Abstract
A hallmark of systemic lupus erythematosus (SLE) is the breaking of B-cell tolerance with the generation of high-affinity autoantibodies; however, the antibody-independent features of the B-cell compartment in SLE are less understood. In this study, we performed an extensive examination of B-cell subsets and their proinflammatory properties in a Chinese cohort of new-onset SLE patients. We observed that SLE patients exhibited an increased frequency of transitional B cells compared with healthy donors and rheumatoid arthritis patients. Plasma from SLE patients potently promoted the survival of transitional B cells in a type I IFN-dependent manner, which can be recapitulated by direct IFN-α treatment. Furthermore, the effect of IFN-α on enhanced survival of transitional B cells was associated with NF-κB pathway activation and reduced expression of the pro-apoptotic molecule Bax. Transitional B cells from SLE patients harbored a higher capacity to produce proinflammatory cytokine IL-6, which was also linked to the overactivated type I IFN pathway. In addition, the frequency of IL-6-producing transitional B cells was positively correlated with disease activity in SLE patients, and these cells were significantly reduced after short-term standard therapies. Thus, the current study provides a direct link between type I IFN pathway overactivation and the abnormally high frequency and proinflammatory properties of transitional B cells in active SLE patients, which contributes to the understanding of the roles of type I IFNs and B cells in the pathogenesis of SLE.
Keywords: Systemic lupus erythematosus, type I interferons, transitional B cells, apoptosis, interleukin 6
Introduction
Systemic lupus erythematosus (SLE) is a chronic and complex autoimmune disease that affects many organs and results in significant organ damage and morbidity.1 B cells play a central role in SLE pathogenesis,2 and B cell-targeting therapies, including B-cell depletion by rituximab3 and B-cell modulation through blocking human B lymphocyte stimulator by belimumab,4,5 represent important advances in SLE treatment. While some B cell-targeting therapies generate promising results, the efficacies of certain trials are variable, indicating a highly heterogeneous feature of the B-cell compartment in SLE.1,6,7
B-cell aberrations in SLE are reflected in multiple aspects. Transitional B (Btr) cells and plasmablasts are greatly expanded while IgM+ memory B cells are decreased in active SLE patients.8,9 CXCR4+ B cells are increased in SLE patients, which is positively correlated with disease activity,10 and GC (germinal center) exclusion of autoreactive B cells is defective in SLE patients, with an expanded autoreactive post-GC IgG+ memory B-cell and plasma cell pool.11,12 In addition to autoantibody generation, B cells can exert antibody-independent functions via cytokine production in autoimmune diseases.13,14 B cells from rheumatoid arthritis and multiple sclerosis patients have been shown to play a pathogenic role as proinflammatory cytokine-producing cells;13 however, their role is much less understood in SLE patients.15
Type I interferons (IFNs) are a family of pleiotropic cytokines that may affect nearly all types of immune cells. Recent studies have highlighted a critical role of type I IFNs in the initiation and progression of SLE, which is closely linked to the dysregulation of the B-cell compartment.16,17 Several studies have indicated a positive correlation between type I IFN signature and autoantibody titers in SLE.18,19 Pro/pre-B cells are significantly reduced while transitional B cells are greatly expanded in the bone marrow of SLE patients with high IFN scores.20 Type I IFNs can reduce the B-cell receptor (BCR) activation threshold of autoreactive B cells21 and license naive B cells to respond against Toll-like receptor 7 ligands.22 Type I IFNs, either directly or indirectly via induction of B-cell activating factor (BAFF), promote B-cell survival, isotype switching and terminal differentiation.23
In the current study, we investigated the distribution and cytokine-producing capacity of different B-cell subsets and their clinical significance in a Chinese cohort of new-onset SLE patients, with a focus on Btr cells, known to be elevated in SLE.24–26 We also explored the biological effects of type I IFNs on several aspects of Btr cells and the possible underlying mechanisms to determine whether there exists a relationship between overactivation of the type I IFN pathway and the abnormalities of Btr cells in SLE.
Materials and methods
Patients and controls
Peripheral blood samples from 64 new-onset SLE patients and 20 new-onset rheumatoid arthritis (RA) patients were obtained from Renji Hospital, Shanghai, China. SLE patients fulfilled the classification criteria of the American College of Rheumatology for SLE. RA patients fulfilled the ACR/EULAR (American College of Rheumatology/European League Against Rheumatism) 2010 criteria. The disease activity of SLE was evaluated by using SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) 2000 criteria.27 Peripheral blood samples from 40 age- and sex-matched healthy donors (HDs) were also collected. Buffy coats were obtained from Blood Center of Changhai Hospital, Shanghai, China. In this study, we also collected peripheral blood samples from 21 new-onset SLE patients 1 month after receiving standard therapy. Table 1 and Supplementary Table 1 summarize the characteristics of all samples. Serum C3 concentration and anti-double-stranded DNA (dsDNA) antibody titers were routinely measured in Renji Hospital by immunoturbidimetry and Farr radioimmunoassay, respectively. For those samples with serum anti-dsDNA antibody titers exceeding the high limit of detection (>100 IU/ml), serial dilutions were performed to obtain the exact antibody titers. This study was approved by the Review Board for Renji Hospital in Shanghai, Republic of China. Informed consent was obtained from all study participants. All studies were performed in accordance with the Declaration of Helsinki.
Table 1.
Demographic data of HDs, new-onset SLE and RA patients in this study
| HDs (n = 40) | SLE (n = 64) | RA (n = 20) | |
|---|---|---|---|
| Age, mean (range) | 35.18 (21–54) | 32.31 (15–54) | 49.74 (20–71) |
| Sex (female/male) | 90% (36:4) | 93.75% (60:4) | 85% (17:3) |
| SLEDAI, mean (range) | NA | 13.35 (2–44) | NA |
| Fever (%) | NA | 1.72% | NA |
| Rash (%) | NA | 52.73% | NA |
| Alopecia, n (%) | NA | 12.73% | NA |
| Arthritis (%) | NA | 58.18% | NA |
| Oral ulcer (%) | NA | 21.82% | NA |
| Photosensitivity (%) | NA | 10.00% | NA |
| Vasculitis (%) | NA | 9.10% | NA |
| Raynaud (%) | NA | 7.55% | NA |
| Serositis (%) | NA | 19.57% | NA |
| Renal (%) | NA | 24.14% | NA |
| Hematology (%) | NA | 69.35% | NA |
| Neurology (%) | NA | 7.27% | NA |
| dsDNA (IU/ml), mean (range) | NA | 417.01 (1.16–3768) | NA |
| C3 (g/liter), mean (range) | NA | 0.53 (0.10–1.54) | NA |
Data are numbers (%) or means (range)
NA not available
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were collected by Lymphoprep (Axis-Shield) density gradient centrifugation of heparinized blood or buffy coats. B cells were isolated by positive selection using anti-CD19 magnetic beads (Miltenyi Biotec). The purity of B cells was greater than 95%. Isolated B cells were further sorted into CD20+IgG−IgA−CD27−CD24hiCD38hi transitional B cells and CD20+IgG−IgA−CD27−CD24intCD38int naive B cells by AriaII (BD Bioscience).
