Abstract
Vascularized composite allotransplants (VCAs) have unique properties because of diverse tissue components transplanted en mass as a single unit. In addition to surgery, this type of transplant also faces enormous immunological challenges that demand a detailed analysis of all aspects of alloimmune responses, organ preservation, and injury, as well as the immunogenicity of various tissues within the VCA grafts to further improve graft and patient outcomes. Moreover, the side effects of long-term immunosuppression for VCA patients need to be carefully balanced with the potential benefit of a non-life-saving procedure. In this review article, we provide a comprehensive update on limb and face transplantation, with a specific emphasis on the alloimmune responses to VCA, established and novel immunosuppressive treatments, and patient outcomes.
Subject terms: Allotransplantation, Peripheral tolerance, Target identification
Introduction
Vascularized composite allotransplants (VCAs) are grafts that are composed of multiple different tissues but are transplanted together as a single unit. A typical example is a hand graft, which consists of muscles, skin, bone, vessels, and nerves. VCAs have gained substantial clinical attention in recent years, especially for patients with injuries that are beyond repair by routine plastic surgery. Unlike solid organ transplants (e.g., liver and heart transplants), VCAs are considered a life-enhancing, rather than a life-saving, procedure, so they face a unique set of concerns and challenges in the field. To date, more than 100 upper extremities, approximately 40 faces, and, more recently, successful genito-uterine transplants have been performed worldwide.1,2 However, there are many issues in the field that deserve immediate attention. Single-center experiences in limb and face transplants are usually small, and the analysis of immune responses remains limited. Moreover, there are individual tissue components in hand and face transplants with strikingly different features and functions. Thus, alloimmune responses against VCA grafts, either acute or chronic, may have very different presentations, especially responses against individual tissue components (e.g., skin tissue versus the bone in the same graft). Additionally, the adaptation of numerous nerves in the VCA grafts and their functional reconstitution from transplant recipients may have an additional impact on graft outcomes. It appears necessary to standardize the analysis of immunologic features by integrating the functionality of different tissue components in VCA grafts. Importantly, a better understanding of the immune responses in VCA may help utilize immunosuppressive drugs in a more tailored fashion.
Immunological challenges of the skin
Skin is a major component of all VCA grafts, and unlike other tissue components, the skin is proven to be extremely immunogenic. In fact, acute rejections occur in ~80% of all face and upper limb transplants during the first year. Notably, the incidence of acute rejections in renal allografts is considerably lower, approximately 10% among kidney transplant patients.3–5 In both clinical and experimental models of VCA, a split tolerance phenomenon has been reported, in which rejection of the skin, but not other components such as muscle or bone, was observed.6 In contrast, neither experimental nor clinical experience has been able to confirm the rejection of muscle tissue in the absence of skin rejection,7 highlighting the specific and high immunogenic properties of the skin tissue in VCA grafts.8–10
It has been shown that a significantly higher number of T cells reside in the skin than in the peripheral circulation.11 In addition, there is a greater representation of T cells with an effector memory phenotype in the skin.11–13 Skin-resident memory T cells bear a diverse T-cell receptor repertoire and have a characteristic Th1 phenotype.11 During inflammation, memory T cells can recruit other key players of innate and adaptive immunity. Skin-resident T cells have the capacity to assume immune responses independent of chemotactic activity.13 Moreover, dermal dendritic cells (DCs) and other antigen-presenting cells (APCs) in the skin can present antigens to skin resident memory T cells directly,14,15 resulting in their activation and effector differentiation.16 This observation provides a novel view to the existing dogma that memory T cells need to be recruited from the circulation to the site of inflammation upon inflammatory stimuli.17 Characteristically, an abundance of CD8+ memory T cells in the skin of VCA have been detected.5,18 Previous studies have considered resident memory T cells the cause of graft versus host disease (GVHD), mainly due to their activation by infiltrating recipient APCs. Notably, GVHD has been detected in dog19 and pig20 models of VCA in combination stem cell transplants.
It should be noted that recipient T cells play a major role in skin allograft rejection. They are activated directly upon recognition of the allogeneic major histocompatibility complex (MHC) antigens presented by donor-derived APCs. Of additional relevance is the indirect allorecognition of processed donor antigens presented by the recipient’s APCs.21,22 In terms of T effector cells, memory Th9 T cells might play a special role in skin rejection, as those cells have been characterized as skin trophic with the capacity to produce tumor necrosis factor-alpha (TNF-α) and granzyme B.17,23 Furthermore, a subset of specialized dendritic epidermal T cells that reside sparsely in human skin represents the first subset of immune cells recruited from the blood into the skin upon immune activation.7,24
Properties of the skin itself seem to play a role in VCAs, thus contributing to increased rejection rates. Interestingly, the microvasculature within the skin has the capacity to induce immune responses.25,26 Endothelial cells of the skin have unique properties to recruit and activate immune cells through the upregulation of MHC class II molecules, inflammatory mediators, adhesion molecules, and vasoactive and costimulatory molecules.27–32 Notably, the width of capillaries within the skin is narrower than the diameter of T cells, leading to endothelial cell-T cell contact.25 This mechanism may cause proinflammatory responses after T-cell infiltration, even in the absence of allorecognition.25,33 Moreover, specific areas of the skin may differ in their capacity to mount immune responses. In an experimental model, it was shown that the infiltration of immune cells and cytokine expression were dependent on allograft properties, with hair-bearing areas showing augmented expression of interleukin (IL)-4, GRO-KC, and monocyte chemoattractant protein-1 in a multicytokine assay.34
The proportion of bone marrow as part of VCA grafts seems to ameliorate rejection processes, with experimental models showing an enhanced acceptance of hemifacial allografts containing vascularized bone marrow.35 In models of VCA flaps,36 however, increasing graft size in the absence of bone marrow has been linked to enhanced rejection. Skin-specific antigens, which are prevalent in the epidermis, may also play a role. Epa-1, a minor histocompatibility antigen, has been identified as a target of cytotoxic T cells37 that causes ulcerative skin lesions and nonspecific tissue damage when injected into Epa-1-positive mice.38
One interesting observation is that direct exposure of hand or face transplants to the environment may provoke rejections. Exposure to cold temperatures during winter months in one patient was linked to reoccurring, histologically confirmed rejections.39 Low humidity and cold temperatures may impair skin barrier function while increasing immune cell chemotaxis.40 A seasonable increase in circulating lymphocytes and immunoglobulins may be of additional relevance.41 (Fig. 1)
Fig. 1.
