Abstract
Donor-specific transplantation tolerance that enables weaning from immunosuppressive drugs but retains immune competence to non-graft antigens has been a lasting pursuit since the discovery of neonatal tolerance. More recently, efforts have been devoted not only to understanding how transplantation tolerance can be induced but also the mechanisms necessary to maintain it as well as how inflammatory exposure challenges its durability. This review focuses on recent advances regarding key peripheral mechanisms of T cell tolerance, with the underlying hypothesis that a combination of several of these mechanisms may afford a more robust and durable tolerance and that a better understanding of these individual pathways may permit longitudinal tracking of tolerance following clinical transplantation to serve as biomarkers. This review may enable a personalized assessment of the degree of tolerance in individual patients and the opportunity to strengthen the robustness of peripheral tolerance.
Keywords: tolerance, TCR avidity, Treg, T cell dysfunction
Subject terms: Allotransplantation, Lymphocyte activation
Introduction
Lifelong immunosuppression is currently necessary to prevent rejection following solid organ transplantation, but it leaves patients with an increased susceptibility to infections and malignancies, as well as subject to the medications’ side effects. Imperfect immunosuppression may also be responsible for the high rates of chronic rejection that limit the half-lives of grafts. Achieving donor-specific tolerance is a possible solution to these problems, as it would enable patients to be weaned from immunosuppression and to retain immune competence against antigens that are not present in the allograft. Transplantation tolerance has been achieved in a few patients, either spontaneously in patients who stopped taking their immunosuppression for various reasons,1 or deliberately in clinical trials using protocols that combined stem cell and kidney transplantation.2–4 However, despite achieving stable transplantation tolerance for many years, a subset of these patients eventually lost their grafts, sometimes after episodes of infections,1,5 suggesting either that not all patients achieve the same robustness of tolerance, or that even robust tolerance can be eroded or disrupted by infections, or both. These clinical observations, as well as results in animal models of tolerance, have led us and others to propose that transplantation tolerance might be more robust if multiple redundant or additive mechanisms to control alloreactive lymphocytes are elicited by the tolerogenic protocol.6,7 A single mechanism of transplantation tolerance, such as deletion or dominant suppression of alloreactive T cells may, in isolation, be sufficient to prevent graft rejection, but the likelihood is rare that such mechanisms completely eliminate alloreactivity. Thus, it is rational that the simultaneous deletion of a majority of alloreactive lymphocytes, together with extrinsic suppression of the remaining alloreactive lymphocytes, would yield a more robust and durable tolerance than either mechanism alone. Additionally, although infections have been confirmed in animal models to be able to precipitate allograft rejection in stable hosts,8 the mechanisms of tolerance that may be more susceptible to erosion by different types of infections are unclear. Therefore, grafts from patients who develop multiple mechanisms of tolerance may have a better chance of withstanding the various infectious assaults that may occur over a lifetime. An improved understanding of the combination of tolerance mechanisms that can better achieve durable transplantation tolerance and of the mechanisms might be affected by inflammatory challenges is important to understand the ground rules of robust tolerance and achieve the desired goal of ‘one transplant for life’.9
Using new techniques to track graft-reactive T cells or graft-infiltrating T cells at the single-cell level, several groups have identified specific mechanisms of tolerance of alloreactive T cells that correlate with long-term graft acceptance. This review will focus on recent data exploring some of these mechanisms, namely preventing the expansion of high avidity alloreactive T cells, and developing the functional hyporesponsiveness of alloreactive T cells either in a cell-extrinsic manner via suppression by FoxP3+ regulatory T cells (Tregs) or regulatory B cells (Bregs), or in a conventional T cell (Tconv)-intrinsic manner.
Preventing the expansion of T cells with high avidity for alloantigen
Constraining the T cell repertoire to Tconvs with lower avidity for alloantigen is emerging as a contributor to transplantation tolerance. Given the vast size and sequence diversity of the T cell repertoire, multiple T cells in an individual are likely to respond to the same alloantigen. T cells with the same specificity may differ in their sensitivity to stimulation, a phenomenon that has been termed functional avidity.10,11 Differences in TCR avidity have been attributed to the affinity and geometry of TCR binding to peptide:MHC (pMHC),12,13 level of TCR14 and co-receptor expression,15 post-translational modifications to the TCR16 and spatial arrangement of TCR components,17,18 whereas exposure to inflammatory cytokines can enhance T cell sensitivity to activation.19 Self-reactivity during thymic development also tunes the sensitivity of a T cell to stimulation by non-self antigens in the periphery through negative regulation of TCR signaling.20 While the TCR sequence largely contributes to affinity for pMHC and tuning of the TCR in the thymus, it is not the sole determinant of avidity because even monoclonal T cells have varied functional avidity.21 Antigen experience also contributes to T cell avidity, as memory T cells are more sensitive to stimulation than naïve or recently stimulated T cells.22,23 Thus, the diversity of the T cell repertoire is not only represented by the range of individual TCR sequences or specificities but also by the combination of parameters that determine avidity for a particular pMHC.
Transplantation tolerance has recently been associated with a restriction in the expansion of high avidity allospecific T cell clones.24 During a productive adaptive immune response, the average functional avidity of responding T cells increases,25–27 an event that has been termed ‘avidity maturation’ at the T cell population level, in contrast to ‘affinity maturation’ at the B cell-intrinsic level. Using the intensity of staining with pMHC multimer as a correlate for functional avidity,27,28 our group showed that allospecific Tconvs in mice rejecting major MHC mismatched heart allografts also experienced a population-wide increase in avidity.24 Increased avidity during allograft rejection was associated with skewing of the TCR sequence representation in the allospecific repertoire but not an increase in other potential contributors to avidity such as co-receptor or TCR expression levels. By contrast, alloreactive Tconvs from mice that were made tolerant to heart allografts with anti-CD154 and donor splenocyte transfer (DST) did not undergo avidity maturation and likewise did not substantially shift their TCR repertoire. The allospecific Tconv population maintained low avidity in tolerant mice even when re-challenged > 30 days later with alloantigen in the absence of additional anti-CD154, indicating that avidity maturation of allospecific Tconvs remained suppressed during the maintenance phase of tolerance. Tregs contributed to the restriction of avidity maturation of Tconvs during the maintenance of tolerance for some but not other allospecificities,24 suggesting that other active or passive suppressive mechanisms of Tconv avidity maturation are likely in place.
Preventing the accumulation of high avidity allospecific Tconvs during tolerance may support graft survival, as high T cell avidity is associated with strong alloimmunity. Indeed, high avidity monoclonal allospecific CD8+ T cells more potently rejected skin allografts than low avidity T cells of the same specificity.24 Others showed that two different allospecific CD4+ T cell clones recognizing the same indirectly presented peptide accelerated minor antigen mismatched heart allograft rejection differently, suggesting that CD4+ T cells with matched specificity can vary in the strength of alloimmunity.29 The functional enhancement of high versus low avidity Tconvs that contributes to their stronger potential for alloimmunity remains unknown, although functional differences between high and low avidity cells have been described. For example, high avidity T cells were shown to produce more cytokines and effector molecules on a per-cell basis in vivo or when antigen was limiting in vitro.12,23,30 Intravital imaging revealed that, compared with low avidity monoclonal CD8+ T cells, high avidity T cells made longer contacts with dendritic cells (DCs) and were held in the lymph nodes longer, allowing greater overall expansion.31 Polyclonal high avidity CD8+ T cells were also better able to lyse target cells in vitro and in vivo,32,33 and they were better able to receive help from CD4+ T cells, further enhancing their function.34 As a result of their enhanced response to antigen, high avidity T cells were more potent than low avidity T cells in mediating anti-tumor immunity as well as autoimmune reactions.12,33 We speculate that a lack of avidity maturation may support graft survival during tolerance by limiting the pool of cells that would be most harmful to the graft if the suppressive mechanisms maintaining tolerance were to be disrupted.
