Significance
Mechanosensory cells convert environmental mechanical stimuli into intracellular signals. This process, termed mechanotransduction, occurs in specialized mechanoreceptive organelles. Using electron tomography we discovered that the mechanoreceptive organelle in fly campaniform mechanoreceptors contains thousands of force-sensitive ion channels that are arranged in a regular pattern, aligned to the intracellular microtubule cytoskeleton. A mechanical model suggests that the pattern is structurally and functionally optimized, because more force-sensitive channels are located at regions that are subject to larger activating forces. We propose that such a pattern enhances the sensitivity and broadens the dynamic range of mechanosensation in this type of mechanoreceptor.
Keywords: NompC, microtubule, mechanoreceptive organelle, electron tomography, mechanoreceptor
Abstract
Mechanoreceptive organelles (MOs) are specialized subcellular entities in mechanoreceptors that transform extracellular mechanical stimuli into intracellular signals. Their ultrastructures are key to understanding the molecular nature and mechanics of mechanotransduction. Campaniform sensilla detect cuticular strain caused by muscular activities or external stimuli in Drosophila. Each campaniform sensillum has an MO located at the distal tip of its dendrite. Here we analyzed the molecular architecture of the MOs in fly campaniform mechanoreceptors using electron microscopic tomography. We focused on the ultrastructural organization of NompC (a force-sensitive channel) that is linked to the array of microtubules in these MOs via membrane-microtubule connectors (MMCs). We found that NompC channels are arranged in a regular pattern, with their number increasing from the distal to the proximal end of the MO. Double-length MMCs in nompC29+29ARs confirm the ankyrin-repeat domain of NompC (NompC-AR) as a structural component of MMCs. The unexpected finding of regularly spaced NompC-independent linkers in nompC3 suggests that MMCs may contain non-NompC components. Localized laser ablation experiments on mechanoreceptor arrays in halteres suggest that MMCs bear tension, providing a possible mechanism for why the MMCs are longer when NompC-AR is duplicated or absent in mutants. Finally, mechanical modeling shows that upon cuticular deformation, sensillar architecture imposes a rotational activating force, with the proximal end of the MO, where more NOMPC channels are located, being subject to larger forces than the distal end. Our analysis reveals an ultrastructural pattern of NompC that is structurally and mechanically optimized for the sensory functions of campaniform mechanoreceptors.
The conversion of mechanical signals into electrical signals in cells, known as mechanotransduction, is required for the perception of sound, touch, and acceleration (1, 2). Mechanotransduction occurs much more rapidly than visual phototransduction or olfactory transduction (2, 3). This suggests that in mechanotransduction mechanical stimuli are directly converted to intracellular signals rather than through a second messenger as in visual and olfactory transduction (1, 2, 4). Based on electrophysiological and mechanical measurements, it has been hypothesized that the mechanotransduction apparatus contains a transduction channel coupled to a molecular spring (3, 5). Mechanical signals are conveyed to the transduction channel by the spring, and the channel responds by changing the opening probability of its pore, through which ion influx initiates electrical signals (1). The molecular spring is a compliant structure and its compliance has two functions. First, it matches the mechanical impedance of rigid structures, such as intracellular cytoskeleton or extracellular matrix, to that of more compliant structures, such as the channel’s gating apparatus. Second, it allows the channel’s gate to fluctuate between open and closed states, thereby encoding incoming stimuli into graded signals. For these reasons, this compliant structure has been termed the “gating spring” (1, 5). A key question is how the mechanotransduction apparatus operates in vivo: How are external forces conveyed to the gating spring and how does the gating spring in turn couple these forces to the channel?
In mechanosensory cells, mechanoreceptive organelles (MOs) are specialized subcellular entities where the transduction apparatuses reside and function. The ultrastructural architectures of MOs have been studied in various model cells, including the hair bundles of inner ear hair cells (4), the microtubule-based dendrites of Caenorhabditis elegans touch cells (6, 7), and the ciliated dendrites of fly type I mechanoreceptors (8–11). These studies provide structural insights into the molecular basis of mechanotransduction in these types of mechanoreceptors.
Campaniform mechanoreceptors are type I insect mechanoreceptors whose dendrites contain a modified cilium (8, 12). They respond to cuticular strain caused by muscular activities or external stimuli to provide mechanosensory feedback during locomotion (13). In Drosophila, campaniform mechanoreceptors at different locations (wing, haltere, leg, etc.) vary in their cuticular, supporting, and neuronal structures (14, 15). This morphological diversity is thought to be important for these receptors to detect different types of cuticular strains caused by their natural stimuli. The MOs of campaniform mechanoreceptors are located at the distal tips of the modified cilia (10, 12). In previous work, the MOs of pedicellar campaniform mechanoreceptors of fly halteres were studied by transmission electron microscopy (TEM) (10, 16), using thin sections and glutaraldehyde fixation. A set of serially connected structures in the MO is thought to form a mechanical signaling pathway that links extracellular structures to the transduction channels (16). In particular, the ankyrin-repeat (AR) domain of NompC (NompC-AR) was found to contribute structurally to a membrane–microtubule connector (MMC). Based on structural and mechanical analyses, we proposed a molecular model in which a transduction channel (i.e., NompC) is connected to a molecular spring (i.e., NompC-AR) (17, 18), as predicted by the “gating-spring” model (1). The finding of this NompC–microtubule complex is consistent with other findings on NompC, including those showing that NompC is a bona fide force-sensitive ion channel (19, 20), that it is mechanically important in fly hearing (9, 21), and that NompC-AR is required for microtubule binding and mechanosensory gating of NompC channels (22, 23). Most recently, Jin et al. (24) reported the atomic structure of NompC resolved by cryo-EM, which showed that NompC is a homotetrameric channel and, most strikingly, that four NompC-ARs form an AR bundle. The new NompC structure raises many questions (25), especially how it relates to the architecture of the transduction apparatus in the MO of fly mechanoreceptors.
Earlier studies of the MOs of campaniform mechanoreceptors by conventional TEM suffered from technical limitations. For example, the glutaraldehyde fixation increased the risk of tissue disruption, in particular to membranes and fine filaments (SI Appendix, Fig. S1). In addition, the low z-resolution of thin-section-based TEM (50 to 100 nm) and the high likelihood that cutting oblique sections (SI Appendix, Fig. S2) made structural measurements inaccurate. These limitations made it difficult to compare the wild-type and mutant structures and thereby precluded more detailed functional and mechanical studies. Therefore, new techniques are required to further determine MO ultrastructure.
In the present study, we used high-pressure freezing (HPF) and dual-axis electron tomography (ET) (26) to analyze the 3D ultrastructure of MOs in fly campaniform mechanoreceptors (SI Appendix, Supplementary Note 1). We found that NompC channels are arranged in a regular pattern on the MO membrane and their number increases from the distal to the proximal end of the MO. Mechanical modeling showed that as a product of the sensillar architectures the MO is strained by a rotational activating force. In this model, the proximal end of the MO, where more NompC are located, receives larger forces than the distal end. Therefore, the spatial pattern of NompC matches the distribution of the activating forces on the MO, suggesting a structural and mechanical optimization for the sensory function of the MOs in fly campaniform mechanoreceptors. Additional structural analysis on nompC mutants confirmed NompC-AR as a component of MMCs and unexpectedly revealed regularly spaced NompC-independent linkers, suggesting that MMCs may also contain non-NompC components.
Results
Sensillar Structures of the Modified Cilium.
