Abstract
Purpose
To evaluate pre-operative and post-operative morphologic characteristics in idiopathic macular epiretinal membrane (ERM) by optical coherence tomography angiography (OCTA).
Methods
Thirty-three subjects with unilateral idiopathic ERM were enrolled and the contralateral eyes served as controls. Vascular parameters including superficial capillary plexus (SCP), deep capillary plexus (DCP), outer capillary plexus (OCP), and choroidal capillary plexus (CCP) were evaluated by OCTA.
Results
The superficial foveal avascular zone (FAZ) was significantly smaller in eyes with ERM (P < 0.0001). The vessel densities (VDs) were significantly increased in the fovea but dramatically decreased in the parafovea in SCP and DCP of ERM eyes (all P < 0.0001), in contrast to those in OCP and CCP. The blood flow was augmented in OCP but declined in choroid compared with the controls. In CCP, the mean foveal VD in ERM was significantly smaller (P = 0.023), whereas parafoveal VD did not significantly change (P = 0.66). At 6 months after surgery, flow area was decreased in OCP (P = 0.0007), and foveal and parafoveal VDs were significantly altered in all layers except the foveal VD in OCP and the choroid (all P < 0.05). The total and inner retinal thickness of the fovea and parafovea were correlated with pre-operative and post-operative visual outcomes, respectively. Smaller FAZ and greater interocular differences between post-operative and fellow eyes in FAZ were associated with worse post-operative visual outcomes.
Conclusions
OCTA provides a better display of the vascular network of the retina and choroid to evaluate the severity and surgical prognosis of ERM patients.
Introduction
Idiopathic macular epiretinal membrane (ERM), one of the most common macular diseases, impairs visual acuity and causes metamorphopsia in elderly people [1, 2]. The visual acuity decreases in eyes with ERM as the severity of disease advances [1, 3]. The macular epiretinal membrane forms along the surface of the internal limiting membrane (ILM), causing vertical traction from the periphery to the center. Histologically, migration of a number of proliferative cells through the minor gaps in ILM to the vitreous cavity probably contributes to the development of ERM [4]. A pars plana vitrectomy (PPV) with epiretinal membrane-peeling surgery is the standard therapy to release the tension and restore the normal structure of the retina in ERM. Although several reports have discussed the microvascular anomalies in eyes with ERM [5–9], the comprehensive exploration into each layer of retina and choroid still needs to be clarified.
Optical coherence tomography (OCT) has been widely applied in the diagnosis and evaluation of retinal diseases since it was invented in the 1990s [10]. Despite its advantages in capturing vivid images of retinal structures, OCT lacks of the detection and visualization of retinal blood flow [11]. Optical coherence tomography angiography (OCTA), designed based on OCT, has been developed as a new method for detecting retinal and choroidal diseases [12–16]. It makes it possible to visualize the vascular network and the blood flow by using an algorithm known as the split-spectrum amplitude decorrelation angiography (SSADA). Furthermore, SSADA can automatically make segmentation of the retina and choroid. Due to the advantages of higher resolution, less time-consuming and non-invasive when compared with FFA, OCTA has been applied to retinal and choroidal diseases, such as aged-related macular degeneration (AMD), choroidal neovascularization (CNV), diabetic retinopathy (DR), and chorioretinopathy [17–20].
This present study sought to explore and quantify the characteristics of all layers of retinal and choroidal vasculatures in ERM patients using OCTA, and to evaluate the surgical prognosis via analyzing the correlations between microvascular changes and visual outcomes at 6 months after surgery.
Methods
Subjects
In this study, 33 subjects (mean age 61.84 ± 9.79 years; range from 50 to 75 years) with a unilateral idiopathic ERM admitted to Zhongshan Ophthalmic Center from September 2016 to May 2018 were enrolled (sample size was estimated on the basis of similar research reported in previous studies [3, 15, 21]). All subjects underwent vitrectomy with epiretinal membrane-peeling surgery between December 2016 and November 2017. The normal contralateral eye served as a control for all participants. Patients with a history of severe cataract, nystagmus, poor eye fixation, significant media opacity or ERM secondary to other retinal diseases were excluded. Those with poor quality of images, signal strength index < 50 and segmentation errors due to severe ERM-induced retinal layer distortion were also excluded. Patients with cataract or cystoid macular edema (CME) did not undergo additional surgeries in combination with ERM removal. All procedures were in compliance with the tenets of the Declaration of Helsinki and the written informed consent was obtained from each participant before the study started. This study obtained formal review and approval from ethics committee of Zhongshan Ophthalmic Center (clinical trial registration number: 2017KYPJ082).
At baseline, the following data were collected for all patients: age, gender, best-corrected visual acuity (BCVA) measured with a Snellen chart, lens status and ophthalmic history. Slit-lamp biomicroscopy and indirect fundus ophthalmoscopy were then performed.
Fundus fluorescein angiography (FFA)
All eyes were evaluated by FFA at baseline and after therapy with a commercial OCT device (Heidelberg Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany). FFA images were collected at the early phase (1–5 min post-injection).
