Significance
Plants respond to herbivory and pathogenic infection with the synthesis of various defense compounds, including volatile compounds that are emitted into the environment. These volatiles can be perceived by neighboring plants and “prime” them for an attack by the specific attacker. We found that whitefly-infested tomato plants release volatiles that prime a defense against pathogens in neighboring plants, which goes at the cost of defenses against insect herbivores, making the neighboring plants more suitable for whitefly development. This apparent ability of whiteflies to manipulate plant defense responses through induced volatile emissions could explain the rapid spread of this invasive pest, and a good understanding of the mechanisms that are involved may lead to novel pest control strategies.
Keywords: herbivore-induced plant volatiles, whiteflies, tomato, salicylic acid, jasmonic acid
Abstract
The whitefly Bemisia tabaci is one of the world’s most important invasive crop pests, possibly because it manipulates plant defense signaling. Upon infestation by whiteflies, plants mobilize salicylic acid (SA)-dependent defenses, which mainly target pathogens. In contrast, jasmonic acid (JA)-dependent defenses are gradually suppressed in whitefly-infested plants. The down-regulation of JA defenses make plants more susceptible to insects, including whiteflies. Here, we report that this host–plant manipulation extends to neighboring plants via airborne signals. Plants respond to insect attack with the release of a blend of inducible volatiles. Perception of these volatiles by neighboring plants usually primes them to prepare for an imminent attack. Here, however, we show that whitefly-induced tomato plant volatiles prime SA-dependent defenses and suppress JA-dependent defenses, thus rendering neighboring tomato plants more susceptible to whiteflies. Experiments with volatiles from caterpillar-damaged and pathogen-infected plants, as well as with synthetic volatiles, confirm that whiteflies modify the quality of neighboring plants for their offspring via whitefly-inducible plant volatiles.
The whitefly Bemisia tabaci is an invasive pest of global importance. As a vector of several destructive plant viruses, this tiny hemipteran insect poses a worldwide threat to the productivity of many crops (1). Several traits have been implicated in its chronic invasiveness (2–5). One of the most intriguing traits is the insect’s apparent ability to induce a plant response that is usually triggered by an attack by a biotrophic pathogen instead of triggering a typical response to insect attack. This leads to the activation of defenses that are effective against pathogens at the cost of defenses that are effective against insects, thereby rendering the plant more suitable and vulnerable to whitefly attack (3, 6). More specifically, upon continuous whitefly infestation, plants predominantly mobilize a defense that is mediated by salicylic acid (SA; refs. 6–8). In some plants, whitefly attack initially also triggers jasmonic acid (JA)-regulated responses (9–11), but in tomato plants JA levels decline within days, whereas the accumulation of SA and the expression of SA-regulated genes gradually increase (10, 11). The reduction in JA-dependent defenses makes the plants more susceptible to insects, including whiteflies (6, 11, 12).
Plants also respond to insect feeding with the production and release of specific blends of volatiles. These herbivore-induced plant volatiles (HIPVs) can serve various functions (13–16), including a direct defense with toxic and repellent effects on the herbivore (17–19) and an indirect defense by attracting natural enemies of herbivores (20–22). More important in the context of this study, HIPVs also serve as signals that provide information on imminent insect attack to undamaged plant tissues (23–26), as well as neighboring plants (27–31). Upon perception of certain HIPVs, neighboring plants prepare for attack, a phenomenon called priming (32–36). When these primed neighbors are attacked themselves by the same herbivore, they will respond more rapidly and strongly with an appropriate defense reaction. It is, as yet, unclear how specific these priming responses are, but it is to be expected that HIPVs will prime for defenses against herbivores and that volatile blends that are triggered by pathogen attack will prime for defenses against pathogens (35, 36). Whitefly infestation results in a blend that is more representative of pathogen attack than of insect attack (7, 8). Here, we show that perception of this whitefly-induced blend by neighboring plants results in a response that renders the neighbors more suitable hosts for whitefly development. This result implies that by manipulating its host’s volatile signals whiteflies may enhance the suitability of neighboring host plants for their offspring.
Results
Exposing Tomato Plants to Whitefly-Induced Plant Volatiles.
In a number of systems, it has been shown that HIPVs can be perceived by neighboring plants and prime these plants to prepare themselves for incoming attack, rendering the neighbors more resistant to the inducing herbivore (27–31). Whitefly-infested plants emit volatile blends that are distinct from volatile blends emitted by plants infested by other arthropods (37), and the induction of these volatiles is mediated by the SA signaling pathway (8). To test if neighboring plants respond to these volatiles, we first conducted a series of experiments to determine if exposure to whitefly-induced volatiles affects a plant’s suitability as a host for whiteflies. Using interconnected glass chambers, each holding two tomato plants, we exposed healthy tomato plants to the volatiles released by whitefly-infested plants (SI Appendix, Fig. S1). The oviposition rates and performance of whiteflies on these exposed plants were compared with control plants that had been exposed to the volatiles of healthy plants.
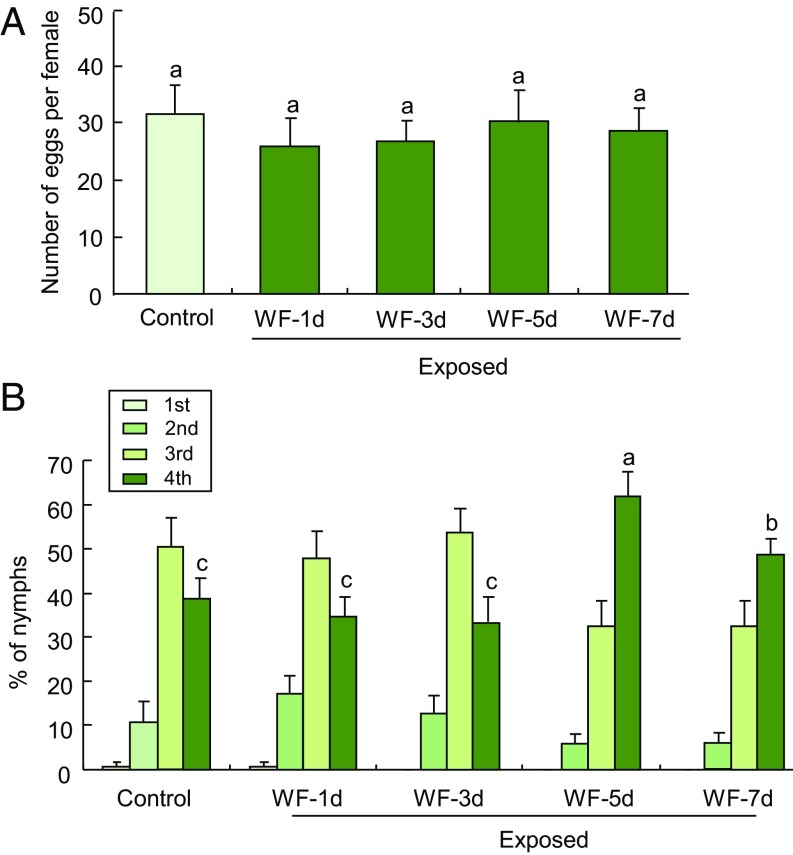
The number of eggs laid by females that were placed on the plants did not significantly differ between plants exposed for 24 h to volatiles from uninfested plants or to volatiles from plants that had been infested by whiteflies for 1, 3, 5, or 7 d (Fig. 1A). However, the development rate of nymphs on these plants was considerably different between the treatments. The proportion of fourth-instar nymphs was significantly higher on plants that had been exposed to volatiles from plants that had been infested by whiteflies for 5 or 7 d, compared with plants that had been exposed to volatiles from uninfested plants (Fig. 1B). This was not the case after exposure to volatiles from infested plants for 1 or 3 d (Fig. 1B). These data imply that tomato plants become more susceptible to B. tabaci after exposure to volatiles from whitefly-infested plants. For subsequent experiments, we used tomato plants that had been infested with B. tabaci for 5 d as source of whitefly-induced volatiles.