Halteres are the fly’s gyroscopes. Forces produced by rotations of the fly body during flight generate stresses at the bases of the rapidly oscillating halteres; the resulting strains are sensed by several arrays of campaniform mechanoreceptors in the pedicel and scabellum segments of the haltere (SI Appendix, Fig. S3). To understand the 3D ultrastructure of MOs in these mechanoreceptors, we applied serial block-face imaging with an FIB/SEM (focused ion beam/scanning electron microscope). Campaniform mechanoreceptors in haltere pedicel and scabellum arrays showed similar morphological organizations but differed in their cuticular, supporting, and neuronal architectures (Fig. 1, SI Appendix, Fig. S4, and Movies S1–S4). These results agree with the previous observations using conventional TEM and SEM but provide 3D structures for the entire sensillum (14, 15).
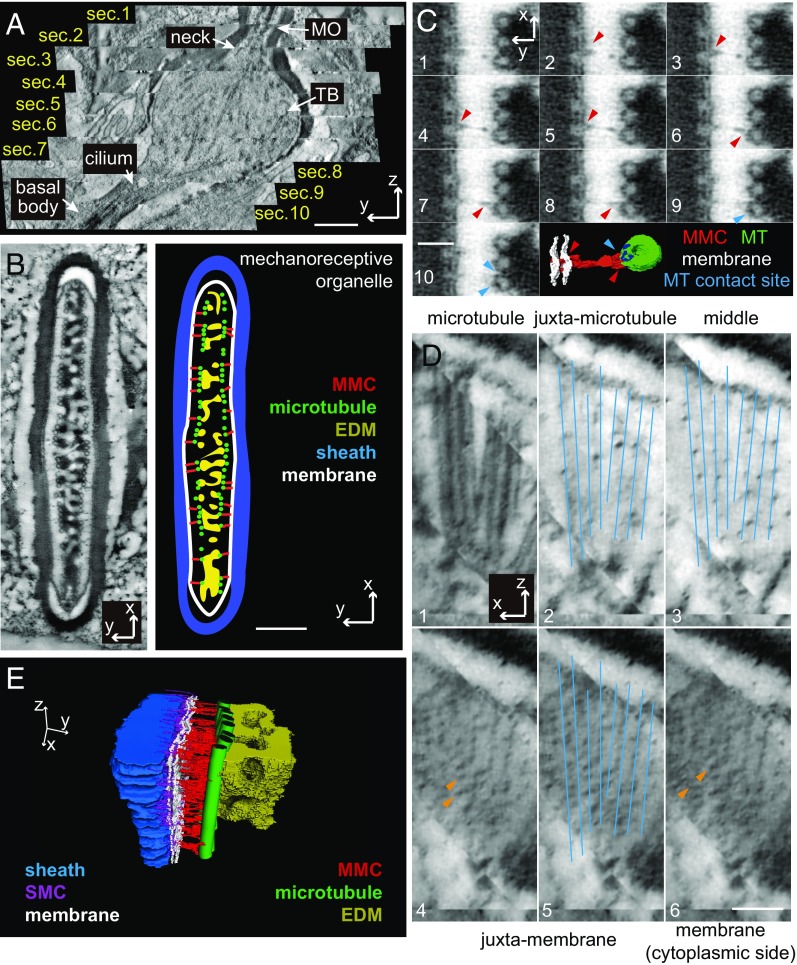
Fig. 1.
Sensillar organization of the modified cilium in a pedicellar campaniform mechanoreceptor of the fly haltere. (A and B) Two representative lateral views of a modified cilium in a campaniform mechanoreceptor taken from the FIB/SEM volume data. The structures are reproduced and presented as cartoon schematics on the right. The colors indicate different structures. The numbers label different segments of the modified cilium (outlined in black). The original volume data are shown in Movie S1. The axes are defined in the main text and shown in each panel. (Scale bars: 1 μm.) (C) The structural model segmented from the volume data. The 3D segmentation is presented in Movie S2. Note that the volume data of a haltere scabellum campaniform mechanoreceptor and the segmented model are also presented (SI Appendix, Fig. S4 and Movies S3 and S4). TB, tubular body. (Scale bar: 1 μm.)
In the present study, we focused on campaniform mechanoreceptors in haltere pedicel arrays (Fig. 1). In this type of mechanoreceptor, the cupola is connected to the cuticle via joint membranes and overlies the septum, dendritic sheath, and MO (Fig. 1 A and B). The dendritic terminal of the sensory cell is specialized into a modified cilium and abuts the cuticular structures. The MO of campaniform mechanoreceptor is located at the distal tip of modified cilium (12). The entire sensillar structure is better visualized in 3D models of the different components (Fig. 1C and Movie S2). The 3D structure of a campaniform mechanoreceptor from the haltere scabellum is shown for morphological comparison (SI Appendix, Fig. S4 and Movies S3 and S4). To better describe the 3D structures, we defined three axes: The z axis (Fig. 1A) is perpendicular to the cuticle surface and is directed into the interior of the tissue; the x axis (Fig. 1A) and y axis (Fig. 1B) are parallel to the longer and shorter axes of the MO. Based on these coordinates, the MO showed a fan shape in the x–z plane (Fig. 1A), a finger shape in the y–z plane (Fig. 1B), and a round-ended rectangular or elliptical shape in the x–y plane (Fig. 2B and Movie S1).
Fig. 2.
Ultrastructure of MO and MMC. (A) A representative lateral view of a modified cilium reconstructed from the ET data. The entire volume data are reconstructed by stitching 10 sections in series. The ET volume data after alignment and stitching are shown in Movie S5. (Scale bar: 0.5 μm.) (B) A representative cross-sectional view of a MO. The structural elements are reproduced and presented in a cartoon schematic (Right). EDM, electron dense materials. (Scale bar: 0.25 μm.) (C) Ten continuous slices (each has a thickness of about 1 nm) showing the morphology of a MMC. The red arrowheads indicate the branched ends of this MMC on both the membrane and microtubule ends. The microtubule contact sites of MMCs are labeled in blue and indicated with blue arrowheads. (Scale bar: 50 nm.) (D) The x–z plane views of a local area in the MO. The visualization planes are shifted from the microtubules to membrane (panels 1–6). Blue lines are microtubule outlines and orange arrowheads indicate the button-shaped structures, together showing that the buttons are aligned to microtubules. Note that in panel 3 the small-density dots represent the cross-sectional views of the MMCs. (Scale bar: 100 nm.) (E) The segmented structural model for a local region in the MO. The 3D model is presented in Movie S6.
Structures of Sheath–Membrane Connectors and MMCs.
To reconstruct the intracellular structures of the modified cilium with higher resolution, we used ET and HPF. We collected tilt-series ET data on 250- or 300-nm tissue sections. Ten sections in series were stitched in 3D space to reconstruct the entire modified cilium (Fig. 2A and Movie S5). Taking advantage of the ET volume data, we corrected the potential problem of oblique sections by adjusting the visualization plane for each structure of interest in 3D space (SI Appendix, Fig. S2).
We focused on the ultrastructure of the MO (Fig. 2B), and in particular the sheath–membrane connectors (SMCs) and MMCs, because these two linkers are likely essential elements in forming the mechanical signaling pathway in the mechanotransduction apparatus of campaniform mechanoreceptors (10). In our ET data, SMCs had a filamentous shape and inserted deeply into the sheath matrix (SI Appendix, Fig. S5); these features differ from the “short column”-like shape observed in our previous study (10). The SMC filaments had a density of about 4,000 to 5,000 μm−2, thereby forming a dense connective layer between the membrane and sheath (SI Appendix, Fig. S5).