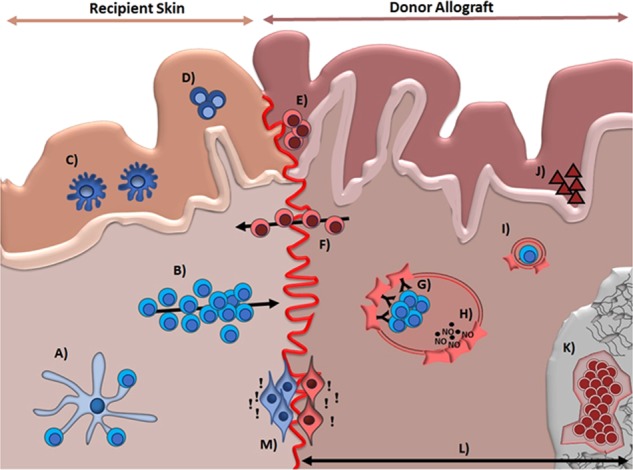
Unique immunological properties of the skin affecting the rejection process. a Skin-resident antigen-presenting cells can directly activate tissue resident memory T cells (TRM) without the need for lymph node homing. b Recipient allogeneic T cells migrating to the transplanted allograft are the main driver of graft rejection in vascularized composite allotransplant (VCA). c) Dendritic epidermal T cells are recruited early from the blood upon tissue damage. d Memory Th9 T cells are skin trophic and produce copious amounts of tumor necrosis factor-alpha and granzyme B. e Injury-induced inflammation occurs in VCA grafts. TRM invasion at the border between allograft and recipient tissues is shown along with high levels of interleukin-1b, interferon-gamma, transforming growth factor-beta, and CCL2-5. f TRM, especially CD8+ T cells, are abundant in the skin. Donor-derived passenger TRM can migrate to surrounding recipient tissue, causing graft versus host disease that may, in turn, contribute to the rejection process. g Endothelial cells can activate lymphocytes through the upregulation of human leukocyte antigen-II and adhesion molecules. h Endothelial cells secrete vasoactive molecules, including NO, bradykinin, and prostacyclin, affecting the inflammatory process. i The width of capillaries within the skin is narrower than the diameter of T cells, leading to contact with molecules upregulated by endothelial cells. j Skin-specific antigens such as Epa-1 and Skn-1 and 2 contribute to the augmented immunogenicity of the skin. k Bone marrow in VCA grafts may ameliorate/modulate rejections. l Size and mass of VCA grafts may interfere with alloimmune responses. m Skin alarmins, released by keratinocytes upon cell death, have chemoattractive abilities. Thus, both adaptive and innate immune cells are recruited to the site of tissue damage
VCAs: a complex interplay between innate and adaptive immunity
As in solid organ transplants, innate and adaptive immune responses are initiated during transplantation. In an ex vivo model of sterile skin injury, a sevenfold increase in inflammatory cytokines has been observed, followed by augmented recruitment of DCs and T cells, thus initiating the proliferation of CD4+ and CD8+ T cells.42 The skin hosts a large number of plasmacytoid DCs that have the capacity to promote IL-17A and IL-22 production, mainly through increased IL-6 secretion upon skin injury.43,44 Moreover, skin-specific DCs may present apoptotic cell-associated antigens that promote CD8+ T-cell cytotoxicity.45,46 In studies that dissected the role of DCs in injury-induced adaptive immune responses, it was shown that skin grafts from K5mOVA mice transgenic for OVA in the skin displayed accelerated rejection, with augmented activation of CD8+ T cells in draining lymph nodes.47 Notably, CD8+ T-cell responses in draining lymph nodes were significantly diminished in a CD8+ DC knockout mouse, supporting a key role for CD8+ DCs in the rejection process.48 Moreover, keratinocytes sorted using laser capture microdissection from human skin displayed an upregulation of CCL20 and IL-23A.49 CCL20 has chemotactic effects for CCR6-positive T cells50 and immature DCs, recruiting them from the periphery to sites of inflammation.51 Elevated levels of CCL20 have also been detected in the skin of psoriasis patients.52 IL-23A secreted by DCs and macrophages residing in the skin activates Th17 cells that play a critical role during allograft rejection.53–55 Comparing syngeneic and allogeneic VCAs,56 CD8+ T cells infiltrated allogeneic groin flaps and accumulated close to the recipient-graft border where tissue damage was most prominent. Both syngeneic and allogeneic skin grafts revealed distinct damage-related immune responses, as shown by the upregulation of IL-1b, interferon-gamma (IFN-γ), transforming growth factor-beta (TGF-β), and IL-10, while the expression of TNF-α, IL-18, and several other cytokines has been linked to the response to allogeneic skin grafts.56
Ischemia reperfusion injury, an inevitable part of the transplant process, causes the release of damage-associated molecular patterns (DAMPs), with the subsequent activation of toll-like receptors fueling allograft rejection.57,58 Skin alarmins, endogenous DAMPs that include IL-1a, IL-33, and several heat shock proteins, are released by keratinocytes and leukocytes subsequent to ischemia reperfusion injury and have chemoattractive abilities to recruit and activate leukocytes of the innate immune system.59–61 Insufficient lymphatic drainage after VCA is likely to further promote rejection processes, as impaired lymphatic drainage may activate DC and T-cell trafficking.7,62 Interestingly, when analyzing subcutaneous fat in a mouse model of reduced lymphatic flow, increased inflammation and fibrosis63 have been observed.
