Abstract
The muscarinic M1 receptor (M1R) is a promising target for treating cognitive impairment associated with cholinergic deficits in disorders such as Alzheimer’s disease and schizophrenia. We previously reported that cooperativity (α-value) was key to lowering the risk of diarrhea by M1R positive allosteric modulators (M1 PAMs). Based on this, we discovered a low α-value M1 PAM, TAK-071 (α-value: 199), and characterized TAK-071 using T-662 as a reference M1 PAM with high α-value of 1786. Both TAK-071 and T-662 were potent and highly selective M1 PAMs, with inflection points of 2.7 and 0.62 nM, respectively. However, T-662 but not TAK-071 augmented isolated ileum motility. TAK-071 and T-662 increased hippocampal inositol monophosphate production through M1R activation and improved scopolamine-induced cognitive deficits in rats at 0.3 and 0.1 mg/kg, respectively. TAK-071 and T-662 also induced diarrhea at 10 and 0.1 mg/kg, respectively, in rats. Thus, taking into consideration the fourfold lower brain penetration ratio of T-662, TAK-071 had a wider margin between cognitive improvement and diarrhea induction than T-662. Activation of M1R increases neural excitability via membrane depolarization, reduced afterhyperpolarization, and generation of afterdepolarization in prefrontal cortical pyramidal neurons. T-662 induced all three processes, whereas TAK-071 selectively induced afterdepolarization. Combining sub-effective doses of TAK-071, but not T-662, with an acetylcholinesterase inhibitor, significantly ameliorated scopolamine-induced cognitive deficits in rats. TAK-071 may therefore provide therapeutic opportunities for cognitive dysfunction related to cholinergic deficits or reduced M1R expression, while minimizing peripheral cholinergic side effects.
Subject terms: Pharmacology, Cognitive neuroscience
Introduction
The muscarinic M1 receptor (M1R) belongs to a subfamily of Gq/11 protein-coupled receptors for the neurotransmitter, acetylcholine (ACh). M1R is predominantly expressed in brain regions involved in cognitive function, such as the frontal cortex, hippocampus, and striatum [1, 2], and M1R knock-out (M1KO) mice show learning deficits [3, 4]. Therefore, the M1R may be a viable target for treatment of cognitive impairment associated with cholinergic deficits or reduced M1R expression.
Alzheimer’s disease (AD) is a progressive neurobiological disorder characterized by dysfunction of pre-synaptic cholinergic neurons [5]. Importantly, post-synaptic neurons are considered to be relatively intact in AD. Acetylcholinesterase inhibitors (AChEIs) such as donepezil and rivastigmine can improve cognitive function in AD by increasing ACh levels in the synaptic cleft; however, they become less effective with disease progression, probably because of the excessive loss of pre-synaptic cholinergic neurons [6]. Moreover, AChEIs can cause severe side effects through non-selective, systemic activation of ACh receptors [7]. Thus, selective stimulation of post-synaptic ACh receptors such as M1R can mitigate these risks.
Schizophrenia is a debilitating disorder comprising three symptomatic domains: positive symptoms, negative symptoms, and cognitive deficits [8]. Postmortem studies have revealed that M1R expression in the dorsolateral prefrontal cortex is decreased in patients with schizophrenia [9, 10]. Importantly, the preferential M1/M4R agonist xanomeline improved cognitive function of patients with AD or schizophrenia [11, 12]. Therefore, selective activation of post-synaptic M1Rs may be an attractive approach to treat cognitive impairment in these disorders.
M1R activators including orthosteric and allosteric agonists, and M1R positive allosteric modulators (M1 PAMs) have been developed. However, they remain challenging, mainly because of their cholinergic side effects such as gastrointestinal (GI) symptoms, hypersalivation, and sweating [13–16]. We previously demonstrated that diarrhea is the major side effect of a highly selective M1 PAM, benzyl quinolone carboxylic acid (BQCA); it did not cause other cholinergic side effects such as lacrimation or salivation in mice [17]. We also found that fine adjustment of the α-value, which represents the binding cooperativity between ACh and an M1R [18], was key to lowering the risk of diarrhea by M1 PAMs [17]. Based on these observations, we discovered a novel low α-value M1 PAM, TAK-071 (4-fluoro-2-[(3S,4S)-4-hydroxytetrahydro-2H-pyran-3-yl]-5-methyl-6-[4-(1H-pyrazol-1-yl)benzyl]-2,3-dihydro-1H-isoindol-1-one). Here, we describe the characterization of TAK-071 using T-662, previously referred to as benzoquinazolinone 12, as a reference M1 PAM with high α-value [19]. We provide evidence that TAK-071, with its lower α-value, has a greater therapeutic window between cognitive improvement and diarrhea induction in rats than T-662. We also show that TAK-071, but not T-662, potentiates the cognitive effect of donepezil in a cholinergic deficit rat model without exacerbating its cholinergic side effects. TAK-071 may offer unique therapeutic opportunities for the central nervous system disorders with cholinergic deficits as a monotherapy or a combination therapy with AChEIs.
Materials and methods
Animals
Male ICR and C57BL/6J mice were supplied by CLEA Japan Inc. (Tokyo, Japan). Male Sprague–Dawley (SD) and Long Evans rats were supplied by Charles River Laboratories, Japan, Inc. (Kanagawa, Japan) and Japan SLC Inc. (Shizuoka, Japan), respectively. Chrm-1tm1 Stl/J wild-type (WT) and KO mice (also known as M1KO mice) on the C57BL/6 background were obtained from the Massachusetts Institute of Technology (Cambridge, MA, USA). The animals were housed in a light-controlled room (12-h light/dark cycle, with lights on at 07:00). After acclimation for at least 1 week, 4- to 32-week-old animals were used for the experiments. The care and use of the animals and the experimental protocols used in this research were approved by the Experimental Animal Care and Use Committee of Takeda Pharmaceutical Company Limited.
Chemicals
TAK-071 (4-fluoro-2-[(3S,4S)-4-hydroxytetrahydro-2H-pyran-3-yl]-5-methyl-6-[4-(1H-pyrazol-1-yl)benzyl]-2,3-dihydro-1H-isoindol-1-one) was synthesized by Takeda Pharmaceutical Company Limited (Kanagawa, Japan). Based on patent information [20], T-662 (3-((1S,2S)-2-hydroxycyclohexyl)-6-((6-(1-methyl-1H-pyrazol-4-yl)pyridin-3-yl)methyl)benzo[h]quinazolin-4(3H)-one) was synthesized by Takeda Pharmaceutical Company Limited. (Kanagawa, Japan). Lithium chloride (LiCl) (Wako Pure Chemical Industries, Ltd., Osaka, Japan), [3H]-pirenzepine (PerkinElmer, Waltham, MA, USA), donepezil hydrochloride (Megafine Pharma (P) Ltd., Mumbai, India), rivastigmine l-Tartrate (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), and scopolamine hydrobromide (Tocris Bioscience, Minneapolis, MN, USA) were used in this study.