In our ET data, MMCs appeared to be thin and filamentous connectors that linked the membrane to the microtubules (Fig. 2C; also see SI Appendix, Fig. S6). They were often branched at both the membrane and microtubule ends (Figs. 2C and 3H and SI Appendix, Fig. S6). Most strikingly, we found that each MMC was linked to a button-shaped structure on the MO membrane (Fig. 2 C and D). Collectively, these buttons were aligned, through MMCs, to the arrayed microtubules and formed a 2D array on the membrane (Fig. 2D). The spatial periodicity between adjacent buttons was ∼28 nm along the x axis, similar to the intermicrotubule distance (10) and ∼18 nm along the longitudinal axis of microtubules (Fig. 3F). The overall density of these buttons on the MO membrane was 2,032 ± 323 μm−2 in wild-type cells (n = 4). A segmented model for the local structures is presented for better visualization (Fig. 2E and Movie S6).
Fig. 3.
Button-shaped structures and MMCs in wild-type and nompC3. (A) The x–z plane views of MO membrane in wild-type (Upper), nompC3 (Middle), and nompC29+29ARs (Lower). The genotype of nompC29+29ARs: nompC2, uas-nompC29+29ARs-GFP/nompC2; +/nompC-gal4. Both nompC3 and nompC2 are null alleles of the nompC gene. Red arrows indicate the button-shaped structures in wild-type and nompC29+29ARs or the dot densities in nompC3. (Scale bar: 100 nm.) (B) The size distributions of button-shaped structures in wild-type and small dots in nompC3. We used the diameters of the bounding circles to quantify the sizes of these structures. (C) Size measurements of NompC transmembrane domains (NompC-TMs). Atomic model: Protein Data Bank ID code 5VKQ. (Left) The channel domain or linker domain of NompC has a size of 6 to 9 nm (edge length of the bounding squares). (Right) The bounding circle of NompC channel domain has a diameter of ∼11 nm. (D) Representative images of MOs in x–y planes show that nearly every microtubule is associated with a MMC in wild-type and nompC3. (Scale bar: 100 nm.) (E) Representative images of the local regions in MOs in x–z planes (between the microtubule plane and membrane plane) show that MMCs (Lower) are aligned to microtubules (Upper). Both wild-type and nompC3 data are presented for comparison. (Scale bar: 100 nm.) (F) The distributions of the spatial interval (d) between adjacent MMCs along microtubules in wild-type and nompC3. (G) The statistical comparison of d in wild-type (black), nompC3 (blue), and nompC29+29ARs (red) strains. (H) The representative x–y plane images for several wild-type MMCs. The last image in each panel contains the segmentation of a typical MMC (red). Note the branched ends of wild-type MMCs on both membrane and microtubule ends (red arrowheads). (Scale bar: 100 nm.) (I) Representative x–y plane images for several linkers in nompC3. The segmentation (red) of a linker is presented in the last image. Note that most of the linkers have no branched ends. (Scale bar: 100 nm.) (J) The morphological classifications of MMCs in wild-type and nompC3. Representative images of wild-type MMCs are shown in the insets (1–4). In these insets, the membrane is on the left side and the microtubule is on the right side. Several other representative images of MMCs in wild-type and nompC3 are presented in SI Appendix, Fig. S6.
NompC Channels Structurally Contribute to the Bulk of Button-Shaped Structures.
Based on the observations described above and in the previous studies (10, 22), we wondered if the button-shaped structures that are linked to MMCs correspond to or contain the channel domains of NompC. In wild-type, these buttons were arranged in an array (Fig. 3A). They had an average diameter of 10.3 ± 1.6 nm (n = 364 buttons from n = 4 cells) (Fig. 3B), similar to the size of NompC channel domain (Fig. 3C). Interestingly, these button-shaped structures were nearly absent in nompC3, a nompC null mutant (Fig. 3A). In nompC3, we observed small dots on the membrane (Fig. 3A). These dots were smaller in size compared with the buttons in wild-type flies (Fig. 3 A and B). We also studied nompC29+29ARs flies in which the wild-type NompC is replaced with one containing two serially linked AR domains on the amino terminus but an unchanged channel domain (22). In this mutant, the button-shaped structures were present, similar to wild-type cells (Fig. 3A). Based on these results, we conclude that the channel domain of NompC structurally contributes to the bulk of membrane-associated button. This observation shows that NompC are arrayed on MO membrane by aligning to the microtubules via MMCs.
NompC-Independent Linkers in nompC3 Are Regularly Spaced but Have Different Structures from Wild-Type MMCs.
We then wondered what the small dots in nompC3 are. Could they possibly correspond to the membrane-contacting structures of the irregularly spaced NompC-independent linkers found in previous studies (10, 22)? Therefore, we checked the linkers in nompC3 in the x–y planes. Surprisingly, we found that in ET data, the membrane-microtubules linkers in nompC3 were regularly spaced (Fig. 3D), different from previous observations in TEM data (10).
To better understand these linkers, we analyzed their spatial distribution and structure. In the x–y plane, nearly every microtubule was associated with an MMC in both wild-type and nompC3 (Fig. 3D). In addition, the spatial density of these linkers along the z axis (Fig. 3 E and F) was similar in wild-type, nompC3, and nompC29+29ARs (Fig. 3G). We then compared the morphology of individual linkers in wild-type and nompC3 (Fig. 3 H–J and SI Appendix, Fig. S6). Wild-type MMCs were morphologically heterogeneous (Fig. 3H), so we classified them into four types (Fig. 3J): Type 1 has no branched ends, type 2 has branched ends only on membrane side, type 3 has branched ends only on microtubule side, and type 4 has branched ends on both sides. In wild-type, over 60% of MMCs were branched at both sides (i.e., type 4) and more than 90% were branched at one end or the other (i.e., type 2, type 3, and type 4) (Fig. 3J). In a sharp contrast, nearly 80% of the linkers in nompC3 fell into type 1 (i.e., no branched ends on either side) (Fig. 3 I and J and SI Appendix, Fig. S6). Therefore, NompC-independent linkers have a spatial distribution similar to wild-type MMCs, but they appear to have a different structure.
We were curious why fewer linkers were observed in nompC3 in previous studies (10, 22). We suspected that these linkers might be more susceptible to the glutaraldehyde-based sample preparation. Therefore, we imaged the glutaraldehyde fixed wild-type and nompC3 samples by ET (SI Appendix, Fig. S7). Interestingly, we found that wild-type samples showed regularly spaced MMCs (SI Appendix, Fig. S7). However, the linkers in nompC3 were nearly absent (SI Appendix, Fig. S7), recapitulating our previous results (10). These observations suggest that the glutaraldehyde fixation-based sample preparation is likely a major reason for the significant disappearance of linkers in nompC3. The susceptibility to the glutaraldehyde-based method suggests that NompC-independent linkers are less stable in comparison with wild-type MMCs.
The Number and Length of MMCs in Wild-Type.
Having confirmed the presence of NompC-independent linkers, we wondered if this observation would argue against the previous conclusion that NompC-AR structurally contributes to MMCs or, on the contrary, suggest that MMCs contain non-NompC components in addition to NompC-AR. To address this issue, we measured the numbers and lengths of MMCs in the MO of wild-type, nompC29+29ARs, and nompC3 cells (Fig. 4 A–C and SI Appendix, Fig. S8).
Fig. 4.