As in solid organ transplants, prolonged ischemic times have been shown to negatively impact VCA outcomes. In a mouse orthotopic hindlimb transplant model, for example, prolonged ischemic times have been associated with diminished graft survival.64 Notably, tolerable ischemic times have not been established for VCA grafts. Utilizing standard preservation methods, cold ischemic times up to 6 h have been cited as an upper threshold in VCAs.65,66 Ex vivo perfusion systems, including a hyperbaric, normothermic perfusion system, have recently been explored for VCAs and showed delayed acute rejection processes of VCA.67
Chronic rejection: a significant challenge in VCA recipients
Chronic rejection was thought to be a rare event in VCAs. Notably, observation times after VCAs are considerably shorter than those after solid organ transplants, and more recently, chronic rejections have also been reported in face and hand transplantation.68–71
In some patients, antibody-mediated processes72 and the formation of tertiary lymphoid organs73 in skin biopsies have been demonstrated. More recently, epidermal thinning and sclerosis have been linked to subclinical inflammation and rejection.74 At the molecular level, proteins of the AP-1 family are also considered to play a role in promoting chronic rejection through collagen accumulation, a process that has been observed for chronic rejection in both solid organ transplants75 and VCAs.69 Clinical histological grading for chronic rejection in VCA remains preliminary and does not include vessel vasculopathy, loss of capillaries, or rejection of the oral mucosa.65,76 An integrative approach involving histological findings and the underlying mechanisms may be helpful in characterizing the conditions.74 Recurrent and insufficiently treated episodes of acute rejection may also contribute to chronic rejection in both solid organ transplants77,78 and VCAs.79 This aspect appears relevant, since nearly 50% of all VCA patients undergo multiple rejection episodes.4 Moreover, the frequency of infections also seems to play a role in chronic VCA rejection.80,81
The premature aging appearance of grafts combined with telangiectasia and mottled leukoderma at suture lines, as well as a reduction of hair follicles, sweat glands, and nerve endings, has also been observed clinically in VCA recipients.71,74 Pathological mechanisms may include sclerotic induration, epidermal thinning, hyperkeratosis, and vessel wall alterations; these changes are often associated with fibrosis or luminal occlusion and collagen type 1 deposition.71,74 It is currently unclear whether the premature aging appearance of grafts represents an aspect of chronic rejection or an entirely different pathophysiology.
Immunosuppression: balancing risks and benefits
As a life-enhancing rather than a life-saving procedure, intense immunosuppression in VCA patients represents a delicate balance. Compliance in taking immunosuppressive drugs is as important in VCA as it is for solid organ transplants.82,83 Most VCA centers utilize immunosuppressive protocols for VCA patients based on experience with conventional immunosuppression used in solid organ transplant patients.10 Approaches include an induction treatment with a polyclonal antithymocyte antibody to deplete T cells and a maintenance triple immunosuppression protocol with tacrolimus, mycophenolate mofetil (MMF), and steroids.5,10 VCA patients often experience high rates of acute rejections, and rescue therapies include pulsed steroids.5 The side effects of immunosuppression in VCA patients are comparable to those in solid organ transplant patients. The increased risks for malignancies84 include Epstein-Barr virus (EBV)-related post-transplant B-cell lymphomas in facial transplant patients85 and squamous cell, lung, colon, and Non-Hodgkin Lymphoma cancers,86,87 in addition to opportunistic infections, such as cytomegalovirus (CMV), EBV, herpes simplex virus, and Pneumocystis jirovecii.81 CMV infections occur most frequently by months 2–6, with viremia arising in nearly every seropositive donor into a seronegative recipient constellation.88 In some cases, CMV infection has been linked to acute graft rejections.80
Bacterial infections have been frequently observed in VCA recipients.89,90 Since the environmentally exposed donor skin is transferred with VCAs, its potentially pathogenic flora consisting of Gram-negative organisms, Staphylococcus aureus, fungi, and anaerobes may play a role. The mucosal tissue of facial VCAs exposes the recipient to donor-derived pathogens, including streptococci, Candida species, and anaerobes.91 Thus, nasal cultures from VCA recipients displaying methicillin-sensitive Staphylococcus aureus, Pseudomonas aeruginosa, and Pseudomonas pneumoniae have been reported.88 Fungal infections at surgical sites have been shown once recipients continue with daily activities exposing them to the environmental flora.91 However, the incidence of invasive candida infections has been low.92 Opportunistic infections specifically associated with face transplantation include superinfected sialocele and parotitis due to remaining donor salivary gland tissue, which can be successfully treated with botulinum toxin injections.93 Clearly, VCAs as non-life-saving procedures require balanced and effective immunosuppression.
