Significance
Histone H2AX elicits proper DNA repair through its phosphorylation by ataxia-telangiectasia mutated (ATM) kinase. While ATM senses reactive oxygen species, the role of H2AX in the maintenance of redox homeostasis remains unknown. Here we establish that H2AX deletion leads to impairment of mitochondrial function and repression of the mitochondrial biogenesis gene PGC-1α. Restoring PGC-1α abrogates mitochondrial deficits and mitigates cell death. This study unveils a role for H2AX in mitochondrial homeostasis associated with neuroprotection.
Keywords: mitochondrial homeostasis, neuroprotection, oxidative stress, histone H2AX, DNA repair
Abstract
Phosphorylation of histone H2AX is a major contributor to efficient DNA repair. We recently reported neurobehavioral deficits in mice lacking H2AX. Here we establish that this neural failure stems from impairment of mitochondrial function and repression of the mitochondrial biogenesis gene PGC-1α. H2AX loss leads to reduced levels of the major subunits of the mitochondrial respiratory complexes in mouse embryonic fibroblasts and in the striatum, a brain region particularly vulnerable to mitochondrial damage. These defects are substantiated by disruption of the mitochondrial shape in H2AX mutant cells. Ectopic expression of PGC-1α restores mitochondrial oxidative phosphorylation complexes and mitigates cell death. H2AX knockout mice display increased neuronal death in the brain when challenged with 3-nitropronionic acid, which targets mitochondria. This study establishes a role for H2AX in mitochondrial homeostasis associated with neuroprotection.
Genome integrity is maintained by a number of tightly regulated pathways that lead to efficient DNA repair in response to endogenous or exogenous genotoxic agents (1). Compromising DNA repair or increasing mutation loads can lead to a broad range of human diseases, including those related to immune deficiency, cancer, inflammation, aging, and developmental disorders affecting the nervous system (2–5). During early development, especially when progenitor cells expand and differentiate into mature neurons, efficient DNA repair machinery is crucial (1). Brain is uniquely vulnerable to genomic instability (1, 6, 7). Defects in DNA repair genes often lead to age-associated neurological disorders, but underlying mechanisms are still obscure (1, 7–9). Several neurological disorders originating from deficient DNA repair often involve oxidative stress (1, 6, 10). DNA repair proteins whose deficiencies are associated with oxidative stress in the brain (3, 11) include ataxia-telangiectasia mutated (ATM) kinase, meiotic recombination 11 (MRE11), Nijmegen breakage syndrome 1 (NBS1), aprataxin (APTX), and tyrosyl-DNA phosphodiesterase 1 (TDP1) (1, 3). Murine ATM models facilitate clarification of links between genomic instability and impaired redox homeostasis. When ATM is absent or mutated, the cell suffers from faulty DNA repair and genomic instability and harbors elevated levels of reactive oxygen species (ROS) originating from the impairment of ROS-sensing functions of ATM (12–14). These deficiencies lead to Ataxia telangiectasia (AT). We have shown that diminution of ROS reduces phenotypic damage associated with AT (15). ATM elicits DNA repair by phosphorylating the histone variant H2AX following double-stranded DNA breaks (16). This process is substantiated by the well-established role of H2AX in DNA damage repair (5). Similarities between ATM and H2AX are evident in the phenotypes of their knockout mouse models. In both instances, males are sterile, and there is increased genomic instability evidenced by abnormalities in chromosome structure, immunodeficiency, and enhanced radiosensitivity (12, 17). These similarities may reflect well their common roles in DNA repair. We recently provided evidence that H2AX facilitates redox homeostasis by controlling an NRF2-regulated antioxidant response pathway (18). Mitochondrial defects are a major source of impaired redox homeostasis and are often associated with age-related neurological diseases (19, 20). A major risk factor for several of these diseases is the concomitant dysfunction of mitochondrial activity leading to increased ROS and decreased DNA repair (21). Here we show that H2AX loss leads to substantially diminished mitochondrial activity associated with disturbed mitochondrial shape. These defects reflect repression of the mitochondrial biogenesis gene peroxisome proliferator-activated receptor gamma coactivator 1-aplha (PGC-1α) and the oxidative phosphorylation subunits. Ectopic expression of PGC-1α partially rescued cells from mitochondrial defects and restored toxicity associated with mitochondrial damage. We also report that H2AX mutant mice were uniquely vulnerable to mitochondrial damage in the brain. These findings identify histone H2AX as a key regulator of mitochondrial homeostasis which promotes neuroprotection and clarify links between genomic instability and redox homeostasis in neurodegeneration and aging.