Fig. 1.
Performance of whiteflies on volatile-exposed tomato plants. (A) Number of eggs laid per female after adults had been introduced on plants for 6 d (n = 10). (B) Percentage of nymphs represented by each instar at 21 d after adults had been added to exposed plants (n = 10–12). Control represents plants that had been exposed for 24 h to the volatiles from undamaged plants; WF-1d, WF-3d, WF-5d, and WF-7d represent the plants that had been exposed for 24 h to the volatiles from plants that had been infested with B. tabaci for 1, 3, 5, or 7 d, respectively. Error bars correspond to SEs. Different letters above bars indicate significant differences between treatments (P < 0.05; for A, Tukey’s multiple comparison test; for B, likelihood ratio test). B presents data for all four nymphal instars, but because the data for the different instars are dependent, we only used the percentage of nymphs that had reached the fourth instar for statistical analyses. The higher the proportion of fourth-instar nymphs found on a plant, the more suited that plant is for whitefly development.
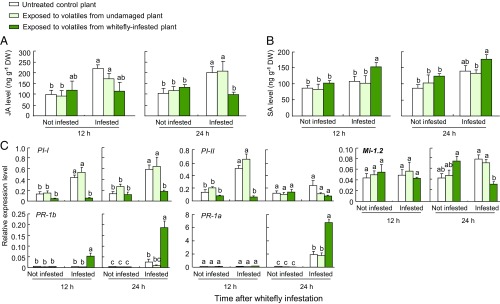
To evaluate the effects of volatiles emitted by B. tabaci-infested plants on the defense responses in neighboring plants, we measured the levels of endogenous JA and SA in plants that had been exposed to the volatiles for 24 h and were then subjected to feeding by B. tabaci adults. Exposed plants that were not infested with whiteflies showed no differences in JA levels when we compared plants exposed to volatiles from uninfested plants and those exposed to volatiles from whitefly-infested plants (Fig. 2A). However, upon infestation with whiteflies for 12 or 24 h, JA levels in plants that had been previously exposed to volatiles from uninfested plants were significantly increased relative to levels before infestation (Fig. 2A). In contrast, plants exposed to volatiles from whitefly-infested plants showed no increase in JA levels at 12 or 24 h after whitefly infestation (Fig. 2A). In these plants, JA levels were only about 50% of the levels in control plants after 24 h of whitefly infestation.
Fig. 2.
Whitefly-induced volatiles prime SA-dependent defenses and suppress JA-dependent defenses in volatile-exposed tomato plants. (A) JA levels in unexposed and exposed plants either without or with subsequent infestation with whiteflies (n = 3). (B) SA levels in unexposed and exposed plants either without or with subsequent infestation with whiteflies (n = 3). (C) Expression of defense genes in unexposed and exposed plants either without or with subsequent infestation with whiteflies (n = 3). Error bars correspond to SEs. Within each graph with six bars, different letters indicate significant differences (P < 0.05; Tukey’s multiple comparison test). DW, dry weight.
SA levels showed the opposite pattern. Again, exposed plants that were not infested showed no difference in SA levels between plants exposed to volatiles from uninfested plants and those exposed to volatiles from whitefly-infested plants (Fig. 2B). Upon whitefly infestation, however, plants exposed to volatiles from uninfested plants showed no increase in SA levels at 12 or 24 h after infestation (Fig. 2B). In contrast, plants exposed to volatiles from whitefly-infested plants showed a significant increase in SA levels at 12 and 24 h after infestation (Fig. 2B).
We next quantified the transcript levels of five defense-related genes in the leaves of exposed plants; the transcript levels were again quantified before and after feeding by B. tabaci adults. Genes encoding for proteinase inhibitors I and II (PI-I and PI-II) are regulated by JA signaling and confer insect resistance in many solanaceous plants, including tomato (38, 39). The tomato gene Mi-1.2 is responsible for resistance against whiteflies (10, 40). PR-1a and PR-1b are known as SA-triggered pathogenesis-related (PR) genes in tomato (41). Without further plant treatment, none of these genes showed an enhanced expression after exposure to plant volatiles, be it from control or whitefly-infested plants (Fig. 2C). This was considerably different for plants that were infested with whitefly adults after the exposure. In infested plants, PI-I showed significant transcriptional induction in plants previously exposed to volatiles from uninfested tomato plants, but not in plants that had been previously exposed to volatiles from whitefly-infested tomato (Fig. 2C). In whitefly-infested plants, PI-II and Mi-1.2 showed similar patterns of suppression after exposure to volatiles from infested plants, but this was only evident after 12 h of infestation for PI-II and after 24 h of infestation for Mi-1.2. PR-1a and PR-1b showed stronger induction after exposure to volatiles from infested plants, but this was only evident after 24 h of infestation for PR-1a and after 12 and 24 h of infestation for PR-1b (Fig. 2C).
Taken together, these results imply that exposure to the volatiles from whitefly-infested plants causes an overall suppression of JA-dependent defense responses in tomato plants, which is only evident when the exposed plants are challenged by whiteflies. This priming effect renders the plants more suitable as hosts for whiteflies. To test if this phenomenon is specific for whitefly infestation, we next conducted a series of experiments to compare the volatile emissions of whitefly-infested tomato plants with volatiles released by plants under attack by caterpillars or a bacterial pathogen (see details in SI Appendix). In addition, we assessed their respective effects on neighboring plants to test our hypothesis that whitefly-induced volatiles are indicative of pathogen infection and therefore prime resistance to pathogens at the cost of defenses against insects.
Volatiles Emitted from Infested or Infected Plants.