Several groups have tested minimization protocols after VCA. Relying on the benefits of steroid-free immunosuppression in solid organ transplants, dual immunosuppression with tacrolimus and mycophenolate after alemtuzumab induction has been tested. This approach, however, has been associated with frequent acute rejection episodes.94 In another clinical series, dual immunosuppression with tacrolimus and MMF was successful when tacrolimus trough levels had been maintained at >5 ng/ml. Nephrotoxic side effects have been more prominent in VCA recipients subsequent to steroid withdrawal.95,96 Moreover, steroid-free maintenance immunosuppression in bilateral arm transplant recipients had been linked to intimal hyperplasia, suggesting that underimmunosuppression may contribute to the development of vasculopathy.94,97
Costimulatory blockade may be a promising addition to maintenance immunosuppression, with potential effects on donor-specific antibodies98 while sparing nephrotoxic side effects.99 While beneficial in some VCA recipients, others developed acute rejections under maintenance immunosuppression with belatacept and tacrolimus monotherapy.100 CD57+ memory T cells lacking CD28 made up a significantly higher proportion in rejecting recipients, suggesting that screening patients for this T-cell subpopulation may be helpful prior to belatacept treatment.100
Topical application of immunosuppression drugs with reduced systemic side effects has been applied successfully in face and upper limb transplantation.101 Lower-grade rejections (Banff grades 1–2) have been successfully treated with topical tacrolimus and clobetasol.102 Interestingly, preclinical studies have shown superior effects of topical compared to systemic immunosuppression in some cases.101 A dichotomous response upon topical tacrolimus treatment has been observed in rats receiving hindlimb transplants; half of the animals rejected the graft after 70 days, similar to untreated controls, while the other half did not show any signs of graft rejection 200 days post transplantation and had significantly lower pathological injury once assessed.101 Topical high-dose application has not been linked to augmented systemic side effects,101 encouraging VCA-specific immunosuppression.
Most recently, a formulation for topical MMF based on the ester prodrug mycophenolic acid was developed, currently allowing the simultaneous topical application of MMF, Tac, and steroids.103 Acute rejections have been treated in most cases with steroid bolus administration; topical immunosuppression and an augmentation of maintenance immunosuppression have also been successful;104 and rare steroid-resistant rejections require polyclonal antibodies (anti-thymocyte globulin).
Tolerance induction protocols: opportunities in VCA
As VCAs are not life-saving transplants and current immunosuppression protocols are lifelong and often produce debilitating side effects in transplant patients, the advantage of immune tolerance in VCA patients is obvious. There are several ongoing tolerance-inducing trials that may benefit VCA patients. Regulatory T cells (Tregs) may represent an opportunity for VCAs.105,106 Through the perforin-dependent lysis of effector T cells, the secretion of immunosuppressive cytokines, including IL-10, IL-35, and TGF-β, and the deprivation of IL-2 through the self-expression of high-affinity IL-2 receptors, Tregs may ameliorate alloimmune responses by inhibiting T effector cells.107 Tregs specific for donor antigens generated through priming with DCs derived from donor skin108 represent promising candidates for alloantigen-specific immunosuppression.
Augmenting autologous Tregs in vivo may be an alternative approach. Experimentally, injections of the IL-2/anti-IL-2 complex increased murine Treg numbers 10-fold,109 leading to prolonged orthotopic forelimb allograft survival, especially when combined with rapamycin.110
Injecting hIL-2/Fc fusion protein, a long-lasting form of IL-2, not only augmented the number of Tregs but also improved suppressive capacities on effector T cells specific for donor antigens in coculture experiments. Notably, responsiveness toward third-party antigens remained intact, indicating a functional immune response.111 Subsequently, measuring FoxP3, GymB, IFN-γ, and Prf1 allowed a prediction of rejection episodes under hIL-2/Fc, ALS, and CsA treatment, enabling the reduction of immunosuppression.111
Injections of donor-derived allogeneic mesenchymal stromal cells after irradiation therapy causing a state of chimerism in recipients have been shown to improve allograft survival of a pig hindlimb.112 In a pig hemifacial model, prolonged allograft survival upon repetitive high-dose application of bone marrow-derived mesenchymal stem cells (MSCs) has been reported.113 Immunosuppression has been reduced to tacrolimus monotherapy in a hand transplant model infusing donor bone marrow after lymphoid depletion.114 MSC infusion may also impact neural regeneration, with improved clinical and electrophysiological outcomes.115–117 Drug interferences between MSCs and immunosuppressants are also relevant,118 as rapamycin and tacrolimus antagonize some of the immunomodulatory effects of MSCs.119
The induction of chimerism in transplant recipients based on T-cell depletion and full body irradiation combined with hematopoietic cell transplantation has shown promising results in a VCA animal model.120 GVHD induced by donor bone marrow infusion, although a theoretical complication,17 has thus far not been reported.121,122
Conclusions
VCAs represent a unique procedure that has unexpected clinical needs; VCAs are life-enhancing for those with irreparable injuries but also come with enormous challenges. Although the current immunosuppression protocols in VCAs are effective in suppressing acute rejection, they produce significant side effects in transplant patients, and the drug-induced toxicity profiles are comparable to those in solid organ transplants. In some patients, however, minimization of immunosuppression protocols has been successfully applied, but the long-term outcomes of those patients remain unclear and require careful follow-ups.
As in other transplants, chronic graft vasculopathy also occurs in VCA patients, and the mechanisms remain to be defined. Another intriguing aspect of VCA patients is the observation of the accelerated aging of VCAs. This phenomenon may reflect aspects of chronic vasculopathy or a yet defined process of “true” accelerated aging. Modern approaches for immune tolerance have been tested in experimental and clinical approaches. While some VCAs may have unique immunological properties with the concomitant transplantation of bone structures, where bone marrow cells may have protolerant features, the field overall is hampered by small numbers of transplants and a lack of clinical consortia (Fig. 2). Clearly, VCAs offer great opportunities in further dissecting the alloimmune responses that not only may facilitate improved treatments of transplant patients but may also help understand aspects of dermatological diseases.
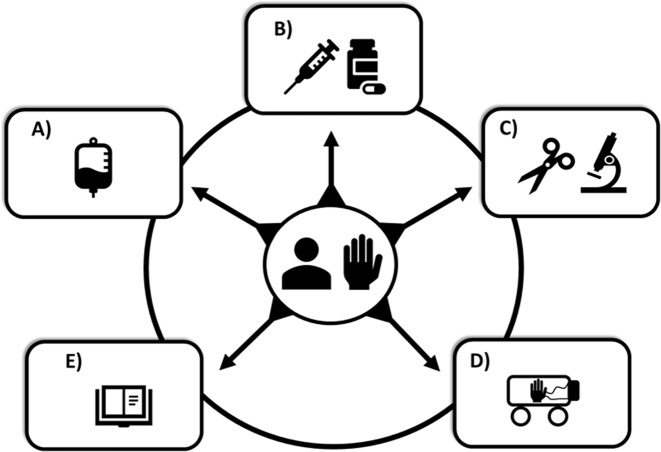
Fig. 2.