Results and Discussion
Histone H2AX Deficiency Leads to Repression of Mitochondrial Biogenesis Genes and Impairment of Oxidative Phosphorylation.
Deficits of DNA repair genes are associated with diverse forms of neurodegeneration (1). Neurologic disorders arising from deficient DNA repair involve oxidative stress as well as the inability to repair oxidative DNA lesions (6, 8). These features are exemplified in AT, a form of progressive cerebellar degeneration associated with oxidative stress (12, 14). We have shown that diminution of ROS reduces phenotypic damage associated with AT (15). ATM kinase elicits DNA repair by phosphorylating the histone variant H2AX following double-stranded DNA breaks, which is consistent with the well-established role of H2AX in DNA damage repair (5). We observed neurobehavioral defects and impaired redox disposition in mice with genetic deletion of H2AX (18). In the present study, we report a role for H2AX in promoting mitochondrial homeostasis and mediating neuronal health.
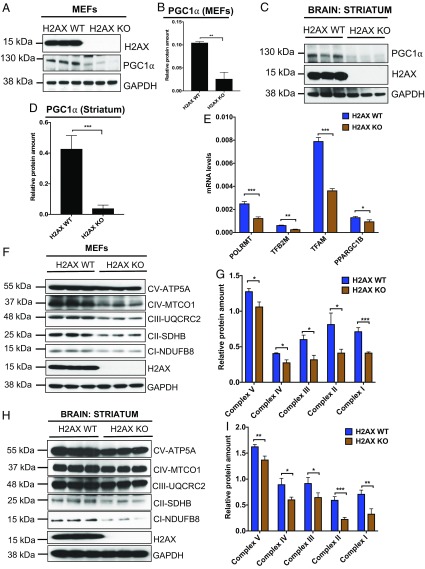
Most mitochondrial proteins are encoded in the nucleus (22). Examples include basal transcription factors such as TFAM, TFB2M, TFB1M, and POLRMT, as well as the mitochondrial biogenesis gene PGC-1α (22, 23). PGC-1α is a transcription coactivator that regulates the expression of TFAM, TFB2M, TFB1M, and POLRMT, as well as the oxidative phosphorylation complex subunits (OXPHOS) through interaction with the transcription factor nuclear respiratory factor 1 (NRF1) (24, 25). It is well established that mitochondrial biogenesis and respiration are stimulated by PGC-1α through induction of NRF1 and NRF2 gene expression (26). Changes in chromatin configuration or alteration of chromatin-based DNA repair pathways are factors in regulating transcription factors (27, 28), but regulatory mechanisms are often unclear. As histone H2AX is a key component of chromatin and an important player in DNA repair pathways, we speculated that loss of H2AX can alter chromatin and DNA damage responses, thereby impacting mitochondrial biogenesis and eliciting oxidative stress. Deletion of H2AX led to pronounced diminution of PGC-1α protein in mouse embryonic fibroblasts (MEFs) as well as the brain (Fig. 1 A–D). Similar decreases occurred for the PGC-1α targets, TFAM, TFB2M, and POLRMT, as well as for PPARGC1B, another homolog of PGC-1α (Fig. 1E). Western blot analysis revealed substantial decreases of the key subunits of the five OXPHOS complexes in both H2AX-deficient MEFs and the brains of mutant mice (Fig. 1 F–I). The reduction in the OXPHOS complexes expression seems more pronounced in the striatum, since only a partial depletion of the OXPHOS complexes I and II was observed in the cortex (SI Appendix, Fig. S1A). Similar pattern of OXPHOS expression was detected in peripheral tissue such as the liver (SI Appendix, Fig. S1B), suggesting an increased vulnerability of the striatum to H2AX loss-induced mitochondrial damage. Taken together, H2AX deletion impairs expression of PGC-1α, the master regulator of mitochondrial biogenesis, leading to alteration of the principal components of mitochondrial function.
Fig. 1.