Low avidity Tconvs, while less potent in mediating alloimmunity, may be more apt at resisting the suppressive mechanisms underlying induced donor-specific tolerance. During central tolerance to self, low avidity self-reactive T cells were more likely to escape thymic deletion and Treg induction compared with high avidity T cells and persisted in the periphery, where they could be activated and mediate autoimmunity following infection.30,35 Most treatments used to induce donor-specific tolerance rely on peripheral tolerance mechanisms, which also exert different effects on T cells depending on the avidity for self. There are conflicting reports on whether high or low avidity T cells are more likely to undergo activation-induced cell death in the periphery.36,37 Interestingly, a study found that both high and low avidity self-reactive CD4+ T cells were susceptible to becoming anergic, but only high avidity anergic cells upregulated regulatory markers such as CTLA-4 and FoxP3 and suppressed the activation of naïve T cells.38
While limiting the expansion of high avidity Tconvs likely supports tolerance, high avidity Tregs may be particularly adept at suppressing alloimmunity. We found that, unlike conventional T cells, allospecific Tregs in mice made tolerant to heart allografts with anti-CD154 and DST were not restricted in ability to avidity mature.24 As multiple mechanisms of Treg-mediated suppression require TCR engagement, high TCR avidity may improve the Treg suppressive capacity and, thereby, promote graft survival. Indeed, human primary Tregs transduced with high-affinity TCRs were more effective than their lower avidity counterparts in suppressing the stimulation of Tconvs with matching specificity in vitro.39 The adoptive transfer of mouse Tregs transduced with a high avidity allospecific TCR was also more effective than the transfer of low avidity T cells in prolonging skin allograft survival after treatment with anti-CD8 monoclonal antibody and rapamycin.40 Thus, strategies to selectively increase the avidity of allospecific Tregs may be advantageous in promoting graft survival and supporting tolerance.
Overall, the effect of constraining Tconv avidity for alloantigen in donor-specific tolerance requires further study. However, models of anti-tumor immunity and autoimmunity clearly show that high avidity T cells are particularly potent in vivo. An increased proliferative potential and per-cell effector response predict that high avidity allospecific Tconvs should be particularly deleterious to the allograft, which we have confirmed experimentally.24 By contrast, low avidity T cells have been shown to escape central and peripheral tolerance mechanisms, leaving the low avidity alloreactive cell population as a weak but potentially uncontrolled source of alloreactivity. Determining whether high and low avidity cells pose unique threats to the allograft is important for understanding the mechanisms and potential shortcomings of tolerance, as well as for developing functional assays to monitor alloreactivity in patients, where it may be advantageous to titrate the dose of allogeneic stimulation to distinguish between high and low avidity T cells. Additionally, whether infections can restore the avidity maturation of T cell populations in tolerant hosts remains to be investigated.
Inducing donor-reactive Tregs
Tregs are critical for the induction and maintenance of peripheral transplantation tolerance in numerous experimental models. Reports that depletion of Tregs results in the inability to develop tolerance,41–44 while the adoptive transfer of Tregs promotes tolerance induction,45–48 point to a necessary and sufficient role for Tregs at the induction phase of tolerance. Furthermore, Tregs preferentially accumulate in tolerant allografts,8,41,44,49–51 and FoxP3−CD4+ Tconvs can convert into FoxP3+ Tregs under select tolerance-inducing therapies,52,53 suggesting that expanded numbers of Tregs are necessary for maintaining tolerance. Indeed, the elimination of Tregs during established tolerance resulted in rejection of the allograft in some models of allograft tolerance.7 However, in other models of tolerance, the elimination of Tregs did not abrogate established tolerance, suggesting that other Treg-independent mechanisms contribute to the maintenance of tolerance. These observations are consistent with our hypothesis that the presence of multiple redundant mechanisms of peripheral tolerance is a necessary feature of robust transplantation tolerance that is able to withstand the pro-inflammatory effects of different types of infections.
Many different mechanisms have been reported to mediate the ability of Tregs to regulate Tconv responses in autoimmunity, infection, tumor immunity and allogeneic transplantation.54 Constitutive expression of CD25 on Tregs suggests that Tregs act as an “IL-2 sink”, thus limiting the access of Tconvs to IL-2.55 Their constitutive CTLA-4 expression is thought to enable Tregs to compete for CD80 and CD86 on antigen-presenting cells (APCs) and reduce their availability to engage CD28 on Tconvs, as well as to stimulate DCs to express indolamine 2,3-dihydrogenase (IDO), which catabolizes tryptophan to kynurenine, thereby inducing Tconv cell death56 and facilitating the generation of iTregs.57 Activated Tregs can upregulate additional suppressive mechanisms that control Tconvs, including the production of immunosuppressive cytokines such as IL-10, IL-35, and TGF-ß; enzymes that deplete pro-inflammatory metabolites such as CD39 and CD73; LAG-3, a CD4-related molecule that binds to MHC class II and to the newly identified ligand fibrinogen like protein 1 (FGL1)58; as well as granzymes and perforin that can directly deplete Tconvs and/or APCs.54,59–61 Finally, Tregs not only modulate the priming phase of the immune response within secondary lymphoid organs, but they can differentiate into specialized subsets that traffic to sites of inflammation where they suppress select immune cell effector functions; thus, Tbet+, IRF4+, STAT-3/RORγt+, Bcl6+ Tregs can inhibit Th1, Th2, Th17, and Tfh responses, respectively.62–69
The specificity of naturally occurring Tregs that limit self-reactivity and modulate alloreactivity has been an area of intense investigation. Thymic Tregs (tTregs) recognizing tissue-restricted self-antigens can be “educated” in the thymus by AIRE-dependent and AIRE-independent mechanisms.70–75 The fraction of “self-reactive” tTregs that have cross-reactive allo-MHC specificity is unknown, although in a recent review, LeGuern and Germana speculated that self-reactive Tregs recognize only a limited set of self-peptides presented on MCH class II, which may be cross-reactive to allogeneic MHC.76 Tregs can also be induced extrathymically at peripheral sites (iTregs) in the presence of TGFβ, IL-2 or other factors.77 Such iTregs can have divergent TCR and antigen specificities from tTregs, but they might be less stable.78–80 As a result, there is tempered enthusiasm for their use in the setting of allograft transplantation.