B-cell culture and treatment
Complete medium consisted of RPMI-1640 containing L-alanyl-L-glutamine dipeptide supplemented with 10% fetal calf serum (Gibco), 5 × 10−5 M of 2-ME (Sigma) and antibiotics (penicillin 100 U/ml, streptomycin 100 μg/ml, Gibco BRL). Purified B cells (1 × 106 cells/ml) were cultured in 96-well plates in complete RPMI-1640 medium and various concentrations of IFN-α (PBL Assay Science) or 25% plasma from individual SLE patients, RA patients or healthy donors. For blocking studies, plasma was pre-incubated with 0.1 μg/ml B18R (type I interferon receptor protein, eBioscience) or 1 μg/ml anti-IFN-γ antibody for 30 min. In some experiments, B cells were incubated with different inhibitors: 1 μM BAY1170-82 (NF-κB inhibitor), 10 μM SB202190 (p38 inhibitor), 10 μM SP600125 (JNK inhibitor) or dimethyl sulfoxide (DMSO) for 30 min before IFN-α treatment.
Cell apoptosis assay
Cell viability and apoptosis were assessed using an apoptosis detection kit with annexin V/propidium iodide according to the manufacturer’s instructions (eBiosciences).
Flow cytometric analysis
PBMCs or enriched B cells were surface stained with fluorochrome-labeled antibodies against CD3 (OKT3), CD20 (SJ25C1), CD24 (ML5), CD27 (O323), CD38 (HIT2), IgM (MHM-88), IgD (IA6-2), IgG (G18-145) and IgA (IS118E10) from eBioscience, BD Biosciences, Biolegend or Miltenyi. In CD3−CD20+ B cells, transitional B cells (Btr), naive B cells (Bn), IgM+ memory B cells (IgM+Bm) and switched memory B cells (Sw Bm) are identified as IgM+IgD+CD27−CD24hiCD38hi, IgM+/D+CD27−CD24+CD38+, IgM+IgD+CD27+, and IgM−IgD−, respectively. For intracellular cytokine staining, cells were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma) plus 1 μg/ml ionomycin (Sigma) in the presence of 5 μg/ml Brefeldin A (Biolegend) for 5 h. Then, cells were washed twice with phosphate-buffered saline and stained with Zombie YellowTM Dye (Biolegend) to eliminate dead cells. After surface staining with antibodies against CD3, CD20, CD24, CD27, CD38, IgM and IgD, cells were fixed, permeabilized and stained for the detection of intracellular cytokines interleukin-6 (IL-6; MQ2-13A5) and tumor necrosis factor-α (TNF-α; MAb11) according to the manufacturer’s instructions (BD Biosciences). Labeled cells were analyzed on an LSRFortessa flow cytometer (BD Biosciences), and data were analyzed by Flowjo software (Tree Star).
Cytokine detection
Concentrations of soluble factors in the plasma and supernatants were detected by the multiplexed Luminex xMAP assay (Cytokine/Chemokine/Growth Factor 45-Plex Human Panel 1 and ProcartaPlex™ Simplex Kits for IL-6, interferon-inducible protein-10 (IP-10) and macrophage inflammatory protein-1β (MIP-1β)) according to the manufacturer’s instructions (eBioscience).
Quantification of gene expression
Total RNA was extracted from freshly sorted or cultured transitional or naive B cells using the RNeasy Plus Micro Kit (Qiagen) according to the manufacturer’s instructions. RNA was reverse transcribed to complementary DNA with the ReverTra Ace qPCR RT Kit (TOYOBO), and gene expression was measured by quantitative reverse transcription-PCR. The primers used are listed in Supplementary Table 2. The expression levels were normalized to GAPDH gene. In addition, IL-6 and TNF-α gene expressions were further normalized to control conditions with no IFN-α treatment.
Statistical analysis
All data were analyzed with Prism (GraphPad software, v7). Differences between groups were assessed by the Mann–Whitney test, Wilcoxon matched pairs test, Kruskal–Wallis test with post hoc analysis by Dunn’s multiple comparison test, or two-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons. Correlations were analyzed by Pearson’s or Spearman’s rank correlation as needed. Multivariate analysis was performed by binary logistic regression analysis. P-values of <0.05 were considered statistically significant.
Results
Plasma from active SLE patients protects transitional B cells from apoptosis
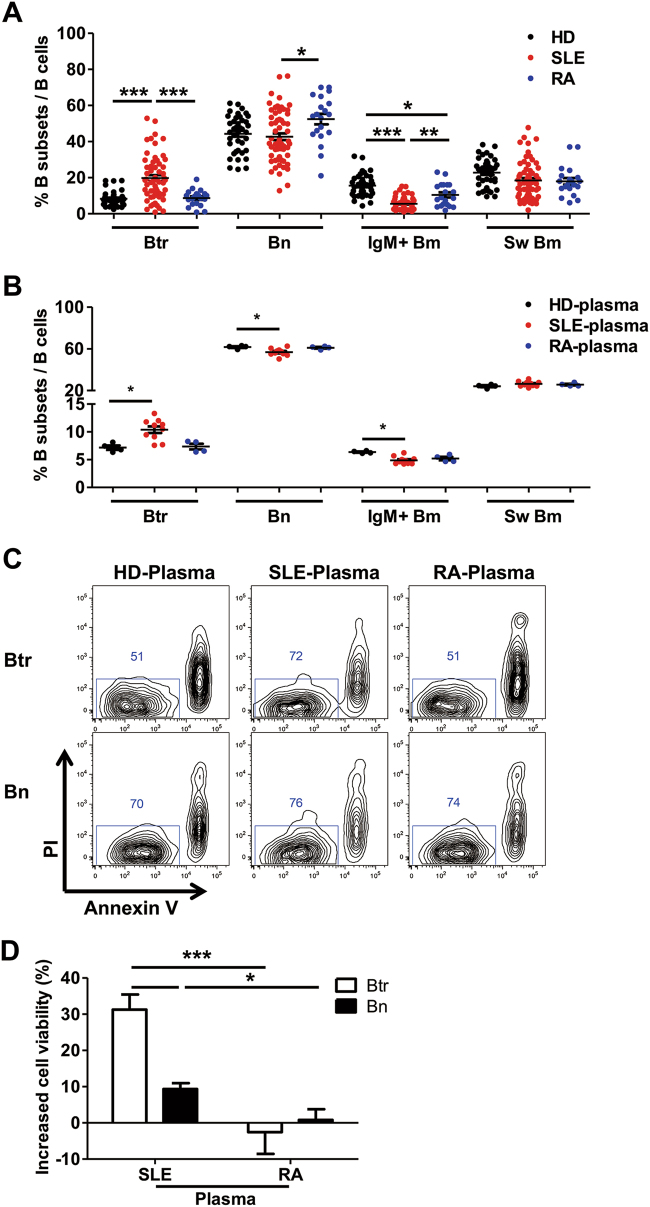
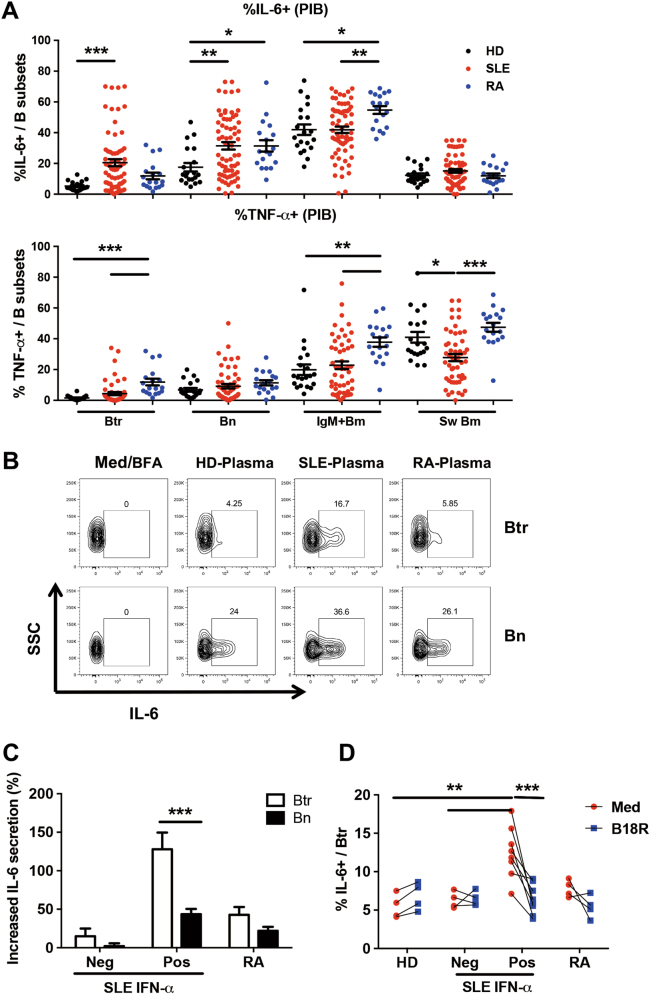
Given the critical role that B cells play in the pathogenesis of SLE, we investigated peripheral B-cell subsets in a Chinese cohort of new-onset SLE patients by polychromatic flow cytometry (Figure S1A). Consistent with the literature,9,24,25 the frequency of transitional B cells (Btr) was significantly increased, while that of IgM+ memory B cells (IgM+ Bm) was strongly decreased in new-onset SLE patients compared with the frequencies measured for HDs and new-onset RA patients (Fig. 1a).