Histone H2AX depletion elicits pronounced diminution of mitochondrial biogenesis genes and reduced expression of OXPHOS complexes. (A–D) H2AX deletion reduces PGC-1α, a major mitochondrial biogenesis gene. Protein lysates from wild-type and H2AX knockout MEFs, as well as lysates from the striatum, were processed for immunoblot detection of H2AX and PGC-1α. Actin was used as a loading control. (A) Representative image of PGC-1α expression in MEFs and (B) quantification. (C) Representative image of PGC-1α expression in the striatum and (D) quantification. Data are means ± SEM (n = 3). Statistical significance was determined by a two-tailed, unpaired Student’s t test. (E) Analysis of POLRMT, TFB2M, TFAM, and PPARGC1B transcript levels in wild-type and H2AX knockout MEFs by RT-PCR. Expression values are relative fold changes for gene transcripts normalized to GAPDH. Error bars represent SEM (n = 3). (F and G) Western blot analysis of OXPHOS subunits in wild-type and H2AX knockout MEFs. We performed the immunoblot detection using total OXPHOS Human WB Antibody Mixture, enabling concomitant analysis of main proteins of each complex in the electron transport chain, including complex I subunit NDUFB8, complex II subunit 30 kDa, complex III subunit Core 2, complex IV subunit I, and ATP synthase subunit alpha; (F) representative image; (G) quantification. Data are means ± SEM (n = 3). (H and I) Western blot analysis of the OXPHOS subunits in the striatum of 4-mo-old wild-type and H2AX knockout mice; (H) representative image; (I) quantification. Data are means ± SEM (n = 3; *P < 0.05; **P < 0.001; ***P < 0.0001).
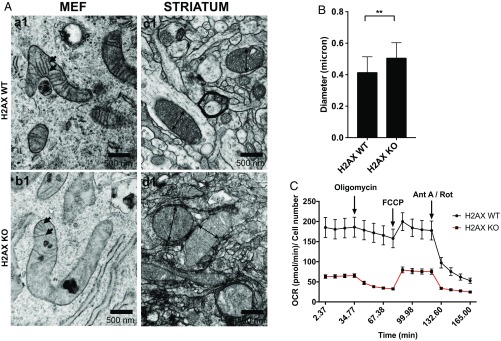
H2AX knockout cells displayed disorganized mitochondrial cristae and slightly enlarged mitochondria (Fig. 2 A and B). Cristae are functionally dynamic compartments, serving as sites for OXPHOS complexes. A substantial amount of the main subunits of the complex III and ATP synthase, and the cytochrome c, are stored in the cristae (29). Shapes of cristae and OXPHOS function are closely linked with direct impact on cellular metabolism (29). The loss of cristae in H2AX mutants fits with the diminished levels of OXPHOS subunits in mutant cells. We monitored OXPHOS function, employing the Seahorse technique. We observed diminished baseline oxygen consumption rate (OCR) in H2AX mutant cells, as well as reduced ATP production and impaired spare respiratory capacity (Fig. 2C).
Fig. 2.
H2AX deletion leads to disorganized cristae, enlarged mitochondria, and impairment of oxidative phosphorylation. (A) Illustrative images of mitochondrial shape of wild-type and H2AX knockout cells in MEFs (a1, b1) and in the striatum (c1, d1) using TEM. Arrows in a1 and b1 indicate mitochondria cristae. Images were taken at 33,000×. (B) Diameter of 30 individual mitochondria in both wild-type and H2AX knockout striatum were quantified. H2AX KO cells display 20% increase in the size of their mitochondrial diameter; **P < 0.001. (C) Significantly higher baseline mitochondrial respiration (0 min to 34 min), ATP production (34.77 min to 70 min), and maximal respiration and sparse respiratory capacity (70 min to 120 min) are observed in H2AX wild-type MEFs compared with H2AX knockout cells. These measurements were assessed using Seahorse MitoStress Kit (n = 5).
Ectopic Expression of PGC-1α Restores OXPHOS Complexes and Reverses Mitochondria Damage-Induced Cytotoxicity.