Key features of transplantation tolerance, including donor-specificity, infectious tolerance, and linked-suppression, are conferred, at least in part, by donor-specific Tregs.44,81 This notion is supported by observations of the superior efficacy of transferred allospecific Tregs over polyclonal Tregs in suppressing alloimmune responses.82–89 Currently, allospecific Tregs are enriched by alloantigen-stimulated expansion in vitro or in vivo; however, the frequency and specificity of endogenous donor-reactive tTregs are likely to vary with each donor-recipient pair, and in vitro stimulation with donor antigen may expand both tTregs and iTregs, both of which may contribute to Treg preparations in variable proportions and, potentially, affect batch-to-batch efficacy. One potential way to circumvent these limitations is the engineered expression of chimeric antigen receptors (CARs) consisting of donor MHC-I-reactive antibodies into polyclonally expanded tTregs.86,90–92 Despite possible pitfalls, the CAR Treg approach has the potential to provide large numbers of stable tTregs with the desired donor-specificity for clinical trials in transplantation.93
Following allograft transplantation, host T cells can recognize intact donor MHC (direct recognition) or donor peptide presented on recipient MHC (indirect recognition), and T cells that are capable of direct recognition are thought to be present at ~100-fold higher frequency than T cells with indirect recognition.94 Furthermore, the proportion of Tconvs and Tregs that have direct versus indirect allo-MHC specificities are comparable. If the anatomy of graft rejection and tolerance proposed by Tang and Vincenti95 is correct, where direct allospecific T cells mediate early events post-transplantation but indirect T cells mediate late events, then direct alloreactive Tregs should promote the induction of tolerance, while Tregs with indirect alloantigen specificity should promote the maintenance of tolerance.45,85,89 Monoclonal antibodies that recognize intact allogeneic MHC-I and -II can be obtained, but mAbs that recognize the dominant donor-peptides presented on recipient MHC are not currently available. Thus, to date, engineering of CAR Tregs will generate Tregs with direct allo-MHC specificity. Whether these CAR Tregs will function preferentially during induction, or also participate in the maintenance of tolerance, remains to be investigated and may depend on their half-life in vivo and schedule of infusions. Because Tregs can mediate infectious tolerance, it is possible that CAR-Tregs with direct donor-MHC specificity might facilitate the in vivo expansion of endogenous Tregs with indirect donor-specificity to promote the maintenance of tolerance.
We recently reported that endogenous Tregs with indirect specificity expanded comparably in untreated transplanted mice undergoing acute rejection and in mice with anti-CD154-induced tolerance,96 resulting in similar absolute Treg numbers in the spleen and infiltrating heart allografts, while the main distinguishing factor between rejection and tolerance was the markedly reduced expansion of donor-reactive Tconvs in tolerance. Likewise, Fan et al.52 had previously used intra-vital microscopy to track ‘color-coded’ Tregs within islet allografts, and they also reported that the increased ratios of Tregs to Tconv in anti-CD154 plus rapamycin-induced tolerance in comparison to rejection were primarily due to a greater influx of Tconvs in rejection. Collectively, these observations underscore the importance of limiting Tconv expansion for achieving high Treg:Tconv ratios, both systemically and in the graft, for Treg therapy to successfully control rejection and promote tolerance.97 If Tconvs are not depleted or if their expansion is not controlled, it would be extremely challenging to achieve high Treg:Tconv ratios in lymphoid organs or in allografts, and for T cell-mediated rejection to be restrained by the transfer of Tregs. The comparable increase in donor-specific Treg numbers in tolerance and rejection suggests that the inflammatory conditions associated with acute allograft rejection did not reduce the rate of Treg accumulation. Nevertheless, Tregs in rejection and tolerance were phenotypically distinct, with significantly higher neuropilin-1 and CD73 levels in Tregs detected during tolerance compared with rejection. Neuropilin-1, a receptor for semaphorins, growth factors and TGFß-1, plays a role in inducing a transcriptome that promotes Treg cell stability and function at inflammatory sites,98–100 whereas CD73 is an ectoenzyme that catabolizes ATP into extracellular adenosine.101,102 Thus, it is tempting to speculate that these phenotypic differences between Tregs in rejection and tolerance indicate potentially enhanced suppressor function by Tregs in tolerance. Further functional and molecular analyses are required to more fully distinguish Tregs in rejection and tolerance; the insights gained by these studies may aid in the generation of the most efficacious Tregs for therapeutic use in the clinic, as well as provide critical information for the development of useful biomarkers for the diagnosis of allograft rejection or tolerance.
Inducing Bregs
In addition to Tregs, Bregs can also mediate allograft tolerance in select experimental models.103,104 Multiple B cell subsets in humans and mice have been implicated to possess regulatory functions, including murine B1a (B220+CD5+), transitional T1 and T2, marginal zone and follicular B cells, and plasma cells. A dominant feature of Bregs is their production of IL-10 or other inhibitory cytokines, such as IL-35 and TGFß, which suppress the function of Tconvs and promote the expansion of Tregs.103,105 More recently, it was reported that Bregs express a common core of inhibitory receptors, such as LAG-3, CD200, PD-L1, and PD-L2 for regulatory plasma cells106 and TIM-1 for Bregs,107,108 similar to core inhibitory receptors expressed in dysfunctional or exhausted T cells.109 As a result of these features, Bregs may directly regulate Tconv and/or DC function through inhibitory cytokines, by depriving access by Tconvs to CD80/CD86 co-stimulation, or through the engagement of co-inhibitory receptors.
In a rat transplant model, it was reported that adoptively transferred donor B cells promoted the survival of a fully MHC mismatched kidney allograft.110 Likewise, donor B cells were reported to be necessary in a mixed chimerism model of transplantation tolerance111 and in cardiac allograft tolerance induced with anti-CD45RB.112 Moreover, Ding et al.113 reported that IL-10-producing Bregs were necessary and sufficient for tolerance to allogeneic islet grafts induced with anti-TIM-1, while Lee et al.114 described a similar requirement for Bregs in tolerance induced with anti-TIM-1 plus anti-CD45RB. Yeung et al.108 showed that TIM-1 was the primary receptor responsible for Breg induction by apoptotic cells, that TIM-1 signaling through its mucin domain played a direct role in stimulating IL-10 production, and that TIM-1 was an inclusive marker of Bregs. More recently, CD9, a tetraspanin-family transmembrane protein, was shown to be a key surface marker on most mouse IL-10+ B cells and their progenitors and to play a role in the suppressive function of IL-10+ B cells that was dependent on B-T cell interactions.115 While these reports clearly implicate a role for Bregs in specific models of tolerance, whether other models of transplantation tolerance induced by reagents that do not directly induce Breg expansion are also dependent on Bregs for tolerance induction or maintenance is currently unclear.
A potential role for Bregs in clinical transplantation was inferred by the early observations of Clatworthy et al.116 that treatment with rituximab (anti-CD20), which depletes B cells, resulted in acute rejection, and by observations of increased frequencies of B cells in the blood of spontaneously tolerant kidney transplant recipients compared with immunosuppressed recipients.117–119 While many other explanations could have been entertained, Bregs quickly became the favored hypothesis, and confirmatory data subsequently emerged that B cells from a small cohort of tolerant renal transplant recipients expressed more IL-10 or granzyme B than B cells from healthy controls.120,121 Despite these intriguing findings, our understanding of Breg biology remains limited because of the lack of a specific marker that could facilitate investigations into their origin, specificity, and function. Indeed, recent observations122,123 raise a cautionary note that the B cell signature of spontaneous tolerance in kidney transplant patients can be biased by immunosuppressive regimens. When the effects of pharmacological immunosuppression were taken into consideration, the resulting signature of tolerance that emerged was no longer significantly enriched for Bregs. While these observations do not definitively negate a role for Bregs in clinical allograft tolerance, they do underscore our incomplete understanding of the biology of Bregs and complexity in states of clinical transplantation tolerance, which makes it unlikely that clinical allograft tolerance can be reliably diagnosed by a single unifying B, or even T, cell biomarker of tolerance. Nevertheless, along with other mechanisms of control of alloreactive Tconv as described in this review, Bregs may further increase the robustness and durability of transplantation tolerance.
Achieving hyporesponsiveness of alloreactive Tconv
Activation and differentiation of Tconvs is a tightly controlled event. A productive response from Tconvs depends on effective presentation of antigen by APCs, including optimal co-stimulatory signals, and a defined antigen load and duration of antigen exposure. Changes in any of these parameters can potentially lead to impairment in Tconv functions. Several states of Tconv-intrinsic dysfunction have been described, including exhaustion (dysfunction arising from chronic stimulation as a result of antigen persistence), anergy (loss of function in response to suboptimal stimulation in the absence of co-stimulation) and senescence (irreversible cell cycle arrest following intermittent repetitive stimulation).124 While efforts are being made to reverse T cell dysfunction in chronic infections and tumor settings, achieving and maintaining the dysfunction of donor-reactive T cells would be desirable in organ transplantation.