Fig. 1. Plasma from SLE patients protects transitional B cells from apoptosis.
a Scatter plots show the percentages of different peripheral B-cell subsets from 40 healthy donors (HDs), 64 SLE patients and 20 RA patients. b–d Purified B cells from healthy donors were cultured with 25% plasma from 4 HDs, 11 SLE patients or 4 RA patients for 24 h. b Scatter plots show the percentages of different B-cell subsets among PI− annexin V− live B cells after different plasma treatment. c Representative flow cytometry plots show the survival of Btr and Bn cells cultured with different plasmas. d Bar plot shows the increased cell viability of Btr and Bn with SLE or RA plasma treatment after normalization to HD plasma. Data are represented as means ± SEM (a, b, d) and are representative of three experiments (b–d). Data were analyzed by the Kruskal–Wallis test with post hoc analysis by Dunn’s multiple comparison test (a, b) and two-way ANOVA with Bonferroni correction for multiple comparisons (d). *P < 0.05, **P < 0.01, ***P < 0.001
To determine whether lupus plasma could affect the homeostasis of the B-cell compartment, we treated healthy donor B cells with plasma from individual new-onset SLE patients, RA patients or HDs for 24 h. After gating on live B cells (Figure S1B), it became apparent that SLE plasma induced a B-cell subset distribution pattern similar to that of active SLE patients: increased frequency of Btr cells and decreased frequency of IgM+ Bm cells (Fig. 1b). Alternatively, when B-cell subsets were gated first and their apoptotic status was analyzed, Btr cells showed a high apoptotic potential in the presence of plasma from HDs or RA patients. In contrast, SLE plasma potently enhanced the survival of Btr cells, while this effect on naive B cells (Bn) and other B-cell subsets was minimal (Fig. 1c, d, and data not shown). In addition, enhanced protection for Btr cell survival by SLE plasma could also be observed as late as 48 h (Figure S1C-D). Thus, our study indicates that SLE plasma could provide a critical signal to promote the survival of Btr cells.
SLE plasma enhances Btr cell survival in a type I IFN-dependent manner
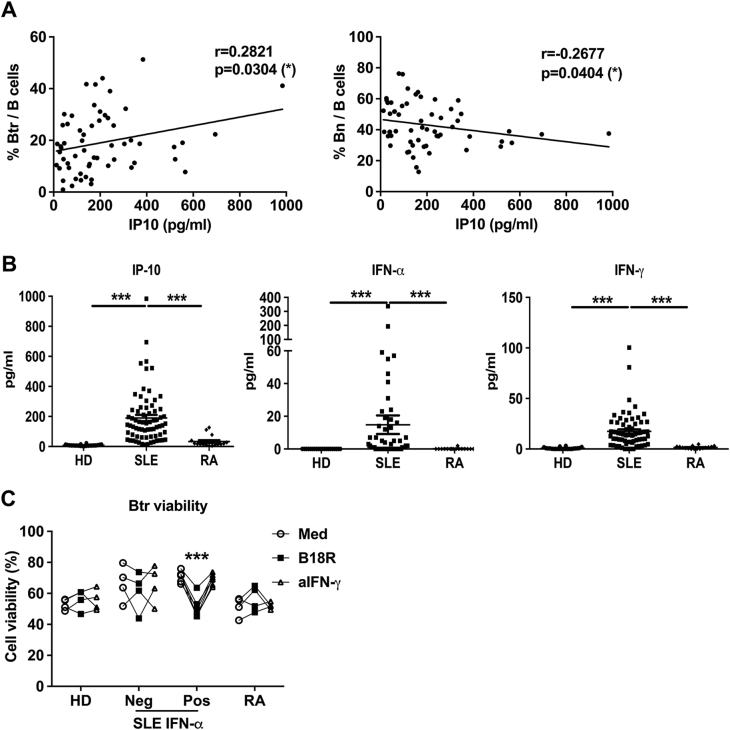
To determine which signal in SLE plasma could play a role in Btr cell survival, we measured a panel of soluble factors in plasma (Supplementary Table 3) and identified a positive correlation between the frequencies of Btr cells and IP-10 concentrations (Fig. 2a). IP-10 has been shown to correlate with disease activity and clinical manifestations in SLE patients.28 IP-10 is mainly induced by IFN-α and IFN-γ, which were also elevated in SLE plasma (Fig. 2b). We found that blocking type I IFN pathway by the soluble type I IFN receptor antagonist B18R could efficiently block the survival-promoting effect of SLE plasma, particularly for samples with detectable IFN-α (Fig. 2c). By contrast, a neutralization antibody against IFN-γ had no effect on Btr cell survival in the presence of SLE plasma, indicating that IFN-γ has no obvious effect on the viability of Btr cell.