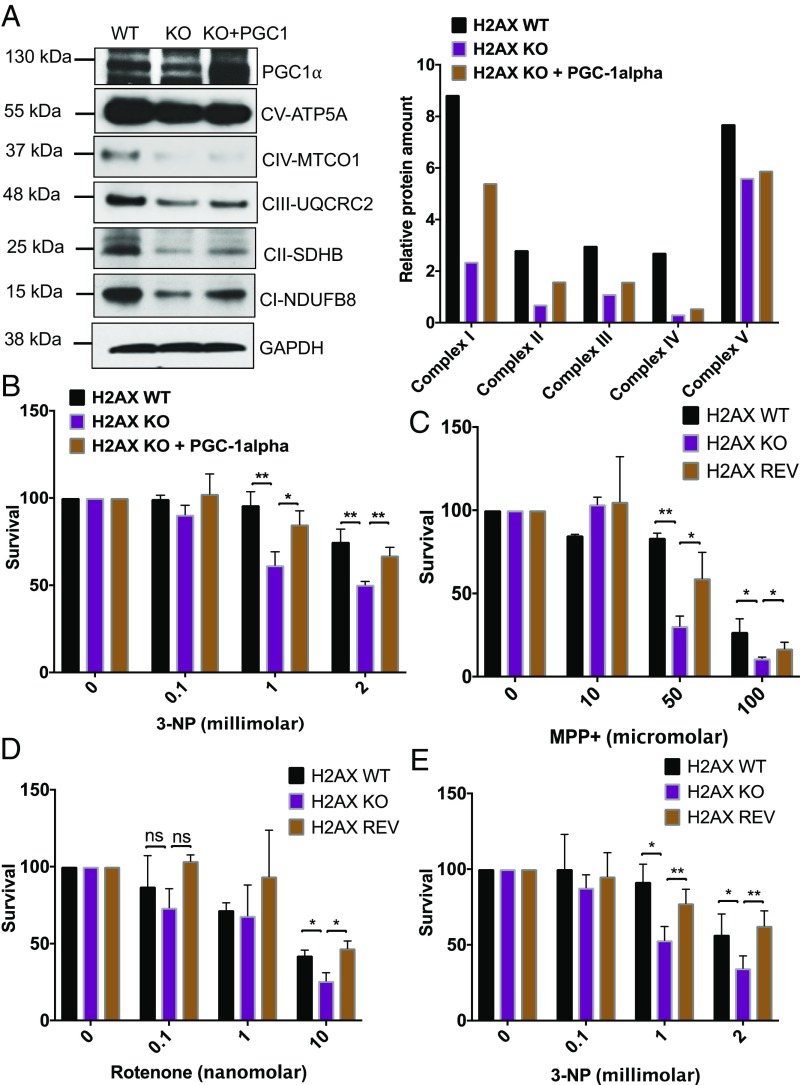
Overexpressing PGC-1α in the H2AX knockout cells partially restored OXPHOS subunit levels (Fig. 3A) and diminished cytotoxicity elicited by the mitochondrial complex II inhibitor 3-nitropropionic acid (3-NP) (Fig. 3B). Enhanced mitochondrial functions, especially those involving the respiratory oxidative phosphorylation complexes, are associated with PGC-1α. PGC-1α is a master integrator of cellular signals that govern mitochondrial biogenesis, oxidative phosphorylation, adaptive thermoregulation, and fatty acid biosynthesis/degradation (22, 30).
Fig. 3.
Ectopic expression of PGC-1α restores OXPHOS subunits and protects from the cytotoxicity induced by mitochondrial damage. (A) Immunoblot analysis of OXPHOS subunits in H2AX wild-type cells (H2AX WT), H2AX knockout cells (H2AX KO), and H2AX knockout cells in which PGC-1α expression was ectopically and transiently restored for 48 h. Actin was used as a loading control. Both wild-type and knockout cells were transfected with control vector. Most of the subunits were partially restored, except for complex IV and complex V. (Left) Representative image. (Right) Quantification. (B) Cells deficient in H2AX are vulnerable to 3-nitropropionic acid (3-NP), an inhibitor of OXPHOS complex II. This cytotoxicity was reversed by ectopic expression of PGC-1α in H2AX knockout cells. Viable cells were quantified using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were treated with increasing doses of 0, 0.1, 1, and 2 mM 3-NP for 48 h before MTT was added. Data are means ± SD; n = 3. Statistical significance was determined by a two-tailed, unpaired Student’s t test. (C–E) Mitochondrial complex inhibitors induce lower survival in H2AX mutant cells, and H2AX ectopic expression mitigates these cytotoxic effects. (C and D) Parental cells (H2AX WT), H2AX knockout cells (H2AX KO), and H2AX knockout cells in which H2AX expression was restored (REV) were treated with increasing concentrations of mitochondrial complex I inhibitors 1-methyl-4-phenylpyridinium (MPP+) and rotenone. Cell survival was estimated 48 h posttreatment using MTT assay. Cells were treated with increasing doses of 0, 10, 50, and 100 μM MPP+, or with 0, 0.1, 1, and 10 nM rotenone for 48 h before MTT was added. (E) Cell vulnerability to 3-nitropropionic acid (3-NP) was analyzed using MTT assay. Cells were treated with increasing doses of 0, 0.1, 1, and 2 mM 3-NP for 48 h before MTT was added. Data are means ± SD; n = 3. Statistical significance was determined by a two-tailed, unpaired Student’s t test; *P < 0.05; **P < 0.001; ns, nonsignificant.