OCTA
The OCTA device (Optovue Inc., Fremont, California, USA) was programmed to repeat B-scans acquired from the same location and decorrelated between B-scans to detect the moving erythrocytes. This instrument has an A-scan rate of 70,000 scans/s, with a wavelength of 840 nm and a bandwidth of 50 nm. By using an algorithm known as SSADA, we were able to visualize the blood flow and microvascular architecture [22, 23]. The SSADA algorithm was utilized calculate the data and then the images were displayed in four layers (supplementary Fig. 1A): superficial capillary plexus (SCP), deep capillary plexus (DCP), outer retina plexus (OCP), and choroidal capillary plexus (CCP). SCP was set from the ILM offset of 3 µm to the inner plexiform layer (IPL) offset of 16 µm. DCP was set from the IPL offset of 16 µm to the IPL offset of 72 µm. OCP was defined from the IPL offset of 72 µm to the retinal pigment epithelium (RPE) offset of 31 µm. And CCP was set from the RPE offset of 31 µm to the RPE offset of 59 µm (supplementary Fig. 1A).
In this study, all participants underwent three different types of scanning: cross-line scans, retina-map scans, and angio-retina scans. The cross-line scans provided vertical and horizontal OCT images, directly showing the location and structure of the lesions. The retina-map scans offered overall details of retinal thickness with a color-coded map to distinguish retinal thickening from thinning. Furthermore, the angio-retina scans covered an area of 3 mm × 3 mm to detect the vasculature of SCP, DCP, OCP, and CCP in the fovea and parafovea (supplementary Fig. 1A).
Blood flow area, non-flow area quantification, and density analyses
The vascular flow area in which vascular signals were detected by the SSADA algorithm was drawn and calculated in a 1-mm radius circle in OCP and CCP (supplementary Fig. 1B). The non-flow area, also known as the foveal avascular zone area (FAZ), is an area without capillaries in the macula. Quantification of the vessel density (VD) is defined as the percentage of an area occupied by vessels. The OCTA instrument covered the areas of 1 mm × 1 mm and 3 mm × 3 mm, representing the fovea and parafovea, respectively. By using the software, the parafoveal region was automatically divided into four fields: the superior, inferior, temporal, and nasal quadrants (supplementary Fig. 2A). The average VD of four quadrants was calculated to analyze parafoveal values.
Central retinal thickness (CRT) assessment
By using the software, the whole retina was automatically segmented into inner and outer portions and then the mean macular thickness in the fovea (within a circle of 1 mm diameter) and the parafovea (within a circle of 3 mm diameter) were calculated. In our study, we analyzed the full, inner and outer central macular thickness to explore the changes pre-operatively and post-operatively.
Interocular differences between post-operative eyes and fellow eyes
Interocular differences in OCTA parameters at 6 months after surgery were defined as follows: (the absolute value of differences between post-operative eye measurement and fellow eye measurement)/fellow eye measurement) × 100%. Pearson correlation analysis was performed to identify the associations between interocular differences in OCTA parameters and post-operative visual outcomes.
Statistical analysis
Data on the blood flow areas, VDs of four layers, BCVA and CRT were recorded by two independent technicians. All data are presented as the means ± stardard deviation (SD). One-way ANOVA test was used to make comparisons among three or more groups, followed by Dunnett’s post hoc test. Pearson correlation analysis was used to analyze the associations between OCTA parameters and pre-operative and post-operative visual outcomes. P < 0.05 was regarded as statistically significant.
Results
A total of 33 eyes (33 patients) with ERM from 50 to 75 years old (mean age: 61.84 ± 9.79 years) were enrolled, including 14 men and 19 women. Approximately 27.27% (9 eyes) of affected eyes were complicated with CME, 90.90% (30 eyes) suffered from cataract, and none of the eyes were pseudophakic at baseline. Patients with cataract and CME did not undergo additional surgeries in combination with membrane peeling. The average logMAR BCVA was 0.11 ± 0.15 (Snellen 20/26 equivalent) in normal controls and 0.82 ± 0.41 (Snellen 20/133 equivalent) in affected eyes (Table 1). And the logMAR BCVA was improved to 0.36 ± 0.28 (Snellen 20/47 equivalent) at 6 months after therapy (Table 1).
Table 1.