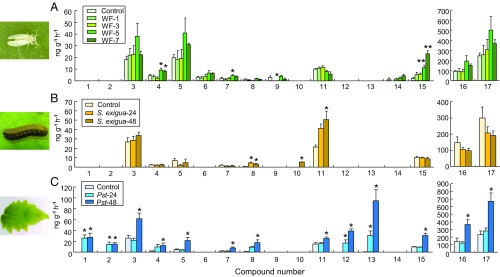
Volatiles were collected from healthy tomato plants and plants that had either been infested by whiteflies (150 adults for 1, 3, 5 or 7 d), infested by caterpillars of the moth Spodoptera exigua (five second-instar caterpillars for 24 or 48 h), or infected by the bacterium Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) for 24 or 48 h. Gas chromatography-mass spectrometry (GC-MS) analysis detected a total of 17 major compounds that were consistently released, depending on the treatments (Fig. 3). Quantitative analysis showed that the amounts of the monoterpene (E)-β-ocimene and of an unknown sesquiterpene released from plants infested with B. tabaci for 1 and 3 d, respectively, were significantly lower than the amounts released from uninfested plants (Fig. 3A and SI Appendix, Table S2). Significant increases in the emission of the monoterpenes β-myrcene and ρ-cymene, and of the sesquiterpene β-caryophyllene, only started 5 d after initial infestation (Fig. 3A). Caterpillar feeding and bacterial infection resulted in considerably faster changes in the release of volatiles by the tomato plants and, surprisingly, all three attackers induced a qualitatively distinct blend. Caterpillar infestation significantly increased the release of (E)-β-ocimene and methyl isonicotinate, and it triggered the release of linalool, which was not detected in the volatiles released by undamaged plants (Fig. 3B and SI Appendix, Table S3). The bacterial infection had the most dramatic effect and resulted in increases in the release of virtually all of the detected volatiles (Fig. 3C and SI Appendix, Table S4). Moreover, the bacterium induced the release of several compounds that were not found in the other treatments, namely (Z)-3-hexenol, 1-hexanol, methyl salicylate, and α-cubebene. Although the bacteria-induced blend shows similarities with the volatile pattern induced by whitefly infestation, the blend did not show the hypothesized resemblance. To test if the caterpillar- and bacteria-induced blends would prime the appropriate defenses in neighboring plants, we conducted an additional series of exposure assays.
Fig. 3.
Compounds identified in blends emitted by whitefly-infested, caterpillar-infested, or pathogen-infected tomato plants. (A) Volatile compounds induced by B. tabaci whiteflies (WF) (n = 6–7). (B) Volatile compounds induced by S. exigua caterpillars (n = 6). (C) Volatile compounds induced by Pst DC3000 bacterial infection (n = 5). Compound numbers represent: 1, (Z)-3-hexenol; 2, 1-hexanol; 3, α-pinene; 4, β-myrcene; 5, γ-terpinene; 6, α-terpinene; 7, ρ-cymene; 8, (E)-β-ocimene; 9, unknown sesquiterpene; 10, linalool; 11, methyl isonicotinate; 12, methyl salicylate; 13, α-cubebene; 14, β-cedrene; 15, β-caryophyllene; 16, 4-carene; 17, β-phellandrene. Error bars correspond to SEs. For individual volatiles, see SI Appendix, Tables S2–S4. Asterisks indicate significant differences from control plants (*P < 0.05; **P < 0.01; Tukey’s multiple comparison test). WF-1, WF-3, WF-5, and WF-7 represent the plants that were infested with B. tabaci for 1, 3, 5, or 7 d, respectively.
Exposure Effects on Plant Resistance.
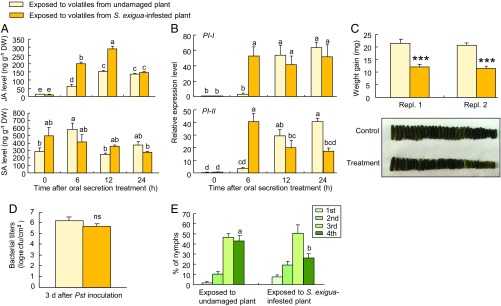
Our hypothesis is that whiteflies manipulate future host plants by triggering their host plants to emit a blend of volatiles that signals the deceptive information to neighboring plants. Indeed, as shown above, the blend emitted by caterpillar-damaged plants was quite different. The question that remains is whether plants that perceive this “correct” blend of volatiles respond in a manner that enhances their resistance against herbivores, including whiteflies. We tested this and indeed found that the priming effects elicited by S. exigua-induced volatiles enhance a defense response that is effective against S. exigua caterpillars and whiteflies (Fig. 4). Plants were first exposed for 24 h to volatiles from caterpillar-infested plants or to volatiles from undamaged plants. Then the exposed plants were damaged with a razor blade and caterpillar regurgitant was applied to the damaged sites to mimic herbivore attack (42). In contrast to whitefly-induced volatiles (Fig. 2B), caterpillar-induced volatiles primed a stronger accumulation of JA levels in exposed plants, whereas SA levels remained largely unaffected in exposed plants that were damaged and treated with caterpillar regurgitant (Fig. 4A). This difference was also found for the expression of genes that are involved in plant defenses (Fig. 4B), with a much higher early expression of PI-I and PI-II in plants that had been exposed to the volatiles of caterpillar-damaged plants. Again, this was the opposite of what was observed for plants exposed to volatiles from whitefly-infested plants, which showed a suppression of all three genes (Fig. 2C). This priming of JA defenses was further reflected in the weight gain of second-instar S. exigua larvae reared on leaves of exposed plants. Weight gain was more than 40% lower on plants that had been exposed to volatiles from S. exigua-infested plants compared with larval weight gain on leaves of plants exposed to volatiles from uninfested plants (Fig. 4C). The priming response elicited by S. exigua-induced volatiles had no apparent effect on a plant’s resistance to the pathogen. Pst DC3000 bacteria grew equally well on the two types of exposed leaves (Fig. 4D). Importantly, in contrast to exposure to volatiles from whitefly-induced plants (Fig. 1C), exposure to caterpillar-induced plant volatiles rendered the exposed plants significantly less suitable for whitefly development (Fig. 4E), further supporting our main hypothesis.
Fig. 4.
Caterpillar-induced volatiles prime JA defenses in volatile-exposed tomato plants. (A) Endogenous JA and SA levels in exposed plants after oral secretion treatment (n = 3). (B) Expression of defense genes in exposed plants after oral secretion treatment (n = 3). (C) Weight gain of S. exigua (n = 31–47; ***P < 0.001; one-way ANOVA). (D) Growth of Pst DC3000 bacteria (n = 3; ns, not significant; one-way ANOVA). (E) Nymphal performance of whitefly (n = 10). Error bars correspond to SEs. Different letters above bars indicate significant differences between treatments (P < 0.05; for A and B, Tukey’s multiple comparison test; for E, likelihood ratio test). DW, dry weight.