To further assess the link between the loss of H2AX and mitochondrial damage, we explored effects of additional inhibitors of mitochondrial complexes on the viability of cells deficient for H2AX. H2AX knockout cells were vulnerable to inhibitors of mitochondrial OXPHOS complexes I and II with toxicity reversed by overexpressing H2AX (Fig. 3 C–E). These observations show that the deficits observed in H2AX mutants are not the result of other unrelated differences between wild-type and mutant cells. Taken together, these findings establish that the mitochondrial defects induced by H2AX deletion lead to increased cytotoxicity which can be reversed by restoring markers of mitochondrial biogenesis. Histone H2AX is a key component of a cluster of proteins involved in genome stability and chromatin remodeling following DNA double-strand breaks. Examples include proteins such as ATM, NBS1, RAD50, and 53BP1, among others. How changes in chromatin dynamic affect the metabolism of mitochondria via regulation of PGC-1α and its transcriptional targets is unclear.
H2AX Deficiency Increases Vulnerability to 3-Nitropropionic Acid That Targets Mitochondria in the Brain: Role in Neuroprotection.
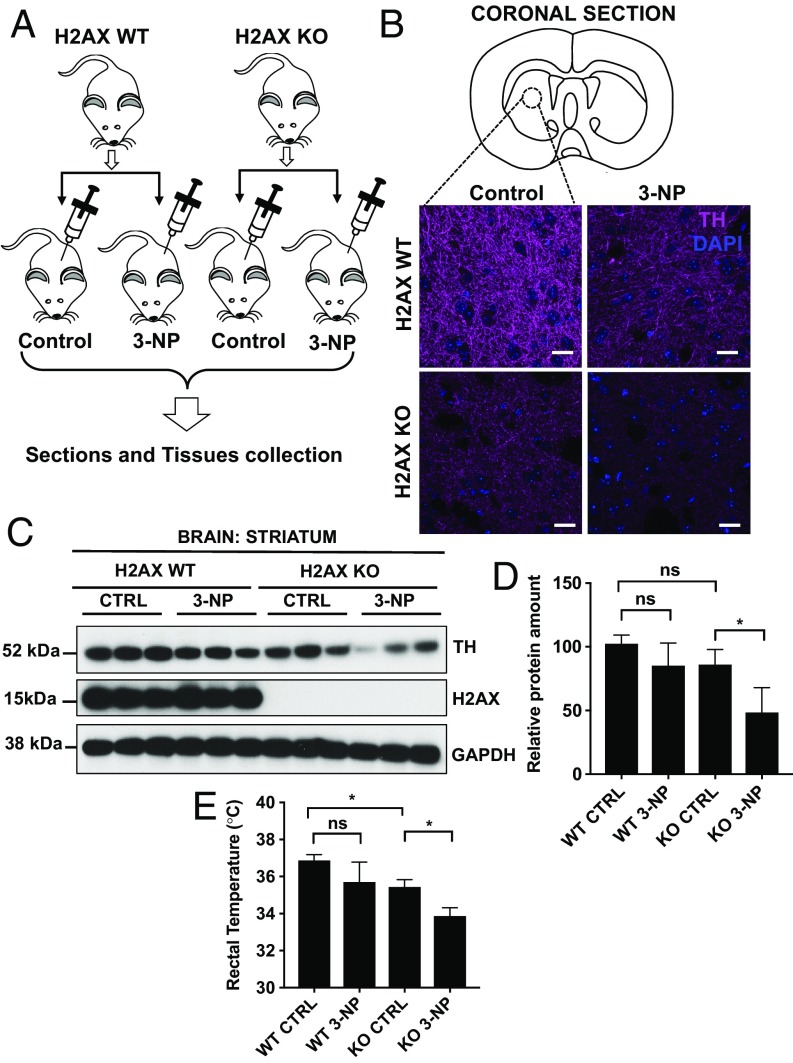
We challenged wild-type and H2AX mutant mice with 3-NP as illustrated in Fig. 4A. In H2AX mutants, acute 3-NP treatment resulted in a 50% loss of striatal dopaminergic nerve terminals as measured by immunostaining and immunoblot analyses of tyrosine hydroxylase (TH) expression (Fig. 4 B–D). These observations were substantiated by signs of systemic mitochondrial impairment in H2AX mutant mice and in mice treated with 3-NP as revealed by measurement of body temperature (Fig. 4E). The damages seem limited to DA neurons, since other populations remain relatively unaffected, as indicated by unchanged NeuN immunoreactivity (SI Appendix, Fig. S2). Several models of neurodegeneration employ drugs that target mitochondrial function (31, 32). For instance, PGC-1α−deficient mice are uniquely vulnerable to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine−induced degeneration of dopamine neurons in the substantia nigra and striatum (25). Overexpressing PGC-1α protects neuronal cells from oxidative stress and cell death (25). Our findings that H2AX knockout mice exhibit increased vulnerability to 3-NP in the striatum suggest enhanced neurodegeneration in mice lacking H2AX following damage to mitochondria, further substantiating evidence that H2AX promotes neuronal health via control of mitochondrial homeostasis.