Fig. 2. SLE plasma enhances Btr cells survival in a type I IFN-dependent manner.
a Scatter plots show the correlation between the frequency of Btr or Bn cells and IP-10 concentration in plasma from 64 new-onset SLE patients, with the correlation analyzed with Spearman’s rank correlation. b Scatter plots show IP-10, IFN-α and IFN-γ levels in plasma from 22 HDs, 64 new-onset SLE patients and 16 RA patients. c Plasma from 4 HDs, 11 SLE patients (Neg: IFN-α was undetectable (4 patients); Pos: IFN-α was detectable (7 patients)), or 4 RA patients was pre-treated with medium, B18R or anti-IFN-γ antibody for 30 min at 4° C. Then, purified B cells from healthy donor were cultured with 25% pre-treated plasma. In addition, after 24 h, PI and annexin V were used to detect their survival. Scatter plots show Btr cell viability after different pre-treated plasma treatment. Data are represented as means ± SEM (b) and are representative of three experiments (c). Data were analyzed by the Kruskal–Wallis test with post hoc analysis by Dunn’s multiple comparison test (b), and two-way ANOVA with Bonferroni correction for multiple comparisons (c). *P < 0.05, ***P < 0.001
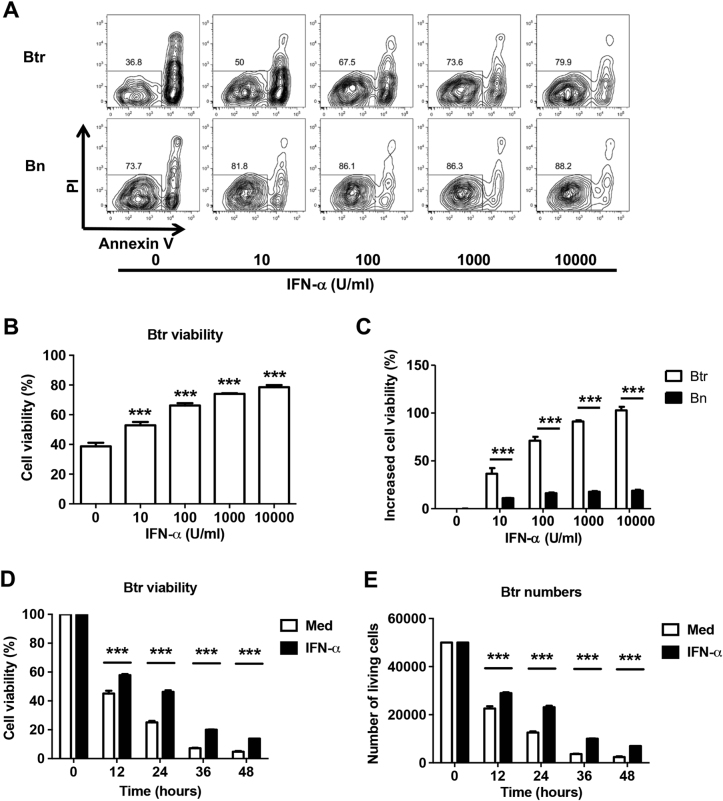
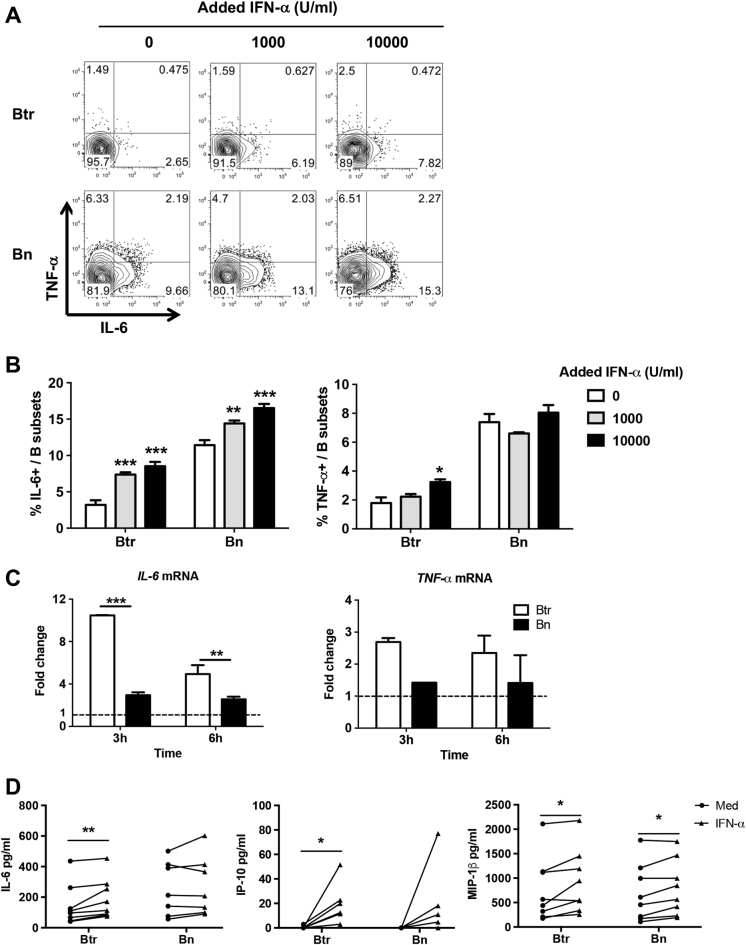
We then investigated the direct effect of IFN-α on the viability of Btr cells. We observed that IFN-α promoted Btr cell survival in a dosage-dependent manner (Fig. 3a–c). IFN-α could also promote Bn (Fig. 3a, c), IgM+ Bm and Sw Bm (Figure S2A) cell survival, but the effects were much less potent. We also performed a kinetic study by using flow cytometry-sorted Btr cells, and as expected, IFN-α treatment significantly increased Btr cell viability and viable cell numbers (Fig. 3d, e). BAFF concentration was also elevated in SLE plasma (Figure S2B), but both IFN-γ and BAFF failed to show obvious effects on Btr cell survival (Figure S2C).
Fig. 3. IFN-α protects Btr cells from spontaneous apoptosis.
a–c Purified B cells from healthy donors were cultured with increasing concentrations of IFN-α for 24 h, and PI and annexin V were used to detect their survival. Representative flow cytometry plots (a) and cumulative data (b) show Btr cell viability. c Bar chart shows the increase in Btr and Bn cell viability with increasing doses of IFN-α normalized to medium alone. d, e Btr cells were sorted from HD B cells, then cultured with or without 1000 U/ml IFN-α. Bar charts show Btr cell viability (PI/Annexin V staining) (d) and viable cell numbers [trypan blue method] (e) at different time points. b–e Data are presented as means ± SEM and are representative of at least three experiments. Data were analyzed by one-way ANOVA with post hoc analysis by Dunnett’s multiple comparison test (b) and two-way ANOVA with Bonferroni correction for multiple comparisons (c–e). ***P < 0.001
NF-κB pathway is involved in IFN-α-mediated Btr cell survival
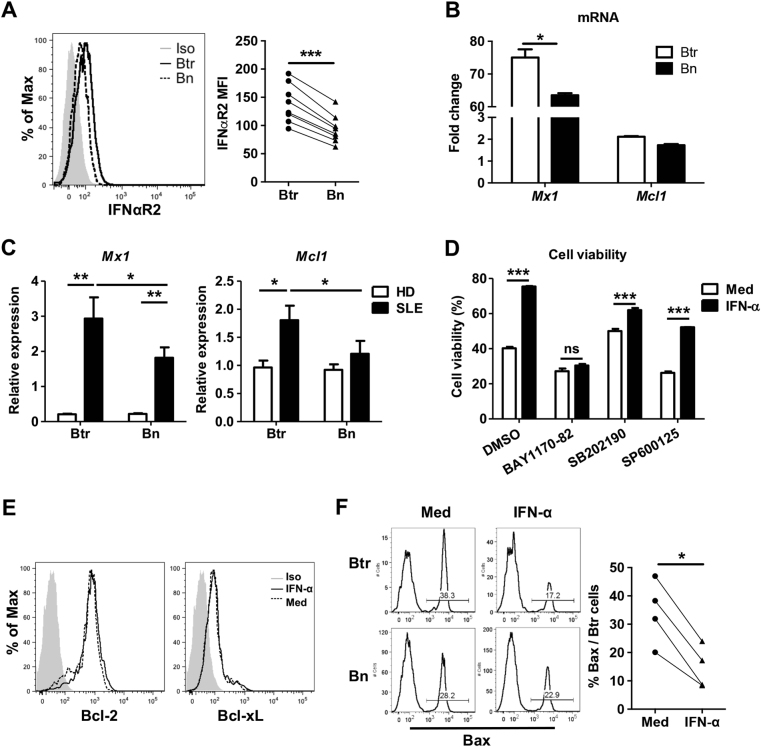
We noted that Btr cells from healthy donors expressed higher amounts of IFN-α receptor 2 than Bn cells did (Fig. 4a). In response to IFN-α treatment, Btr cells expressed significantly higher type I IFN-targeted gene Mx1 than Bn cells did (Fig. 4b). In addition, type I IFN-targeted genes Mx1 and Mcl1 were significantly upregulated in Btr cells from SLE patients compared with those in Btr cells of healthy donors and Bn cells from SLE patients (Fig. 4c). The above-mentioned results suggest that Btr cells constantly receive type I IFN signaling in vivo, which may lead to enhanced survival in an apoptosis-prone environment.