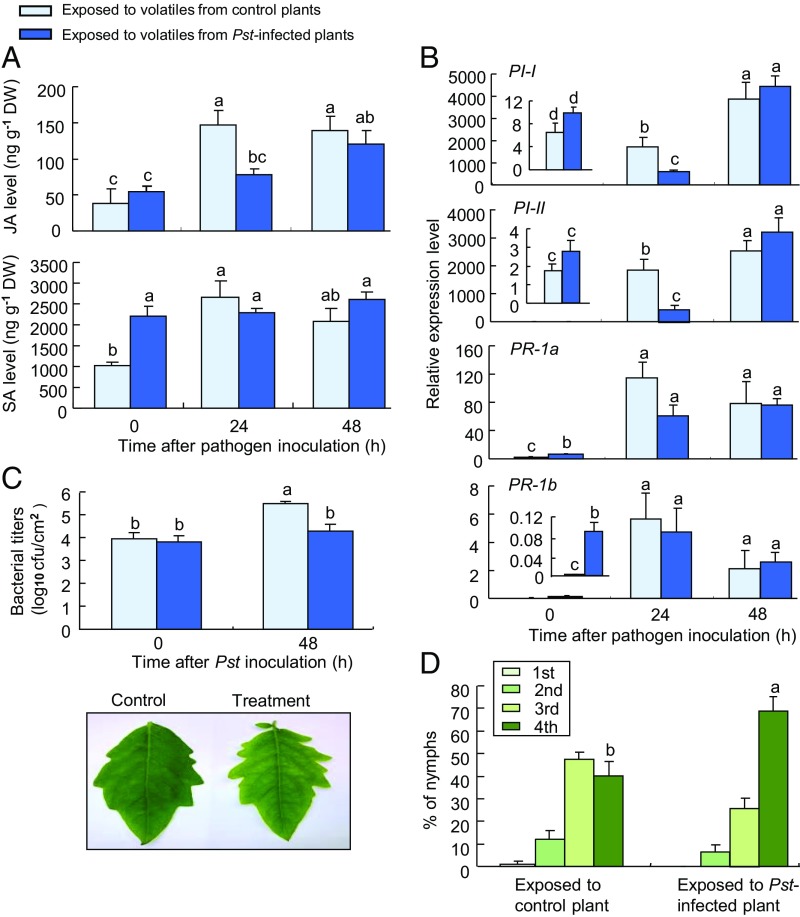
A similar series of experiments was conducted with tomato plants that were exposed to volatiles of Pst DC3000-infested plants for 24 h. Control plants were exposed to the volatiles of healthy plants. A subset of these exposed plants was then inoculated with the pathogen. The results further support our hypothesis, with the plants that were exposed to bacteria-induced volatiles showing greatly suppressed inducible JA levels, whereas the production of SA was found to be enhanced at the time of inoculation, but not affected at later time points (Fig. 5A).
Fig. 5.
Pathogen-induced volatiles prime SA defenses in volatile-exposed tomato plants. (A) Endogenous JA and SA levels in exposed plants after Pst DC3000 infection (n = 3). (B) Expression of defense genes in exposed plants after Pst DC3000 infection (n = 3). (C) Bacterial growth of Pst DC3000 (n = 3). (D) Nymphal performance of whiteflies (n = 10). Error bars correspond to SEs. Different letters above bars indicate significant differences between treatments (P < 0.05; A–C, Tukey’s multiple comparison test; D, likelihood ratio test). DW, dry weight.
Gene expression patterns also showed similarities to exposure to whitefly-induced volatiles (Fig. 2C), with suppressed expression of the JA-related genes PI-I and PI-II in inoculated plants that had been previously exposed to pathogen-induced volatiles and an early increased expression of PR-1a and PR-1b before inoculation (Fig. 5B). That these responses resulted in enhanced resistance to the pathogen was reflected in bacterial titers, which were significantly lower 48 h after inoculation of plants that had been exposed to pathogen-induced volatiles (Fig. 5C). Most importantly, as with plants that had been exposed to whitefly-induced volatiles (Fig. 1C), exposure to pathogen-induced volatiles rendered the plants significantly more suitable for whitefly development (Fig. 5D).
Exposure to Synthetic Compounds.
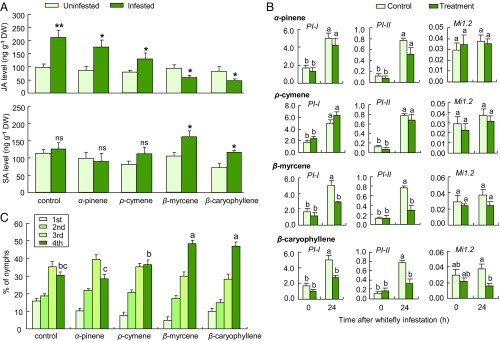
We next conducted a number of exposure experiments with synthetic compounds that were characteristic for the blend induced by whiteflies. This allowed us to confirm that these volatiles differentially prime JA- and SA-dependent defenses. We first quantified JA and SA levels in tomato plants that were exposed to the volatiles β-myrcene, ρ-cymene, and β-caryophyllene, with α-pinene as a control volatile that was not enhanced by whitefly infestation. Plants were exposed to a realistic dose of one of these compounds for 24 h and then, either were left unharmed or they were infested with whitefly adults for 24 h. Upon infestation, plants exposed to only the solvent (control), α-pinene, or ρ-cymene showed a significant increase in JA levels (Fig. 6A), whereas plants exposed to β-myrcene or β-caryophyllene showed a significant decrease in JA levels (Fig. 6A). Upon whitefly infestation, control plants exposed to α-pinene and ρ-cymene still showed no increase in SA level (Fig. 6A), but plants exposed to β-myrcene and β-caryophyllene showed a significant increase in SA levels in response to whitefly infestation (Fig. 6A).
Fig. 6.
Exposure to whitefly-induced volatile compounds suppresses JA-dependent defenses and accelerates whitefly performance. (A) Endogenous JA and SA levels in uninfested and whitefly-infested plants that had been preexposed for 24 h to the solvent dichloromethane (control) or synthetic volatile compounds (n = 3; *P < 0.05; **P < 0.01; one-way ANOVA). (B) Expression of defense genes in plants that had been preexposed for 24 h to only the solvent dichloromethane (control) or synthetic volatile compounds (treatment; n = 3). (C) Nymphal performance of whiteflies (n = 10). Error bars correspond to SEs. Different letters above bars indicate significant differences between treatments (P < 0.05; for B, Tukey’s multiple comparison; for C, likelihood ratio test). DW, dry weight.
After 24 h of exposure and without subsequent B. tabaci infestation, exposure to α-pinene, β-myrcene, β-caryophyllene, or ρ-cymene did not affect the expression of four defense genes (Fig. 6B). Upon subsequent B. tabaci infestation, exposure to α-pinene or ρ-cymene did not affect the expression of four defense genes (Fig. 6B). In contrast, exposure to β-myrcene suppressed the expression of PI-I and PI-II, and exposure to β-caryophyllene suppressed the expression of all four genes in infested leaves (Fig. 6B). These responses are indicative of suppressed JA-dependent defenses.
We also assessed the performance of whitefly nymphs on plants exposed to the synthetic volatiles, using the same performance criteria as for the previous bioassays. The percentages of nymphs that had reached the fourth instar were significantly higher on plants exposed to β-myrcene and β-caryophyllene than on control plants (Fig. 6C). In contrast, the percentage of fourth-instar nymphs on plants exposed to α-pinene or ρ-cymene was not different from that on control plants (Fig. 6C). Overall, these results match the expected induction patterns and whitefly performance, based on the results from the exposures to natural volatiles. However, it should be noted that bacterial infection induced the emission of α-pinene (Fig. 3), which was not the case for whitefly infestation, nor for caterpillar infestation.