Drug treatment
TAK-071 and T-662 suspended in 0.5% (w/v) methylcellulose in distilled water, and donepezil dissolved in distilled water were administered per orally (p.o.). Rivastigmine dissolved in saline was injected intraperitoneally (i.p.). Scopolamine and LiCl dissolved in saline were injected subcutaneously (s.c.). Additional detailed methods are described in the Supplementary Information.
Ca2+ flux assay
Chinese hamster ovary (CHO-K1) cells stably expressing human M1R were stimulated with test compounds. Calcium flux was measured by a fluorescence-based assay using the Ca2+-responsive dye, Fluo-4. Effects of test compounds on other muscarinic receptor subtypes (M2–M5R) were also assessed using similar methods. Additional detailed methods are described in the Supplementary Information.
Binding modulation assay
Cell membranes from FreeStyle™ 293 cells transiently expressing human M1R were incubated with various concentrations of test compounds and ACh, and 4 nM [3H]-pirenzepine in assay buffer. Radioactivity was measured by Topcount® (PerkinElmer) as previously described [17]. Additional detailed methods are described in the Supplementary Information.
Data analysis of in vitro M1 PAM parameters
Radioligand binding data were globally fitted to the allosteric ternary complex model as previously described [21, 22]. Additional detailed methods are described in the Supplementary Information.
Extracellular field recording
Horizontal brain slices containing the hippocampus were prepared from male SD rats (20- to 30-day-old). Extracellular field recordings were made in the stratum pyramidale of the CA3c region. The areas under the curve of power spectra in the gamma frequency range (20–80 Hz) were used to quantify the strength of gamma oscillations. Additional detailed methods are described in the Supplementary Information.
In vivo inositol monophosphate (IP1) production
To assess in vivo phosphoinositide hydrolysis, we used a homogeneous time-resolved fluorescence (HTRF)-based assay system, the IP-One HTRF® assay kit (Cisbio Bioassays, Codolet, France) [23]. The in vivo IP1 level was calculated as the ratio of the concentration of IP1 to that of total protein and was represented as a percentage of the vehicle-treated control group. Details are described in the Supplementary Information.
Spontaneous ileum contraction in the Magnus assay
Longitudinal ileums of male ICR mice were mounted in organ baths and their contractile response was continuously recorded. The maximum strength from the baseline obtained by cumulative application of the compounds was recorded. Additional detailed methods are described in the Supplementary Information.
Slice patch-clamp recording
Experiments were performed on coronal brain slices of the medial prefrontal cortex (mPFC) from 4-week-old male C57BL/6J mice as previously reported [24]. Whole-cell current clamp recordings were carried out in visually identified pyramidal neurons of mPFC layer 5. Detailed methods are described in the Supplementary Information.
Novel object recognition test (NORT)
The cognitive efficacy of drugs was represented by the novelty discrimination index (NDI), which was the ratio of interaction time with a novel object to total time. Additional methods are described in the Supplementary Information.
Assessment of peripheral cholinomimetic effects
Animals were observed for 4 to 6 h after administration of M1 PAMs or AChEIs to assess their peripheral cholinomimetic effects. Details are described in the Supplementary Information.
Extracellular field electrophysiology for long-term depression (LTD) in layer 5 pyramidal neurons of the mouse PFC
Methods are described in the Supplementary Information.
[3H]N-methylscopolamine ([3H]NMS) binding assay
Methods are described in the Supplementary Information.
Analysis of phosphorylated cyclic AMP response element–binding protein (pCREB), brain-derived neurotrophic factor (BDNF) production, and cell proliferation in the mouse brain
Methods are described in the Supplementary Information.
Statistical analysis
Statistical methods are described in the figure legends. Additional detailed information is described in the Supplementary Information.
Results
TAK-071 and T-662 selectively activated M1R and induced IP1 production in the rodent brain
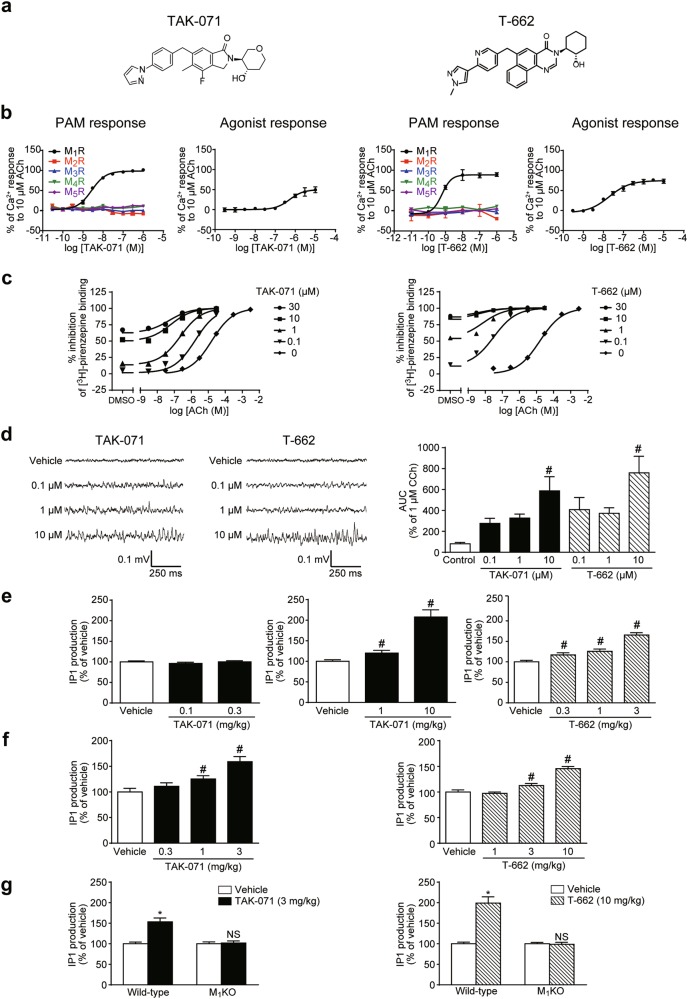
PAM activities of TAK-071 and T-662 (Fig. 1a) for M1R and other muscarinic receptor subtypes were measured using Ca2+ flux assays in CHO-K1 cells expressing human M1–M5R. Inflection point (IP) values of TAK-071 and T-662 for M1R activation were 2.7 and 0.62 nM, respectively (Fig. 1b), while their IP values for M2–M5R activation were >1000 nM (Fig. 1b). Thus, TAK-071 and T-662 had >370-fold and >1600-fold M1R selectivity over other muscarinic receptors, respectively. EC50 values of TAK-071 and T-662 for M1R agonist activities were 520 and 20 nM, respectively (Fig. 1b). Margin between PAM and agonist response of TAK-071 is wider than that of T-662 (192-fold vs. 32-fold). In LTD analysis with or without a muscarinic agonist, carbachol (CCh), using mouse PFC slices, TAK-071 at 10 μM started to show only PAM response, while T-662 at 10 μM started to show both agonist and PAM responses (p ≤ 0.05, Supplementary Figure S1a). TAK-071 may have less agonistic profile compared with T-662. TAK-071 at 10 µM showed no significant binding activity (≥50% inhibition) to any relevant receptors, ion channels or enzymes, except for the adrenergic α1 receptor (52% inhibition of ligand binding) (Supplementary Table S1, assays performed by Eurofins Panlabs Taiwan Ltd., Taipei, Taiwan).