The numbers and lengths of MMCs in wild-type and mutant MOs. (A–C) The x–y plane views of distal (Upper), middle (Middle), and proximal (Lower) regions of a representative MO in wild-type (A), nompC29+29ARs (B), and nompC3 (C). Note in the wild-type MO the membrane is loosely connected to the sheath in the distal region (Upper) and at two sides of MO (along the x axis) in all three planes. Another set of raw ET images for each genotype is provided in SI Appendix, Fig. S8. (Scale bars: 100 nm.) (D) The number of MMCs is lowest at the distal region and increases toward the proximal end in wild-type (n = 4, black), nompC3 (n = 4, blue), and nompC29+29ARs (n = 5, red). The data are presented as mean ± SD. (E) The length distribution of wild-type MMCs. (F) The lengths of wild-type MMCs at different positions along x axis in the distal, middle, and proximal regions of the MO (blue circles: distal; red squares, middle; green triangles, proximal). The lines are fitting curves to show the location-dependent length change. (G) The average lengths of MMCs change with their z-positions in wild-type (n = 4, black), nompC3 (n = 4, blue), and nompC29+29ARs (n = 5, red). The data are presented as mean ± SD. Note that the average lengths of MMCs in nompC29+29ARs and nompC3 in all z-positions are longer than wild-type MMCs.
The number of MMCs in the MOs varied with z-position, reflecting the MO geometry. The distal region had the least number of MMCs. Toward the proximal side, the number of MMCs first increased then stayed relatively constant through the depth of the MO and only slightly decreased near the proximal end (Fig. 4D). Both nompC29+29ARs and nompC3 had numbers of MMCs similar to wild-type (Fig. 4D).
The mean length of wild-type MMCs was 42.7 ± 13.5 nm (n = 416 MMCs from four cells). The length distribution was broad, ranging from 20 to 80 nm (Fig. 4E). The large variation was primarily because MMCs at different locations had different lengths. There were two regular patterns. First, in x–y planes, the lengths of MMCs in the middle region of MO were longer than those on two sides (Fig. 4 A and F and SI Appendix, Fig. S8). Second, along the z axis, the average length of MMCs first increased and then stayed relatively constant until the proximal end of the MO (Fig. 4G). We noticed that at all z-positions MMCs showed a similar range of lengths (Fig. 4F and SI Appendix, Fig. S8), suggesting that the increase in the mean length of MMCs is primarily due to the increase in the ratio of longer MMCs.
We also noticed a spatial correlation between the length of MMCs and the local membrane–sheath contact. In the distal part of the MO, the membrane only loosely connected to the sheath (Fig. 4A) and appeared to be closer to the microtubules. In these regions, the MMCs were mostly shorter. On the contrary, in the proximal part of the MO, where most of the membrane was tightly attached to the sheath (Fig. 4A), many more MMCs were longer. Similarly, in the x–y planes at all z-positions the membrane on two sides of the MO (x axis) only loosely connected to or even detached from the sheath. In these regions, the MMCs were generally shorter than those in the middle region, where the sheath had a tighter contact with the membrane (Fig. 4A). Thus, the proximity between the sheath and membrane varies with both the z- and x-positions.
MMCs Are Longer in nompC29+29ARs and nompC3.
Having measured the length of wild-type MMCs, we then analyzed the MMCs in nompC29+29ARs. If NompC-AR contributes to the MMCs, then doubling the length of NompC-AR should increase the length of the MMCs. The length distribution of MMCs in nompC29+29ARs showed a location-dependent pattern, similar to wild-type MMCs (Fig. 4B and SI Appendix, Fig. S8). The mean lengths of MMCs in nompC29+29ARs were twice as long as wild-type MMCs at nearly all z-positions (Fig. 4G). The maximum width of MO increased from 268.3 ± 55.2 nm (n = 4 cells) in wild-type to 406.6 ± 64.9 nm (n = 5 cells) in nompC29+29ARs. Two controls supported this finding. First, to rule out the possibility that the expansion of MOs might be an artifact caused by ET sample preparation, we imaged live campaniform receptors in freshly dissected halteres using spinning-disk confocal microscopy (SI Appendix, Fig. S9). In confocal images, control MOs showed a solid stripe, while the nompC29+29ARs MOs showed an oval ring shape (SI Appendix, Fig. S9) in which the membranes of MOs could be resolved. In the confocal data, the maximum width of MOs in nompC29+29ARs was 405.5 ± 54.4 nm (haltere pedicel, n = 7 cells), consistent with the ET data. Second, to check if the widening of MOs could be caused by the Gal4 expression system, we measured the MO membrane areas in both wild-type and nompC29+29ARs samples. They were nearly the same [wild type: 1.13 ± 0.15 μm2 (n = 4 cells); nompC29+29ARs: 1.09 ± 0.14 μm2 (n = 5 cells)]. Given the similar density of MMCs in these two strains (Fig. 3F), the total number of MMCs should be similar (also shown in Fig. 4D). Therefore, the Gal4 expression system in nompC29+29ARs did not increase the MO membrane area or the number of MMCs. Finally, we do not believe that GFP fusion on the carboxyl terminus of NompC29+29ARs causes the widening of the MOs. GFP molecules have a size of about 3∼4 nm and cannot account for the 40-nm increase in the mean length of MMCs. Thus, the doubling of MMC lengths in nompC29+29ARs reflects the doubling of NompC-AR in this mutant, consistent with the previous conclusion that the NompC-AR structurally contributes to MMCs.
We also analyzed the lengths of MMCs in nompC3. If NompC-AR is not a component of MMCs, then the absence of NompC is not expected to alter the MMC. On the contrary, if MMCs contain both NompC-AR and non-NompC component, the loss of NompC may change the structure and mechanical properties of MMCs, in turn causing length changes. We found that the length distribution of MMCs was location-dependent in nompC3, similar to wild-type MMCs (Fig. 4C and SI Appendix, Fig. S8). However, the mean lengths of NompC-independent linkers in nompC3 were longer than wild-type MMCs at all z-positions (Fig. 4G). The maximum width (y axis) of MOs in nompC3 [301.2 ± 57.2 nm (n = 4 cells)] was also larger than that in wild-type cells. These results, together with the morphological analysis (Fig. 3J), showed that the NompC-independent linkers distribute in a pattern similar to wild-type MMCs, but their structures appear to be changed due to the loss of NompC.
In summary, our observations in nompC29+29ARs confirm that NompC-AR is a structural component of MMCs that spans the gap between membrane and microtubule. In addition, structural analysis on NompC-independent linkers in nompC3 suggests that MMCs may contain a non-NompC component.
A Candidate Mechanism: MMCs May Bear Tensions.
Previous studies showed that NompC, as a membrane protein, could bind to microtubules in the absence of other binding partners (24). Such microtubule-binding ability relies on the NompC-AR domain (22, 23). Therefore, NompC-AR is expected to span the gap between the MO membrane (channel domain) and microtubules (NompC-AR). MMCs are the only structures in the cytoplasm of MO that may morphologically fit or contain NompC-ARs. Furthermore, the double-length MMCs observed in nompC29+29ARs suggest that NompC-AR is a component of MMCs. Finally, structural analysis of linkers in nompC3 suggests that MMCs may contain NompC-AR and non-NompC components. Based on these results, our observations raise a question: Why are MMCs in nompC29+29ARs and nompC3 longer than wild-type MMCs?