Novel approaches in improving vascularized composite allotransplant outcomes. a Tolerance protocols involving regulatory T cells or mesenchymal stromal cells. b Immunosuppression minimization protocols, including topical intragraft application of immunosuppressive drugs (e.g., tacrolimus/mycophenolate mofetil). c Minimally invasive microsurgical techniques. d Novel preservation approaches involving ex vivo hypo- or norm preservation. e Refined rejection criteria and guidelines to assess outcomes
Acknowledgements
Dr. Stefan G. Tullius currently serves as an Einstein-BIH visiting fellow. This study was supported by the Einstein-BIH Visiting Fellow Program (to S.G.T., M.M. and I.M.S.) and the Biomedical Education Program (BMEP) of the German Academic Exchange Service (DAAD to J.I.).
Competing interests
The authors declare no competing interests.
References
- 1.Shores JT, Brandacher G, Lee WP. Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast. Reconstr. Surg. 2015;135:351e–360e. doi: 10.1097/PRS.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 2.Brannstrom M. Womb transplants with live births: an update and the future. Expert. Opin. Biol. Ther. 2017;17:1105–1112. doi: 10.1080/14712598.2017.1347633. [DOI] [PubMed] [Google Scholar]
- 3.Sinha I, Pomahac B. Split rejection in vascularized composite allotransplantation. Eplasty. 2013;13:e53. [PMC free article] [PubMed] [Google Scholar]
- 4.Petruzzo, P. & Dubernard, J. M. The International Registry on Hand and Composite Tissue allotransplantation. Clin. Transpl. 247–253 (2011) PMID: 22755418. [PubMed]
- 5.Fischer S, et al. Acute rejection in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2014;19:531–544. doi: 10.1097/MOT.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 6.Mathes DW, et al. Split tolerance to a composite tissue allograft in a swine model. Transplantation. 2003;75:25–31. doi: 10.1097/00007890-200301150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman CL, et al. Immunobiology in VCA. Transpl. Int. 2016;29:644–654. doi: 10.1111/tri.12764. [DOI] [PubMed] [Google Scholar]
- 8.Kanitakis J. The challenge of dermatopathological diagnosis of composite tissue allograft rejection: a review. J. Cutan. Pathol. 2008;35:738–744. doi: 10.1111/j.1600-0560.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 9.Kueckelhaus M, et al. Utility of sentinel flaps in assessing facial allograft rejection. Plast. Reconstr. Surg. 2015;135:250–258. doi: 10.1097/PRS.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 10.Kueckelhaus M, et al. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl. Int. 2016;29:655–662. doi: 10.1111/tri.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark RA, et al. The vast majority of CLA + T cells are resident in normal skin. J. Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 12.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J. Invest. Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Olshansky M, Carbone FR, Ma JZ. Transcriptional analysis of T cells resident in human skin. PLoS ONE. 2016;11:e0148351. doi: 10.1371/journal.pone.0148351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark RA, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci. Transl. Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, et al. Skin infection generates non-migratory memory CD8 + T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egawa G, Kabashima K. Skin as a peripheral lymphoid organ: revisiting the concept of skin-associated lymphoid tissues. J. Invest. Dermatol. 2011;131:2178–2185. doi: 10.1038/jid.2011.198. [DOI] [PubMed] [Google Scholar]
- 17.Chadha R, Leonard DA, Kurtz JM, Cetrulo CL., Jr The unique immunobiology of the skin: implications for tolerance of vascularized composite allografts. Curr. Opin. Organ Transplant. 2014;19:566–572. doi: 10.1097/MOT.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 18.Lian CG, et al. Biomarker evaluation of face transplant rejection: association of donor T cells with target cell injury. Mod. Pathol. 2014;27:788–799. doi: 10.1038/modpathol.2013.249. [DOI] [PubMed] [Google Scholar]
- 19.Mathes DW, et al. Tolerance to vascularized composite allografts in canine mixed hematopoietic chimeras. Transplantation. 2011;92:1301–1308. doi: 10.1097/TP.0b013e318237d6d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hettiaratchy S, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004;77:514–521. doi: 10.1097/01.tp.0000113806.52063.42. [DOI] [PubMed] [Google Scholar]
- 21.Bhan AK, Mihm MC, Jr., Dvorak HF. T cell subsets in allograft rejection. In situ characterization of T cell subsets in human skin allografts by the use of monoclonal antibodies. J. Immunol. 1982;129:1578–1583. [PubMed] [Google Scholar]
- 22.Sarhane KA, et al. Diagnosing skin rejection in vascularized composite allotransplantation: advances and challenges. Clin. Transplant. 2014;28:277–285. doi: 10.1111/ctr.12316. [DOI] [PubMed] [Google Scholar]
- 23.Schlapbach C, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci. Transl. Med. 2014;6:219ra218. doi: 10.1126/scitranslmed.3007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laggner U, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J. Immunol. 2011;187:2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issa F. Vascularized composite allograft-specific characteristics of immune responses. Transpl. Int. 2016;29:672–681. doi: 10.1111/tri.12765. [DOI] [PubMed] [Google Scholar]
- 26.Taflin C, Charron D, Glotz D, Mooney N. Immunological function of the endothelial cell within the setting of organ transplantation. Immunol. Lett. 2011;139:1–6. doi: 10.1016/j.imlet.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 28.Pober JS, Kluger MS, Schechner JS. Human endothelial cell presentation of antigen and the homing of memory/effector T cells to skin. Ann. N. Y. Acad. Sci. 2001;941:12–25. doi: 10.1111/j.1749-6632.2001.tb03706.x. [DOI] [PubMed] [Google Scholar]