OCTA parameters measured in contralateral control eyes, pre-operative ERM eyes and post-operative eyes
| Contralateral eyes (n = 33) | Pre-operative eyes with ERM (n = 33) | Differencea (95% CI) | P valuea | Six months post-operative (n = 33) | Differenceb (95% CI) | P valueb | |
|---|---|---|---|---|---|---|---|
| BCVA, logMAR | 0.11 ± 0.15 | 0.82 ± 0.41 | 0.71 (0.48 to 0.93) | <0.0001* | 0.36 ± 0.28 | −0.45 (−0.714 to −0.20) | 0.0001* |
| OCTA parameters | |||||||
| SCP | |||||||
| FAZ area (mm2) | 0.37 ± 0.085 | 0.12 ± 0.12 | −0.25 (−0.37 to −0.14) | <0.0001* | 0.26 ± 0.21 | 0.14(0.014 to 0.26) | 0.025* |
| Foveal VDs (%) | 26.62 ± 4.84 | 39.09 ± 7.06 | 12.47(8.09 to 16.85) | <0.0001* | 32.01 ± 4.75 | −7.08 (−11.91 to −2.25) | 0.002* |
| Parafoveal VDs (%) | 51.97 ± 4.76 | 42.6 ± 4.78 | −9.37(−13.03 to −5.71) | <0.0001* | 47.15 ± 4.66 | 4.55(0.51 to 8.59) | 0.023* |
| DCP | |||||||
| Foveal VDs (%) | 23.56 ± 5.38 | 42.53 ± 7.91 | 18.98(14.10 to 23.86) | <0.0001* | 36.86 ± 5.24 | −5.67(−11.05 to −0.29) | 0.036* |
| Parafoveal VDs (%) | 58.74 ± 4.57 | 48.51 ± 5.78 | −10.23(−14.67 to −5.78) | <0.0001* | 53.94 ± 7.17 | 5.43(0.52 to 10.33) | 0.026* |
| OCP | |||||||
| Outer retina flow area (mm2) | 0.95 ± 0.26 | 1.14 ± 0.13 | 0.18(0.0071 to 0.35) | 0.039* | 0.83 ± 0.26 | −0.31(−0.50 to −0.11) | 0.0007* |
| Foveal VDs (%) | 49.95 ± 10.25 | 38.16 ± 6.91 | −11.79(−19.20 to −4.38) | 0.0008* | 32.71 ± 11.39 | −5.45(−13.62 to 2.73) | 0.29 |
| Parafoveal VDs (%) | 38.14 ± 5.51 | 42.75 ± 3.92 | 4.61(0.94 to 8.29) | 0.0094* | 38.45 ± 4.54 | −4.31(−8.36 to −0.25) | 0.034* |
| CCP | |||||||
| Choroid flow area (mm2) | 1.89 ± 0.081 | 1.78 ± 0.15 | −0.11 (−0.21 to −0.011) | 0.025* | 1.74 ± 0.16 | −0.041(−0.15 to 0.069) | 0.74 |
| Foveal VDs (%) | 63.89 ± 4.70 | 57.67 ± 6.50 | −6.23(−11.76 to −0.69) | 0.023* | 54.70 ± 10.36 | −2.97(−9.08 to 3.14) | 0.55 |
| Parafoveal VDs (%) | 64.91 ± 1.96 | 63.70 ± 5.16 | −1.21 (−4.07 to 1.64) | 0.66 | 62.16 ± 3.52 | −1.54(−4.70 to 1.62) | 0.55 |
| Thickness measured at foveal centre, µm | |||||||
| Total | 241.40 ± 19.23 | 506.90 ± 139.90 | 265.50(197.40 to 333.60) | <0.0001* | 358.10 ± 71.24 | −148.80(−225.10 to −72.51) | <0.0001* |
| Inner retina | 62.32 ± 10.08 | 167.60 ± 24.96 | 105.30(87.67 to 123.0) | <0.0001* | 115.90 ± 31.97 | −51.70(−71.15 to −32.24) | <0.0001* |
| Outer retina | 178.70 ± 12.29 | 368.20 ± 97.05 | 189.50(141.90 to 237.20) | <0.0001* | 242.30 ± 45.79 | −125.90(−178.50 to −73.36) | <0.0001* |
| Thickness measured at parafovea, µm | |||||||
| Total | 309.80 ± 15.95 | 451.50 ± 110.60 | 141.70(89.97 to 193.40) | <0.0001* | 338 ± 39.17 | −113.50(−171.40 to −55.54) | <0.0001* |
| Inner retina | 121.80 ± 11.89 | 164.60 ± 22.07 | 42.76(28.31 to 57.21) | <0.0001* | 124.20 ± 22.08 | −40.38(−56.32 to −24.44) | <0.0001* |
| Outer retina | 187.30 ± 8.75 | 302.20 ± 90.52 | 114.90(73.62 to 156.30) | <0.0001* | 209.9 ± 13.83 | −92.34(−137.90 to −46.77) | <0.0001* |
All data are presented as the means ± SD
OCTA optical coherence tomography angiography, ERM epiretinal membrane, BCVA best-corrected visual acuity, SCP superficial capillary plexus, FAZ foveal avascular zone, VDs vessel densities, DCP deep capillary plexus, OCP outer retinal capillary plexus, CCP choroidal capillary plexus
*The value was statistically significant (P < 0.05)
aComparison between pre-operative ERM eyes and control eyes
bComparison between post-operative and pre-operative eyes
Interocular comparisons at baseline
Table 1 presents interocular differences between the normal contralateral eyes and eyes with ERM. In the SCP, eyes with ERM had a smaller area of FAZ than the contralateral eyes (P < 0.0001). The foveal VDs were increased whereas the parafoveal VDs were significantly decreased in both SCP and DCP (all P < 0.0001, difference 95% CI referred to Table 1). Mean flow area in OCP was markedly larger than that of unaffected contralateral eyes (P = 0.039), in contrast to the tendency in CCP (P = 0.025). Our results also found that eyes with ERM had a lower foveal VD (P = 0.0008, difference 95% CI [−11.79% (−19.20% to −4.38%)] but a higher parafoveal VD in OCP (P = 0.0094). With respect to the CCP, VD in the fovea showed a significant decrease in comparison with the controls (P = 0.023, difference 95% CI [−6.23%(−11.76% to −0.69%)]), but the parafoveal one did not alter (P = 0.66). The CRT in both foveal and parafoveal regions was markedly increased in eyes with ERM (both P < 0.0001).
