Natural killer (NK) cells are innate lymphocytes that specialize in discriminating and killing altered self-cells, such as malignant and virus-infected cells. However, during pregnancy, local uterine NK cells do not exhibit this killing capacity and display an important regulatory role by promoting trophoblast invasion and spiral artery remodeling and ensuring correct placentation.1 Over the past decade, it has become clear that NK cells possess adaptive properties and innate immune memory.2 In humans, memory NK cells have mainly been described as expansions of NKG2C+ and LILRB1+ peripheral blood NK cells (pbNK),3 and pbNK cell subpopulation expansions expressing self-specific killer-immunoglobulin-like receptors (KIR)4 following cytomegalovirus (CMV) infection.
It is known that pregnancy complications such as preeclampsia and intrauterine growth restriction affect subsequent pregnancies less frequently compared to first pregnancies.5,6 Pregnancy in repeated pregnancies is also enhanced in terms of more extensive and earlier trophoblast invasion, vascularization, and angiogenesis.7 Since 70% of first trimester lymphocytes in the placenta are NK cells and play such a crucial role in proper placentation, it is plausible that pregnancy could induce NK cell memory. The induction of such pregnancy trained NK cells could play a beneficial role in a more efficient placentation in future gestations.
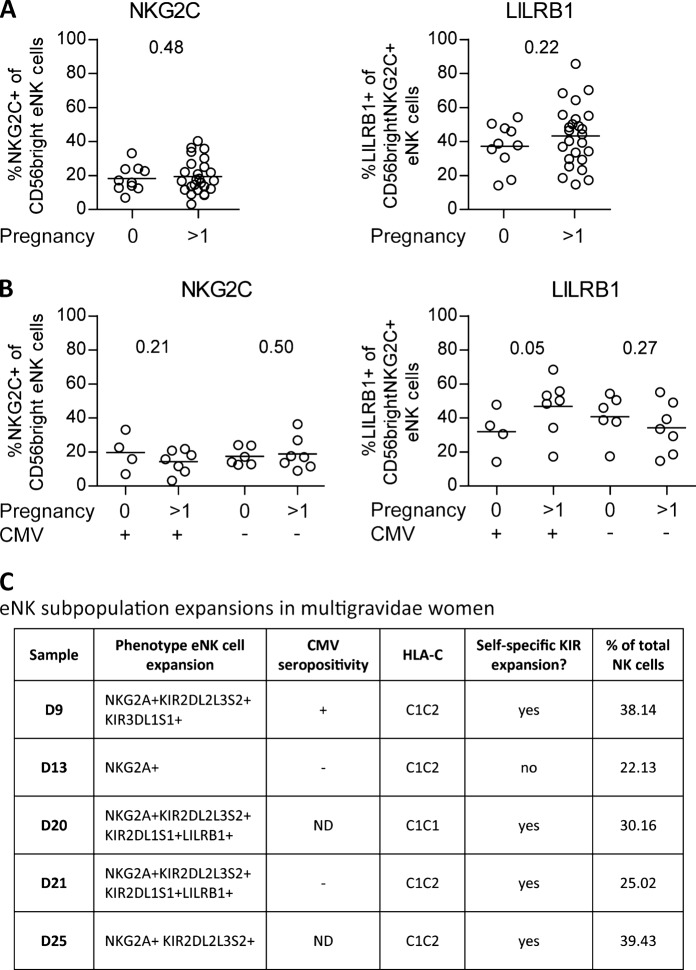
Recently, Gamliel et al.8 described the presence of pregnancy trained NK cells in the non-pregnant uterus, i.e. the endometrium, of women who have experienced pregnancy before. However, in this article, all samples were derived from women who were CMV positive (CMVpos). From studies reporting expansions of memory NK cells in response to viral infections such as hantavirus, HIV, and hepatitis C virus, it became clear that these expansions were in part driven by underlying CMV co-infection.9,10 This raises the question as to whether pregnancy trained NK cells also exist in the endometrium of CMV negative (CMVneg) women. To address this, we phenotyped NK cells from the non-pregnant endometrium of both nulli- (n = 10; women who have never been pregnant) and multigravidae (n = 25; women who have been pregnant before) women. We also determined the presence of anti-CMV IgG antibodies in the plasma (n = 11 CMVpos, n = 13 CMVneg, and n = 11 not determined; methods described in ref. 11). Interestingly, we did not see an increase in the percentage of NKG2C+ endometrial NK (eNK) cells and the percentage of LILRB1 expression on NKG2C+ eNK cells in multigravidae compared to nulligravidae women, irrespective of CMV status (Fig. 1a). However, when separating our cohort based on CMV status, we indeed observed a trend towards a higher percentage of LILRB1 expression on NKG2C+ eNK cells in the multigravidae samples of CMVpos women (Fig. 1b). This increase was not observed in the CMVneg multigravidae women. Additionally, no difference in the percentage of NKG2C+ eNK cells was observed when comparing nulli - and multigravidae CMVpos samples.