Tconv dysfunction has primarily been studied in CD8+ T cells in settings of chronic infections and tumors.125–127 Upon chronic stimulation, CD8+ T cells have been shown to lose effector functions over time and acquire a stable dysfunctional state associated with unique transcriptional, epigenetic and metabolic signatures. An important phenotypic feature of dysfunctional CD8+ T cells is the overexpression of several inhibitory receptors, including PD-1, LAG-3, TIM-3, and others, which appears to be important in maintaining dysfunction. For instance, blocking the inhibitory PD-1 axis can reinvigorate CD8+ T cells, resulting in a reduction of the viral load or restoration of anti-tumor immunity. These results suggest that dysfunction may be reversible, and targeting these inhibitors in cancer settings has provided remarkable efficacy in the clinic.
Dysfunctional CD4+ Tconvs have also been observed in models of chronic lymphocytic choriomeningitis virus (LCMV) infection, and these cells, like dysfunctional CD8+ T cells, displayed reduced production of effector cytokines and elevated levels of inhibitory receptors.128–132 In contrast to CD8+ T-cells, loss of effector cytokines by CD4+ T-cells was not progressive but was rapidly (9 days post-infection) induced and sustained, coinciding with the increased viral load.131,132 In this infectious model, CD4+ T-cells showed abortive differentiation despite intact priming by APCs and without enhanced Treg activity or differentiation.131 CD4+ T-cells in chronic infection models could gain novel functions, such as production of the cytokines IL-10 and IL-21.132–134 Additionally, CD4+ T-cells were observed to overexpress a variety of inhibitory receptors that are biased toward activated CD4+ T cells (i.e., BTLA, CD200) or shared with CD8+ T cells (i.e., PD-1, CTLA-4), and agonistic engagement of inhibitory receptors could participate in the development of dysfunction.132 Functional defects paralleled changes in transcriptional profiles, and exhausted CD4+ T-cells were enriched in the expression of the transcription factors Blimp1, Eomes and Helios, but downregulated T-bet.132 Overall, exhausted CD4+ T-cells in chronic infection lost the Th1 phenotype but acquired an alternate functional state that might still be able to provide B cell help, and they displayed core programs that were unique as well as core programs that were shared with dysfunctional CD8+ T-cells.
T cell dysfunction has been less studied in transplantation settings. Importantly, transplantation tolerance differs from immune tolerance in chronic infection and tumor settings in that it is induced therapeutically rather than developing spontaneously, by exposing T cells to donor antigens while using immunosuppressive drugs to reduce signals from the TCR, costimulatory molecules or cytokines. In particular, short-course blockade of the CD40-CD154 costimulatory axis, using anti-CD154 antibody along with donor-specific transfusion (DST), or short-course blockade of CD3 signaling using non-depleting anti-CD3 mAb, have been successful in animal models at inducing donor-specific tolerance of select organs, where recipients accept primary and secondary donor-matched allografts but retain immune competence against other antigens.51,135–137 In these models, alloreactive T cells might acquire a dysfunctional state, as their restimulation with alloantigen often reveals reduced cytokine production and proliferation. However, initial studies did not have the technical ability to track graft-reactive T cells, such that it was not always clear whether hyporesponsiveness to alloantigen rechallenge was due to true cell-intrinsic dysfunction of alloreactive Tconv, to their suppression by Tregs or other inhibitory cell subsets, or to the fact that they had been partially deleted.
The use of adoptively transferred TCR-transgenic T cells specific for graft-expressed antigens as tracers of the alloimmune response has enabled more precise insights into the fate and function of alloreactive Tconvs in transplant rejection and tolerance. Interestingly, not all graft-reactive Tconvs appear to become dysfunctional in models of transplantation tolerance. In one model, skin from CB6F1 (progeny of C57BL/6 and Balb/c) mice was transplanted into RAG-deficient C57BL/6 recipients that lacked T and B cells and received as alloreactive tracers graft-reactive TCR‐transgenic CD4+ TEa T-cells, which recognize the donor-derived Eαd52–68 peptide presented by recipient I-Ab.138 These skin grafts survived long-term following anti-CD154/DST treatment, and TEa T-cells showed an early abortive expansion that resulted in reduced accumulation of these cells, with the residual TEa T-cells appearing hyporesponsive on day 7, with poor proliferation and secretion of IL-2 and IFNγ after antigen-specific restimulation in vitro. Moreover, these cells rejected grafts significantly more slowly when transferred into secondary RAG-deficient C57BL/6 recipients that received CB6F1 skins. By contrast, in mice treated with a similar anti-CD154/DST regimen but transferred, in the absence of a graft, with TCR‐transgenic CD4+ TCR75 T cells, which recognize the donor-derived Kd54–68 peptide presented by recipient I-Ab, the TCR75 CD4+ T cells were shown to remain functionally competent despite undergoing abortive expansion.139 Whether the presence of a graft, in addition to the DST and anti-CD154, would have yielded a different functional outcome was not investigated. Abortive proliferation of TCR75 in this model was dependent on exogenous Tregs rather a TCR75 cell-intrinsic dysfunction. These 2 contrasting models suggest that long-term graft acceptance might be achieved by distinct mechanisms that both result in the abortive expansion of the tracked alloreactive T cells. In a full-mismatch pancreatic islet transplant mouse model (Balb/c into C57BL/6), abortive expansion of CD4+ Tconvs after administration of anti-CD3 antibody also occurred and was shown to depend on Tregs.137 The importance of Tregs in curbing the proliferation of allogeneic Tconvs during the induction of transplantation tolerance44,137,139–141 suggests that Tregs and Tconvs are differentially affected by the tolerogenic regimens and may have differential activation requirements.
The mechanisms by which Tconvs acquire cell-intrinsic dysfunction during the induction of transplantation tolerance are incompletely understood. Inhibition of the transcription factor IRF4 may be one such mechanism for CD4+ Tconvs. Indeed, mice harboring T-cells that were deficient in the transcription factor IRF4 accepted cardiac allografts indefinitely in the absence of immunosuppression, and depletion of Tregs during the peri-transplant period did not precipitate graft rejection.142 It should be noted that the authors used PC61, an anti-CD25 antibody that leads to partial Treg depletion, such that a role for remaining Tregs cannot be conclusively ruled out. Nevertheless, PC61 treatment is sufficient in other models to prevent the induction of tolerance,143 suggesting that the absence of IRF4 may have profound cell-intrinsic effects in CD4+ Tconvs. In fact, this study showed that IRF4-deficient CD4+ T-cells were epigenetically skewed to overexpress an array of inhibitory receptors including PD-1, and combined blockade of PD-1 and CTLA-4 pathways early post-transplantation led to the restoration of proliferation and IFNγ production in allograft-reactive CD4+ T-cells and to allograft failure.142 Of note, the reduction of IRF4 may not lead to dysfunction of CD8+ T cells, as IRF4+/- CD8+ T cells that expressed reduced IRF4 did not acquire dysfunction in a chronic LCMV model and instead secreted higher amounts of IL-2, IFNγ, and TNFα than WT CD8+ T cells upon ex vivo rechallenge with antigen.144 Why expression of IRF4 would promote exhaustion in CD8+ T cells and prevent it in CD4+ T cells remains to be determined, but one might speculate that dysfunctional IRF4-deficient CD4+ T cells might not provide adequate help to IRF4-deficient CD8+ T cells to enable their rejection of allografts.