Comparisons between post-operative and pre-operative OCTA analyses
As shown in Table 1, OCTA parameters of the affected eyes were statistically compared at baseline and 6 months after therapy. At post-operative 6 months, eyes had a larger area of FAZ compared with that at baseline (P = 0.025). Additionally, foveal VDs were significantly decreased and parafoveal VDs were obviously increased in both SCP and DCP (SCP: foveal VD P = 0.002; parafoveal VD P = 0.023; DCP: foveal VD P = 0.036; parafoveal VD P = 0.026). Mean value of flow area in OCP was markedly decreased from (1.14 ± 0.13) mm2 to (0.83 ± 0.26) mm2 (P = 0.0007), whereas that in CCP did not significantly change (P = 0.74). In OCP, VD in the parafovea at 6-month follow-up visit was significantly smaller than that at baseline (P = 0.034), but the foveal VD remained unchanged after therapy (P = 0.29). With respect to the CCP, VD did not significantly change in both foveal and parafoveal regions in eyes with ERM after surgery (both P = 0.55). Furthermore, post-operative CRT became significantly thinner in all layers in both foveal and parafoveal regions (both P < 0.0001).
Correlations in OCTA parameters and visual outcomes
OCTA parameters measured in SCP, DCP, OCP, and CCP were not correlated with pre-operative LogMAR BCVA (Table 2). By contrast, total and inner CRT measured at fovea and parafovea were significantly positively correlated with LogMAR BCVA (Fig. 1 A to D, Table 2), but no significant association was identified between outer CRT (fovea and parafovea) and pre-operative visual outcome (P > 0.05, Table 2). These findings indicate that epiretinal membrane-induced retinal thickening in macula contributes to worse visual acuity, and inner retina rather than outer portion is responsible for the retinal thickening.
Table 2.
Pearson correlation analysis of the pre-operative and post-operative BCVA and Optical Coherence Tomography Angiography Parameters in Patients with Epiretinal Membrane
| BCVA, logMAR | ||||
|---|---|---|---|---|
| Pre-operative | Post-operative | |||
| Pearson r value | P value | Pearson r value | P value | |
| SCP | ||||
| FAZ area (mm2) | 0.13 | 0.61 | −0.53 | 0.0014* |
| Foveal VDs (%) | −0.19 | 0.42 | 0.30 | 0.28 |
| Parafoveal VDs (%) | 0.16 | 0.52 | 0.11 | 0.69 |
| DCP | ||||
| Foveal VDs (%) | −0.36 | 0.13 | −0.075 | 0.79 |
| Parafoveal VDs (%) | −0.15 | 0.54 | 0.27 | 0.34 |
| OCP | ||||
| Outer retina flow area (mm2) | 0.25 | 0.30 | −0.31 | 0.25 |
| Foveal VDs (%) | 0.32 | 0.19 | −0.071 | 0.80 |
| Parafoveal VDs (%) | 0.083 | 0.74 | −0.10 | 0.72 |
| CCP | ||||
| Choroid flow area (mm2) | −0.20 | 0.42 | 0.13 | 0.63 |
| Foveal VDs (%) | −0.20 | 0.42 | 0.18 | 0.53 |
| Parafoveal VDs (%) | 0.0082 | 0.97 | 0.13 | 0.64 |
| Thickness measured at foveal centre, µm | ||||
| Total | 0.73 | <0.0001* | 0.59 | 0.0003* |
| Inner retina | 0.51 | 0.0032* | 0.48 | 0.0045* |
| Outer retina | 0.25 | 0.31 | 0.49 | 0.062 |
| Thickness measured at parafovea, µm | ||||
| Total | 0.38 | 0.033* | 0.80 | <0.0001* |
| Inner retina | 0.70 | <0.0001* | 0.42 | 0.015* |
| Outer retina | 0.27 | 0.26 | 0.33 | 0.24 |
OCTA opticalcoherence tomography angiography, ERM epiretinal membrane,
BCVA best-corrected visual acuity, SCP superficial capillary plexus, FAZ foveal avascular zone, VDs vessel densities, DCP deep capillary plexus, OCP outer retinal capillary plexus, CCP choroidal capillary plexus
*The value was statistically significant (P < 0.05)
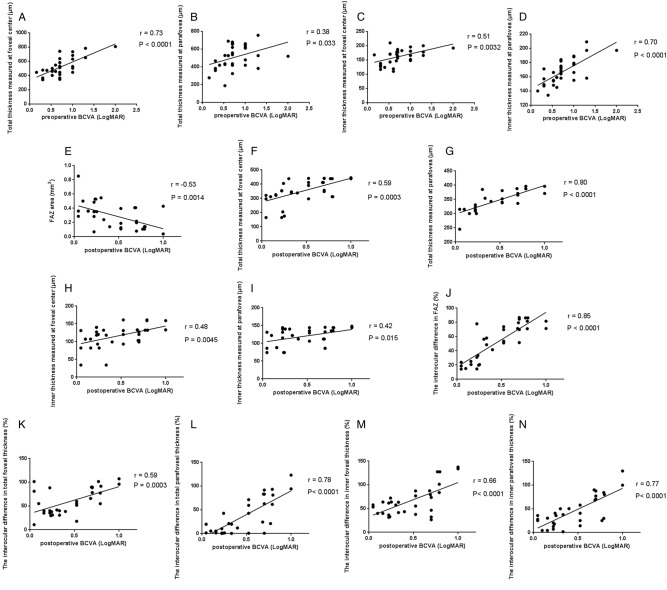
Fig. 1.