Among several alternative possibilities (Discussion), a simple mechanism to account for the length change of mutant MMCs is that MMCs bear tension. Several observations in the present study agree with this hypothesis. First, most wild-type MMCs (404 of 416 MMCs) are longer than purified NompC-AR (∼20 nm) in vitro (24), consistent with NompC-AR’s being a component of MMCs and MMCs’ being stretched. Second, if MMCs bear tension, then sheath–membrane contact would be important for the stability of this tension as it holds the membrane side of MMCs. In the case of loose sheath–membrane contact, MMCs are expected to be less stretched and appear to be shorter. This agrees with the correlation between the MMC lengths and the tightness of sheath–membrane contact (Fig. 4A). Third, if MMCs are considered as spring-like structures, doubled NompC-ARs in nompC29+29ARs can be thought of as two serially linked springs, with half of the stiffness of a single NompC-AR (κ2AR = 0.5κAR). Upon the same stretching force (fex), the length changes of doubled NompC-ARs should also double (Δl2AR = 2ΔlAR). Therefore, the MMC length is expected to be doubled in nompC29+29ARs [l2AR+Δl2AR = 2 (lAR+ΔlAR)], consistent with our observations (Fig. 4G). Fourth, if MMCs are compound structures of NompC-AR and non-NompC component, the loss of NompC-AR is expected to reduce the stiffness of individual MMCs (κMMC = κNompC-AR + κnon-NompC). Given that the number of linkers is nearly unchanged in nompC3 (Fig. 4D), the collective stiffness of NompC-independent linkers in the entire MO is expected to be reduced. In this case, the linkers are expected to be stretched more (Δllinker = fex/κnon-NompC > fex/κMMC) and appear to be longer than wild-type MMCs. This agrees with our observations in nompC3.
What is the source of tension in MMCs? We suspected that it may be extracellular forces that stretch the MO membrane through the sheath–membrane contact and then in turn stretch the MMCs (Fig. 5A). Are such forces present? To test this hypothesis, we performed localized laser ablation experiments on freshly dissected halteres. The mechanical response of the tissue to laser ablation should test whether the MOs are under tension. We first ablated a single MO in wild-type samples. The structures (cuticle and the adjacent MOs) next to the ablated MO showed a rapid expansion (Fig. 5B, Upper and Movie S7), recapitulating a typical elastic relaxation process and thereby suggesting the presence of tension in the MO. Similar observations were made in nompC29+29ARs (Fig. 5B, Middle and Movie S8), suggesting that tension is also present when the NompC-AR is doubled. We then reasoned that if such extracellular forces act in every receptor, there would be tissue tensions across the entire array of campaniform mechanoreceptors (Fig. 5A). We tested this by using a laser to ablate a bigger area (around two MOs). The surrounding tissue relaxed rapidly (Fig. 5B, Lower and Movie S9) after the ablation. Interestingly, we noticed that the surrounding tissue expanded along both the x and y axes, suggesting the presence of tissue tension in both directions.
Fig. 5.
Tissue tension across the haltere pedicel receptor field. (A, Left) A cartoon schematic showing the potential extracellular tension (fEX) on the MO along the y axis. (A, Right) A y–z plane image showing the directions of the extracellular tension in the receptor array. (Scale bar: 1 μm.) (B) Laser ablation experiments: both wild-type and nompC29+29ARs were used in this experiment. The observations on two strains are similar. Note that in wild-type we used the auto-fluorescence of haltere cuticle to locate the focus plane for visualization and laser ablation. In the nompC29+29ARs strain, the pedicellar MOs are visible due to the expression of NompC29+29ARs-GFP. (Upper) Single MO ablation on a wild-type haltere (Movie S7). (Middle) Single MO ablation on a nompC29+29AR haltere (Movie S8). (Lower) Two MOs ablation on a nompC29+29ARs haltere (Movie S9). The cuticular structures surrounding the ablated MOs in haltere receptor field show instantaneous expansion in all three experiments. The white arrows indicate the moving directions of adjacent MOs after the laser ablation. In the merged images, the red channels are the images of the MOs taken right before the ablation and the green channels are immediately taken after the ablation. The mismatch of red and green channels in the merged channel indicates the structural movements after the laser ablation. Note that in the case of two MOs ablation in nompC29+29ARs, the shift away of the adjacent MOs occurs along both x and y axes, suggesting the presence of extracellular tension throughout the receptor array in both directions. (Scale bars: 2 μm.)
Modeling Analysis: The Moment Activation Mechanism of the MO.
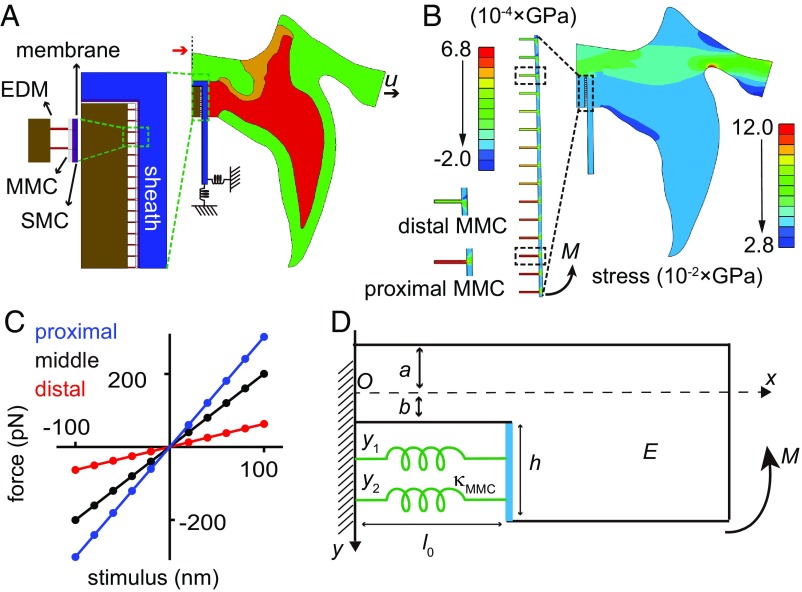
To further understand the mechanics of the MO, we took a theoretical approach, building a mechanical model using the finite element method (Fig. 6A). In this model, geometric information of the large structures was based on the FIB/SEM volume data (Fig. 1). SMCs and MMCs were incorporated based on the structural analysis on ET data (Fig. 2). The mechanical property (Young’s modulus or stiffness) of each component was primarily taken from the literatures (SI Appendix, Table S1). The simulation results showed that the pedicellar campaniform sensillum converted cuticle deformation (Fig. 6A) into rotational forces (i.e., a moment, M) on the MO membrane, with a positive stress indicating tension (Fig. 6B). This appears to be due to the architecture and mechanical properties of supporting structures. As a result, the proximal end of the MO received a greater moment than the distal end and was strained to a greater extent (Fig. 6 B and C). We termed such a mechanical process “moment activation mechanism.” The resulting forces on MMCs were linearly proportional to cuticular deformation (Fig. 6C). With a cuticular deformation at the nanometer scale, the force on each MMC was at the piconewton level. Because of the uncertainty in estimating the mechanical properties, we simulated different conditions to explore variation in several parameters (i.e., septum, joint membrane, MMC, and membrane). We found that the Young’s moduli of the supporting materials (i.e., joint membrane and septum) and the stiffness of the MMCs were important for determining the resulting forces on MMCs, while the membrane contributed little to the overall stiffness (SI Appendix, Fig. S10). However, in all simulation conditions, the proximal region of MO received larger forces than the distal region, in agreement with the moment activation mechanism (SI Appendix, Fig. S10).
Fig. 6.