Irrespective of the transcriptional programs that drive the hyporesponsiveness of alloreactive Tconvs, induction of transplantation tolerance independent of Tregs may be explained in part by enhanced inhibitory signaling in Tconvs. This appears to be the case in IRF4-deficient CD4+ T cells.142,145 Moreover, mice that are genetically deficient in fucosyltransferase-VII (Fut7), an important enzyme for the biosynthesis of selectin ligands, exhibited long-term cardiac allograft survival associated with overexpression of PD-1 on CD4+ T-cells, with PD-1-blockade but not Treg depletion early post-transplantation restoring IFNγ and IL-17A production and leading to graft rejection.146 As another example, in wild-type mice transplanted with allogeneic pancreatic islets, both CD4+ and CD8+ T cells upregulated PD-1 after anti-CD3 administration, and PD-1 blockade prevented the induction of T-cell dysfunction and triggered graft rejection.136 CD4+ Tconv dysfunction with hallmarks of anergy was also thought to play a role in the maintenance of tolerance in this model, as graft-infiltrating CD4+ Tconvs, which are thought to be enriched in graft-reactive cells, did not produce IL-2 and IFNγ upon ex-vivo stimulation with PMA/ionomycin and expressed high PD-1 and the markers CD73hiFR4hi137 that have been described to characterize anergic T cells.147 Moreover, PD-1 blockade, but not Treg depletion, in FoxP3-DTR mice with diphtheria toxin at the maintenance phase of tolerance precipitated rejection,137 further suggesting a role for Tconv dysfunction in the maintenance phase of tolerance.
Several pathways of tolerance may coexist with Tconv dysfunction during the maintenance phase of tolerance, thus enabling more robust tolerance. For instance, in the mice with IRF4-deficient T cells discussed above, blockade of PD-1 and CTLA-4, which could prevent tolerance induction, did not precipitate acute rejection when administered on day 30 post-cardiac transplantation.142 It is possible that IRF4-deficient CD4+ T cells regained some effector functions after double checkpoint blockade but that other functions remained suppressed by Tregs or were intrinsically lost, but this model appears to reveal a redundant mechanism of tolerance at the maintenance phase of graft acceptance. This result is consistent with our own data in a model of fully MHC mismatched heart allograft tolerance induced with anti-CD154/DST, as single interventions such as depletion of Tregs, blockade of PD-1 or addition of TCR75 cells to increase the precursor frequency of graft-reactive T cells were each sufficient to prevent the induction of transplantation tolerance when administered at the time of transplantation, but unsuccessful at breaking tolerance when given 60 days post-transplantation.143 Only when all three interventions were combined on day 60 could they precipitate rejection, suggesting that multiple peripheral mechanisms coexist and act in a redundant way during the maintenance phase of tolerance, making it more robust. Whether T cell-intrinsic dysfunction of TCR75 cells plays a role in the maintenance of tolerance in this model remains to be definitively determined. By contrast, other models of long-term graft acceptance rely on single mechanisms of tolerance, as exemplified in a full mismatch skin transplant model, where skin grafts were indefinitely accepted following the administration of non-depleting anti-CD4, anti-CD8 and anti-CD40L antibodies.44 In this case, depletion of Tregs without parallel targeting of any inhibitory pathway around day 50 post-transplantation led to graft rejection, perhaps revealing a tolerance that is less robust and more easily broken.
In addition to the global readout of graft acceptance and functional inhibition of alloreactive Tconvs at the population level, another level of complexity lies in the heterogeneous fate of individual T cells. In mouse models of chronic LCMV infection and tumor, single-cell analyses have revealed that the pool of dysfunctional T-cells is heterogeneous and consists of distinct subpopulations, perhaps due to the different levels and durations of antigen exposure experienced by each cell.132,148–153 Little is known about whether dysfunctional Tconv populations in transplantation tolerance are also heterogeneous. In the islet transplantation and anti-CD3 administration model, single cell multiplex PCR showed that the graft-infiltrating dysfunctional CD8+ T cells were either Perforin 1 (Prf1)+ or Fas ligand (FasL)+, but they both co-expressed Granzyme B (Gzmb).136 The majority of the CD8+ T-cells were FasL+, as anti-CD3 selectively but partially depleted the cytotoxic Prf1+ subset. Interestingly, these subsets lost expression of Prf1 and FasL with time but maintained Gzmb (Prf1-veFasl-veGzmb+). Whether Prf1-veFasl-veGzmb+ cells were more dysfunctional and represented a distinct state remains to be determined, as blockade of PD-1 signaling precipitated allograft rejection at both the induction and maintenance phases of tolerance. By contrast, CD4+ T-cells infiltrating the allograft were either Gzmb+Tbx21+ or did not express these molecules.137 Both subsets were hypo-functional and enriched in the anergic markers FR4 and CD73, but whether they expanded differentially in response to blockade of the PD1 pathway was not reported.
Thus, dysfunction of alloreactive Tconvs in transplantation tolerance is a complex phenomenon, possibly comprising cells with different levels of dysfunction marked by unique phenotypic markers and those that respond differently to Treg suppression or inhibitory receptor engagement. It is possible that these subsets are exclusive and not inter-related, or they may represent distinct differentiation stages in the process of acquiring terminal dysfunction. Temporal high-throughput molecular and functional analyses, as well as single-cell analyses, may be needed to convincingly define different states of dysfunctional alloreactive Tconvs. Finally, whether or when Tregs may play a role in facilitating Tconv dysfunction needs to be clarified.
Concluding remarks
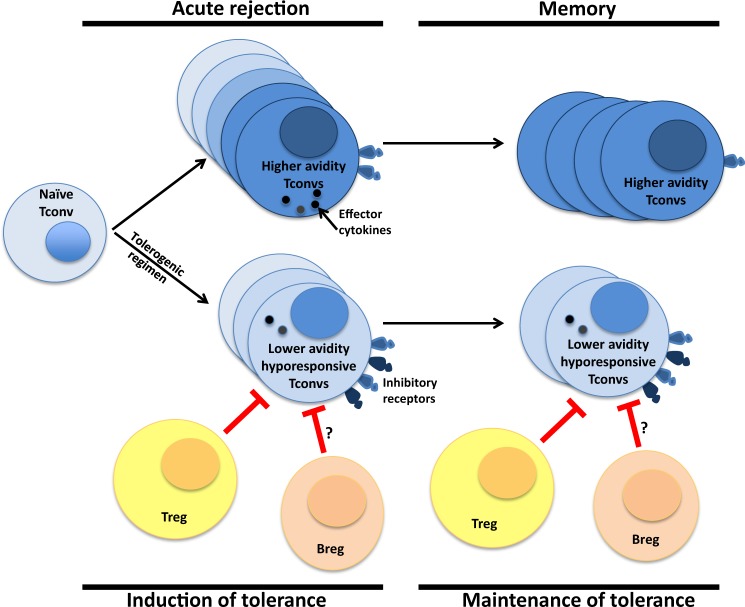
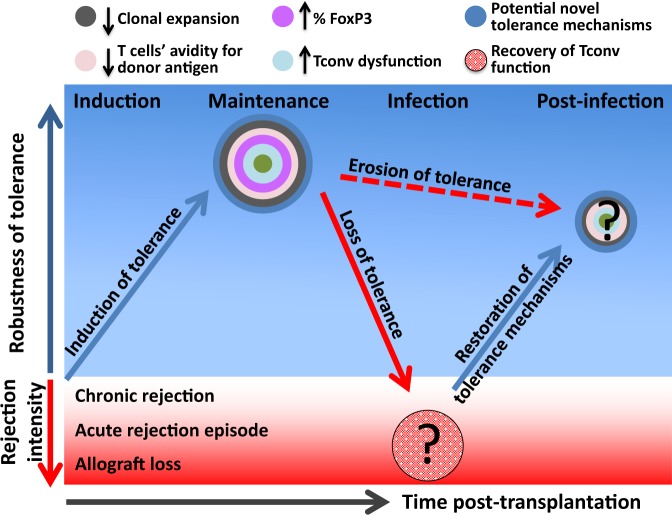
The induction of transplantation tolerance that is durable and resistant to inflammatory challenges may result in life-long allograft acceptance that avoids many of the limitations of current immunosuppressive regimens. Mechanisms of peripheral T cell tolerance that have been associated with transplantation tolerance in animal models include the abortive proliferation of alloreactive Tconvs, an occurrence that can be Treg-dependent or -independent and that may prevent the accumulation of high avidity Tconv clones that would otherwise occur in transplant rejection, thus maintaining low avidity alloreactive Tconv populations (Fig. 1). Moreover, cell-intrinsic hyporesponsiveness of residual Tconvs that appears to depend on engagement of inhibitory receptors on alloreactive Tconvs can also contribute to long-term graft acceptance (Fig. 1). Although mechanisms of tolerance can be sequential in some models, where Tregs are required early and engagement of inhibitory receptors is required late,136 they can be cumulative in others, thus providing redundant layers of tolerance that may be important for durability143 (Fig. 2). However, even in the anti-CD154/DST model of cardiac transplantation tolerance, which currently seems to be one of the most robust animal models of tolerance, infection with Listeria monocytogenes (Lm) could precipitate allograft loss in a subset of mice that had developed stable donor-specific transplantation tolerance8 and erode the tolerance of the remaining mice42 (Fig. 2). Surprisingly, tolerance could be spontaneously restored following resolution of the infection, allowing acceptance of a second donor-matched cardiac allograft in the absence of immunosuppression. This restored tolerance appeared to be less robust because the depletion of Tregs could precipitate rejection of the second heart in previously infected but not uninfected hosts.51 This ‘memory’ of tolerance that resurfaces following successful graft rejection was also recently reported in mice with IRF4-deficient T cells,145 providing a possible mechanistic pathway for this phenomenon.