In complementary experiments, we used several volatiles that were more typical for the volatile blends induced by the other two attackers and studied their effects on defense gene expression. As representative of caterpillar infestation, we used linalool and methyl isonicotinate, whereas we used 1-hexanol, (Z)-3-hexenol, and methyl salicylate as representative for plants with bacterial infection (Fig. 3). Plants that had been exposed for 24 h to linalool or methyl isonicotinate and were subsequently damaged and treated with caterpillar regurgitant showed greatly increased expression of the genes PI-I and PI-II, and to some extent PR-1a, compared with plants exposed to the solvent only (SI Appendix, Fig. S2). Exposure for 24 h to 1-hexanol, (Z)-3-hexenol, and methyl salicylate and subsequent bacterial infection for 24 h resulted in greatly enhanced expression of PR-1b and PI-I, compared with plants exposed to the solvent (SI Appendix, Fig. S3). The expression of PR-1a was only enhanced in plants that were exposed to (Z)-3-hexenol or methyl salicylate. These results do not entirely match the induction patterns observed for the natural blends and, therefore, imply that the studied volatiles are not all responsible for the observed response differences.
Discussion
Despite their immobility, plants are capable of resisting most of their numerous attackers. They mainly rely on a diverse arsenal of defense mechanisms that have antiherbivore and/or antimicrobial properties. However, many specialized herbivores and pathogens are able to overcome and sometimes manipulate these defenses and can thrive on their specific host plants. Here, we show that volatile emissions are also vulnerable to manipulation. The generalist whitefly B. tabaci induced plants into producing a volatile blend that evokes neighboring plants to down-regulate JA-dependent defenses, and this coincides with the enhanced performance of B. tabaci nymphs. We also observed these plants to display a stronger SA response, which is assumed to cause a down-regulation of JA responses (6, 8), but this is not always evident (43) and remains to be determined for tomato. We conducted a supplementary experiment that excludes the possibility that odor emitted from whiteflies themselves is involved in the suppression of JA-dependent defenses and the induction of SA-dependent defenses (SI Appendix, Fig. S6). Taken together, these findings imply that the whitefly-induced volatiles affect neighboring tomato plants in a way that makes them more suitable as a host for the next generation of whiteflies.
Interestingly, the volatile blends induced by the three attackers were all distinctly different, but the three volatiles that were significantly induced by whitefly infestation were also present in the pathogen-induced blend while they were not induced by the caterpillars (Fig. 3). We found these compounds also in the blends released by two other tomato varieties in response to whitefly infestation (SI Appendix, Fig. S4 and Table S5). Exposure to these compounds (β-myrcene or β-caryophyllene) indeed enhanced SA-dependent responses and suppressed JA-dependent responses upon insect attack (Fig. 6). It should be noted that these compounds are constitutively emitted by tomato plants and that the release of β-caryophyllene in particular is highly variable among tomato lines (18, 44). Our study confirms this with the variety Moneymaker having low constitutive emissions (1.9 ng·h−1·g−1) and the two other studied varieties having higher constitutive emission of β-caryophyllene (Zhefen 302: 18.6 ng·h−1·g−1; Zheza 809: 19.8 ng·h−1·g−1). Despite this variability, it seems to be a reliable “priming” signal, as each of the three varieties significantly increased its release of β-caryophyllene in response to whitefly infestation (Fig. 3 and SI Appendix, Fig. S4B), whereas caterpillar attack had no effect on β-caryophyllene emission (Fig. 3B). It appears that β-caryophyllene itself does not serve as a defense against whiteflies (45) but does provide protection against a bacterial pathogen (46). It will be worth testing if β-caryophyllene–mediated priming provides protection against the viruses transmitted by whiteflies.
Similar to β-caryophyllene, β-myrcene is known to be controlled by the JA pathway in other plant species. For instance, in Arabidopsis thaliana, it is a product of At-TPS 10 and is up-regulated upon treatment with Me-JA, but also SA or whitefly infestation (8, 47). In tomato, however, LeMTS2, which was identified as a β-myrcene synthase, is not affected by JA treatment (48). This is consistent with our results, implying that it is controlled by SA and serves as a priming signal in tomato that is characteristic for pathogen infection. Exposure to other volatiles that were typical for pathogen induction also enhanced SA-dependent defenses (SI Appendix, Fig. S3), whereas caterpillar feeding induced the release of volatiles that primed the JA pathway (Fig. 4 and SI Appendix, Fig. S2).
These findings have major implications for our understanding of plant–insect and plant–pathogen interactions. They demonstrate high specificity in the information carried by the inducible volatile blends and indicate that plants use this information to launch a specific response to their attackers. Apparently, B. tabaci is able to interfere with this information transfer, which could be an important reason why this and other whitefly species have been so successful as invasive pests. The plant response may, however, be adaptive if priming of the SA pathway enhances its resistance to the harmful viruses that are commonly transmitted by whiteflies (4, 5). This remains to be determined. It can also not be fully excluded that microorganisms, e.g., bacteria, are involved in the induction of the typical SA up-regulation in response to whitefly infestation. However, each of these possible induction scenarios clearly favors the performance and propagation of the whitefly and may have facilitated its invasiveness and rapid spread. Finally, it would be interesting to study if the original host plants of B. tabaci, which so far remain unknown (49), have coevolved adaptations and show different, perhaps more fitting responses to whitefly attacks.
The observed specificity in odor-mediated defense responses also has important applied implications, as a thorough understanding of the mechanisms and signals that are involved in these plant–plant interactions may lead to strategies that exploit the odorous alert signals to enhance crop resistance (50). For instance, selecting crop varieties that have a more appropriate response to whitefly-induced volatiles may greatly reduce their susceptibility to whiteflies. Alternatively, breeding or otherwise creating varieties that, in responses to whitefly attack, give off volatiles that trigger JA-dependent defenses in nearby leaves may show enhanced resistance against whiteflies.
This study also shows that different pests trigger the release of distinct volatile “odor-prints.” This high specificity provides information that is of use to the plant and associated organisms, but this specificity could also be exploited for crop monitoring if we can develop odor sensors that can capture this specificity (16). Considering the very rapid changes in volatile blends, such sensory systems may alert farmers to the presence of a pest or disease long before any damage is visible and control measures would still be effective. Similar to whiteflies, we may be able to exploit the sophisticated odorous language of plants to optimize the quality of crop plants for consumption by future generations.
Materials and Methods
Plants and Insects.
Tomato plants (Solanum lycopersicum; cv. Moneymaker) were grown in 500-mL pots containing a commercial potting mix (Fafard Growing Mix 1) and were kept in a climate-controlled room [25 ± 2 °C, 60–70% relative humidity (RH), 10-h light:14-h dark photoperiod]. Six-week-old plants with four to five fully expanded leaves were used for experiments. A colony of virus-free (SI Appendix, Fig. S5) B. tabaci (Gennadius) MEAM1 (Hemiptera: Aleyrodidae) was maintained on tomato plants in a separate climate-controlled room (25 ± 2 °C, 50–60% RH, 10-h light:14-h dark photoperiod).
Exposing Plants to Volatiles Emitted from Whitefly-Infested, Caterpillar-Infested, and Bacteria-Infected Plants.
Tomato plants were infested with B. tabaci by placing 150 adult whiteflies on each plant in a ventilated cage (20 × 20 × 40 cm). For the first exposure experiment, the adults were allowed to feed on the plant for 1, 3, 5, or 7 d. Control plants were placed in cages and left uninfested for the same time periods. Subsequent experiments were conducted with plants that were infested with whiteflies for 5 d.