Fig. 4.
H2AX deficiency leads to increased neuronal damage following mitochondrial damage. (A) Wild-type and H2AX knockout mice were injected with either 3-nitropropionic acid or PBS following instructions described in Materials and Methods. After 24 h, mice were killed, and brain tissues were either fixed in NBF or collected for biochemical analyses. (B) Coronal sections of the brain were taken through the striatum and analyzed for TH-positive cells by immunohistochemistry. Purple, TH; blue, DAPI. (Scale bars, 100 μm.) (C and D) Western blot analysis of TH in the striatum. (C) Representative image; (D) quantification. Data are means ± SD; n = 4 mice per group. Statistical significance was determined by a two-tailed, unpaired Student’s t test. (E) H2AX loss and 3-nitropionic acid treatment cause reduction of body temperature. We measured the rectal temperature in wild-type and H2AX knockout mice. H2AX mutant mice display a significant reduction in body temperature, and this reduction progresses with 3-nitropropionic acid treatment. These changes are reflective of lower mitochondrial activity. Data are means ± SD; n = 4 mice per group. Statistical significance was determined by a two-tailed, unpaired Student’s t test; *P < 0.05; ns, nonsignificant.
In summary, the present study demonstrates that H2AX, a histone variant and classic DNA repair protein, promotes mitochondrial biogenesis and mediates responses to neurotoxic drugs targeting mitochondria. In addition, we show that cells lacking H2AX have impaired oxygen consumption, as well as disturbances in mitochondrial shape. Histone variant H2AX has been studied primarily as a mediator of DNA repair (5). The present study extends the role of this protein to the arena of mitochondrial function and neuronal health. Our findings reveal how alterations in the nuclear genome influence mitochondrial activity and cell metabolism. Other reports describe concomitant defects in DNA repair and mitochondrial activity in neurodegenerative diseases such as AT (7, 33). Several features of aging and neurodegeneration in AT are suppressed by complementation with antiaging compounds or selective activators of mitochondrial biogenesis (7). One of the hallmarks of neurodegeneration is the elevated risk of impaired mitochondrial function combined with the cell’s inability to properly handle DNA lesions. These phenomena occur in diverse diseases as well as during aging (21, 34). Our finding that deficiency of the DNA repair gene H2AX leads to impaired mitochondrial homeostasis and increased neuronal damage reflects a key role for histone H2AX and chromatin-based DNA repair in neurodegenerative diseases and aging. Accordingly, global genomic instability resulting from mutations or deficiencies in DNA repair genes may impact mitochondrial function leading to neurodegeneration.
Materials and Methods
Cell Culture.
MEF from both wild-type and H2AX mutant mice were obtained from the Developmental Therapeutics Branch, Laboratory of Molecular Pharmacology, National Cancer Institute, National Institutes of Health. Cells were grown at 37 °C with 5% CO2 in DMEM (Invitrogen), supplemented with 10% FBS (Atlanta Biologicals). All media were supplemented with 2 mM glutamine, penicillin, and streptomycin (Invitrogen).
Animals.