Fig. 4. NF-κB pathway is involved in IFN-α-mediated Btr cell survival.
a Representative flow cytometry plot and cumulative data show the expression of IFNαR2 in healthy donor Btr and Bn cells. b Purified Btr and Bn from healthy donor B cells were stimulated with 1000 U/ml IFN-α for 3 h. Bar plots show fold changes of Mx1 and Mcl1 mRNA expression of Btr or Bn cells after IFN-α stimulation. c Bar plots show the relative expression of genes Mxl and Mcl1 in purified Btr and Bn cells from 5 healthy donors and 5 SLE patients. d Healthy donor B cells were pre-treated with DMSO or different inhibitors for 30 min before being cultured with 1000 U/ml IFN-α for 24 h. Bar chart shows Btr viability under different conditions. e, f Purified B cells from healthy donors were cultured with or without 1000 U/ml IFN-α for 24 h, and Bcl-2, Bcl-xL and Bax were measured by intracellular staining. e Representative flow cytometry plots show Bcl-2 and Bcl-xL expression in Btr cells. f Representative flow cytometry plot and cumulative data show the expression of Bax in healthy donor Btr and Bn cells. Data are presented as means ± SEM (b–d) and are representative of at least three experiments (b–d). Data were analyzed by two-way ANOVA with Bonferroni correction for multiple comparisons (c, d) and the Wilcoxon matched pairs test (a–c, f). *P < 0.05, **P < 0.01, ***P < 0.001
IFN-α could activate multiple pathways, and by using several kinase inhibitors, we found that the nuclear factor (NF)-κB pathway is critical for IFN-α-mediated Btr cell survival (Fig. 4d). Interestingly, there was minimal upregulation of anti-apoptotic molecules Bcl-2 and Bcl-xL in IFN-α-treated Btr cells (Fig. 4e); however, the pro-apoptotic protein Bax was strongly downregulated in Btr cells after IFN-α treatment (Fig. 4f). It has been reported that Bax expression is inhibited by NF-κB activation;29 thus, our study suggests that the survival-promoting effect of IFN-α on Btr cells could be mediated by the activation of the NF-κB pathway first, followed by the inhibition of Bax expression.
Enhanced proinflammatory properties of transitional B cells in active SLE patients
Next, we investigated the cytokine-producing capacity of different B-cell subsets in SLE patients, which is not well understood in SLE.13 The IL-10-positive signals were too low to be reliably detected under our 5 h PMA plus Ionomycin stimulation condition. By contrast, IL-6- and TNF-α-producing B cells were readily detected by flow cytometry (Figure S3A). Compared with those from healthy donors, B cells from SLE patients did not show an enhanced capacity to produce IL-6 (Figure S3B), and the frequency of TNF-α-producing B cells tended to be even lower (Figure S3C).
Interestingly, unlike B cells, Btr cells and to a lesser extent Bn cells from SLE patients showed a much higher frequency of IL-6-producing cells than those from healthy donors (Fig. 5a). In contrast, a significant decrease in TNF-α-producing Sw Bm cells was observed in SLE patients (Fig. 5a). We also observed that several B-cell subsets from RA patients harbored a high capacity to produce IL-6 (Bn and IgM+ Bm cells) and TNF-α (Btr and IgM+ Bm) (Fig. 5a). Collectively, B-cell subsets from SLE patients exhibited a unique cytokine-producing profile unlike that of HD or RA patients.
Fig. 5. Proinflammatory potency of transitional B cells in active SLE patients.
a PBMC from 22 HDs, 64 SLE patients and 20 RA patients were stimulated with PMA/Ionomycin in the presence of Brefeldin A for 5 h, and intracellular IL-6 and TNF-α were detected by flow cytometry. Scatter plots show the frequencies of IL-6 and TNF-α production in different B-cell subsets. b, c Purified B cells from healthy donors were cultured with 25% plasma from 4 healthy donors or 11 SLE patients (Neg: IFN-α was undetectable (4 patients); Pos: IFN-α was detectable (7 patients)) for 24 h. PMA/Ionomycin and Brefeldin A were added in the last 5 h. b Representative flow cytometry plots show IL-6 production in Btr and Bn cells in different plasmas. c Bar plots show increased IL-6 secretion in Btr cells with SLE or RA plasma treatment after normalization to healthy donor plasma treatment. d Plasma was pre-treated with medium or B18R for 30 min at 4 °C. Then, purified B cells from healthy donors were cultured with 25% pre-treated plasma. Scatter plots show the frequency of IL-6-producing Btr cells after different pre-treated plasma treatment. Data are presented as means ± SEM (a, c) and are representative of three experiments (b–d). Data were analyzed by the Kruskal–Wallis test with post hoc analysis by Dunn’s multiple comparison test (a) and two-way ANOVA with Bonferroni correction for multiple comparisons (c, d). *P < 0.05, **P < 0.01, ***P < 0.001
Plasma from SLE patients promotes the IL-6-producing capacity of Btr cells in a type I IFN-dependent manner
B-cell activities can be strongly influenced by extrinsic factors; therefore, we tested whether plasma from active SLE patients could affect the cytokine-producing capacity of Btr cells. Healthy donor B cells were treated with plasma from new-onset SLE patients, RA patients and healthy donors for 24 h. We found that plasma from SLE patients had a strong capacity to induce IL-6 production compared with plasma from RA patients or healthy donors (Fig. 5b). We observed that only SLE plasma with detectable IFN-α efficiently stimulated Btr cells to produce IL-6 (Fig. 5c), suggesting that IFN-α may play an important role in triggering IL-6 expression in Btr cells. The type I IFN dependence was confirmed by using the soluble type I IFN receptor antagonist B18R (Fig. 5d). By contrast, neutralization of IFN-γ had no obvious effect on IL-6 production by Btr cells (Figure S4). We also found that other cytokines, namely, BAFF, APRIL and IL-6, did not play a role in the generation of IL-6-producing Btr cells (data not shown).
Next, we examined whether IFN-α could directly enhance the cytokine-producing capacity of Btr cells. The results showed that IFN-α can strongly increase the capacity of Btr cells to produce IL-6 at both the protein and messenger RNA (mRNA) levels (Fig. 6a–c). IFN-α can also promote IL-6 expression in Bn cells; however, its effect is less dramatic, particularly at the mRNA level (Fig. 6a–c). TNF-α expression in Btr cells was less affected following IFN-α stimulation, and only a high concentration of IFN-α exhibited a promoting effect (Fig. 6a–c). Moreover, IFN-α stimulation also promoted the secretion of IL-6, as well as that of IP-10 and MIP-1β, from Btr cells (Fig. 6d), while TNF-α was undetectable in our experiments. These results indicate that IFN-α can directly promote IL-6 expression in Btr cells, which explains the enhanced IL-6 production observed in SLE Btr cells that are likely to be conditioned by a type I IFN-rich environment.