Fig. 1.
TAK-071 and T-662, selective M1 PAMs with low and high α-values, respectively, induced in vitro hippocampal gamma oscillations and in vivo IP1 production through M1R activation. a Chemical structures of TAK-071 and T-662. b Effects of TAK-071 and T-662 on Ca2+ responses in the presence (PAM response) or absence (agonist response) of EC20 ACh in CHO-K1 cells expressing human recombinant M1–M5R. Data are expressed as percent activation in response to 10 µM ACh (n = 2–4). c Effects of TAK-071 and T-662 on the binding affinity between ACh and M1R measured by the displacement of [3H]-pirenzepine binding to FreeStyle™ 293 cell membranes expressing human recombinant M1R. Data are expressed as percent inhibition of [3H]-pirenzepine binding (n = 2). d Effects of TAK-071 and T-662 on gamma frequency oscillations in region CA3 of rat hippocampal slices. Representative traces by application of each compound are shown (left). The AUC in the gamma frequency range (20–80 Hz) were used to quantify the strength of the network oscillations. The AUC data were normalized to that of carbachol (1 µM for 10 min) obtained prior to compound application (right). Data are expressed as mean + SEM (n = 3–7); #p ≤ 0.05 compared with the vehicle-treated control group by a two-tailed Dunnett’s multiple comparison test. e Effects of TAK-071 and T-662 on IP1 production in the rat hippocampus. Data are expressed as mean + SEM (n = 6). The IP1 production level is represented as a percentage of the vehicle-treated control group; #p ≤ 0.05 compared with the vehicle-treated control group by a two-tailed Shirley–Williams’ test (middle) or Williams’ test (right). f Effects of TAK-071 and T-662 on IP1 production in the mouse hippocampus. Data are expressed as mean + SEM (n = 6); #p ≤ 0.05 compared with the vehicle-treated control group by a two-tailed Williams’ test. g Effects of TAK-071 and T-662 on IP1 production in the hippocampus of WT and M1KO mice. Values are expressed as mean + SEM (n = 10); *p ≤ 0.05 compared with the vehicle-treated group in WT mice using the Aspin–Welch t-test; NSp > 0.05 compared with the vehicle-treated group of mice using the Aspin–Welch t-test. For the in vivo IP1 assay, TAK-071 and T-662 were orally administered 3 h prior to killing. See also Supplementary Figure S1b–e and Supplementary Table S3b. ACh acetylcholine, AUC areas under the curve, CCh carbachol, CHO-K1 Chinese hamster ovary, IP1 inositol monophosphate, M1R M1 receptor, M1KO M1R knock-out, NS not significant, PAM positive allosteric modulator, SEM standard error of the mean, WT wild-type
The binding modulation assay revealed that TAK-071 had an α-value of 199 and T-662 had an α-value of 1786 (Fig. 1c).
M1R activation induces gamma oscillatory activity in rat hippocampal slices [25, 26]. Both TAK-071 and T-662 at 10 µM significantly produced gamma oscillations in the CA3 region (p ≤ 0.05, Fig. 1d).
M1R activation generates inositol trisphosphate (IP3) through activation of phospholipase C [27]. IP3 is rapidly degraded to IP1, followed by further degradation to myo-inositol by inositol monophosphatase [23]. Lithium inhibits inositol monophosphatase, thus, activation of M1R can be assessed by measuring IP1 accumulation in the presence of lithium. TAK-071 at 1 and 10 mg/kg and T-662 at 0.3‒3 mg/kg, together with 10 mmol/kg of LiCl, significantly increased IP1 content in the rat hippocampus (p ≤ 0.05, Fig. 1e). In rats, the Kp-values (ratios of hippocampal to plasma concentrations) of TAK-071 at 1 mg/kg and T-662 at 0.3 mg/kg were 0.20 (122 ng/g vs. 609 ng/ml) and 0.05 (2 ng/g vs. 44 ng/ml), respectively (Supplementary Table S2a).
TAK-071 at 1 and 3 mg/kg and T-662 at 3 and 10 mg/kg also significantly induced IP1 production in the mouse hippocampus (p ≤ 0.05, Fig. 1f) and TAK-071 at 3 mg/kg significantly increased IP1 production in the mouse PFC and striatum (p ≤ 0.05, Supplementary Figure S1b). Thus, 3 mg/kg of TAK-071 was used to assess IP1 production in various brain regions of WT and M1KO mice. In WT mice, TAK-071 (3 mg/kg) significantly induced IP1 production in brain regions where M1R is highly expressed (hippocampus, PFC, and striatum), but not in the brainstem, where there is little M1R expression (Fig. 1g and Supplementary Figure S1c). Moreover, TAK-071 (3 mg/kg) did not induce IP1 production in any brain regions of M1KO mice (Fig. 1g and Supplementary Figure S1c). T-662 (10 mg/kg) also induced hippocampal IP1 production in WT mice but not in M1KO mice (Fig. 1g). No significant differences in pharmacokinetic profiles of these compounds were observed between WT and M1KO mice (Supplementary Table S3a). Therefore, both TAK-071 and T-662 induced IP1 production through M1R activation in mice.
Both TAK-071 and T-662 are activators of M1R, and chronic administration risks M1R desensitization. Repeated pre-administration of TAK-071 (3 mg/kg) or T-662 (10 mg/kg) to mice for 13 days (days 1‒13) did not change the basal IP1 levels (Supplementary Table S3b) or TAK-071-induced or T-662-induced IP1 production (Supplementary Figure S1d, e) in mouse brain regions on the next (final) day of administration (day 14); note that vehicle was administered to mice before tissue sampling on day 14. TAK-071 or T-662 concentrations in plasma and brain were similar after single and repeated administration (Supplementary Table S3c). Thus, TAK-071 and T-662 may have a lower risk of M1R desensitization in mice.