Animals were housed on a 12-h light−dark schedule and received food and water ad libitum. William Bonner and Andre Nussenzweig (National Cancer Institute/National Institutes of Health) kindly provided the H2AX heterozygote mice. All animals were treated in accordance with the recommendations of the National Institutes of Health and approved by the Johns Hopkins University Committee on Animal Care.
Four to five-month-old animals were used to analyze effects of 3-Nitropropionic acid (3-NP). Injections were based on protocols previously reported for mice (32). In brief, 3-NP (Sigma) was dissolved at 10 mg/mL in sterile 0.1 ml PBS and adjusted to pH 7.4 with sodium hydroxide. For TH detection in the striatum, mice received i.p. injections of 60 mg/kg of 3-NP twice, with 2 h between injections, and tissues were collected 24 h postinjection.
Real-Time PCR.
Total RNA was extracted from cells using RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Quality of RNA preparation, based on the 28S/18S ribosomal RNAs ratio, was assessed using the RNA 6000 Nano Lab-On-chip (Agilent Technologies). Reverse transcription and real-time PCR (RT-PCR) were performed as previously described (35). Oligonucleotides were predesigned and validated, and were considered to be proprietary information by Thermo Fisher Scientific. However, the assays IDs are available and are referenced as follow: PPARGC1B (Mm00504730_m1), TFAM (Mm00447485_m1), TFB2M (Mm01620397_s1), and POLRMT (Mm00553272_m1)
Mitochondrial Stress Assay Using Seahorse.
Mitochondrial function was determined by measuring OCR of each cell line using XF Cell Mito Stress Test Kit (Agilent Technologies). Wild-type and H2AX knockout MEFs were seeded in an XF96 cell culture microplate. Media were prepared by adding 1 mmol/L of pyruvate, 2 mmol/L of glutamine, and 10 mmol/L of glucose and stored as per the manufacturer’s instructions. Seahorse assay was run in XF96 Extracellular Flux Analyzer (Agilent Technologies). Following three baseline OCR measurements, cells were exposed sequentially to oligomycin (0.5 μmol/L), carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP; 1 μmol/L), and rotenone/antimycin A (0.5 μmol/L). Oligomycin inhibits ATP synthase (complex V), and the decrease in OCR following injection of oligomycin correlates with the mitochondrial respiration associated with cellular ATP production. FCCP is an uncoupling agent that collapses the proton gradient and disrupts the mitochondrial membrane potential, allowing cells to achieve maximal OCR. As a result, electron flow through the electron transfer chain is uninhibited, and oxygen is maximally consumed by complex IV. The FCCP-stimulated OCR can then be used to calculate spare respiratory capacity, defined as the difference between maximal respiration and basal respiration. Spare respiratory capacity is a measure of the ability of the cell to respond to increased energy demand. The third injection is a mix of rotenone, a complex I inhibitor, and antimycin A, a complex III inhibitor. This combination shuts down mitochondrial respiration and enables the calculation of nonmitochondrial respiration driven by processes outside the mitochondria. Data were normalized to total cell survival. The assay results were analyzed using Wave program 2.3.0 (Seahorse Bioscience), and data were exported in GraphPad Prism 7.
Brain Tissue Processing and Immunostaining.
Mice were anesthetized by i.p. injection of sodium pentobarbital (80 mg/kg), then the heart was exposed, and transcardiac perfusion was performed using PBS for 5 min followed by ice-cold 10% neutral buffered formalin (NBF; vol/vol) for 30 min (using 3 mL/min rate of perfusion) and maintained in NBF for 24 h. Tissues were transferred to 30% sucrose and maintained at 4 °C for 24 h. Brains were sectioned on a freezing stage sliding microtome into a series of 35-μm sections. Sections were permeabilized with 0.5% Triton X-100 in Tris buffered saline, then blocked with 5% normal goat serum. Mouse anti-TH was used as a primary antibody at the dilution of 1:1,000. Anti-mouse IgG (H+L) Alexa Fluor 647 conjugate was used as a secondary antibody. ZEISS LSM 800 confocal microscopy was used to image immunostained sections.
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism 7 software (GraphPad Software) and Microsoft Excel 2010. Parametric data were analyzed using a two-tailed t test. A value of P < 0.05 was considered statistically significant. Data are presented as mean ± SD or as mean ± SEM.