Fig. 6. IFN-α promotes IL-6 expression in transitional B cells.
a, b Purified B cells from healthy donors were cultured with increasing concentrations of IFN-α for 24 h, and PMA/Ionomycin was added in the last 5 h in the presence of Brefeldin A. IL-6- and TNF-α-producing B-cell subsets were measured by flow cytometry. Representative flow cytometry plots (a) and cumulative data (b) show the frequencies of IL-6- and TNF-α-producing Btr and Bn cells. c Purified Btr and Bn cells from healthy donor B cells were stimulated with 10,000 U/ml IFN-α for 3 and 6 h. Bar plots show fold changes of IL-6 and TNF-α mRNA expression of Btr or Bn cells after IFN-α stimulation. d Purified Btr and Bn cells from healthy donor B cells were stimulated with or without 10,000 U/ml IFN-α in the presence of 100 ng/ml PMA and 2 μg/ml Ionomycin for 24 h. Scatter plots show concentrations of IL-6, IP-10 and MIP-1β levels in the supernatants of Btr or Bn cells. Data are represented as means ± SEM and are representative of three experiments (b, c). Data were analyzed by two-way ANOVA with Bonferroni correction for multiple comparisons (b, c) and the Wilcoxon matched pairs test (d). *P < 0.05, **P < 0.01, ***P < 0.001
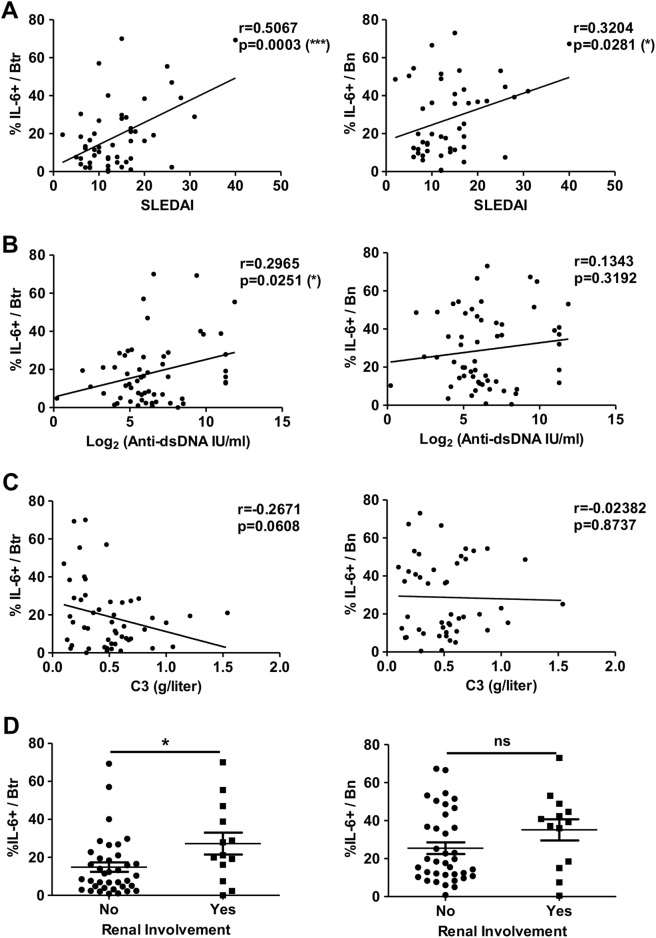
The frequency of IL-6-producing Btr cells is strongly correlated with disease activities in SLE patients
Given the increased frequency of IL-6-producing Btr cells in active SLE patients, we next explored the possible clinical relevance of these cells. We found a highly positive correlation between the frequency of IL-6-producing Btr cells and SLEDAI scores (Fig. 7a). The frequency of IL-6-producing Btr cells was also positively correlated with the titers of serum anti-dsDNA autoantibody (Fig. 7b) and tended to be inversely associated with serum C3 levels (Fig. 7c). Furthermore, SLE patients with lupus nephritis (LN) showed significantly higher frequencies of IL-6-producing Btr cells than those without LN (Fig. 7d). By contrast, the frequency of IL-6-producing Bn cells was much lower or was not associated with disease activity, including SLEDAI scores, serum anti-dsDNA autoantibody, serum C3 levels or association with renal involvement (Fig. 7a–d). When SLE patients were divided into two groups according to SLEDAI scores (low disease activity, SLEDAI <8; high disease activity, SLEDAI ≥8), the frequency of IL-6-producing Btr cells was still significant following multivariate analysis (P = 0.042) (Supplementary Table 4), suggesting that the frequency of IL-6-producing Btr cells is a robust parameter for indicating lupus disease activity.
Fig. 7. The frequency of IL-6-producing Btr cells is strongly correlated with disease activities in SLE patients.
Scatter plots show the correlations between the frequency of IL-6-producing Btr (left) or Bn (right) cells and SLEDAI (a), serum anti-dsDNA titers (b) or serum complement C3 levels (c) in new-onset SLE patients. Data were analyzed with Pearson’s correlation. d Scatter plots show the frequency of IL-6-producing Btr (left) or Bn (right) cells in SLE patients with (Yes) or without (No) lupus nephritis. Data are presented as means ± SEM and were analyzed by the Mann–Whitney test. *P < 0.05, ***P < 0.001
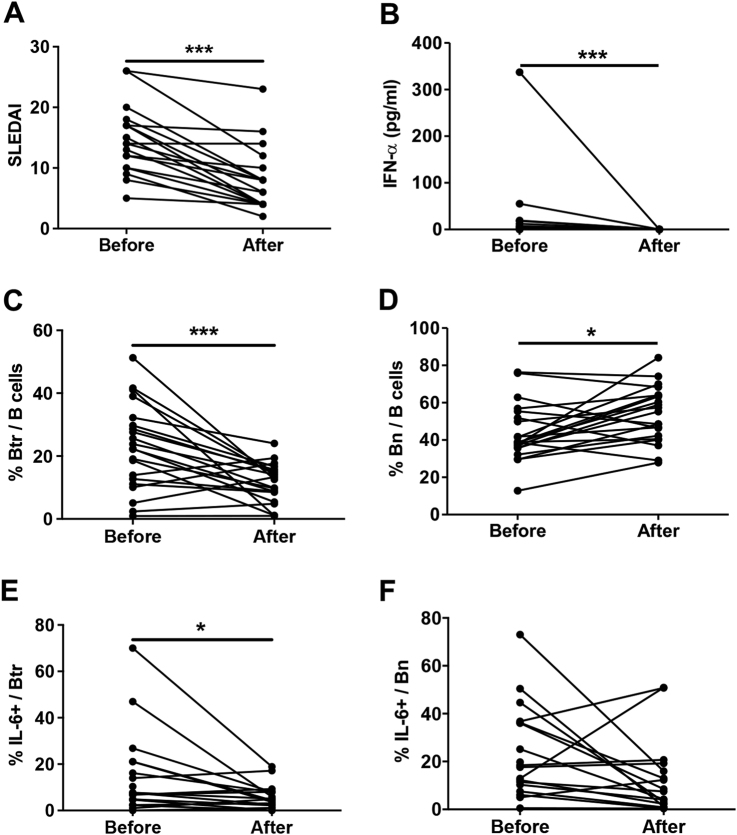
We also checked several parameters in this cohort of SLE patients who have received a short-term (1 month) standard therapy. We found that SLEDAI scores (Fig. 8a), plasma IFN-α concentrations (Fig. 8b) and the frequencies of Btr cells (Fig. 8c) and IL-6-producing Btr cells (Fig. 8e) were significantly reduced after treatment. In contrast, the frequency of Bn cells (Fig. 8d) was increased, and no significant change in IL-6-producing Bn cells (Fig. 8f) was observed after therapy. The frequencies of TNF-α-producing Btr and Bn cells also did not show significant changes (data not shown). These results clearly indicate that the frequency of IL-6-producing Btr cells closely reflects disease status in active SLE.