Ca2+ functions as a second messenger in a variety of cellular processes, including for the CREB [28]. M1R activation by TAK-071 increased in intracellular Ca2+ (Fig. 1b). We surmised this may be followed by pCREB and consequent activation of downstream signaling, such as induction of BDNF expression and hippocampal cell proliferation. As expected, TAK-071 at 3 and 10 mg/kg or T-662 at 3‒30 mg/kg significantly increased pCREB levels (p ≤ 0.05, Supplementary Figure S2a) and repeated administration of TAK-071 at 10 mg/kg or T-662 at 30 mg/kg for 15 days stimulated BDNF production (p ≤ 0.05, Supplementary Figure S2b) and increased in 5-bromo-2′-deoxy-uridine (BrdU)-positive cells (p ≤ 0.05, Supplementary Figure S2c) in the mouse hippocampus.
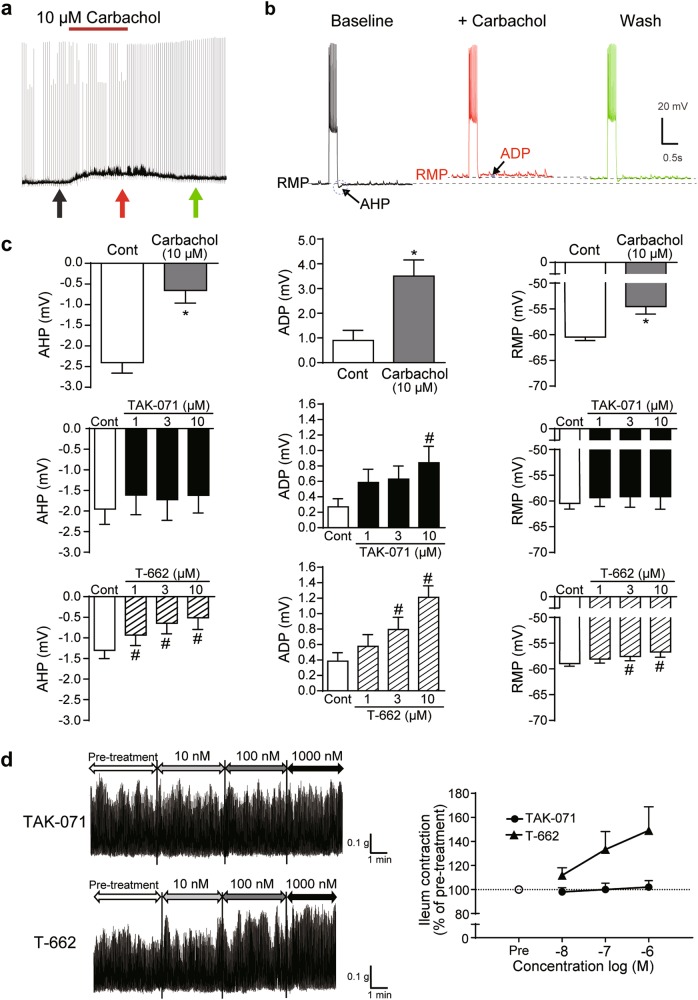
Unlike T-662, TAK-071 selectively induced afterdepolarization in layer 5 pyramidal neurons and did not cause mouse ileum contractions in vitro
M1R activation contributes to three cholinergic effects in layer 5 pyramidal neurons of the mouse PFC: depolarization of the resting membrane potential (RMP), and the two physiological responses, suppression of afterhyperpolarization (AHP) and induction of afterdepolarization (ADP) that typically follow brief periods of action potential generation [29]. To investigate effects of M1 PAMs with different α-values on brain function, we assessed cholinergic excitation by TAK-071 and T-662. We first tested CCh at 10 µM for 10 min (Fig. 2a, b; CCh was reported to induce neural excitation through activation of M1R under these experimental conditions [29]). CCh suppressed AHP following depolarizing current injections, produced an ADP potential, and induced depolarization of the RMP in layer 5 pyramidal neurons (Fig. 2a‒c). TAK-071 at 10 µM generated significant ADP, but did not induce either RMP depolarization or AHP suppression (Fig. 2c). In contrast, T-662 generated subthreshold changes in RMP, suppressed AHP, and generated ADP (Fig. 2c).
Fig. 2.
Unlike T-662, TAK-071 selectively induced afterdepolarization in layer 5 pyramidal neurons and did not increase spontaneous ileum motility in vitro. a Chart recording of the membrane potential of a layer 5 pyramidal neuron with bath-applied carbachol (10 µM for 10 min). Brief current steps (~450 pA) delivered at 20 s intervals generated short periods of activity. b Individual responses to current injections shown at the times indicated by arrows in a, with expanded views of the AHPs and ADPs superimposed. c Summary graph comparing mean changes in AHPs, ADPs, and RMPs following the bath application of carbachol, TAK-071, or T-662 for 10 min. Data are expressed as mean ± SEM (n = 5); *p ≤ 0.05 compared with the vehicle (veh)-treated group by paired t-test; #p ≤ 0.05 compared with the veh-treated group by a paired Dunnett’s multiple comparison test. d Effects of TAK-071 and T-662 on spontaneous ileum motility in mice. Data are shown as representative traces (left) and the percent change of ileum contraction from the baseline measured at pre-treatment (right, n = 8). ADP afterdepolarization, AHP afterhyperpolarization, RMP resting membrane potential, SEM standard error of the mean
We previously showed that low α-value M1 PAMs might have a lower risk of inducing ileum contraction and diarrhea in mice [17]. T-662, but not TAK-071, increased spontaneous ileum motility in a concentration-dependent manner (Fig. 2d). Compared with T-662, TAK-071 may have a lower impact on ileum motility.