A setup of interconnected glass chambers was used to expose healthy tomato plants to volatiles emitted from whitefly-infested plants. Two emitter plants and two receiver plants were placed in “upwind” and “downwind” glass chambers (23 cm diameter, 40 cm high), respectively. The upwind chamber was connected with a Teflon tube (diameter 7 mm) to the downwind chamber. A small cotton swab, which had no effect on the airflow of the setup, was placed in the Teflon tube connecting the two chambers to prevent the whiteflies from moving from the upwind to the downwind chamber. Charcoal-purified air was pumped into the system at 300 mL·min−1, passing the airflow from the upwind chamber with the two infested plants into the downwind chamber with the two undamaged tomato plants (receiver plants), for 24 h under the growth conditions described above. For control plants, two undamaged plants were used as emitter plants in the upwind chamber.
Similar procedures were followed to expose plants to volatiles from caterpillar-infested and bacteria-infected plants. Briefly, two plants that had been infested with S. exigua (five second-instar larvae per plant) for 24 h or two plants that had been inoculated with P. syringae pv. tomato DC3000 (Pst DC3000; OD600 = 0.1) for 48 h were used as emitter plants. Two undamaged tomato plants were exposed to volatiles from S. exigua-infested or Pst DC3000-infected plants for 24 h. During the exposure, S. exigua larvae were kept on the leaves. In all cases, control plants were exposed to the volatiles from two undamaged plants.
Whitefly, Caterpillar, and Pathogen Treatments.
After 24-h exposure to volatiles emitted from plants preinfested with B. tabaci, exposed plants were kept uninfested, or were infested with B. tabaci (150 adult whiteflies per plant) for 12 or 24 h. Control plants exposed to volatiles emitted from undamaged plants were subject to the same treatments without or with B. tabaci infestation.
Plants exposed for 24 h to volatiles from S. exigua-infested plants were either left undamaged or they were scratched with a razor blade and caterpillar regurgitant was applied to the damaged sites (6 μL per plant), and measurements were taken 6, 12, and 24 h after treatment. Control plants exposed for 24 h to volatiles from undamaged plants were subject to the same treatments without or with damage and caterpillar regurgitant.
Plants exposed for 24 h to volatiles from Pst DC3000-infected plants were either left unharmed or were spray-inoculated with the Pst DC3000 (OD600 = 0.1; see SI Appendix, SI Materials and Methods for details) for 24 and 48 h. Control plants exposed for 24 h to volatiles from undamaged plants were subject to the same treatments without or with Pst DC3000 infection.
For each treatment, leaf tissues collected from two plants were pooled as one sample. Leaf samples were directly frozen in liquid nitrogen and stored at −80 °C for subsequent gene-expression and phytohormone analysis. Each experiment was repeated with three biological replicates.
Insect Preference and Performance Experiments.
To determine the effect of the volatiles from B. tabaci-infested tomato plants on insect resistance in neighboring plants, we assessed the oviposition rates of adults, as well as developmental rates of nymphs on plants exposed for 24 h to volatiles from either B. tabaci-infested or uninfested plants. For the oviposition experiment, 10 B. tabaci adults (five males and five females; 48 h after emergence) were introduced at 0900 into a clip cage that was secured to the abaxial surface of a plant leaf. Fecundity of female adults was recorded 6 d after insect release by counting the number of eggs laid on the leaves under a microscope.
In a separate experiment, 100 B. tabaci adults (a mixture of females and males; 48 h after emergence) were released and allowed to feed on plants exposed for 24 h to volatiles from either B. tabaci-infested or uninfested plants. After 48 h of feeding, adults were removed from the plants by aspiration. The exposed plants that now carried nymphs (produced by the added adults) were exposed again to the volatiles from B. tabaci-infested or undamaged plants for 24 h at day 7 and day 14, after the adults had been added. At 21 d since addition of adults, the number of nymphs and their developmental stages (first through fourth instars) were recorded. Developmental rates on the two treatments were compared by calculating the proportion of fourth-instar nymphs (red-eye stage) on each plant (number of fourth instars/total number of nymphs). Each treatment was represented by 10 replicate plants.
Quantification of Endogenous JA and SA.
Endogenous JA and SA were extracted and quantified as described by Engelberth et al. (51) with modification. In brief, plant material (250–300 mg) was frozen and ground in liquid nitrogen. For quantification purposes, [9, 10]-dihydro-JA (15 ng; Sigma-Aldrich) and D6-SA (20 ng) were added as internal standards with 2 mL of 80% methanol. JA, SA, and the internal standards were partitioned to an aqueous phase by centrifugation and vaporization. Subsequently, they were extracted from the aqueous phase with an equal volume of ethyl acetate and then dried. The dried extract was resuspended in 0.1 M acetic acid and loaded onto a C18 column (Waters Company). The C18 column was sequentially eluted with a series of solvent mixtures [acetic acid/methanol (vol/vol) at 83/17, 60/40, and 40/60]. The effluents of the last 4 mL in 40% methanol and the first 3 mL in 60% methanol were collected (52). After evaporation of the solvent and esterification of the residue using excess ethereal diazomethane. Samples were analyzed using a gas chromatograph coupled to a mass selective detector (6890N/5973 MSD; Agilent Technologies, Inc.), which was operated in electron impact ionization mode. Chemicals were separated on an HP-5-MS column (30 m × 0.25 mm × 0.25 mm; 19091S-433; J&W Scientific, Agilent Technologies). Esterification products of endogenous JA, SA, and their internal standards were analyzed in selected-ion monitoring. The product and precursor ions are m/z 120 and 152 for MeSA, and 123 and 156 for (D4-MeSA) respectively; ions m/z 151 and 224 for MeJA, and m/z 153 and 226 for dh-MeJA. SA and JA were quantified by correlating the peak area (extracted ion) of the compound with the peak area of the respective internal standard. Measurement of D4-MeSA instead of D6-MeSA was due to loss of two deuterium ions during sample preparation (51). All solvents used during extraction procedures were analytical grade (Sigma-Aldrich).
RNA Extraction and cDNA Synthesis.
To minimize wounding- and dehydration-induced gene expression, all leaf samples from different treatments were directly frozen in liquid nitrogen and stored at −80 °C for further RNA extraction. Leaf tissues from two plants were pooled as one sample. Samples were ground to a fine powder in liquid nitrogen with a pestle and mortar. Total RNA was extracted from 100 mg of each leaf sample using a plant RNA isolation kit (Axygen), in accordance with the manufacturer’s instructions. RNA concentration and purity were determined using a NanoDropTM Spectrophotometer ND-2000 (Thermo Scientific), and the integrity of RNA was assessed by 1% agarose gel electrophoresis and ethidium bromide staining. First-stand cDNA was synthesized from 200 ng of RNA using a First-Strand cDNA Synthesis Kit (TaKaRa) in accordance with the manufacturer’s instructions.
Quantitative Real-Time PCR.