Fig. 1.
Mechanisms of T cell tolerance associated with transplantation tolerance. Following transplantation, naïve graft-reactive Tconvs expand, with preferential accumulation of T cell clones with higher avidity for alloantigens, and persistence of these clones into the memory phase of the alloresponse. By contrast, following a tolerogenic regimen, graft-reactive T cells undergo abortive proliferation, an event that can be Treg-dependent or -independent. This leads to the accumulation of fewer alloreactive T cell clones of lower avidity for alloantigen. The lower avidity profile persists during the maintenance phase of tolerance, with T cells of some but not all specificities constrained by Tregs. In addition, alloreactive T cells overexpress inhibitory receptors and become dysfunctional, resembling exhausted or anergic T cells, and they can sometimes recover function upon blockade of the inhibitory receptors. Bregs may also contribute to the suppression of alloreactivity, although the specific Tconv functions inhibited and at what phases and location of the alloresponse remain to be clarified
Fig. 2.
Tolerance is a dynamic state. Transplantation tolerance can exist at different levels of robustness depending on the redundancy of mechanisms of T cell tolerance achieved by the tolerogenic regimen, and the degree of robustness may vary over time. A robust tolerance might be more resistant to inflammatory challenges, but tolerance can be lost or eroded following infection, presumably because of a reduction in the quantity or quality of T cell mechanisms of tolerance. Some mechanisms of tolerance can be restored after the infection is cleared, enabling the acceptance of second donor-matched allografts, though the restored tolerance may be less robust after compared with before infection
How Lm or other infections during the maintenance phase of tolerance impact the low avidity profile of alloreactive Tconvs, the number or function of graft-reactive Tregs and Bregs or the possible dysfunction of alloreactive Tconvs remains to be elucidated. Because infections have preceded graft losses in patients who developed tolerance to their allograft, it is likely that inflammatory challenges also affect mechanisms of transplantation tolerance in the clinic. Whether infections caused by different pathogens have differential impacts on distinct mechanisms of tolerance or whether successive infections will progressively erode simultaneous pathways of tolerance are open questions for future analyses. Interestingly, it may be possible to retain immune competence to infections and tolerance to an allograft, as recently reported in mice bearing a deletion of Coronin-1 in T cells.154
Being able to track alloreactive Tconvs and Tregs and evaluate their discrete functions and numbers, as well as TCR avidity profiles, may allow clinicians and researchers to assess mechanisms of tolerance that are induced in the clinic and evaluate their persistence over time. A better understanding of the inflammatory challenges that can revert each tolerance mechanism, and a better identification of the therapeutic interventions that can reinduce select tolerance mechanisms may help ensure the long-term maintenance of robust and persistent tolerance in transplant recipients.
Finally, memory T cells are known to be more resistant to costimulation blockade and Treg suppression.155 The ability of tolerogenic regimens to limit avidity maturation of memory alloreactive Tconv populations, as well as to induce Tconv cell-intrinsic dysfunction, remains to be investigated and is important for the clinical applicability of tolerogenic therapies.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Brouard S, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am. J. Transplant. 2012;12:3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scandling JD, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 4.Leventhal J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci. Transl. Med. 2012;4:124ra128. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am. J. Transplant. 2014;14:1599–1611. doi: 10.1111/ajt.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller ML, Chong AS, Alegre ML. Fifty shades of tolerance. Curr. Transplant. Rep. 2017;4:262–269. doi: 10.1007/s40472-017-0166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You Sylvaine, Chatenoud Lucienne. The Concerted Action of Multiple Mechanisms to Induce and Sustain Transplant Tolerance. OBM Transplantation. 2018;2(4):1–1. [Google Scholar]
- 8.Wang T, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am. J. Transplant. 2010;10:1524–1533. doi: 10.1111/j.1600-6143.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai T, et al. Tolerance: one transplant for life. Transplantation. 2014;98:117–121. doi: 10.1097/TP.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 11.Honjo K, Xu XY, Bucy RP. Heterogeneity of T cell clones specific for a single indirect alloantigenic epitope (I-Ab/H-2Kd54-68) that mediate transplant rejection. Transplantation. 2000;70:1516–1524. doi: 10.1097/00007890-200011270-00020. [DOI] [PubMed] [Google Scholar]
- 12.Zhong S, et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc. Natl Acad. Sci. USA. 2013;110:6973–6978. doi: 10.1073/pnas.1221609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krogsgaard M, et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol. Cell. 2003;12:1367–1378. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 14.Labrecque N, et al. How much TCR does a T cell need? Immunity. 2001;15:71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 15.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8αβ versus CD8αα expression. J. Immunol. 2001;167:2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 16.Kuball J, et al. Increasing functional avidity of TCR-redirected T cells by removing defined glycosylation sites in the TCR constant domain. J. Exp. Med. 2009;206:463–475. doi: 10.1084/jem.20082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minguet S, Swamy M, Alarcón B, Luescher IF, Schamel WWA. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14:135–143. [PubMed] [Google Scholar]
- 19.Richer MJ, Nolz JC, Harty JT. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity. 2013;38:140–152. doi: 10.1016/j.immuni.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat. Immunol. 2014;15:266–274. doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannidou K, et al. Heterogeneity assessment of functional T cell avidity. Sci. Rep. 2017;7:44320–44320. doi: 10.1038/srep44320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Essen MR, Kongsbak M, Geisler C. Mechanisms behind functional avidity maturation in T cells. Clin. Dev. Immunol. 2012;2012:163453. doi: 10.1155/2012/163453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesse MD, Karulin AY, Boehm BO, Lehmann PV, Tary-Lehmann MAT. Cell clone’s avidity is a function of its activation state. J. Immunol. 2001;167:1353–1361. doi: 10.4049/jimmunol.167.3.1353. [DOI] [PubMed] [Google Scholar]
- 24.Miller ML, et al. Distinct graft-specific TCR avidity profiles during acute rejection and tolerance. Cell Rep. 2018;24:2112–2126. doi: 10.1016/j.celrep.2018.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 26.Slifka MK, Whitton JL. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat. Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 27.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutoit V, et al. Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–5856. [PubMed] [Google Scholar]
- 29.Honjo K, Yan XuX, Kapp JA, Bucy RP. Evidence for cooperativity in the rejection of cardiac grafts mediated by CD4+ TCR Tg T cells specific for a defined allopeptide. Am. J. Transplant. 2004;4:1762–1768. doi: 10.1046/j.1600-6143.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 30.Enouz S, Carrie L, Merkler D, Bevan MJ, Zehn D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J. Exp. Med. 2012;209:1769–1779. doi: 10.1084/jem.20120905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozga AJ, et al. pMHC affinity controls duration of CD8+ T cell–DC interactions and imprints timing of effector differentiation versus expansion. J. Exp. Med. 2016;213:2811–2829. doi: 10.1084/jem.20160206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeh HJ, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 34.Zhu Z, et al. CD4+ T cell help selectively enhances high-avidity tumor antigen-specific CD8+ T Cells. J. Immunol. 2015;195:3482–3489. doi: 10.4049/jimmunol.1401571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderton SM, Fillatreau S. Activated B cells in autoimmune diseases: the case for a regulatory role. Nat. Clin. Pract. Rheumatol. 2008;4:657–666. doi: 10.1038/ncprheum0950. [DOI] [PubMed] [Google Scholar]