Compared with T-662, TAK-071 had a wider margin between doses leading to cognitive improvement and diarrhea induction
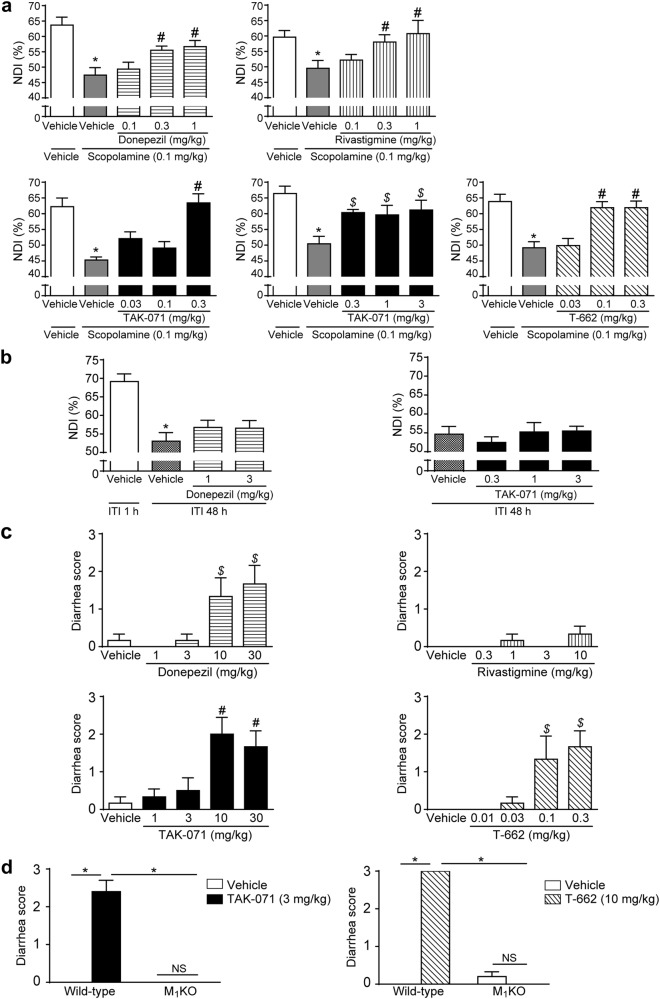
We next assessed the effects of TAK-071 and T-662 on object recognition memory in rats, with AChEIs as a control, using the NORT. Scopolamine, which has been used to induce cognitive impairment associated with cholinergic deficits in animals and humans [30, 31], was used. No significant differences in total exploration time to objects were observed among groups in the acquisition trial (Supplementary Figure S3a). Both donepezil and rivastigmine significantly increased the NDI in retention trials, suggesting the amelioration of scopolamine-induced cognitive deficits (p ≤ 0.05 at 0.3 and 1 mg/kg, Fig. 3a). TAK-071 at 0.3‒3 mg/kg and T-662 at 0.1 and 0.3 mg/kg also significantly ameliorated scopolamine-induced cognitive deficits (p ≤ 0.05, Fig. 3a).
Fig. 3.
TAK-071 had a wider margin between doses leading to cognitive improvement and diarrhea induction than T-662. a Effects of donepezil, rivastigmine, TAK-071, and T-662 on scopolamine-induced memory deficits in the novel object recognition test in rats. Data are represented as mean + SEM (n = 7–9); *p ≤ 0.05 compared with the vehicle-treated control group (Aspin–Welch t-test); #p ≤ 0.05 compared with the vehicle-scopolamine-treated group (two-tailed Williams’ test) or $p ≤ 0.05 (two-tailed Shirley–Williams’ test). b Effects of donepezil and TAK-071 on time-dependent memory decay in naive rats. Data are expressed as mean + SEM (n = 10); *p ≤ 0.05 between ITIs of 1 and 48 h in the vehicle-treated group (Aspin–Welch t-test). c Effects of donepezil, rivastigmine, TAK-071, and T-662 on diarrhea induction in rats, and d effects of TAK-071 (3 mg/kg) and T-662 (10 mg/kg) on diarrhea induction in wild-type and M1KO mice. The severity of diarrhea was scored as follows: 0, normal pellets; 1, wet but formed feces; 2, loose or mucous feces; 3, severe watery diarrhea. The maximum score obtained during observation up to 4 or 6 h was adopted. Data are expressed as mean + SEM (n = 6–10). For c and d, #p ≤ 0.05 compared with the vehicle-treated group by a two-tailed Williams’ test; $p ≤ 0.05 compared with the vehicle-treated group by a two-tailed Shirley–Williams’ test; *p ≤ 0.05 compared by the Aspin–Welch t-test; NSp > 0.05 compared by the Aspin–Welch t-test. ITI intertrial interval, NDI novelty discrimination index, NS not significant, SEM standard error of the mean
To examine whether both M1 PAMs interact with orthosteric site or have negative cooperativity with scopolamine, we performed [3H]NMS binding assays. TAK-071 and T-662 inhibited [3H]NMS binding to M1R at concentrations >3 and 0.1 μM, respectively (Supplementary Figure 3b).
In the time-dependent forgetting paradigm in NORT, no significant differences in total exploration time to objects were observed among groups (Supplementary Figure S3c). Rats in the 48 h intertrial interval (ITI) group, but not in the 1 h ITI control group, failed to discriminate the novel object, suggesting time-dependent memory decay. In this paradigm, neither donepezil at 1 or 3 mg/kg nor TAK-071 at 0.3‒3 mg/kg improved the memory decay (Fig. 3b).
We characterized cholinomimetic effects, such as GI dysfunctions, lacrimation, salivation, miosis, and fasciculation in rats. Donepezil at ≥10 mg/kg and rivastigmine at ≥3 mg/kg induced almost all cholinergic signs, while TAK-071 at up to 30 mg/kg and T-662 at up to 0.3 mg/kg induced only diarrhea (Table 1). The effects of AChEIs and M1 PAMs on diarrhea induction in rats were further examined. Donepezil at 10 and 30 mg/kg, TAK-071 at 10 and 30 mg/kg, and T-662 at 0.1 and 0.3 mg/kg significantly increased diarrhea scores in a dose-dependent manner (p ≤ 0.05, Fig. 3c). Furthermore, TAK-071 at 3 mg/kg and T-662 at 10 mg/kg, which induced diarrhea in WT mice, did not induce diarrhea in M1KO mice (Fig. 3d). Thus, both M1 PAMs induced diarrhea through M1R activation in mice.
Table 1.