Mechanical modeling of the pedicellar campaniform sensillum. (A) Modeling schematics; all structural components are indicated (see SI Appendix for modeling details). The mechanical rigidities used in the initial simulations: cuticle, 2 GPa; sheath, 2 GPa; SMC, 2 GPa; membrane, 30 kT; MMC, 16 pN/nm; septum, 0.15 GPa; joint membrane, 0.5 GPa. The mechanical constraint from tubular body is modeled as elastic springs in three axes (only two of the three springs are shown here). The deformation stimulus is indicated as u. The red arrow indicates the symmetry axis. (B) A representative simulation result in which color encodes stress (gigapascals) on each point. The MO is strained by a mechanical moment (M, i.e., a rotational force). Color bars used for the complete sensillum (Right) and the enlarged MO (Left) indicate different scales. A distal MMC and a proximal MMC (indicated by two dashed boxes in the enlarged MO) are further enlarged, showing that the proximal one withstands a larger force. Note that the MO can be strained by stretching or compressive forces. A typical simulation with a stretching force (u = 60 nm) is shown here as an example. (C) The deformation-force curves of the most distal (blue), the middle (red), and the most proximal (black) MMCs in the MO are shown. Note that the proximal end withstands larger forces. The horizontal axis represents stimulus (nanometers) and the vertical axis represents the resulting forces on the MMC (piconewtons). (D) A simplified spring-beam model to account for the moment activation mechanism. The x axis is the neutral axis and O is the origin of the neutral axis. Further modeling details are provided in SI Appendix. Note that this cartoon is only 2D, so w (i.e., thickness of the beam) is not labeled in this schematic.
Based on these numerical simulations, we derived a simplified “spring-beam” model to account for the mechanical mechanism underlying the operation of the MO (Eq. 1) (Fig. 6D). Model details are provided in SI Appendix. Briefly, in this model, yi is the vertical position of the springs (i.e., MMCs) relative to the neutral axis, E is the Young’s modulus of the beam (cuticle, joint membrane, and septum), M is the torque produced by the supporting structures due to cuticle deformation, l0 is the initial length of MMCs, κMMC is the stiffness of MMC, w is the thickness of the beam, and fi is the resulting force on MMC. In this model, cuticular deformation is converted to a torque (M), which creates a larger force (fi) on the proximal spring and a smaller force on the distal spring, consistent with the finite element simulation results and essentially accounting for the moment activation mechanism. This equation shows that the geometry (a, b, w) and Young’s modulus (E) of the beam (i.e., the cuticle, joint membrane, and septum) (Eq. 1) are important for extracellular mechanics. It provides a possible explanation for the diversities in the cuticular structures of different campaniform mechanoreceptors, namely that the receptors with different supporting structures are designed for sensing different forces. In addition, the stiffness (κMMC), distribution (yi) and geometry (l0) of MMCs are important for intracellular mechanics (Eq. 1), demonstrating that the spatial distribution of NompC and the molecular composition of MMCs are both key factors in understanding the gating mechanics of NompC in vivo.
| [1] |
Discussion
In the present study, we reconstructed the 3D structures of MOs in fly campaniform mechanoreceptors. The MO comprises regularly arranged mechanotransduction apparatuses, each of which is composed of an MMC which terminates in a button-shaped structure in the membrane. The AR domain of the NompC protein contributes to the MMC and the channel component of the protein likely contributes to the button (Fig. 7). In addition, the MMC may contain a NompC-independent component. Mechanical modeling of the campaniform sensillum shows that cuticular deformation is converted to a rotational force that acts on the MO (Fig. 7). Our structural and mechanical analyses show that the predicted force distribution on the MO matches well with the spatial distribution of NompC channels, suggesting that the MO may have an optimized structural–mechanical design. We now justify these conclusions and their potential implications for understanding fly mechanotransduction.
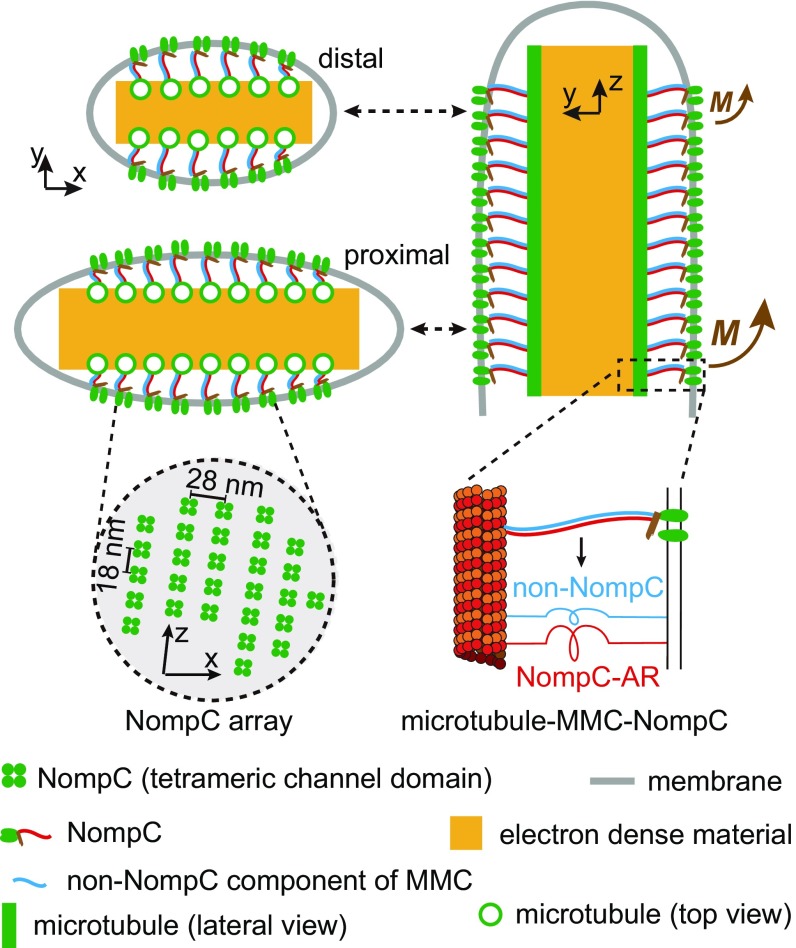
Fig. 7.
The spatial distribution of NompC matches the moment activation mechanism. NompC channels form an array on the MO membrane (Lower Left). Due to the MO geometry, more NompC channels are located on the proximal end than on the distal end (Upper Left). Based on modeling analysis, we propose a moment activation mechanism in which the proximal part of the MO receives larger forces than the distal part (Upper Right). Therefore, the spatial distribution of NompC matches the force distribution on the MO. In addition, we also observed non-NompC linkers in the nompC3 mutant, suggesting that MMCs may contain non-NompC components in addition to the NompC-AR (Lower Right).
Ultrastructural Distribution of NompC in the MO.
We observed a 2D array of button-shaped structures on the MO membrane (Figs. 2D and 7). Based on the size comparison and mutant analysis, we conclude that the channel domains of NompC structurally contribute to the bulk of these buttons. This implies that the density of NompC in MOs is about 2,000 μm−2 (Fig. 7). Such a high density of ion channels is similar to that of voltage-gated channels at the axon initial segment and likely to be functionally important in ensuring that the electrical responses are large and that the signaling is robust.
We found that, due to the MO geometry, the number of NompC channels first increases from the distal end of the MO and then stays relatively constant from the middle region to the proximal end (Fig. 7). Modeling analysis shows that cuticular deformation is converted to a mechanical moment that creates a larger force on the proximal end than on the distal end of the MO (Fig. 7). Therefore, our results suggest that more NompC channels are placed in the regions that receive larger forces, which we propose increases the sensitivity of the MO. However, the NompC channels on the distal region could make additional responses in case of stronger stimuli, thereby conferring a broad dynamic range to the MO. In this way, the MOs could make graded responses to a wide range of stimuli.
MMCs Have both NompC-AR and Non-NompC Components.