Fig. 8. The frequency of IL-6-producing Btr cells is decreased in SLE patients after short standard therapy.
Scatter plots show the SLEDAI scores (a), the concentration of plasma IFN-α (b), the frequencies of Btr cells (c) and Bn cells (d), and the frequencies of IL-6-producing Btr cells (e) and Bn cells (f) before and after 1-month standard therapy in 21 SLE patients. Data were analyzed by the Wilcoxon matched pairs test (a–f)
Discussion
Overactivation of the type I IFN pathway and dysregulation of the B-cell compartment are two salient features of SLE, and several studies have reported that the two abnormalities are intimately associated.1,2,30 Here, we provide strong evidence that a close link exists between type I IFN signaling and enhanced survival and the IL-6-producing capacity of circulating transitional B cells in a Chinese cohort of new-onset SLE patients. In addition, the frequency of IL-6-secreting Btr cells closely reflects disease activity in SLE patients, supporting the notion that this frequency could be a new parameter for monitoring disease progress.
One feature of the B-cell compartment in active SLE patients is the expanded circulation of Btr cells,24,25 but how this phenomenon is established remains poorly understood. Increased bone marrow output could be one reason, as one study has provided an association between expansion of Btr cells and type I IFN-induced BAFF and APRIL within human bone marrow.20 However, Btr cells are prone to apoptosis in the absence of stimulation;25 therefore, this population should be stably maintained by a given signal. In the current study, we first found a correlation between Btr cell frequency and IP-10, an IFN-induced chemokine, in a population of new-onset SLE patients. Although we were unable to conduct a direct correlation study between Btr cell frequency and IFN-α as the latter is undetectable in many lupus samples, we demonstrated the role of the type I IFN pathway in promoting Btr cell survival by a specific type I IFN antagonist B18R or direct treatment.
Previous studies have demonstrated a role of type I IFNs in promoting B-cell survival,31–33 and the effect of type I IFNs has been attributed to the phosphatidylinositol-3-kinase (PI3K) and NF-κB pathways, particularly anti-apoptotic molecules Bcl-2 and Bcl-xL.32,34 In our study, we also found that NF-κB activation is required for IFN-α to promote Btr cell survival. However, we failed to observe a clear difference in Bcl-2 and Bcl-xL expression with or without IFN-α treatment. Instead, we observed that pro-apoptotic molecule Bax expression is downregulated in Btr cells following IFN-α treatment. Thus, an NF-κB-Bax axis is likely operative in IFN-α-treated Btr cells. IFN-α is still able to enhance Btr cell survival at later time points (36–48 h, Fig. 3d, e) compared with medium alone; however, the effect becomes modest. This finding is most likely due to the nature of the transient activation of the NF-κB pathway. Compared with naive B cells, transitional B cells express higher levels of type I IFN receptor and show higher responsiveness to type I IFNs by increasing the expression of several IFN-targeted genes. This mechanism may explain the preferential effects of type I IFNs on transitional B cells. Taken together, type I IFNs are likely to affect Btr cells in several respects, including increased bone marrow output and enhanced peripheral survival.
It should be noted that under our experimental conditions, we failed to find a role of BAFF in promoting Btr survival. BAFF is a critical cytokine for regulating B-cell development and differentiation and promotes the survival of immature/transitional B cells in mice.35,36 To our knowledge, few studies have directly verified BAFF ability to promote immature/transitional B-cell survival in humans. While BAFF enhances human mature B-cell survival,25,37 Sims et al.25 failed to observed a pro-survival effect of BAFF on immature B cells. Our result is consistent with this finding. The reason is not known, but species differences and/or different experimental conditions could provide an explanation. Possibly, additional signals such as BCR or other cytokine signals are required to synergize with BAFF to function on human immature/transitional B cells.
B cells exert their functions via antibody-dependent and antibody-independent pathways. In recent years, the antibody-independent roles of B cells have attracted great attention,6,38 particularly in the context of autoimmune diseases. B cells from patients with rheumatoid arthritis or multiple sclerosis have shown enhanced production of proinflammatory cytokines IL-6 and TNF-α.39–41 The effectiveness of B-cell depletion therapy in the two diseases has been linked to the elimination of proinflammatory cytokine-producing B cells.13 However, the antibody-independent role of B cells in SLE patients is still unclear. In one study, B cells from SLE patients did not show an increased capacity to produce IL-6 and TNF-α,42 and in another study, B cells from SLE patients produced lower levels of proinflammatory cytokines than those measured for healthy controls.15 Consistent with these studies, we found that B cells overall do not exhibit a proinflammatory feature in active SLE patients.
Insight into B-cell subsets revealed that the frequency of IL-6-producing Btr cells in SLE patients is significantly higher than that of healthy controls. Human Btr cells have been identified as an important source of IL-10-producing regulatory B cells; however, the regulatory activity of these cells is abolished in SLE43 and RA patients.44,45 The underlying mechanism for the loss of regulatory activity in Btr cells is not clear, although chronic stimulation by type I IFNs has been proposed.17 Interestingly, our results suggest an additional proinflammatory feature of Btr cells in SLE patients with a significant link to enhanced type I IFN activity. Serum and urinary IL-6 levels are increased in active SLE patients,46 and IL-6 is well known to promote plasma cells and T helper 17 differentiation.47 Several clinical trials aiming to block IL-648,49 or IL-6 receptor50 have shown positive effects. Thus, IL-6 has been suggested to play a pathogenic role in SLE. Whether the IL-6-producing Btr cells described in the current study contribute to auto-inflammation is an open question; however, given the close correlation between these cells and disease activity, IL-6-producing Btr cells may become a useful biomarker in SLE. The memory B-cell compartment of SLE patients painted a different picture, and TNF-α-producing isotype-switched memory B cells were significantly decreased in lupus patients. This result was most likely due to the “exhausted” memory B-cell expansion in SLE patients,24,51,52 an issue to be addressed in our future study.
In conclusion, we observed that type I IFNs critically contribute to the abnormalities of transitional B cells by promoting their survival and proinflammatory activities in active SLE patients. Our study provides new insight into the heterogeneous nature of the immune disorders in SLE. Given the promising as well as challenging results gathered from targeted therapies to type I IFN pathway,53 B cells6 and IL-6,48,49 our study further stresses the importance of personalized immunomonitoring and tailored immunotherapy in tackling SLE, a yet uncured disease.
Electronic supplementary material
Acknowledgements
This work was supported by the National Basic Research Program of China (No. 2014CB541904); the National Natural Science Foundation of China (Nos. 31470879 81571575 8171101311 and 31770960); the Interdisciplinary Innovation Team, External Cooperation Program (No. GJHZ201312) and Key Project QYZDB-SSW-SMC036; and the Strategic Priority Research Program (No. XDPB0303), Chinese Academy of Sciences.