M1 PAMs had a lower risks of cholinergic side effects than acetylcholinesterase inhibitors in rats
| Drug | Dose (mg/kg) | Loose or mucous stool, or diarrhea | Lacrimation | Salivation | Miosis | Fasciculation |
|---|---|---|---|---|---|---|
| (a) | ||||||
| Vehicle | 0 | 0/6 (0%) | 0/6 (0%) | 1/6 (17%) | 0/6 (0%) | 0/6 (0%) |
| Donepezil | 1 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| Donepezil | 3 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| Donepezil | 10 | 3/6 (50%) | 0/6 (0%) | 1/6 (17%) | 5/6 (83%) | 6/6 (100%) |
| Donepezil | 30 | 3/6 (50%) | 0/6 (0%) | 4/6 (67%) | 6/6 (100%) | 6/6 (100%) |
| (b) | ||||||
| Vehicle | 0 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| Rivastigmine | 0.3 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 1/6 (17%) | 0/6 (0%) |
| Rivastigmine | 1 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| Rivastigmine | 3 | 0/6 (0%) | 0/6 (0%) | 3/6 (50%) | 5/6 (83%) | 6/6 (100%) |
| Rivastigmine | 10 | 0/6 (0%) | 3/6 (50%) | 6/6 (100%) | 6/6 (100%) | 6/6 (100%) |
| (c) | ||||||
| Vehicle | 0 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| TAK-071 | 1 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| TAK-071 | 3 | 1/6 (17%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| TAK-071 | 10 | 5/6 (83%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| TAK-071 | 30 | 4/6 (67%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| (d) | ||||||
| Vehicle | 0 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| T-662 | 0.01 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| T-662 | 0.03 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| T-662 | 0.1 | 3/6 (50%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| T-662 | 0.3 | 4/6 (67%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
Data are represented as the total number of incidences for all rats (n = 6). The incidence ratio (%) is noted in parentheses
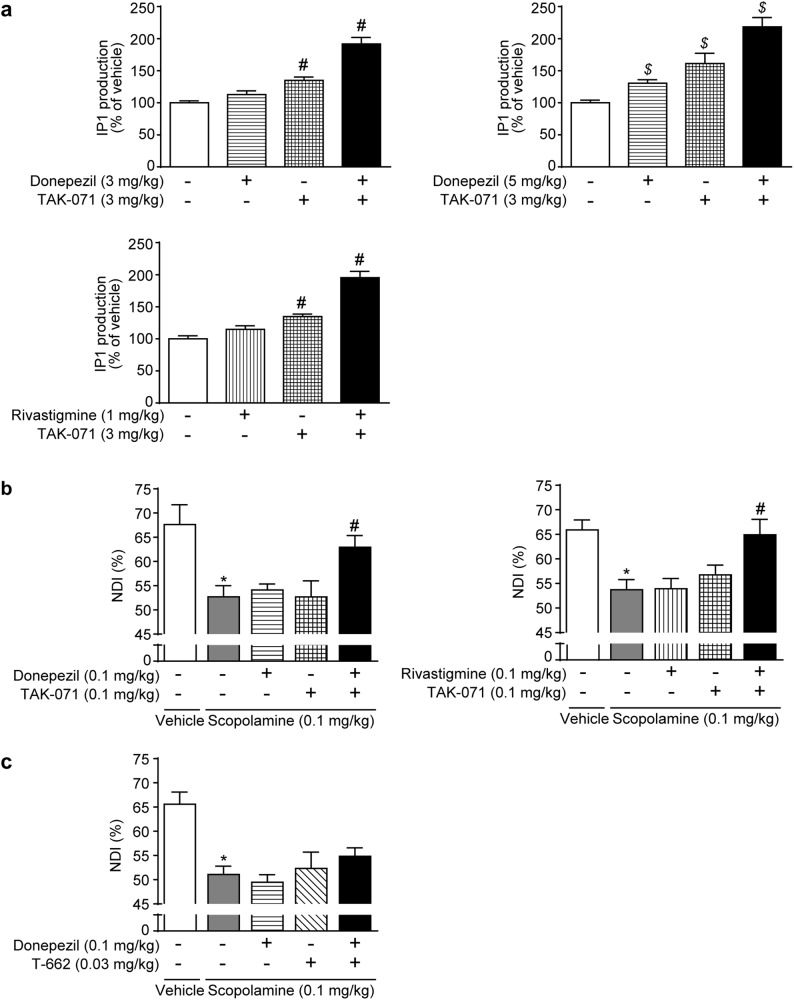
Donepezil combined with TAK-071 but not T-662 improved scopolamine-induced cognitive deficits in NORT without exacerbating cholinomimetic effects in rats
The increased ACh levels by AChEIs and the potentiation of M1R signaling by M1 PAMs can produce synergistic effects. Donepezil at 0.3‒3 mg/kg and rivastigmine at 0.1‒1 mg/kg induced little IP1 production (Supplemental Figure S4a, b). The combination of TAK-071 (3 mg/kg) with donepezil (3 or 5 mg/kg) or rivastigmine (1 mg/kg) induced more robust IP1 production in the rat brain than TAK-071 alone (Fig. 4a, Supplementary Figure S4c, d). Interestingly, the combination of sub-effective doses of TAK-071 (0.1 mg/kg) and donepezil (0.1 mg/kg) or rivastigmine (0.1 mg/kg) significantly suppressed scopolamine-induced cognitive deficits in rats (p ≤ 0.05, Fig. 4b). No significant differences in pharmacokinetic profiles of donepezil and TAK-071 were observed for each compound alone or the combination of both compounds (Supplementary Figure S4e and Supplementary Table S2b, c). Thus, TAK-071 may have a synergistic effect with AChEIs against the scopolamine-induced cognitive deficits. The combination of effective doses of TAK-071 (1 mg/kg) and donepezil (1 mg/kg) did not attenuate the efficacy of each drug alone (Supplementary Figure S4f); note that these experimental conditions might not be suitable for detection of synergistic/additive effects because of ceiling effects in cognitive function. In contrast, either combinations of TAK-071 (1 or 3 mg/kg) with donepezil (0.3 mg/kg), or TAK-071 (0.1 mg/kg) with rivastigmine (0.1 mg/kg) did not exacerbate cholinergic side effects (Supplementary Table S4). To our surprise, the combination of sub-effective doses of T-662 (0.03 mg/kg) and donepezil (0.1 mg/kg) did not show any synergistic/additive effects against scopolamine-induced cognitive deficits in rats (Fig. 4c).
Fig. 4.
The combination of donepezil with TAK-071 but not with T-662 improved scopolamine-induced cognitive deficits in the novel object recognition test (NORT) in rats. a IP1 levels in the hippocampus were measured. Data show mean + SEM and are presented as a percentage of the vehicle-treated control group (n = 6); #p ≤ 0.05 compared with the vehicle-treated control group by a two-tailed Dunnett’s multiple comparison test; $p ≤ 0.05 compared with the vehicle-treated control group by Steel’s multiple comparison test. b, c Effects on scopolamine-induced memory deficits in the NORT in rats were measured. Data are presented as mean + SEM (n = 7 or 8); *p ≤ 0.05 compared with the vehicle (p.o. or i.p.) + vehicle (s.c.) control group by the Aspin–Welch t-test; #p ≤ 0.05 compared with the vehicle (p.o. or i.p.) + scopolamine (s.c.)-treated group by a two-tailed Dunnett’s multiple comparison test. IP1 inositol monophosphate, NDI novelty discrimination index, NORT novel object recognition test, SEM standard error of the mean
Discussion
Cholinergic deficits are involved in the cognitive dysfunction of various dementias, including AD, dementia with Lewy body (DLB), and schizophrenia [5, 32–35]. Several studies have suggested the pivotal role of M1R on cognitive function [3, 4]. Thus, selective M1R activation may be a promising approach to treat cognitive impairment related to cholinergic dysfunction. However, an M1R selective PAM, BQCA induced diarrhea in WT mice but not in M1KO mice [17]. To solve this issue, we found that low α-value M1 PAMs might have a lower risk of inducing ileum contraction and diarrhea in mice [17].