Our results provide several lines of evidence to confirm a previously proposed argument that NompC-AR contributes structurally to the MMCs (10, 22). First, the button-shaped structures on MO membrane resemble the channel domain of NompC in size, are absent in the nompC3, and are rescued in nompC29+29ARs, suggesting that the channel domains of NompC structurally contribute to the bulk of these buttons. These buttons are aligned to the arrayed microtubules in MO cytoplasm through MMCs. This is consistent with the molecular picture in which the channel domain and the AR domain of NompC contribute to the button-shaped structure and MMC, respectively (Fig. 7). Second, doubling the AR domain in nompC29+29ARs doubles the MMC lengths. In a previous study, the length of MMCs in nompC29+29ARs (18 ± 5 nm) was found to be only slightly longer than wild-type MMCs (15 ± 5 nm) (22). This is likely due to the differences in sample preparation and imaging methods. Double-length MMCs reflect the doubling of NompC-AR and confirm that NompC-AR structurally contributes to the entire length of MMCs. Third, most wild-type MMCs show filamentous shapes and have branched ends. However, the length and morphology of linkers are altered in nompC3. This correlates with the loss of NompC-ARs.
NompC-independent linkers are poorly preserved in glutaraldehyde-fixed samples, so in the previous work we were not sure if they were random denatured protein filaments or real component of MMCs (10). The observation of regularly spaced NompC-independent linkers in ET data suggests that they are not random denatured protein. It is formally possible that these linkers are non-NompC molecules that “invade” the MOs only in the absence of NompC. However, their similar density to wild-type MMCs suggests a similar binding pattern to the arrayed microtubules in the MO. Therefore, the presence of non-NompC linkers suggests that MMCs may contain non-NompC components. Based on our previous immunostaining data using an antibody against the amino terminus of NompC (12), we think that these linkers are not fragments or other isoforms of NompC that may be expressed in nompC3.
NompC molecules can bind to microtubules on their own and this interaction depends on NompC-AR (23, 24). Interestingly, NompC-independent linkers show direct connections to both membrane and microtubule. If NompC-independent linkers represent the non-NompC component of MMCs, this would suggest a molecular picture in which NompC-AR and the non-NompC linkers together form MMCs (Fig. 7). In this model, NompC, in addition to being a mechanotransduction channel, contributes to the mechanical coupling in the mechanical signaling pathway. In the absence of NompC, mechanical coupling between membrane and microtubule could still occur, but likely would be less stiff. Therefore, if there are additional mechanically sensitive channels, as is the case in the fly’s auditory cells (27, 28), mechanosensitivity in nompC null mutants might be additionally reduced due to effects on mechanical coupling. Future work to identify the molecules that form NompC-independent linkers and to dissect the structural-mechanics of the MO in fly’s auditory cells is necessary.
MMCs May Be Under Tension.
Alternative mechanisms.
We found that wild-type MMCs in vivo are generally longer than NompC-AR in vitro (24), MMCs are longer in nompC3 and nompC29+29ARs, and MMCs show location-dependent lengths. To understand these findings, we proposed a model in which MMCs in vivo are under tension and thereby are stretched. We now discuss some alternative mechanisms.
First, could it be possible that NompC-AR in purified NompC is compressed due to the presence of nanodisc, while MMCs in vivo are unstressed so that they appear to be longer? Reconstitution in nanodisc stabilizes the transmembrane domains of membrane proteins. However, NompC-ARs are cytoplasmic domains and thereby not likely to be axially compressed. Furthermore, MMCs in vivo being unstressed would predict that they do not contribute to the mechanical feedback in keeping the membrane–microtubule distances in the MO. If this were the case, one would not expect any changes in the MMC lengths and MO sizes in nompC3 and nompC29+29ARs. Therefore, MMCs’ being unstressed in vivo is inconsistent with our observations.
Second, could the increases in the lengths of MMCs in nompC mutants be the secondary effects of dysfunctional mechanotransduction (e.g., disrupting NompC function)? Our observations in nompC3 and nompC29+29ARs do not support this hypothesis. NompC29+29ARs was reported to have a similar mechanosensory response to mechanical stimuli in comparison with wild-type NompC (22). We confirmed that the number of NompC29+29ARs in the MO of nompC29+29ARs cells is similar to wild-type. Using a behavioral assay, we found that NompC29+29ARs rescues, to a large extent, the phenotype observed in nompC3: nompC29+29ARs flies could walk fairly well and showed moderate flying ability (SI Appendix, Fig. S11). If MMCs’ being longer is a nonspecific consequence of disrupting NompC function, one would expect minor structural changes in nompC29+29ARs (partial rescue) and greater structural changes in nompC3 (loss of function). However, this is inconsistent with our observations that structural changes in the MMCs and MOs in nompC29+29ARs are greater than in nompC3.
Third, could the MMCs at different locations have different molecular compositions, for example more or fewer repeating units, so they show different lengths? This would require MMCs with different compositions to be patterned in the tiny space of the MO (0.5 × 1.0 × 0.3 μm3) along both x and z axes. Precisely arranged structural (e.g., cytoskeleton- or matrix-associated) cues might contribute to such differential molecular specification. However, membrane–microtubule distance is unlikely the underlying cue as this distance can be changed when the components (e.g., NompC-AR) of MMCs are altered. We also do not think chemical cues (small diffusive molecules) can be the patterning signals as free diffusion would rapidly eliminate the gradient in this small space.
Structural and functional implications.
Given the stiffness of each NompC-AR to be at the order of 1 pN/nm, 20-nm extension predicts the stretching force to be around 20 pN. Such a force is able to straighten the 24 ankyrin repeats in human Ankyrin-R, while larger forces can partially unfold the ankyrin repeats (29). Our analysis raises a question of how the NompC-AR bundle responds structurally to this force. One possibility is that the AR domains of NompC in vivo are present in a straightened or partially unstructured form and undergo stretching/relaxing cycles while halteres are rapidly beating. In this view, the NompC-AR would behave similarly to other mechanosensitive proteins, like talin in the focal adhesion complex (30) and titin in muscle (31).
Another interesting issue is how tension may regulate the gating of NompC. We consider two possible scenarios. First, if the NompC channel is sensitive to pulling or pushing forces from MMCs along the axial direction, the resting tension may mechanically offset the channels’ sensitivity to gating forces. Second, if the NompC channel is sensitive to changes in membrane tension, the channels’ responses will be symmetric if there is zero resting tension in the MMC because both pulling and pushing forces will lead to an increase in membrane tension. The presence of resting tension in MMCs will make the channels’ responses asymmetric, so these channels can be both excited and inhibited.
Moment Activation Mechanism.
Our modeling studies, based on the architecture and the mechanical properties of the supporting structures, suggest that cuticular deformation will lead to rotation of the campaniform mechanoreceptor so that the strain on the MO is not uniform but is larger at the proximal end. We term this the moment activation mechanism. This conclusion holds over a wide range of mechanical parameters. One caveat, however, is that our numerical simulations and mechanical model are essentially static. Haltere campaniform mechanoreceptors, which detect forces during flight, sense highly dynamic signals. Therefore, our modeling analysis on MO mechanics cannot fully reveal the complexity of dynamics at haltere, sensillar, and subcellular levels but provide an intuitive and qualitative description on how the MO in fly haltere campaniform mechanoreceptors may operate.
Materials and Methods
Flies.
All flies were maintained on standard medium at 23 to 25 °C. w1118 was used as wild-type strain. nompC3 strain was provided by Martin Göpfert, University of Göttingen, Göttingen, Germany. nompC2, nompC-gal4, and nompC29+29ARs strains were provided by Wei Zhang, Tsinghua University, Beijing, China, originally from the Jan laboratory, University of California, San Francisco (22).
Laser Ablation.
Laser ablation experiment was performed using the Micropoint system (a 435-nm laser; Andor) added on the Andor spinning-disk confocal microscope.
Structure Visualization.
The atomic structure of NompC was visualized and analyzed using Chimera (University of California, San Franciso) (32).