Correlations between OCTA parameters and visual outcomes. Scatter plots showing the associations between the a total foveal CRT, b total parafoveal CRT, c inner foveal CRT, d inner parafoveal CRT and pre-operative LogMAR BCVA, respectively. e FAZ area in the treated eyes was negatively correlated with post-operative LogMAR BCVA. Positive associations between f total foveal CRT, g total parafoveal CRT, h inner foveal CRT, i inner parafoveal CRT and post-operative LogMAR BCVA, respectively. Post-operative interocular differences (%) in j FAZ area, k total foveal, l total parafoveal CRT, (m) inner foveal and (n) parafoveal CRT were positively associated with LogMAR BCVA at 6 months after surgery. Pearson’s correlation coefficient (r) and p values for the slope of the regression line are provided in the figure. OCTA optical coherence tomography angiography, CRT central retinal thickness, BCVA best-corrected visual acuity, FAZ foveal avascular zone
Among post-operative OCTA parameters, FAZ area was negatively correlated with post-operative LogMAR BCVA (r = −0.53, P = 0.0014, Fig. 1e and Table 2), implying that smaller post-operative FAZ area may have no significant improvement on visual acuity in ERM eyes at post-operative 6 months. Similar to findings in pre-operative correlation, positive correlations were identified between CRT (total and inner, fovea and parafovea) and post-operative LogMAR BCVA (Fig. 1f to i, Table 2), indicating that recovery in macular thickness, especially inner retinal thickness, is a pivotal predictor in surgical prognosis of visual acuity, which is consistent with the study of Joe et al. [24] but in contrast to the findings of Arichika et al. [21].
Furthermore, interocular differences between post-operative eyes and fellow control eyes were also evaluated. Interestingly, similar to post-operative findings, interocular differences in FAZ area, CRT measured in the fovea and parafovea (total and inner) showed significant correlations with post-operative LogMAR BCVA (Fig. 1j–n, Table 3), suggesting that eyes with larger interocular differences in FAZ area and macular CRT, compared to the control contralateral eyes, had worse visual outcomes at 6 months after surgery. These findings provide another aspect of OCTA parameters in predicting surgical prognosis of ERM patients.
Table 3.
Pearson correlation analysis of the post-operative BCVA and interocular differences of Optical Coherence Tomography Angiography Parameters in Patients underwent epiretinal membrane revomal
| Interocular differences (%) | Post-operative BCVA,LogMAR | ||
|---|---|---|---|
| Pearson r value | P value | ||
| SCP | |||
| FAZ area | 53.91 ± 31.25 | 0.85 | <0.0001* |
| Foveal VDs | 27.39 ± 20.18 | 0.27 | 0.35 |
| Parafoveal VDs | 12.68 ± 8.53 | −0.16 | 0.58 |
| DCP | |||
| Foveal VDs | 70.89 ± 42.68 | −0.2 | 0.5 |
| Parafoveal VDs | 10.67 ± 11.94 | −0.32 | 0.27 |
| OCP | |||
| Outer retina flow area | 60.56 ± 104.1 | 0.14 | 0.64 |
| Foveal VDs | 41.32 ± 23.52 | −0.13 | 0.67 |
| Parafoveal VDs | 17.77 ± 13.58 | 0.22 | 0.46 |
| CCP | |||
| Choroid flow area | 10.32 ± 6.99 | −0.32 | 0.26 |
| Foveal VDs | 16.80 ± 13.40 | −0.33 | 0.26 |
| Parafoveal VDs | 5.57 ± 4.38 | −0.27 | 0.35 |
| Thickness measured at foveal centre | |||
| Total | 55.18 ± 25.59 | 0.59 | 0.0003* |
| Inner retina | 65.41 ± 31.35 | 0.66 | <0.0001* |
| Outer retina | 42.05 ± 23.67 | 0.33 | 0.25 |
| Thickness measured at parafovea | |||
| Total | 35.31 ± 36.83 | 0.78 | <0.0001* |
| Inner retina | 45.15 ± 32.50 | 0.77 | <0.0001* |
| Outer retina | 13.61 ± 8.55 | 0.3 | 0.29 |
Interocular differences at 6 months after surgery were defined as follows: (the absolute value of differences between post-operative eye measurement and fellow eye measurement)/fellow eye measurement) × 100%
OCTA optical coherence tomography angiography, ERM epiretinal membrane, BCVA best-corrected visual acuity, SCP superficial capillary plexus, FAZ foveal avascular zone, VDs vessel densities, DCP deep capillary plexus, OCP outer retinal capillary plexus, CCP choroidal capillary plexus
*The value was statistically significant (P < 0.05)
Case
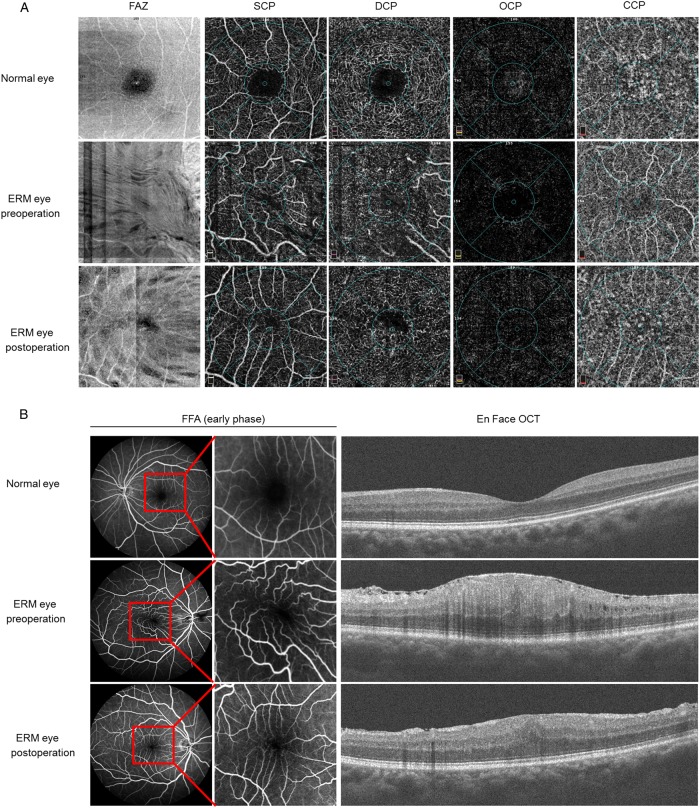