The transcript levels of defense-related genes PI-I, PI-II, Mi-1.2, PR-1a, and PR-1b in samples were quantified by real-time quantitative RT-PCR (qRT-PCR). The qRT-PCR was carried out on an ABI 7500 Real Time PCR System with a 96-well rotor. The amplification reactions were performed in a final volume of 20 μL that contained 10 μL of iQ SYBR supermix (Bio-Rad), 0.8 μL of forward primer (5 µM) and reverse primer (5 µM) pairs (SI Appendix, Table S1), and 2 μL of cDNA first-strand template. Thermal cycling conditions were 5 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 15 s at 55–62 °C, and 30 s at 72 °C. Subsequently, a melting curve was recorded between 60 °C and 95 °C with the hold every 5 s. Primers used for qRT-PCR are listed in SI Appendix, Table S1. All reactions were run in duplicate technical replicates, and average values were used in the analysis. Normalized gene expression was calculated using the 2−∆Ct method with GAPDH as an endogenous control gene, and values were subsequently log2 transformed for data analysis.
Volatile Collections and Analysis.
We collected and analyzed the volatile blends of plants infested with B. tabaci or S. exigua, and those infected with Pst DC3000. For the whitefly treatments, plants were infested with B. tabaci (150 adults per plant) for 1, 3, 5, and 7 d, and control plants were left uninfested. For the caterpillar treatment, plants were infested with S. exigua (five second-instar larvae per plant) for 24 and 48 h, and control plants were left uninfested. For the pathogen treatment, plants were spray-inoculated with the Pst DC3000 (OD600 = 0.1) and left for 24 and 48 h, and control plants were sprayed with MgCl2 solution (10 mM). Six-week-old plants with four to five fully expanded leaves were used for volatile collections.
Headspace volatile samples were collected as described in detail by Zhang et al. (53). In brief, two plants of the same treatment were placed together in a 5-L glass jar. Purified air (filtered through silica, a molecular sieve, and activated charcoal) was pulled through the jar at 100 mL·min−1 with a vacuum pump, and volatiles were trapped in a glass tube (10 cm long, 5 mm diameter) that contained 50 mg of 80/100 mesh Porapak-Q (Altech Assoc.). The air-inlet, air-outlet, filter and sampling glass jar were connected with Teflon tubing. After 3 h of trapping under continuous light (4,750 ± 86 Lx), the trap was rinsed with 200 μL of dichloromethane, and 300 ng of nonyl acetate (Sigma) was added as internal standard. For each treatment, volatile collection was repeated five to seven times, and collections were made for each treatment in parallel on each experimental day (replication).
Samples were analyzed with a Shimadzu GC-2010 plus GC-MS (Shimadzu) equipped with an Rxi-5MS (30 m–0.32 mm i.d., 0.25-µm film thickness) column. Column effluent was ionized by electron impact ionization (70 eV). Mass scanning was done from 33 to 300 m/z. The temperature programs of the GC were as follows: 40 °C (3-min hold), 6 °C·min−1 to 220 °C (5-min hold). Compounds were identified by comparing the mass spectra with those of authentic standards or with NIST 08 spectra. Quantification of identified compounds was based on comparison of their peak areas with the internal standard.
Exposing Plants to Synthetic Volatile Compounds.
Plants were also exposed to individual volatile compounds that dominate the blends emitted by B. tabaci-infested plants. For this, β-caryophyllene (purity ≥ 80%; Sigma-Aldrich), β-myrcene (purity ≥ 95%; Sigma-Aldrich), ρ-cymene (purity ≥ 99.5%; Sigma-Aldrich), and α-pinene (purity ≥ 98%; Sigma-Aldrich) were dissolved in dichloromethane at 2 μg/μL, generating four test volatile solutions. To obtain precise release rates, we used dispensers as described by von Mérey et al. (54). Each dispenser consisted of a glass vial with 100 mg of glass wool. A 100-μL volume of a particular solution was pipetted onto the glass wool and then the vials were sealed with a screwcap that had a pierceable septum. Next, a glass capillary (Drummond, Sigma-Aldrich) was inserted through the septum. Depending on the length and internal diameter of the capillary, different release rates from the capillary opening can be obtained. After evaluating different versions, we chose to use dispensers with a 5-µL capillary that was shortened to 4 cm. With these we obtained release rates of β-caryophyllene, β-myrcene, ρ-cymene, and α-pinene of, respectively, 137.7 ± 43.5, 122.9 ± 30.8, 78.0 ± 24.1, 314.3 ± 53.6 ng/h (mean ± SE, n = 4), which were 1.2, 1.3, 1.7, and 0.8 times the release rates of these compounds from two odor-source plants (approximately 10.5 g). To start an experiment, two intact plants and a volatile dispenser loaded with a given compound were placed in the glass vessel (23 cm diameter, 40 cm high) for 24 h, during which charcoal-purified air was pumped into the system at 300 mL·min−1 and passed over the two undamaged tomato plants. Control plants were placed in vessels with dispensers that only contained 100 μL of pure dichloromethane.
Statistical Analysis.
The number of eggs laid by females, and differences in volatile emissions, gene expression, and phytohormone levels were analyzed by one-way ANOVA. If treatments were significant (P < 0.05), Tukey’s multiple-comparison tests were used to analyze significant differences between pairs. Performance of S. exigua and Pst DC3000 on different plants were analyzed with one-way ANOVA. The percentages of fourth instars of B. tabaci on different plants were analyzed using the generalized linear model with binomial distribution and link function logit. Results are presented as the likelihood ratio statistics of the χ2 distribution (55).
Supplementary Material
Acknowledgments
We thank Thomas Degen and Prof. Le Kang for their comments on the manuscript and Prof. Zi-Hong Ye for her advice on statistical analysis. P.-J.Z. received support from National Key R&D Program of China Grant 2017YFD0200400 and National Natural Science Foundation of China Grant 31471779, and T.C.J.T. received support from Swiss National Science Foundation Grant 31003A-122132 and European Research Council Advanced Grant 788949.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818599116/-/DCSupplemental.