Generation of cells expressing H2AX-WT [H2AX-rescued cells or revertant (REV)], Western blots, cell viability assay, Transmission Electron Microscopy (TEM), Mitochondrial extraction, and immunoblot can be found in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Roxanne Barrow and Lauren Albacarys of The Solomon H. Snyder Department of Neuroscience (Johns Hopkins University) for help with experiments. We thank Barbara Smith (TEM Specialist at Johns Hopkins School of Medicine) for her help in preparation and imaging of TEM samples. This work was supported by the National Institutes of Health and United States Public Health Service (USPHS) Grants MH18501 and DA000266.
Footnotes
Conflict of interest statement: U.W. and O.A.M. are coauthors on a 2016 article; they did not collaborate directly on the paper. The authors declare no competing financial interests.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820245116/-/DCSupplemental.
References
- 1.McKinnon PJ. DNA repair deficiency and neurological disease. Nat Rev Neurosci. 2009;10:100–112. doi: 10.1038/nrn2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10:S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 3.McKinnon PJ. Maintaining genome stability in the nervous system. Nat Neurosci. 2013;16:1523–1529. doi: 10.1038/nn.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 5.Bonner WM, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subba Rao K. Mechanisms of disease: DNA repair defects and neurological disease. Nat Clin Pract Neurol. 2007;3:162–172. doi: 10.1038/ncpneuro0448. [DOI] [PubMed] [Google Scholar]
- 7.Fang EF, et al. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canugovi C, Misiak M, Ferrarelli LK, Croteau DL, Bohr VA. The role of DNA repair in brain related disease pathology. DNA Repair (Amst) 2013;12:578–587. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan SF, et al. ATM-dependent phosphorylation of MEF2D promotes neuronal survival after DNA damage. J Neurosci. 2014;34:4640–4653. doi: 10.1523/JNEUROSCI.2510-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Souza AD, Parish IA, Krause DS, Kaech SM, Shadel GS. Reducing mitochondrial ROS improves disease-related pathology in a mouse model of ataxia-telangiectasia. Mol Ther. 2013;21:42–48. doi: 10.1038/mt.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fakouri NB, et al. Toward understanding genomic instability, mitochondrial dysfunction and aging. FEBS J. September 20, 2018 doi: 10.1111/febs.14663. [DOI] [PubMed] [Google Scholar]
- 12.Barlow C, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 13.Barlow C, et al. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci USA. 1999;96:9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 15.Weyemi U, et al. NADPH oxidase 4 is a critical mediator in ataxia telangiectasia disease. Proc Natl Acad Sci USA. 2015;112:2121–2126. doi: 10.1073/pnas.1418139112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 17.Celeste A, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weyemi U, et al. Histone H2AX deficiency causes neurobehavioral deficits and impaired redox homeostasis. Nat Commun. 2018;9:1526. doi: 10.1038/s41467-018-03948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Cho DH, Lipton SA. Redox regulation of protein misfolding, mitochondrial dysfunction, synaptic damage, and cell death in neurodegenerative diseases. Exp Neurol. 2012;238:12–21. doi: 10.1016/j.expneurol.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura T, Lipton SA. ‘SNO’-storms compromise protein activity and mitochondrial metabolism in neurodegenerative disorders. Trends Endocrinol Metab. 2017;28:879–892. doi: 10.1016/j.tem.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Leigh-Brown S, Enriquez JA, Odom DT. Nuclear transcription factors in mammalian mitochondria. Genome Biol. 2010;11:215. doi: 10.1186/gb-2010-11-7-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruas JL, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 27.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weyemi U, et al. The histone variant H2A.X is a regulator of the epithelial-mesenchymal transition. Nat Commun. 2016;7:10711. doi: 10.1038/ncomms10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cogliati S, Enriquez JA, Scorrano L. Mitochondrial cristae: Where beauty meets functionality. Trends Biochem Sci. 2016;41:261–273. doi: 10.1016/j.tibs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 30.LeBleu VS, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 32.Mealer RG, Subramaniam S, Snyder SH. Rhes deletion is neuroprotective in the 3-nitropropionic acid model of Huntington’s disease. J Neurosci. 2013;33:4206–4210. doi: 10.1523/JNEUROSCI.3730-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Browne SE, et al. Treatment with a catalytic antioxidant corrects the neurobehavioral defect in ataxia-telangiectasia mice. Free Radic Biol Med. 2004;36:938–942. doi: 10.1016/j.freeradbiomed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Sedelnikova OA, et al. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100. doi: 10.1111/j.1474-9726.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- 35.Weyemi U, et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.