Figure 2 showed OCTA and FFA parameters in a case of ERM before and after surgery. A 67-year-old female with ERM in her right eye received a complete ophthalmic examination, a bilateral OCTA evaluation and FFA measurement before and after surgery. The visual acuity was 20/20 in her left eye and 20/60 in her right eye, and the OCT scans showed a remarkable formation of epiretinal membrane along the surface of ILM (Fig. 2b). The pre-operative OCTA indicated that the area of superficial FAZ was markedly decreased in the eye with ERM in comparison with the normal contralateral eye (Fig. 2a). Vessel distortion and vascular tortuosity were observed in OCTA and FFA in the affected eye due to the contraction of epiretinal membrane (Fig. 2a, b). Compared to FFA, OCTA images could provide more details in the microvasculature of the retina. No leakage was observed at the early phase of FFA in the pre-operative eye and fellow eye. The FAZ zone, vessel distribution and VD were greatly improved at 6 months after vitrectomy and membrane-peeling surgery. Furthermore, visual acuity was improved from 20/60 to 20/40 and total foveal CRT was decreased from 589 to 441 µm at post-operative 6 months.
Fig. 2.
Pre-operative and post-operative OCTA and FFA analyses of an ERM patient. a Representative images were shown from the normal contralateral eye and the affected eye in a patient with ERM before and after surgery using OCTA. The overall OCTA morphology of the fovea was presented as follows: SCP, DCP, OCP, and OCP. Post-operative evaluation was performed at 6 months after therapy. b Representative images of FFA and En Face OCT images were shown in a case of pre-operative and post-operative ERM eye and normal fellow eye. FFA images were collected at the early phase post dye injection and the macula was magnified to show vascular distortion as in the figure. No leakage was found in pre-operative and post-operative eyes at the early stage of FFA. OCTA optical coherence tomography angiography, SCP superficial capillary plexus, DCP deep capillary plexus, OCP outer retinal capillary plexus, CCP choroidal capillary plexus
Discussion
ERM, one of the most common retinal diseases, destroys visual acuity and causes metamorphosis due to traction of the membrane. Further comprehensive investigations in the microvasculature of ERM have aroused more and more attention from ophthalmologists. Although accumulating studies have focused on the blood flow and vascular characteristics in eyes with ERM before and after surgery [5, 8, 9], specific and comprehensive analyses at different depths of the retina and choroid using OCTA are still in need. We therefore explored deeper into the blood flow, VDs and CRT in eyes with ERM via OCTA pre- and post-operatively.
FFA is a common instrument to explore vascular characteristics in retinal diseases. Macular vessel distortion and vascular tortuosity were identified on both FFA and OCTA images of eyes with ERM. Compared with FFA, OCTA is non-invasive and it provides high-resolution images, making it possible to quantify vascular characteristics in multiple diseases. Using OCTA, we demonstrated interocular differences between eyes with ERM and the unaffected eyes in terms of SCP, DCP, OCP, and CCP. In comparison with the fellow eyes, eyes with ERM had a smaller superficial FAZ area, indicating that the contraction from the periphery to the center of the membrane contributes to the decrease in FAZ, in accordance with results from Kim et al. [9]. Interestingly, foveal VDs were notably increased but parafoveal VDs were significantly decreased in both SCP and DCP, in contrast to the tendency in OCP and CCP. The imbalanced distribution of foveal/parafoveal VDs in different segmentation of ERM indicates that ERM-associated traction causes different vessel distortion in retina and choroid. It is plausible to assume that vessels in SCP and DCP are augmented due to the centripetal force of ERM, which may subsequently impair the microvasculature in OCP and CCP. Kim et al. [9] reported that eyes with ERM had lower parafoveal VDs in SCP and DCP, but he only focused on the inner retinal layer (SCP and DCP) and did not evaluate VDs in the fovea as well as the blood flow. In this investigation, we explored the blood flow in both eyes and our results showed that the flow area was larger in OCP, but smaller in CCP compared with that of the unaffected eye. Since we and other scientists [21] uncovered that the outer retina was thickened in the foveal region in ERM, we therefore make an assumption that the enlargement of the flow area in OCP attributes to the thickening of the outer retina as more blood flow is needed to satisfy the metabolic demand. However, the precise mechanisms whereby ERM plays different roles in the flow area in OCP and CCP remain to be elucidated by subsequent research.