References
- 1.Jones DR. Plant viruses transmitted by whiteflies. Eur J Plant Pathol. 2003;109:195–219. [Google Scholar]
- 2.Liu SS, et al. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science. 2007;318:1769–1772. doi: 10.1126/science.1149887. [DOI] [PubMed] [Google Scholar]
- 3.Walling LL. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008;146:859–866. doi: 10.1104/pp.107.113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang T, et al. Begomovirus-whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol Ecol. 2012;21:1294–1304. doi: 10.1111/j.1365-294X.2012.05457.x. [DOI] [PubMed] [Google Scholar]
- 5.Luan JB, et al. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol Lett. 2013;16:390–398. doi: 10.1111/ele.12055. [DOI] [PubMed] [Google Scholar]
- 6.Zarate SI, Kempema LA, Walling LL. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007;143:866–875. doi: 10.1104/pp.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang PJ, et al. Whiteflies interfere with indirect plant defense against spider mites in lima bean. Proc Natl Acad Sci USA. 2009;106:21202–21207. doi: 10.1073/pnas.0907890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang PJ, et al. Phloem-feeding whiteflies can fool their host plants, but not their parasitoids. Funct Ecol. 2013;27:1304–1312. [Google Scholar]
- 9.Su Q, et al. The whitefly-associated facultative symbiont Hamiltonella defensa suppresses induced plant defences in tomato. Funct Ecol. 2015;29:1007–1018. [Google Scholar]
- 10.Rodríguez-Álvarez CI, López-Climent MF, Gómez-Cadenas A, Kaloshian I, Nombela G. Salicylic acid is required for Mi-1-mediated resistance of tomato to whitefly Bemisia tabaci, but not for basal defense to this insect pest. Bull Entomol Res. 2015;105:574–582. doi: 10.1017/S0007485315000449. [DOI] [PubMed] [Google Scholar]
- 11.Zhang PJ, He YC, Zhao C, Ye ZH, Yu XP. Jasmonic acid-dependent defenses play a key role in defending tomato against Bemisia tabaci nymphs, but not adults. Front Plant Sci. 2018;9:1065. doi: 10.3389/fpls.2018.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang PJ, et al. Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J Chem Ecol. 2013;39:612–619. doi: 10.1007/s10886-013-0283-2. [DOI] [PubMed] [Google Scholar]
- 13.Gershenzon J. Plant volatiles carry both public and private messages. Proc Natl Acad Sci USA. 2007;104:5257–5258. doi: 10.1073/pnas.0700906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Mescher MC, De Moraes CM. Role of plant sensory perception in plant-animal interactions. J Exp Bot. 2015;66:425–433. doi: 10.1093/jxb/eru414. [DOI] [PubMed] [Google Scholar]
- 16.Turlings TCJ, Erb M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu Rev Entomol. 2018;63:433–452. doi: 10.1146/annurev-ento-020117-043507. [DOI] [PubMed] [Google Scholar]
- 17.De Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 18.Bleeker PM, et al. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009;151:925–935. doi: 10.1104/pp.109.142661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veyrat N, Robert CAM, Turlings TCJ, Erb M. Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J Ecol. 2016;104:591–600. [Google Scholar]
- 20.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 21.Turlings TCJ, Wäckers FL. Recruitment of predators and parasitoids by herbivore-damaged plants. In: Cardé RT, Millar J, editors. Advances in Insect Chemical Ecology. Cambridge Univ Press; Cambridge, UK: 2004. pp. 21–75. [Google Scholar]
- 22.Dicke M, van Loon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol. 2009;5:317–324. doi: 10.1038/nchembio.169. [DOI] [PubMed] [Google Scholar]
- 23.Arimura G, et al. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 24.Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost CJ, et al. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett. 2007;10:490–498. doi: 10.1111/j.1461-0248.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Saona CR, Rodriguez-Saona LE, Frost CJ. Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. J Chem Ecol. 2009;35:163–175. doi: 10.1007/s10886-008-9579-z. [DOI] [PubMed] [Google Scholar]
- 27.Dicke M, Bruin J. Chemical information transfer between plants: Back to the future. Biochem Syst Ecol. 2001;29:981–994. [Google Scholar]
- 28.Karban R, Shiojiri K. Self-recognition affects plant communication and defense. Ecol Lett. 2009;12:502–506. doi: 10.1111/j.1461-0248.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 29.Karban R, Yang LH, Edwards KF. Volatile communication between plants that affects herbivory: A meta-analysis. Ecol Lett. 2014;17:44–52. doi: 10.1111/ele.12205. [DOI] [PubMed] [Google Scholar]
- 30.Erb M, et al. Indole is an essential herbivore-induced volatile priming signal in maize. Nat Commun. 2015;6:6273. doi: 10.1038/ncomms7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erb M. Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling. Curr Opin Plant Biol. 2018;44:117–121. doi: 10.1016/j.pbi.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA. 2004;101:1781–1785. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kost C, Heil M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol. 2006;94:619–628. [Google Scholar]
- 34.Ton J, et al. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- 35.Conrath U, et al. Prime-A-Plant Group Priming: Getting ready for battle. Mol Plant Microbe Interact. 2006;19:1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- 36.Frost CJ, Mescher MC, Carlson JE, De Moraes CM. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008;146:818–824. doi: 10.1104/pp.107.113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva DB, Weldegergis BT, Van Loon JJA, Bueno VH. Qualitative and quantitative differences in herbivore-induced plant volatile blends from tomato plants infested by either Tuta absoluta or Bemisia tabaci. J Chem Ecol. 2017;43:53–65. doi: 10.1007/s10886-016-0807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson R, Narvaez J, An G, Ryan C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: Effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci USA. 1989;86:9871–9875. doi: 10.1073/pnas.86.24.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Williams MM, Loh YT, Lee GI, Howe GA. Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 2002;130:494–503. doi: 10.1104/pp.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nombela G, Williamson VM, Muñiz M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant Microbe Interact. 2003;16:645–649. doi: 10.1094/MPMI.2003.16.7.645. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs JM, et al. Ralstonia solanacearum requires PopS, an ancient AvrE-family effector, for virulence and to overcome salicylic acid-mediated defenses during tomato pathogenesis. MBio. 2013;4:e00875-13. doi: 10.1128/mBio.00875-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alborn HT, et al. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- 43.Moreira X, Abdala-Roberts L, Castagneyrol B. Interactions between plant defence signalling pathways: Evidence from bioassays with insect herbivores and plant pathogens. J Ecol. 2018;106:2353–2364. [Google Scholar]
- 44.Degenhardt DC, Refi-Hind S, Stratmann JW, Lincoln DE. Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry. 2010;71:2024–2037. doi: 10.1016/j.phytochem.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Sadeh D, et al. Whitefly attraction to rosemary (Rosmarinus officinialis L.) is associated with volatile composition and quantity. PLoS One. 2017;12:e0177483. doi: 10.1371/journal.pone.0177483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang M, et al. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012;193:997–1008. doi: 10.1111/j.1469-8137.2011.04001.x. [DOI] [PubMed] [Google Scholar]
- 47.Li R, et al. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell. 2014;26:4991–5008. doi: 10.1105/tpc.114.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Schie CCN, Haring MA, Schuurink RC. Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol Biol. 2007;64:251–263. doi: 10.1007/s11103-007-9149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown JK, Frohlich DR, Rosell RC. The sweetpotato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex? Annu Rev Entomol. 1995;40:511–534. [Google Scholar]
- 50.Pickett JA, Khan ZR. Plant volatile-mediated signalling and its application in agriculture: Successes and challenges. New Phytol. 2016;212:856–870. doi: 10.1111/nph.14274. [DOI] [PubMed] [Google Scholar]
- 51.Engelberth J, et al. Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal Biochem. 2003;312:242–250. doi: 10.1016/s0003-2697(02)00466-9. [DOI] [PubMed] [Google Scholar]
- 52.Wei J, et al. Antagonism between herbivore-induced plant volatiles and trichomes affects tritrophic interactions. Plant Cell Environ. 2013;36:315–327. doi: 10.1111/j.1365-3040.2012.02575.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang PJ, et al. Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. New Phytol. 2013;197:1291–1299. doi: 10.1111/nph.12106. [DOI] [PubMed] [Google Scholar]
- 54.von Mérey G, et al. Dispensing synthetic green leaf volatiles in maize fields increases the release of sesquiterpenes by the plants, but has little effect on the attraction of pest and beneficial insects. Phytochemistry. 2011;72:1838–1847. doi: 10.1016/j.phytochem.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Zerbino MS, Altier NA, Panizzi AR. Performance of nymph and adult of Piezodorus guildinii (Westwood) (Hemiptera: Pentatomidae) feeding on cultivated legumes. Neotrop Entomol. 2016;45:114–122. doi: 10.1007/s13744-015-0345-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.