- 29.Karmann K, Hughes CC, Fanslow WC, Pober JS. Endothelial cells augment the expression of CD40 ligand on newly activated human CD4 + T cells through a CD2/LFA-3 signaling pathway. Eur. J. Immunol. 1996;26:610–617. doi: 10.1002/eji.1830260316. [DOI] [PubMed] [Google Scholar]
- 30.Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc. Natl Acad. Sci. USA. 1995;92:4342–4346. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol. Rev. 1990;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 32.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu. Rev. Immunol. 1992;10:333–358. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- 34.Hautz T, et al. The impact of skin type and area on skin rejection in limb transplantation. VCA. 2014;1:42–49. [Google Scholar]
- 35.Barth RN, et al. Vascularized bone marrow-based immunosuppression inhibits rejection of vascularized composite allografts in nonhuman primates. Am. J. Transplant. 2011;11:1407–1416. doi: 10.1111/j.1600-6143.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez AE, et al. A novel rat full-thickness hemi-abdominal wall/hindlimb osteomyocutaneous combined flap: influence of allograft mass and vascularized bone marrow content on vascularized composite allograft survival. Transpl. Int. 2014;27:977–986. doi: 10.1111/tri.12364. [DOI] [PubMed] [Google Scholar]
- 37.Snider ME, Armstrong L, Hudson JL, Steinmuller D. In vitro and in vivo cytotoxicity of T cells cloned from rejecting allografts. Transplantation. 1986;42:171–177. doi: 10.1097/00007890-198608000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Noble RL, Steinmuller D. Blocking of interleukin-2 production, but not the tissue destruction induced by cytotoxic T cells, by cyclosporine. Transplantation. 1989;47:322–326. doi: 10.1097/00007890-198902000-00027. [DOI] [PubMed] [Google Scholar]
- 39.Lopdrup RG, et al. Seasonal variability precipitating hand transplant rejection? Transplantation. 2017;101:e313. doi: 10.1097/TP.0000000000001877. [DOI] [PubMed] [Google Scholar]
- 40.Engebretsen KA, Johansen JD, Kezic S, Linneberg A, Thyssen JP. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016;30:223–249. doi: 10.1111/jdv.13301. [DOI] [PubMed] [Google Scholar]
- 41.MacMurray JP, Barker JP, Armstrong JD, Bozzetti LP, Kuhn IN. Circannual changes in immune function. Life Sci. 1983;32:2363–2370. doi: 10.1016/0024-3205(83)90767-1. [DOI] [PubMed] [Google Scholar]
- 42.Valvis SM, Waithman J, Wood FM, Fear MW, Fear VS. The immune response to skin trauma is dependent on the etiology of injury in a mouse model of burn and excision. J. Invest. Dermatol. 2015;135:2119–2128. doi: 10.1038/jid.2015.123. [DOI] [PubMed] [Google Scholar]
- 43.Gregorio J, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J. Exp. Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guiducci C, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desch AN, et al. CD103 + pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J. Exp. Med. 2011;208:1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Q, et al. CD103 + dendritic cells elicit CD8 + T cell responses to accelerate kidney injury in adriamycin nephropathy. J. Am. Soc. Nephrol. 2016;27:1344–1360. doi: 10.1681/ASN.2015030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azukizawa H, et al. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur. J. Immunol. 2003;33:1879–1888. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty R, et al. CD8( + ) lineage dendritic cells determine adaptive immune responses to inflammasome activation upon sterile skin injury. Exp. Dermatol. 2018;27:71–79. doi: 10.1111/exd.13436. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy-Crispin M, et al. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J. Invest. Dermatol. 2012;132:105–113. doi: 10.1038/jid.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paradis TJ, Cole SH, Nelson RT, Gladue RP. Essential role of CCR6 in directing activated T cells to the skin during contact hypersensitivity. J. Invest. Dermatol. 2008;128:628–633. doi: 10.1038/sj.jid.5701055. [DOI] [PubMed] [Google Scholar]
- 51.Le Borgne M, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8 + T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Zaba LC, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 54.Oberhuber R, et al. CD11c + dendritic cells accelerate the rejection of older cardiac transplants via interleukin-17A. Circulation. 2015;132:122–131. doi: 10.1161/CIRCULATIONAHA.114.014917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee E, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedman O, et al. Immunological and inflammatory mapping of vascularized composite allograft rejection processes in a rat model. PLoS ONE. 2017;12:e0181507. doi: 10.1371/journal.pone.0181507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alegre ML, Goldstein DR, Chong AS. Toll-like receptor signaling in transplantation. Curr. Opin. Organ Transplant. 2008;13:358–365. doi: 10.1097/MOT.0b013e3283061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J. Clin. Invest. 2003;111:1571–1578. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 60.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv. Exp. Med. Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 61.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 2012;33:271–280. doi: 10.1016/j.it.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Zampell JC, et al. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast. Reconstr. Surg. 2012;129:825–834. doi: 10.1097/PRS.0b013e3182450b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Datta N, Devaney SG, Busuttil RW, Azari K, Kupiec-Weglinski JW. Prolonged cold ischemia time results in local and remote organ dysfunction in a murine model of vascularized composite transplantation. Am. J. Transplant. 2017;17:2572–2579. doi: 10.1111/ajt.14290. [DOI] [PubMed] [Google Scholar]