Fig. 1.
Pregnancy trained endometrial natural killer cells. a, b Percentage of NKG2C expression on CD56bright endometrial natural killer (eNK) cells and the percentage of LILRB1 expression on CD56brightNKG2C+ eNK cells. a Comparison of eNK cells from women with (>1) and without (0) previous pregnancies. b Comparison of eNK cells from CMVpos (CMV+) and CMVneg (CMV−) women, with (>1) and without (0) previous pregnancies. P-values determined with the Mann–Whitney one-tail test. c Characteristics of eNK subpopulation expansions in multigravidae samples. ND not determined
These results imply that the increase of pregnancy-induced LILRB1 expression on NKG2C+ eNK cells only occurs in CMVpos and not in CMVneg women, suggesting that CMV seropositivity might be a prerequisite for the induction of these pregnancy-induced memory eNK cells. Although CMV seropositivity itself does not lead to an induction of memory-like eNK cells, the possible molecular changes (epigenetic and transcriptional) induced by CMV on eNK cells could make them more receptive towards pregnancy-induced training.
In addition to the expansion of LILRB1-expressing NKG2C+ NK cells, it is also known that CMV infection can induce the expansion of pbNK cell subpopulations that express KIR receptors specific for self-HLA-C.4 We questioned whether pregnancy would induce such expansions of eNK cell subpopulations. We performed an outlier analysis on eNK cell subpopulations derived from all our nulli- and multigravidae women (methods described in ref. 11). We found that 5 out of 25 multigravidae endometrial samples (20%) show eNK cell subpopulation expansions, while none of the nulligravidae samples showed expansions (Fig. 1c). Notably, the majority of the expansions in the multigravidae samples express KIR receptors specific for the woman’s own HLA-C. Our outlier analysis data suggests that the expansions in multigravidae eNK cells were not restricted to CMV seropositivity since these expansions were present in both CMVpos and CMVneg donors (Fig. 1c; 1 CMVpos, 2 CMVneg, 2 not determined), suggesting that CMV might not be a prerequisite for pregnancy-induced eNK cell subpopulation expansions.
In conclusion, our data on eNK cells from CMVpos and CMVneg women suggests that priming by CMV infection might be a prerequisite for the presence of pregnancy-induced LILRB1-expressing NKG2C+ eNK cells in multigravidae women. However, our results on pregnancy-induced self-HLA-C specific eNK subpopulation expansions imply that pregnancy can also shape the eNK cell population regardless of CMV seropositivity. Further research is needed to determine how CMV primes the induction of pregnancy trained LILRB1-expressing NKG2C+ eNK cells and whether the observed expansions expressing self-specific KIR would alter NK cell function in a manner beneficial for future gestations.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J. Clin. Invest. 2014;124:1872–1879. doi: 10.1172/JCI68107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity. 2015;43:634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guma M, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 4.Beziat V, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozuki N, et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13(Suppl 3):S2. doi: 10.1186/1471-2458-13-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prefumo F, Ganapathy R, Thilaganathan B, Sebire NJ. Influence of parity on first trimester endovascular trophoblast invasion. Fertil. Steril. 2006;85:1032–1036. doi: 10.1016/j.fertnstert.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 8.Gamliel M, et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity. 2018;48:951–62 e5. doi: 10.1016/j.immuni.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Adams NM, et al. NK cell responses redefine immunological memory. J. Immunol. 2016;197:2963–2970. doi: 10.4049/jimmunol.1600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peppa D, et al. Adaptive reconfiguration of natural killer cells in HIV-1 infection. Front. Immunol. 2018;9:474. doi: 10.3389/fimmu.2018.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feyaerts D, et al. Endometrial natural killer (NK) cells reveal a tissue-specific receptor repertoire. Hum. Reprod. 2018;33:441–451. doi: 10.1093/humrep/dey001. [DOI] [PubMed] [Google Scholar]