HPF and Freeze Substitution.
HPF fixation of fly haltere and freeze substitution methods were developed based on a previous protocol (33). The detailed protocols are described in SI Appendix, Supplementary Methods.
ET.
The serial sections (250 or 300 nm) were prepared with a Leica Ultracut UCT or Leica EM UC7 microtome (Leica) and collected on Formvar-coated copper slot grids. Poststaining was performed with 2% UA in 70% methanol, followed by 0.4% lead citrate (EMS 17900). Gold nanoparticles (15 nm) (EMGC15; BBI Solutions) were added to both sides of the sections as the fiducial markers. The dual-axis tilt series ranging from −60° to +60° were acquired with a FEI Tecnai F30 or FEI Tecnai F20 electron microscope (Thermo Fisher Scientific). FEI Tecnai F30 electron microscope was equipped with an Axial Gatan US1000 CCD camera and controlled with SerialEM automated acquisition software (34). An FEI Tecnai F20 electron microscope was equipped with a Gatan US4000 (895) CCD camera and controlled with FEI Xplore 3D TEM tomography software.
Serial Block-Face Imaging Using FIB/SEM.
The sample preparation for FIB/SEM was similar to that used for ET imaging except for the embedding medium (Durcupan ACM, 44610; Sigma). For serial FIB milling and SEM imaging, a layer of block surface was milled by gallium ion beam and then the block surface was imaged using an electron beam with 2-kV acceleration voltages, 0.4-nA current, and 8-μs dwell time on a FEI Helios NanoLab G3 UC FIB-SEM. After volume data collection, the images were imported into Amira, aligned using DualBeam 3D Wizard module, and exported as a stack of images in TIF format. The image stacks were then used for structural segmentation.
Structure Reconstruction, Segmentation, and Measurement.
Tomograms were reconstructed with the IMOD software package (v4.7) (35). To stitch the adjacent sections in the z axis, the microtubules were traced and used as landmarks (SI Appendix, Fig. S12). These microtubules were first traced using cylinder correlation and trace correlation lines modules in Amira (Thermo Fisher Scientific) (SI Appendix, Fig. S12). The alignment and stitching were performed using the SerialSectionStack module in Amira (SI Appendix, Fig. S12). The structural segmentation and 3D surface generation for both ET and FIB/SEM data were performed in Amira. All structural measurements in 3D space were performed in Amira.
Supplementary Material
Acknowledgments
We thank Jonathon Howard for the initial support of this work and his comments on the manuscript; Tobias Fürstenhaupt for technical assistance in ET; Martin Göpfert for the nompC3 strain; Wei Zhang, Yuhnung Jan, and Lily Jan for the nompC2, nompC-gal4, and nompC29+29ARs strains; and the electron microscopy facility of Tsinghua University and the electron microscopy facility of MPI-CBG. This work was supported by National Key R&D Program of China Grant 2017YFA0503502; National Natural Sciences Foundation of China Grants 31671389, 31801129, and 11620101001; Qingdao National Laboratory for Marine Science and Technology Grant QNLM2016ORP0301; and startup funding from Tsinghua University Grant 20151080423. X.L. was supported by Max Planck Partner Group Program and Tsinghua-Peking Joint Center for Life Sciences. L.S. was supported by a postdoctoral fellowship from Tsinghua-Peking Joint Center for Life Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.B.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819371116/-/DCSupplemental.
References
- 1.Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- 2.Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- 3.Corey DP, Hudspeth AJ. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983;3:962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillespie PG, Müller U. Mechanotransduction by hair cells: Models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markin VS, Hudspeth AJ. Gating-spring models of mechanoelectrical transduction by hair cells of the internal ear. Annu Rev Biophys Biomol Struct. 1995;24:59–83. doi: 10.1146/annurev.bb.24.060195.000423. [DOI] [PubMed] [Google Scholar]
- 6.O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 7.Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J Neurosci. 2007;27:14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keil TA. Functional morphology of insect mechanoreceptors. Microsc Res Tech. 1997;39:506–531. doi: 10.1002/(SICI)1097-0029(19971215)39:6<506::AID-JEMT5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 9.Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Göpfert MC. Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat Neurosci. 2012;15:1198–1200. doi: 10.1038/nn.3175. [DOI] [PubMed] [Google Scholar]
- 10.Liang X, et al. A NOMPC-dependent membrane-microtubule connector is a candidate for the gating spring in fly mechanoreceptors. Curr Biol. 2013;23:755–763. doi: 10.1016/j.cub.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 11.Liang X, Sun L, Liu Z. Mechanosensory Transduction in Drosophila Melanogaster. Springer; Singapore: 2017. [Google Scholar]
- 12.Liang X, Madrid J, Saleh HS, Howard J. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton (Hoboken) 2011;68:1–7. doi: 10.1002/cm.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pringle JWS. The gyroscopic mechanism of the halteres of Diptera. Philos Trans R Soc Lond B Biol Sci. 1948;233:347–384. [Google Scholar]
- 14.Grünert U, Gnatzy W. Campaniform sensilla of Calliphora vicina (Insecta, Diptera) Zoomorphology. 1987;106:320–328. [Google Scholar]
- 15.Cole ES, Palka J. The pattern of campaniform sensilla on the wing and haltere of Drosophila melanogaster and several of its homeotic mutants. J Embryol Exp Morphol. 1982;71:41–61. [PubMed] [Google Scholar]
- 16.Liang X, Madrid J, Howard J. The microtubule-based cytoskeleton is a component of a mechanical signaling pathway in fly campaniform receptors. Biophys J. 2014;107:2767–2774. doi: 10.1016/j.bpj.2014.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanini D, Göpfert MC. Mechanosensation: Tethered ion channels. Curr Biol. 2013;23:R349–R351. doi: 10.1016/j.cub.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Howard J, Bechstedt S. Hypothesis: A helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 2004;14:R224–R226. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 19.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 20.Yan Z, et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Göpfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 2006;9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, et al. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell. 2015;162:1391–1403. doi: 10.1016/j.cell.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin P, et al. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature. 2017;547:118–122. doi: 10.1038/nature22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang X, Howard J. Structural biology: A force-sensitive ion channel springs to life. Curr Biol. 2017;27:R1017–R1020. doi: 10.1016/j.cub.2017.07.065. [DOI] [PubMed] [Google Scholar]
- 26.O’Toole E, Müller-Reichert T. Electron tomography of microtubule end-morphologies in C. elegans embryos. Methods Mol Biol. 2009;545:135–144. doi: 10.1007/978-1-60327-993-2_8. [DOI] [PubMed] [Google Scholar]
- 27.Effertz T, Wiek R, Göpfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 2011;21:592–597. doi: 10.1016/j.cub.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 28.Lehnert BP, Baker AE, Gaudry Q, Chiang AS, Wilson RI. Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron. 2013;77:115–128. doi: 10.1016/j.neuron.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure. 2005;13:669–682. doi: 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Yao M, et al. Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Sci Rep. 2014;4:4610. doi: 10.1038/srep04610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, et al. Dynamics of equilibrium folding and unfolding transitions of titin immunoglobulin domain under constant forces. J Am Chem Soc. 2015;137:3540–3546. doi: 10.1021/ja5119368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersen EF, et al. UCSF Chimera–A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 33.Bechstedt S, et al. A doublecortin containing microtubule-associated protein is implicated in mechanotransduction in Drosophila sensory cilia. Nat Commun. 2010;1:11. doi: 10.1038/ncomms1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Mastronarde DN. Dual-axis tomography: An approach with alignment methods that preserve resolution. J Struct Biol. 1997;120:343–352. doi: 10.1006/jsbi.1997.3919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.