- 37.Black CM, Armstrong TD, Jaffee EM. Apoptosis-regulated low-avidity cancer-specific CD8(+) T cells can be rescued to eliminate HER2/neu-expressing tumors by costimulatory agonists in tolerized mice. Cancer Immunol. Res. 2014;2:307–319. doi: 10.1158/2326-6066.CIR-13-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallone R, et al. Functional avidity directs T-cell fate in autoreactive CD4+ T cells. Blood. 2005;106:2798–2805. doi: 10.1182/blood-2004-12-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh WI, et al. Avidity and bystander suppressive capacity of human regulatory T cells expressing de novo autoreactive t-cell receptors in Type 1 diabetes. Front. Immunol. 2017;8:1313. doi: 10.3389/fimmu.2017.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsang JY, et al. The potency of allospecific Tregs cells appears to correlate with T cell receptor functional avidity. Am. J. Transplant. 2011;11:1610–1620. doi: 10.1111/j.1600-6143.2011.03650.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee I, et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young JS, et al. Erosion of transplantation tolerance after infection. Am. J. Transplant. 2017;17:81–90. doi: 10.1111/ajt.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francis RS, et al. Induction of transplantation tolerance converts potential effector T cells into graft-protective regulatory T cells. Eur. J. Immunol. 2011;41:726–738. doi: 10.1002/eji.201040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kendal AR, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J. Exp. Med. 2011;208:2043–2053. doi: 10.1084/jem.20110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brennan TV, et al. Requirements for prolongation of allograft survival with regulatory T cell infusion in lymphosufficient hosts. J. Surg. Res. 2011;169:e69–e75. doi: 10.1016/j.jss.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin CY, Graca L, Cobbold SP, Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat. Immunol. 2002;3:1208–1213. doi: 10.1038/ni853. [DOI] [PubMed] [Google Scholar]
- 47.Graca L, et al. Both CD4(+)CD25(+) and CD4(+)CD25(−) regulatory cells mediate dominant transplantation tolerance. J. Immunol. 2002;168:5558–5565. doi: 10.4049/jimmunol.168.11.5558. [DOI] [PubMed] [Google Scholar]
- 48.Feng G, et al. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3 + regulatory T cells. Eur. J. Immunol. 2008;38:2512–2527. doi: 10.1002/eji.200838411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L, et al. TLR engagement prevents transplantation tolerance. Am. J. Transplant. 2006;6:2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 51.Miller ML, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat. Commun. 2015;6:7566. doi: 10.1038/ncomms8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan Z, et al. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat. Med. 2010;16:718–722. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regateiro FS, et al. Foxp3 expression is required for the induction of therapeutic tissue tolerance. J. Immunol. 2012;189:3947–3956. doi: 10.4049/jimmunol.1200449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3( ) regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Thornton AM, Shevach EM. CD4 + CD25 + immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 57.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J. Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;176:1–14. doi: 10.1016/j.cell.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 60.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 2013;34:74–80. doi: 10.1016/j.it.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levine AG, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017;546:421–425. doi: 10.1038/nature22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohnmacht C, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 67.Sefik E, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;349:993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malchow S, et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity. 2016;44:1102–1113. doi: 10.1016/j.immuni.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kieback E, et al. Thymus-derived regulatory T cells are positively selected on natural self-antigen through cognate interactions of high functional avidity. Immunity. 2016;44:1114–1126. doi: 10.1016/j.immuni.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 73.Legoux FP, et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity. 2015;43:896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire + medullary thymic epithelial cells. Nat. Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 75.Malhotra D, et al. Tolerance is established in polyclonal CD4( + ) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat. Immunol. 2016;17:187–195. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LeGuern C, Germana S. On the elusive TCR specificity of thymic regulatory T cells. Am. J. Transplant. 2019;19:15–20. doi: 10.1111/ajt.15165. [DOI] [PubMed] [Google Scholar]
- 77.Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A. Induced regulatory T cells: their development, stability, and applications. Trends Immunol. 2016;37:803–811. doi: 10.1016/j.it.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Bailey-Bucktrout SL, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ezzelarab MB, et al. Regulatory T cell infusion can enhance memory T cell and alloantibody responses in lymphodepleted nonhuman primate heart allograft recipients. Am. J. Transplant. 2016;16:1999–2015. doi: 10.1111/ajt.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 81.Graca L, et al. Donor-specific transplantation tolerance: the paradoxical behavior of CD4+CD25+ T cells. Proc. Natl Acad. Sci. USA. 2004;101:10122–10126. doi: 10.1073/pnas.0400084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sagoo P, et al. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carvalho-Gaspar M, et al. Location and time-dependent control of rejection by regulatory T cells culminates in a failure to generate memory T cells. J. Immunol. 2008;180:6640–6648. doi: 10.4049/jimmunol.180.10.6640. [DOI] [PubMed] [Google Scholar]
- 84.Golshayan D, et al. In vitro-expanded donor alloantigen-specific CD4+ CD25 + regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109:827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 85.Joffre O, et al. Prevention of acute and chronic allograft rejection with CD4 + CD25 + Foxp3 + regulatory T lymphocytes. Nat. Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacDonald KG, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore C, et al. Alloreactive regulatory T cells generated with retinoic acid prevent skin allograft rejection. Eur. J. Immunol. 2015;45:452–463. doi: 10.1002/eji.201444743. [DOI] [PubMed] [Google Scholar]
- 88.Sanchez-Fueyo A, et al. Specificity of CD4+CD25 + regulatory T cell function in alloimmunity. J. Immunol. 2006;176:329–334. doi: 10.4049/jimmunol.176.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsang JY, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J. Clin. Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noyan F, et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am. J. Transplant. 2017;17:917–930. doi: 10.1111/ajt.14175. [DOI] [PubMed] [Google Scholar]
- 91.Boardman DA, et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transplant. 2017;17:931–943. doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- 92.Pierini A, et al. T cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight. 2017;2:92865. doi: 10.1172/jci.insight.92865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Q, et al. Chimeric Antigen Receptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance. Front. Immunol. 2018;9:2359. doi: 10.3389/fimmu.2018.02359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood. 2011;118:5671–5680. doi: 10.1182/blood-2011-02-337097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang Q, Vincenti F. Transplant trials with Tregs: perils and promises. J. Clin. Invest. 2017;127:2505–2512. doi: 10.1172/JCI90598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Young JS, Yin D, Vannier AGL, Alegre ML, Chong AS. Equal expansion of endogenous transplant-specific regulatory T cell and recruitment into the allograft during rejection and tolerance. Front. Immunol. 2018;9:1385. doi: 10.3389/fimmu.2018.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: how many cells do we need? Curr. Opin. Organ Transplant. 2012;17:349–354. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 98.Hansen W, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Delgoffe GM, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Glinka Y, Prud’homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bono MR, Fernandez D, Flores-Santibanez F, Rosemblatt M, Sauma D. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015;589:3454–3460. doi: 10.1016/j.febslet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 102.Jin D, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stolp J, Turka LA, Wood KJ. B cells with immune-regulating function in transplantation. Nat. Rev. Nephrol. 2014;10:389–397. doi: 10.1038/nrneph.2014.80. [DOI] [PubMed] [Google Scholar]