- 65.Tasigiorgos S, et al. Face transplantation-current status and future developments. Transpl. Int. 2018;31:677–688. doi: 10.1111/tri.13130. [DOI] [PubMed] [Google Scholar]
- 66.Landin L, et al. Outcomes with respect to disabilities of the upper limb after hand allograft transplantation: a systematic review. Transpl. Int. 2012;25:424–432. doi: 10.1111/j.1432-2277.2012.01433.x. [DOI] [PubMed] [Google Scholar]
- 67.Fries CA, et al. A hyperbaric warm perfusion system preserves tissue composites ex vivo and delays the onset of acute rejection. J. Reconstr. Microsurg. 2018;35:97–107. doi: 10.1055/s-0038-1667298. [DOI] [PubMed] [Google Scholar]
- 68.Morris P, et al. Face transplantation: a review of the technical, immunological, psychological and clinical issues with recommendations for good practice. Transplantation. 2007;83:109–128. doi: 10.1097/01.tp.0000254201.89012.ae. [DOI] [PubMed] [Google Scholar]
- 69.Petruzzo P, et al. Clinicopathological findings of chronic rejection in a face grafted patient. Transplantation. 2015;99:2644–2650. doi: 10.1097/TP.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 70.Morelon E, et al. Face transplantation: partial graft loss of the first case 10 years later. Am. J. Transplant. 2017;17:1935–1940. doi: 10.1111/ajt.14218. [DOI] [PubMed] [Google Scholar]
- 71.Kanitakis J, et al. Chronic rejection in human vascularized composite allotransplantation (hand and face recipients): an update. Transplantation. 2016;100:2053–2061. doi: 10.1097/TP.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 72.Weissenbacher A, et al. Antibody-mediated rejection in hand transplantation. Transpl. Int. 2014;27:e13–e17. doi: 10.1111/tri.12233. [DOI] [PubMed] [Google Scholar]
- 73.Hautz T, et al. Lymphoid neogenesis in skin of human hand, nonhuman primate, and rat vascularized composite allografts. Transpl. Int. 2014;27:966–976. doi: 10.1111/tri.12358. [DOI] [PubMed] [Google Scholar]
- 74.Krezdorn, N. et al. Chronic rejection of human face allografts. Am. J. Transplant. 18, 1–10 (2018). [DOI] [PMC free article] [PubMed]
- 75.Bakker RC, et al. Early interstitial accumulation of collagen type I discriminates chronic rejection from chronic cyclosporine nephrotoxicity. J. Am. Soc. Nephrol. 2003;14:2142–2149. doi: 10.1097/01.asn.0000077345.81206.00. [DOI] [PubMed] [Google Scholar]
- 76.Cendales LC, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am. J. Transplant. 2008;8:1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- 77.Lindholm A, et al. The impact of acute rejection episodes on long-term graft function and outcome in 1347 primary renal transplants treated by 3 cyclosporine regimens. Transplantation. 1993;56:307–315. doi: 10.1097/00007890-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 78.Matas AJ, Gillingham KJ, Payne WD, Najarian JS. The impact of an acute rejection episode on long-term renal allograft survival (t1/2) Transplantation. 1994;57:857–859. doi: 10.1097/00007890-199403270-00015. [DOI] [PubMed] [Google Scholar]
- 79.Unadkat JV, et al. Composite tissue vasculopathy and degeneration following multiple episodes of acute rejection in reconstructive transplantation. Am. J. Transplant. 2010;10:251–261. doi: 10.1111/j.1600-6143.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- 80.Schneeberger S, et al. Cytomegalovirus-related complications in human hand transplantation. Transplantation. 2005;80:441–447. doi: 10.1097/01.tp.0000168454.68139.0a. [DOI] [PubMed] [Google Scholar]
- 81.Barker JH, et al. Investigation of risk acceptance in facial transplantation. Plast. Reconstr. Surg. 2006;118:663–670. doi: 10.1097/01.prs.0000233202.98336.8c. [DOI] [PubMed] [Google Scholar]
- 82.Lopez MM, et al. Long-term problems related to immunosuppression. Transpl. Immunol. 2006;17:31–35. doi: 10.1016/j.trim.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 83.Pomahac, B., Gobble, R. M. & Schneeberger, S. Facial and hand allotransplantation. Cold Spring Harb. Perspect. Med. 4 (2014). [DOI] [PMC free article] [PubMed]
- 84.Brenner MJ, Tung TH, Jensen JN, Mackinnon SE. The spectrum of complications of immunosuppression: is the time right for hand transplantation? J. Bone Joint Surg. Am. 2002;84-a:1861–1870. [PubMed] [Google Scholar]
- 85.Conrad A, et al. Epstein-Barr virus-associated smooth muscle tumors in a composite tissue allograft and a pediatric liver transplant recipient. Transpl. Infect. Dis. 2013;15:E182–E186. doi: 10.1111/tid.12126. [DOI] [PubMed] [Google Scholar]
- 86.Madani H, Hettiaratchy S, Clarke A, Butler PE. Immunosuppression in an emerging field of plastic reconstructive surgery: composite tissue allotransplantation. J. Plast. Reconstr. Aesthet. Surg. 2008;61:245–249. doi: 10.1016/j.bjps.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 87.Siemionow M, Ozturk C. Face transplantation: outcomes, concerns, controversies, and future directions. J. Craniofac. Surg. 2012;23:254–259. doi: 10.1097/SCS.0b013e318241b920. [DOI] [PubMed] [Google Scholar]
- 88.Knoll BM, et al. Infections following facial composite tissue allotransplantation–single center experience and review of the literature. Am. J. Transplant. 2013;13:770–779. doi: 10.1111/ajt.12013. [DOI] [PubMed] [Google Scholar]
- 89.Gordon CR, Avery RK, Abouhassan W, Siemionow M. Cytomegalovirus and other infectious issues related to face transplantation: specific considerations, lessons learned, and future recommendations. Plast. Reconstr. Surg. 2011;127:1515–1523. doi: 10.1097/PRS.0b013e318208d03c. [DOI] [PubMed] [Google Scholar]
- 90.Hammond SP. Infections in composite tissue allograft recipients. Infect. Dis. Clin. North Am. 2013;27:379–393. doi: 10.1016/j.idc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Broyles JM, et al. Characterization, prophylaxis, and treatment of infectious complications in craniomaxillofacial and upper extremity allotransplantation: a multicenter perspective. Plast. Reconstr. Surg. 2014;133:543e–551e. doi: 10.1097/PRS.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 92.Avery RK. Update on infections in composite tissue allotransplantation. Curr. Opin. Organ Transplant. 2013;18:659–664. doi: 10.1097/MOT.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 93.Barret JP, et al. Full face transplant: the first case report. Ann. Surg. 2011;254:252–256. doi: 10.1097/SLA.0b013e318226a607. [DOI] [PubMed] [Google Scholar]