Competing interests
The authors declare no competing interests.
Contributor Information
Qiong Fu, Email: fuqiong@renji.com.
Xiaoming Zhang, Email: xmzhang@ips.ac.cn.
Supplementary information
Supplementary information accompanies this paper at (10.1038/s41423-018-0010-6).
References
- 1.Kaul A, et al. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 2.Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res. Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duxbury B, Combescure C, Chizzolini C. Rituximab in systemic lupus erythematosus: an updated systematic review and meta-analysis. Lupus. 2013;22:1489–1503. doi: 10.1177/0961203313509295. [DOI] [PubMed] [Google Scholar]
- 4.Navarra SV, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 5.Furie R, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorner T, Lipsky PE. Beyond pan-B-cell-directed therapy - new avenues and insights into the pathogenesis of SLE. Nat. Rev. Rheumatol. 2016;12:645–657. doi: 10.1038/nrrheum.2016.158. [DOI] [PubMed] [Google Scholar]
- 7.Sanz I. Systemic lupus erythematosus: extent and patterns of off-label use of rituximab for SLE. Nat. Rev. Rheumatol. 2016;12:700–702. doi: 10.1038/nrrheum.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobi AM, et al. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2010;69:305–308. doi: 10.1136/ard.2008.096495. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Bayona B, Ramos-Amaya A, Perez-Venegas JJ, Rodriguez C, Brieva JA. Decreased frequency and activated phenotype of blood CD27 IgD IgM B lymphocytes is a permanent abnormality in systemic lupus erythematosus patients. Arthritis Res. Ther. 2010;12:R108. doi: 10.1186/ar3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao LD, et al. Contribution and underlying mechanisms of CXCR4 overexpression in patients with systemic lupus erythematosus. Cell. Mol. Immunol. 2017;14:842–849. doi: 10.1038/cmi.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappione A, 3rd, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tipton CM, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 2015;16:755–765. doi: 10.1038/ni.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lino AC, Dorner T, Bar-Or A, Fillatreau S. Cytokine-producing B cells: a translational view on their roles in human and mouse autoimmune diseases. Immunol. Rev. 2016;269:130–144. doi: 10.1111/imr.12374. [DOI] [PubMed] [Google Scholar]
- 14.Cho Kyung-Ah, Lee Jun-Kyu, Kim Yu-Hee, Park Minhwa, Woo So-Youn, Ryu Kyung-Ha. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cellular & Molecular Immunology. 2017;14(11):895–908. doi: 10.1038/cmi.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieber J, et al. Active systemic lupus erythematosus is associated with a reduced cytokine production by B cells in response to TLR9 stimulation. Arthritis Res. Ther. 2014;16:477. doi: 10.1186/s13075-014-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crow MK. Type I interferon in the pathogenesis of lupus. J. Immunol. 2014;192:5459–5468. doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon M, Blair PA, Isenberg DA, Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity. 2016;44:683–697. doi: 10.1016/j.immuni.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirou KA, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 19.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 20.Palanichamy A, et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J. Immunol. 2014;192:906–918. doi: 10.4049/jimmunol.1302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uccellini MB, et al. Autoreactive B cells discriminate CpG-rich and CpG-poor DNA and this response is modulated by IFN-alpha. J. Immunol. 2008;181:5875–5884. doi: 10.4049/jimmunol.181.9.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekeredjian-Ding IB, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 23.Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Role of type I interferons in the activation of autoreactive B cells. Immunol. Cell Biol. 2012;90:498–504. doi: 10.1038/icb.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wehr C, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin. Immunol. 2004;113:161–171. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Sims GP, et al. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J. Immunol. 2009;182:4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 27.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002;29:288–291. [PubMed] [Google Scholar]
- 28.Kong KO, et al. Enhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosus. Clin. Exp. Immunol. 2009;156:134–140. doi: 10.1111/j.1365-2249.2009.03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bentires-Alj M, et al. Inhibition of the NF-kappa B transcription factor increases Bax expression in cancer cell lines. Oncogene. 2001;20:2805–2813. doi: 10.1038/sj.onc.1204343. [DOI] [PubMed] [Google Scholar]
- 30.Baechler EC, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruuth K, Carlsson L, Hallberg B, Lundgren E. Interferon-alpha promotes survival of human primary B-lymphocytes via phosphatidylinositol 3-kinase. Biochem. Biophys. Res. Commun. 2001;284:583–586. doi: 10.1006/bbrc.2001.5025. [DOI] [PubMed] [Google Scholar]
- 32.Badr G, et al. Type I interferon (IFN-alpha/beta) rescues B-lymphocytes from apoptosis via PI3Kdelta/Akt, Rho-A, NFkappaB and Bcl-2/Bcl(XL) Cell. Immunol. 2010;263:31–40. doi: 10.1016/j.cellimm.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Chang NH, et al. Interferon-alpha induces altered transitional B cell signaling and function in systemic lupus erythematosus. J. Autoimmun. 2015;58:100–110. doi: 10.1016/j.jaut.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Yang CH, et al. IFNalpha/beta promotes cell survival by activating NF-kappa B. Proc. Natl. Acad. Sci. USA. 2000;97:13631–13636. doi: 10.1073/pnas.250477397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batten M, et al. BAFF mediates survival of peripheral immature B lymphocytes. J. Exp. Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R. BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J. Immunol. 2010;185:4570–4581. doi: 10.4049/jimmunol.1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodland RT, et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X. Regulatory functions of innate-like B cells. Cell. Mol. Immunol. 2013;10:113–121. doi: 10.1038/cmi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeo L, et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann. Rheum. Dis. 2011;70:2022–2028. doi: 10.1136/ard.2011.153312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adlowitz DG, et al. Expansion of activated peripheral blood memory B cells in rheumatoid arthritis, impact of B cell depletion therapy, and biomarkers of response. PLoS ONE. 2015;10:e0128269. doi: 10.1371/journal.pone.0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barr TA, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleischer V, et al. Epratuzumab inhibits the production of the proinflammatory cytokines IL-6 and TNF-alpha, but not the regulatory cytokine IL-10, by B cells from healthy donors and SLE patients. Arthritis Res. Ther. 2015;17:185. doi: 10.1186/s13075-015-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blair PA, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Flores-Borja F, et al. CD19+ CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 2013;5:173ra123. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 45.Daien CI, et al. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol. 2014;66:2037–2046. doi: 10.1002/art.38666. [DOI] [PubMed] [Google Scholar]
- 46.Peterson E, Robertson AD, Emlen W. Serum and urinary interleukin-6 in systemic lupus erythematosus. Lupus. 1996;5:571–575. doi: 10.1177/096120339600500603. [DOI] [PubMed] [Google Scholar]
- 47.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rovin BH, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 2016;68:2174–2183. doi: 10.1002/art.39722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace DJ, et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann. Rheum. Dis. 2017;76:534–542. doi: 10.1136/annrheumdis-2016-209668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Illei GG, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei C, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 52.Fleischer SJ, et al. Increased frequency of a unique spleen tyrosine kinase bright memory B cell population in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:3424–3435. doi: 10.1002/art.38854. [DOI] [PubMed] [Google Scholar]
- 53.Crow MK, Olferiev M, Kirou KA. Targeting of type I interferon in systemic autoimmune diseases. Transl. Res. J. Lab. Clin. Med. 2015;165:296–305. doi: 10.1016/j.trsl.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.