- 104.Fillatreau S. Regulatory plasma cells. Curr. Opin. Pharmacol. 2015;23:1–5. doi: 10.1016/j.coph.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 105.Shen P, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lino AC, et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity. 2018;49:120–133. doi: 10.1016/j.immuni.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao S, Brooks CR, Sobel RA, Kuchroo VK. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J. Immunol. 2015;194:1602–1608. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yeung MY, et al. TIM-1 signaling is required for maintenance and induction of regulatory B cells. Am. J. Transplant. 2015;15:942–953. doi: 10.1111/ajt.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chihara N, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558:454–459. doi: 10.1038/s41586-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan Y, et al. Postoperative administration of donor B cells induces rat kidney allograft acceptance: lack of association with Th2 cytokine expression in long-term accepted grafts. Transplantation. 2002;73:1123–1130. doi: 10.1097/00007890-200204150-00020. [DOI] [PubMed] [Google Scholar]
- 111.Fehr T, et al. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells, and MHC class II. J. Immunol. 2008;181:165–173. doi: 10.4049/jimmunol.181.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deng S, et al. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J. Immunol. 2007;178:6028–6032. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 113.Ding Q, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee KM, et al. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am. J. Transplant. 2012;12:2072–2078. doi: 10.1111/j.1600-6143.2012.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun J, et al. Transcriptomics identify CD9 as a marker of murine IL-10-competent regulatory B cells. Cell Rep. 2015;13:1110–1117. doi: 10.1016/j.celrep.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clatworthy MR, et al. B-cell-depleting induction therapy and acute cellular rejection. N. Engl. J. Med. 2009;360:2683–2685. doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pallier A, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 118.Sagoo P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J. Clin. Invest. 2010;120:1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Newell KA, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J. Clin. Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nova-Lamperti E, et al. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4 + T-cell responses. Sci. Rep. 2016;6:20044. doi: 10.1038/srep20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chesneau M, et al. Tolerant kidney transplant patients produce B Cells with regulatory properties. J. Am. Soc. Nephrol. 2015;26:2588–2598. doi: 10.1681/ASN.2014040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rebollo-Mesa I, et al. Biomarkers of tolerance in kidney transplantation: are we predicting tolerance or response to immunosuppressive treatment? Am. J. Transplant. 2016;16:3443–3457. doi: 10.1111/ajt.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bottomley MJ, Chen M, Fuggle S, Harden PN, Wood KJ. Application of operational tolerance signatures are limited by variability and type of immunosuppression in renal transplant recipients: a Cross-Sectional Study. Transplant. Direct. 2017;3:e125. doi: 10.1097/TXD.0000000000000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thommen DS, Schumacher TN. T Cell Dysfunction in Cancer. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hashimoto M, et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu. Rev. Med. 2018;69:301–318. doi: 10.1146/annurev-med-012017-043208. [DOI] [PubMed] [Google Scholar]
- 127.Wang C, Singer M, Anderson AC. Molecular Dissection of CD8(+) T-Cell Dysfunction. Trends Immunol. 2017;38:567–576. doi: 10.1016/j.it.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 129.Ciurea A, Hunziker L, Klenerman P, Hengartner H, Zinkernagel RM. Impairment of CD4(+) T cell responses during chronic virus infection prevents neutralizing antibody responses against virus escape mutants. J. Exp. Med. 2001;193:297–305. doi: 10.1084/jem.193.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 131.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crawford A, et al. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 136.Baas M, et al. TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. eLife. 2016;5:e08133. doi: 10.7554/eLife.08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Besancon A, et al. The induction and maintenance of transplant tolerance engages both regulatory and anergic CD4(+) T cells. Front. Immunol. 2017;8:218. doi: 10.3389/fimmu.2017.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quezada SA, et al. Mechanisms of donor-specific transfusion tolerance: preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003;102:1920–1926. doi: 10.1182/blood-2003-02-0586. [DOI] [PubMed] [Google Scholar]
- 139.Chai JG, et al. Allospecific CD4(+) T cells retain effector function and are actively regulated by Treg cells in the context of transplantation tolerance. Eur. J. Immunol. 2015;45:2017–2027. doi: 10.1002/eji.201545455. [DOI] [PubMed] [Google Scholar]
- 140.Ferrer IR, et al. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc. Natl. Acad. Sci. USA. 2011;108:20701–20706. doi: 10.1073/pnas.1105500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang X, et al. Cardiac allograft acceptance induced by blockade of CD40-CD40L costimulation is dependent on CD4+ CD25+ regulatory T cells. Surgery. 2011;149:336–346. doi: 10.1016/j.surg.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 142.Wu J, et al. Ablation of Transcription Factor IRF4 Promotes Transplant Acceptance by Driving Allogenic CD4(+) T Cell Dysfunction. Immunity. 2017;47:1114–1128. doi: 10.1016/j.immuni.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miller ML, et al. Tracking of TCR-transgenic T cells reveals that multiple mechanisms maintain cardiac transplant tolerance in mice. Am. J. Transplant. 2016;16:2854–2864. doi: 10.1111/ajt.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Man K, et al. Transcription factor IRF4 promotes CD8(+) T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity. 2017;47:1129–1141. doi: 10.1016/j.immuni.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 145.Zhang H, et al. Ablation of interferon regulatory factor 4 in T cells induces “memory” of transplant tolerance that is irreversible by immune checkpoint blockade. Am. J. Transplant. 2018;3:1–10. doi: 10.1111/ajt.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sarraj B, et al. Impaired selectin-dependent leukocyte recruitment induces T-cell exhaustion and prevents chronic allograft vasculopathy and rejection. Proc. Natl Acad. Sci. USA. 2014;111:12145–12150. doi: 10.1073/pnas.1303676111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kalekar LA, et al. CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat. Immunol. 2016;17:304–314. doi: 10.1038/ni.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Paley MA, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc. Natl Acad. Sci. USA. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.He R, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 151.Im SJ, et al. Defining CD8+T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Singer M, et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell. 2016;166:1500–1511. doi: 10.1016/j.cell.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sakuishi K, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jayachandran R, et al. Disruption of Coronin 1 signaling in T cells promotes allograft tolerance while maintaining anti-pathogen immunity. Immunity. 2019;50:152–165. doi: 10.1016/j.immuni.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 155.Yang J, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl Acad. Sci. USA. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]