- 94.Cavadas PC, Ibanez J, Thione A, Alfaro L. Bilateral trans-humeral arm transplantation: result at 2 years. Am. J. Transplant. 2011;11:1085–1090. doi: 10.1111/j.1600-6143.2011.03503.x. [DOI] [PubMed] [Google Scholar]
- 95.Hricik DE, et al. Long-term graft outcomes after steroid withdrawal in African American kidney transplant recipients receiving sirolimus and tacrolimus. Transplantation. 2007;83:277–281. doi: 10.1097/01.tp.0000251652.42434.57. [DOI] [PubMed] [Google Scholar]
- 96.Augustine JJ, Hricik DE. Are maintenance corticosteroids no longer necessary after kidney transplantation? Clin. J. Am. Soc. Nephrol. 2012;7:383–384. doi: 10.2215/CJN.01020112. [DOI] [PubMed] [Google Scholar]
- 97.Kaufman CL, et al. Graft vasculopathy in clinical hand transplantation. Am. J. Transplant. 2012;12:1004–1016. doi: 10.1111/j.1600-6143.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 98.Kim EJ, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am. J. Transplant. 2014;14:59–69. doi: 10.1111/ajt.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vincenti F, et al. Belatacept and long-term outcomes in kidney transplantation. N. Engl. J. Med. 2016;374:333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 100.Grahammer J, et al. Benefits and limitations of belatacept in 4 hand-transplanted patients. Am. J. Transplant. 2017;17:3228–3235. doi: 10.1111/ajt.14440. [DOI] [PubMed] [Google Scholar]
- 101.Olariu R, et al. Intra-graft injection of tacrolimus promotes survival of vascularized composite allotransplantation. J. Surg. Res. 2017;218:49–57. doi: 10.1016/j.jss.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 102.Ravindra KV, et al. Hand transplantation in the United States: experience with 3 patients. Surgery. 2008;144:638–643. doi: 10.1016/j.surg.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 103.Feturi FG, et al. Mycophenolic acid for topical immunosuppression in vascularized composite allotransplantation: optimizing formulation and preliminary evaluation of bioavailability and pharmacokinetics. Front. Surg. 2018;5:20. doi: 10.3389/fsurg.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diaz-Siso JR, et al. Initial experience of dual maintenance immunosuppression with steroid withdrawal in vascular composite tissue allotransplantation. Am. J. Transplant. 2015;15:1421–1431. doi: 10.1111/ajt.13103. [DOI] [PubMed] [Google Scholar]
- 105.Sakaguchi S. Naturally arising CD4 + regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 106.Issa F, Wood KJ. The potential role for regulatory T-cell therapy in vascularized composite allograft transplantation. Curr. Opin. Organ Transplant. 2014;19:558–565. doi: 10.1097/MOT.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 107.Yang JH, Eun SC. Therapeutic application of T regulatory cells in composite tissue allotransplantation. J. Transl. Med. 2017;15:218. doi: 10.1186/s12967-017-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sagoo P, et al. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Webster KE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu H, et al. Utility of IL-2 complexes in promoting the survival of murine orthotopic forelimb vascularized composite allografts. Transplantation. 2018;102:70–78. doi: 10.1097/TP.0000000000001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jindal R, et al. Spontaneous resolution of acute rejection and tolerance induction with IL-2 fusion protein in vascularized composite allotransplantation. Am. J. Transplant. 2015;15:1231–1240. doi: 10.1111/ajt.13118. [DOI] [PubMed] [Google Scholar]
- 112.Kuo YR, et al. Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T-cell regulation in a swine hind-limb model. Plast. Reconstr. Surg. 2011;127:569–579. doi: 10.1097/PRS.0b013e318200a92c. [DOI] [PubMed] [Google Scholar]
- 113.Kuo YR, et al. Immunomodulatory effects of bone marrow-derived mesenchymal stem cells in a swine hemi-facial allotransplantation model. PLoS ONE. 2012;7:e35459. doi: 10.1371/journal.pone.0035459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee WP. Hand transplantation: evolution of a personal outlook. J. Hand Surg. Am. 2017;42:286–290. doi: 10.1016/j.jhsa.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 115.Carriel V, et al. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J. Neural Eng. 2013;10:026022. doi: 10.1088/1741-2560/10/2/026022. [DOI] [PubMed] [Google Scholar]
- 116.Lopatina T, et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE. 2011;6:e17899. doi: 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu G, et al. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int. J. Mol. Med. 2011;28:565–572. doi: 10.3892/ijmm.2011.725. [DOI] [PubMed] [Google Scholar]
- 118.Plock JA, Schnider JT, Solari MG, Zheng XX, Gorantla VS. Perspectives on the use of mesenchymal stem cells in vascularized composite allotransplantation. Front. Immunol. 2013;4:175. doi: 10.3389/fimmu.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Buron F, et al. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: an in vitro study using human cells. Transplant. Proc. 2009;41:3347–3352. doi: 10.1016/j.transproceed.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 120.Leonard DA, et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. Am. J. Transplant. 2014;14:343–355. doi: 10.1111/ajt.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2013;368:1850–1852. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scandling JD, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am. J. Transplant. 2012;12:1133–1145. doi: 10.1111/j.1600-6143.2012.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]