In this study, we carefully characterized M1R selectivity of low and high α-value M1 PAMs, TAK-071 (α-value: 199), and T-662 (α-value: 1786), to compare their pharmacological properties (Fig. 1c). TAK-071 and T-662 had >370-fold and >1600-fold selectivity for human M1R over other muscarinic receptors, respectively (Fig. 1b), showed similar efficacy against physiological rat M1R as measured by gamma frequency oscillations in rat hippocampal slices (Fig. 1d), and induced IP1 production and diarrhea via M1R activation (Figs. 1g and 3d). In line with previous observations, TAK-071 had much smaller effects on ileum motility than T-662 using the in vitro Magnus method (Fig. 2d). Moreover, TAK-071 and T-662 improved scopolamine-induced cognitive deficits at 0.3 and 0.1 mg/kg, respectively, whereas TAK-071 and T-662 induced diarrhea at 10 and 0.1 mg/kg, respectively (Fig. 3a, c). Therefore, TAK-071 and T-662 showed a 33-fold difference in the margin between doses leading to cognitive improvement and diarrhea induction in rats. If cognitive improvement and diarrhea induction by M1 PAMs are caused through central and peripheral effects, respectively, brain penetration of these compounds could also affect their safety margins. Brain permeability of TAK-071 (Kp-value: 0.2) was fourfold greater than that of T-662 (Kp-value: 0.05) in rats (Supplementary Table S2a). Even taking this difference into consideration, TAK-071 had an eightfold wider safety margin than T-662 for diarrhea.
Recently, lower agonistic profile of M1 PAMs has been reported to be associated with not only the lower adverse effects, but also superior pharmacological effects [36–38]. TAK-071 showed lower agonistic profile compared with T-662 in the calcium flux assay using M1R-expressing cells (Fig. 1b) and LTD analysis with mouse PFC (Supplementary Figure S1a). Further studies would be needed to understand the relationship between α-value, agonistic profile, and pharmacological profile of M1 PAMs.
Similar to donepezil, TAK-071 improved scopolamine-induced cognitive deficits in rats, but not time-dependent memory decay of naive rats, in the NORT (Fig. 3a, b). Donepezil has been reported to slightly but significantly worsen cognitive function in healthy young and elderly subjects, which was possibly caused by perturbation of a rigorously optimized cholinergic system in the healthy participants [39, 40]. Therefore, the scopolamine-induced memory deficit model would be suitable for evaluation of cognitive efficacy in cholinergic dysfunction. TAK-071 and T-662 significantly inhibited [3H]NMS binding to M1R at concentrations >3 and 0.1 μM, respectively, suggesting their interaction with orthosteric site or negative cooperativity with scopolamine (Supplementary Figure S3b). In pharmacokinetic analysis that included Kp-value in rats, the brain concentrations of TAK-071 and T-662 at effective dose in NORT (0.3 mg/kg) were estimated as 0.11 and 0.0062 μM, respectively (Supplementary Table S2d). Thus, both compounds improved the scopolamine-induced cognitive deficits at >10-fold lower concentration than those for the inhibition of [3H]NMS binding to M1R. TAK-071 and T-662 might improve scopolamine-induced cognitive deficits through M1R activation, but not through inhibition of scopolamine binding to M1R.
TAK-071, but not T-662, with donepezil at sub-effective doses improved cognitive function (Fig. 4b, c). This superior pharmacological profile of TAK-071 may be due to its selective induction of ADP generation in the electrophysiological analysis using mouse brain slices (Fig. 2c); T-662 generated subthreshold changes in the RMP, suppressed AHP, and produced ADP. ACh release in the PFC and hippocampus is reported to be strictly regulated during cognitive tasks [41]. Thus, spatially and temporally regulated cholinergic stimulation might be required to exert cognitive efficacy. Low α-value M1 PAMs like TAK-071 could selectively potentiate physiologically regulated cholinergic stimulation without potentiating “noise signals” like depolarized RMP, even under conditions with high ACh levels by co-administration of donepezil. On the contrary, M1 PAMs with high α-value can robustly enhance the binding affinity between ACh and M1R that result in the induction of agonist-like response (activation of the resting receptors) in the brain. Note that the concentration of TAK-071 (10 μM) used for the electrophysiological analysis was 100-fold higher than the estimated brain concentration of 0.11 μM for cognitive improvement in NORT (Supplementary Table S2d). Further studies are needed to clarify the relationship between selective ADP induction and cognitive efficacy of TAK-071.
Interestingly, in contrast to donepezil and rivastigmine, neither TAK-071 nor T-662 induced salivation, fasciculation, or miosis at doses within 10-fold of the minimal effective dose (Fig. 3a; Table 1). Importantly, the combination of TAK-071 and donepezil did not exacerbate any cholinergic side effects (Supplementary Table S4). Most patients with AD take AChEIs, including donepezil, thus the synergistic cognitive improvement promoted by TAK-071 and donepezil or rivastigmine would be beneficial for clinical use. Unfortunately, enhancement of pCREB and BDNF levels, and cell proliferation in the hippocampus by TAK-071 and T-662 were observed at doses at which they showed higher risks of diarrhea induction (Supplementary Figure S2).
In conclusion, TAK-071 is a low α-value M1 PAM with the potential for treatment of cognitive dysfunction associated with cholinergic deficiency and low impact on GI function. TAK-071 is currently being evaluated in a Phase 1 clinical trial (ClinicalTrials.gov, Identifier: NCT02769065).
Electronic supplementary material
Acknowledgements
We thank Dr. Koji Murakami, Ms. Noriko Suzuki, and Mr. Yuuichi Arakawa for performing the NORT; Dr. Takayuki Niimura and Dr. Katsuya Sakimura for performing IP1 screening; Mr. Yuto Hiura, Ms. Maki Miyamoto, Mr. Yasuyuki Debori, Mr. Akihiko Goto, and Dr. Hisao Shimizu for performing the pharmacokinetic analysis of test compounds; Dr. Hiroki Sakamoto for helpful discussions regarding synthesis of compounds; Mr. Minoru Nakamura for providing chemical compounds; and Dr. Ceri H Davies for helpful discussions regarding electrophysiology assay design. The authors are employees of Takeda Pharmaceutical Company Limited. This work was supported by Takeda Pharmaceutical Company Limited.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuu Sako, Emi Kurimoto
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0168-8).