Our study also disclosed post-operative features of OCTA in ERM. Post-operatively, eyes had a larger area of FAZ, a lower rate of foveal VD and a higher rate of parafoveal VD in both SCP and DCP. In the OCP, both the flow area and the parafoveal VD had statistically significant alterations after therapy, whereas the foveal VD remained unchanged. However, eyes did not show any alterations after therapy in parameters detected in CCP. All these findings detected by OCTA determines that membrane-peeling surgery not only effectively improves morphologic characteristics of the macula, but also normalizes the blood flow and VDs in the fovea and parafovea.
Furthermore, our study identified several correlations between OCTA parameters and visual outcomes. Firstly, both foveal and parafoveal CRT were correlated with pre-operative visual acuity, indicating that eyes with thicker epiretinal membrane had worse visual outcomes. Interestingly, the inner retina thickness rather than the outer portion was revealed to be significantly correlated with pre-operative visual acuity, indicating a crucial role of inner retina in predicting visual outcomes of eyes affected by ERM.
Our study also disclosed that post-operative eyes with larger FAZ area had a better prognosis of visual outcomes. One possible explanation is that epiretinal membrane removal relieves retinal mechanical traction from periphery to center and improves macular vessel distortion, which enlarges FAZ area, normalizes macular morphology and improves BCVA. Similarly, foveal and parafoveal CRT were both correlated with post-operative visual outcomes, emphasizing the significance of macular CRT in the prognosis of ERM.
Notably, positive correlations were identified in interocular differences between OCTA parameters and post-operative visual outcomes. In other words, post-operative eyes with greater differences in FAZ area, foveal and parafoveal CRT, especially the inner portion had worse visual prognosis at 6 months after therapy compared with the fellow eyes. All these findings suggest that OCTA parameters, especially FAZ area and CRT (total and inner), could be used to evaluate the surgical prognosis of ERM in various aspects.
There were several limitations in our study. Firstly, a small number of participants were recruited in this study, which might yield false-negative or false-positive results. Secondly, different locations of ERM probably affect these measurements and a more specific classification needs to be carried out. Additionally, we also excluded the cases presenting with severe retinal distortion and segmentation errors because severe epiretinal membrane could confound OCTA signals and measurements, which might induce a selection bias.
In conclusion, we comprehensively illustrate the microvascular characteristics before and after epiretinal membrane surgery by OCTA in terms of the blood flow and VDs at different depths of the retina and choroid. Our study also discloses that ERM not only causes vascular distortion in the inner retina, but also affects the outer retina and even choroid to some extent, providing a solid foundation for the microvascular pathogenesis of ERM. The OCTA analyses also predict the surgical prognosis as well as pave the way for clinicians to comprehensively understand the vessel and blood flow changes after therapy.
Summary
What was known before
The traction of epiretinal membrane leads to vessel distortion in macula, mainly affecting superficial vessel densities in the foveal region.
What this study adds
This study systematically and comprehensively evaluated OCTA parameters in eyes with epiretinal membrane, such as the blood flow as well as vessel densities in the fovea and parafovea at different layers of retina and choroid.
And it also compared pre- and post-operative vascular parameters to determine surgical prognosis.
Electronic supplementary material
Acknowledgements
This work was supported by the Fundamental Research Fund of the State Key Laboratory of Ophthalmology under Grant (No.30306020240020128); and the Developmental Education Fund of Guangdong Province for Sun Yat-sen University under Grant (No.83000-3050057).
Compliance with ethical standards
Conflict of interest
The funding organization had no role in the design or conduct of this research. All authors declare that they have no conflict of interest.
Contributor Information
Wei Chi, Phone: +8613710616456, Email: chiwei@mail.sysu.edu.cn.
Shaochong Zhang, Phone: +8613902217605, Email: zhangshaochong@gzzoc.com.
Electronic supplementary material
The online version of this article (10.1038/s41433-018-0272-3) contains supplementary material, which is available to authorized users.
