee MC, Wiech K, Bingel U, Tracey I (2010) Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A 107, 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lancaster JL, Summerin JL, Rainey L, Freitas CS, Fox PT (1997) The Talairach Daemon, a database server for Talairach Atlas Labels. Neuroimage 5, S633. [Google Scholar]
- [79].Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.