References
- 1.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–43. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–8. doi: 10.1016/0024-3205(93)90300-R. [DOI] [PubMed] [Google Scholar]
- 3.Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, et al. Role for the M1 muscarinic acetylcholine receptor in top-down cognitive processing using a touchscreen visual discrimination task in mice. ACS Chem Neurosci. 2015;6:1683–95. doi: 10.1021/acschemneuro.5b00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, et al. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- 5.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–47. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deardorff WJ, Feen E, Grossberg GT. The use of cholinesterase inhibitors across all stages of Alzheimer’s disease. Drugs Aging. 2015;32:537–47. doi: 10.1007/s40266-015-0273-x. [DOI] [PubMed] [Google Scholar]
- 7.Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2007;127:45–63. [PubMed]
- 8.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 9.Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7:1083–91. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- 10.Scarr E, Dean B. Muscarinic receptors: do they have a role in the pathology and treatment of schizophrenia? J Neurochem. 2008;107:1188–95. doi: 10.1111/j.1471-4159.2008.05711.x. [DOI] [PubMed] [Google Scholar]
- 11.Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–9. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 12.Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–73. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 13.Fisher A, Heldman E, Gurwitz D, Haring R, Karton Y, Meshulam H, et al. M1 agonists for the treatment of Alzheimer’s disease. Novel properties and clinical update. Ann N Y Acad Sci. 1996;777:189–96. doi: 10.1111/j.1749-6632.1996.tb34418.x. [DOI] [PubMed] [Google Scholar]
- 14.Nathan PJ, Watson J, Lund J, Davies CH, Peters G, Dodds CM, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16:721–31. doi: 10.1017/S1461145712000752. [DOI] [PubMed] [Google Scholar]
- 15.Uslaner JM, Kuduk SD, Wittmann M, Lange HS, Fox SV, Min C, et al. Preclinical to human translational pharmacology of the novel M1 positive allosteric modulator MK-7622. J Pharmacol Exp Ther. 2018;365:556–66. doi: 10.1124/jpet.117.245894. [DOI] [PubMed] [Google Scholar]
- 16.Wienrich M, Meier D, Ensinger HA, Gaida W, Raschig A, Walland A, et al. Pharmacodynamic profile of the M1 agonist talsaclidine in animals and man. Life Sci. 2001;68:2593–2600. doi: 10.1016/S0024-3205(01)01057-8. [DOI] [PubMed] [Google Scholar]
- 17.Kurimoto E, Matsuda S, Shimizu Y, Sako Y, Mandai T, Sugimoto T, et al. An approach to discovering novel muscarinic M1 receptor positive allosteric modulators with potent cognitive improvement and minimized gastrointestinal dysfunction. J Pharmacol Exp Ther. 2018;364:28–37. doi: 10.1124/jpet.117.243774. [DOI] [PubMed] [Google Scholar]
- 18.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdul-Ridha A, Lane JR, Mistry SN, Lopez L, Sexton PM, Scammells PJ, et al. Mechanistic insights into allosteric structure-function relationships at the M1 muscarinic acetylcholine receptor. J Biol Chem. 2014;289:33701–11. doi: 10.1074/jbc.M114.604967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuduk SD, Beshore DC, Di Marco CN, Greshock TJ. PCT international applications. 2010. WO 2010/059773 A1 20100527.
- 21.Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30:148–55. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci. 2007;28:382–9. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Trinquet E, Fink M, Bazin H, Grillet F, Maurin F, Bourrier E, et al. d-myo-inositol 1-phosphate as a surrogate of d-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation. Anal Biochem. 2006;358:126–35. doi: 10.1016/j.ab.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol. 2014;1183:221–42. doi: 10.1007/978-1-4939-1096-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–9. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 26.Spencer JP, Middleton LJ, Davies CH. Investigation into the efficacy of the acetylcholinesterase inhibitor, donepezil, and novel procognitive agents to induce gamma oscillations in rat hippocampal slices. Neuropharmacology. 2010;59:437–43. doi: 10.1016/j.neuropharm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Caulfield MP, Birdsall NJ. International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–90. [PubMed] [Google Scholar]
- 28.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–61. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 29.Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci. 2009;29:9888–902. doi: 10.1523/JNEUROSCI.1366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartus RT, Dean RL, Pontecorvo MJ, Flicker C. The cholinergic hypothesis: a historical overview, current perspective, and future directions. Ann N Y Acad Sci. 1985;444:332–58. doi: 10.1111/j.1749-6632.1985.tb37600.x. [DOI] [PubMed] [Google Scholar]
- 31.Buccafusco JJ. The revival of scopolamine reversal for the assessment of cognition-enhancing drugs. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. Boca Raton: CRC Press; 2009. [PubMed]
- 32.Fujishiro H, Umegaki H, Isojima D, Akatsu H, Iguchi A, Kosaka K. Depletion of cholinergic neurons in the nucleus of the medial septum and the vertical limb of the diagonal band in dementia with Lewy bodies. Acta Neuropathol. 2006;111:109–14. doi: 10.1007/s00401-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 33.Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J Neurol Sci. 1977;34:247–65. doi: 10.1016/0022-510X(77)90073-9. [DOI] [PubMed] [Google Scholar]
- 34.Terry AV., Jr. Role of the central cholinergic system in the therapeutics of schizophrenia. Curr Neuropharmacol. 2008;6:286–92. doi: 10.2174/157015908785777247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang SW, Lai MK, Kirvell S, Francis PT, Esiri MM, Hope T, et al. Impaired coupling of muscarinic M1 receptors to G-proteins in the neocortex is associated with severity of dementia in Alzheimer’s disease. Neurobiol Aging. 2006;27:1216–23. doi: 10.1016/j.neurobiolaging.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Davoren JE, Garnsey M, Pettersen B, Brodney MA, Edgerton JR, Fortin JP, et al. Design and synthesis of gamma- and delta-Lactam M1 positive allosteric modulators (PAMs): convulsion and cholinergic toxicity of an M1-selective PAM with weak agonist activity. J Med Chem. 2017;60:6649–63. doi: 10.1021/acs.jmedchem.7b00597. [DOI] [PubMed] [Google Scholar]
- 37.Moran SP, Dickerson JW, Cho HP, Xiang Z, Maksymetz J, Remke DH, et al. M1-positive allosteric modulators lacking agonist activity provide the optimal profile for enhancing cognition. Neuropsychopharmacology. 2018;43:1763–71. [DOI] [PMC free article] [PubMed]
- 38.Bradley SJ, Molloy C, Bundgaard C, Mogg AJ, Thompson KJ, Dwomoh L, et al. Bitopic binding mode of an M1 muscarinic acetylcholine receptor agonist associated with adverse clinical trial outcomes. Mol Pharmacol. 2018;93:645–56. doi: 10.1124/mol.118.111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beglinger LJ, Gaydos BL, Kareken DA, Tangphao-Daniels O, Siemers ER, Mohs RC. Neuropsychological test performance in healthy volunteers before and after donepezil administration. J Psychopharmacol. 2004;18:102–8. doi: 10.1177/0269881104040248. [DOI] [PubMed] [Google Scholar]
- 40.Beglinger LJ, Tangphao-Daniels O, Kareken DA, Zhang L, Mohs R, Siemers ER. Neuropsychological test performance in healthy elderly volunteers before and after donepezil administration: a randomized, controlled study. J Clin Psychopharmacol. 2005;25:159–65. doi: 10.1097/01.jcp.0000155822.51962.b4. [DOI] [PubMed] [Google Scholar]
- 41.Teles-Grilo Ruivo LM, Baker KL, Conway MW, Kinsley PJ, Gilmour G, Phillips KG, et al. Coordinated acetylcholine release in prefrontal cortex and hippocampus is associated with arousal and reward on distinct timescales. Cell Rep. 2017;18:905–17. doi: 10.1016/j.celrep.2016.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.