References
- 1.Inoue M, Kadonosono K. Macular diseases: epiretinal membrane. Dev Ophthalmol. 2014;54:159–63. doi: 10.1159/000360462. [DOI] [PubMed] [Google Scholar]
- 2.Dupas B, Tadayoni R, Gaudric A. Epiretinal membranes. J Fr d’ophtalmologie. 2015;38:861–75. doi: 10.1016/j.jfo.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Nishi Y, Shinoda H, Uchida A, Koto T, Mochimaru H, Nagai N, et al. Detection of early visual impairment in patients with epiretinal membrane. Acta Ophthalmol. 2013;91:e353–357. doi: 10.1111/aos.12060. [DOI] [PubMed] [Google Scholar]
- 4.Nam DH, Desouza PJ, Hahn P, Tai V, Sevilla MB, Tran-Viet D, et al. Intraoperative spectral domain optical coherence tomography imaging after internal limiting membrane peeling in idiopathic epiretinal membrane with connecting strands. Retina. 2015;35:1622–30. doi: 10.1097/IAE.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Y, Teng Y, Gao M, Liu X, Chen J, Liu W. Quantitative choriocapillaris perfusion before and after vitrectomy in idiopathic epiretinal membrane by optical coherence tomography angiography. Ophthalmic Surg, Lasers Imaging Retin. 2017;48:906–15. doi: 10.3928/23258160-20171030-06. [DOI] [PubMed] [Google Scholar]
- 6.Mastropasqua Leonardo, Borrelli Enrico, Carpineto Paolo, Toto Lisa, Di Antonio Luca, Mattei Peter A., Mastropasqua Rodolfo. Microvascular changes after vitrectomy with internal limiting membrane peeling: an optical coherence tomography angiography study. International Ophthalmology. 2017;38(4):1465–1472. doi: 10.1007/s10792-017-0608-1. [DOI] [PubMed] [Google Scholar]
- 7.Lei J, Durbin MK, Shi Y, Uji A, Balasubramanian S, Baghdasaryan E, et al. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography En face images. JAMA Ophthalmol. 2017;135:1092–8. doi: 10.1001/jamaophthalmol.2017.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitagawa Yorihisa, Shimada Hiroyuki, Shinojima Ari, Nakashizuka Hiroyuki. FOVEAL AVASCULAR ZONE AREA ANALYSIS USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY BEFORE AND AFTER IDIOPATHIC EPIRETINAL MEMBRANE SURGERY. Retina. 2019;39(2):339–346. doi: 10.1097/IAE.0000000000001972. [DOI] [PubMed] [Google Scholar]
- 9.Kim YJ, Kim S, Lee JY, Kim JG, Yoon YH. Macular capillary plexuses after epiretinal membrane surgery: an optical coherence tomography angiography study. Br J Ophthalmol. 2018;102:1086–91. [DOI] [PubMed]
- 10.Ibne Mokbul M. Optical Coherence Tomography: Basic Concepts and Applications in Neuroscience Research. J Med Eng. 2017;2017:3409327. doi: 10.1155/2017/3409327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koustenis A, Jr., Harris A, Gross J, Januleviciene I, Shah A, Siesky B. Optical coherence tomography angiography: an overview of the technology and an assessment of applications for clinical research. Br J Ophthalmol. 2017;101:16–20. doi: 10.1136/bjophthalmol-2016-309389. [DOI] [PubMed] [Google Scholar]
- 12.Chan SY, Wang Q, Wei WB, Jonas JB. Optical coherence tomographic angiography in central serous chorioretinopathy. Retina. 2016;36:2051–8. doi: 10.1097/IAE.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 13.Coscas G, Lupidi M, Coscas F. Optical Coherence Tomographic Angiography in Diabetic Macular Ischemia: A New Step Forward. JAMA Ophthalmol. 2016;134:373–4. doi: 10.1001/jamaophthalmol.2015.4821. [DOI] [PubMed] [Google Scholar]
- 14.Dansingani KK, Inoue M, Engelbert M, Freund KB. Optical coherence tomographic angiography shows reduced deep capillary flow in paracentral acute middle maculopathy. Eye (Lond, Engl) 2015;29:1620–4. doi: 10.1038/eye.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durbin MK, An L, Shemonski ND, Soares M, Santos T, Lopes M, et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135:370–6. doi: 10.1001/jamaophthalmol.2017.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita K, Kawamura A, Yuzawa M. Choriocapillaris changes imaged by OCT angiography after half-dose photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmic Surg, Lasers Imaging Retin. 2017;48:302–10. doi: 10.3928/23258160-20170329-04. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Desai R, Nesper P, Gill M, Fawzi A, Skondra D. Optical coherence tomographic angiography imaging in age-related macular degeneration. Ophthalmol Eye Dis. 2017;9:1179172116686075. doi: 10.1177/1179172116686075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malihi M, Jia Y, Gao SS, Flaxel C, Lauer AK, Hwang T, et al. Optical coherence tomographic angiography of choroidal neovascularization ill-defined with fluorescein angiography. Br J Ophthalmol. 2017;101:45–50. doi: 10.1136/bjophthalmol-2016-309094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ting DSW, Tan GSW, Agrawal R, Yanagi Y, Sie NM, Wong CW, et al. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol. 2017;135:306–12. doi: 10.1001/jamaophthalmol.2016.5877. [DOI] [PubMed] [Google Scholar]
- 20.Quaranta-El Maftouhi M, El Maftouhi A, Eandi CM. Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. Am J Ophthalmol. 2015;160:581–7. doi: 10.1016/j.ajo.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Arichika S, Hangai M, Yoshimura N. Correlation between thickening of the inner and outer retina and visual acuity in patients with epiretinal membrane. Retina. 2010;30:503–8. doi: 10.1097/IAE.0b013e3181bd2d65. [DOI] [PubMed] [Google Scholar]
- 22.Spaide Richard F., Fujimoto James G., Waheed Nadia K., Sadda Srinivas R., Staurenghi Giovanni. Optical coherence tomography angiography. Progress in Retinal and Eye Research. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan A C S, Tan G S, Denniston A K, Keane P A, Ang M, Milea D, Chakravarthy U, Cheung C M G. An overview of the clinical applications of optical coherence tomography angiography. Eye. 2017;32(2):262–286. doi: 10.1038/eye.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joe SG, Lee KS, Lee JY, Hwang JU, Kim JG, Yoon YH. Inner retinal layer thickness is the major determinant of visual acuity in patients with idiopathic epiretinal membrane. Acta Ophthalmol. 2013;91:e242–3. doi: 10.1111/aos.12017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.