Abstract
Background
Referrals to paediatric endocrine clinics for short stature are common. Height velocity (HV) is an essential component of the evaluation of short stature as growth deceleration often reflects an underlying paediatric endocrine diagnosis (PED). Access to previous measurements facilitates prompt calculation of HV.
Objective
To determine the availability of previous measurements at time of referral for short stature and to determine predictors of a PED.
Methods
A retrospective chart review was performed on all referrals for short stature to a single paediatric endocrinologist between January 2008 and December 2014. Standard practice following receipt of a referral for short stature included repeated requests to the referring physician for previous measurements.
Results
A total of 324 charts of patients aged 11 months to 18 years were reviewed and 286 were eligible for inclusion. Previous measurements were available in 72.4%, and 44.8% of these were found to have a PED. There was a significant relation between HV<25th percentile and a PED (P<0.0001) and between height deficit (HD) and a PED (P<0.0001). Logistic regression analysis showed that a HV<25th percentile and a HD>2 standard deviations, increased the odds of PED by a factor of 5.12 (P<0.001) and 1.39 (P<0.005), respectively.
Conclusion
HV is a significant predictor of a PED. Our higher rate of previous measurement availability is likely due to our effective referral screening protocol. The availability of these measurements, which are essential for HV calculation, are likely to reduce delays in diagnosis and management.
Keywords: Growth charts, Paediatric endocrine diagnosis, Short stature referrals
Growth is an important indicator of health, and a growth chart (GC) is a fundamental element of evaluation of overall health. Monitoring of growth with serial measurements has been recommended in routine healthcare visits from infancy to adolescence (1). Referrals to paediatric endocrine clinics for short stature are common. Short stature is defined as either a height that is two standard deviations (SDs) below the mean height for age and sex (less than third percentile), or two SDs below the mid-parental height (MPH). The MPH is calculated by addition of the mother’s and father’s height, and then addition of either 13 cm for boys, or subtraction of 13 cm for girls, all of which is then divided by two (Table 1). Height deficit (HD) is calculated as the difference in the mid-parental height z-score and the patient’s height z-score (Table 1).
Table 1.
HV, MPH and HD calculations
| HV | |
| MPH | |
| HD |
HD Height deficit; HV Height velocity; MPH Mid-parental height.
In our study, a paediatric endocrine diagnosis (PED) was defined as an underlying endocrine disorder causing short stature including growth hormone deficiency (GHD), idiopathic short stature (identified by Health Canada criteria), small for gestational age and syndromic -Turner, Noonan, Prader-Willi. ‘No PED’ included the diagnoses of familial short stature, constitutional growth delay or children who were not short as defined above.
Height velocity (HV) is an essential component of the evaluation of short stature as growth deceleration often reflects an underlying PED (2). HV is calculated by (a) subtracting the current measured height from a previously measured height, (b) then dividing by the time period (months) between measurements and (c) multiplying by 12 months to give a result in cm/year (Table 1). Access to previous measurements facilitates prompt calculation of HV and prevents delay in investigation as well as any required treatment. A consensus statement on GHD concluded that ‘the single most useful parameter in the assessment of the child with growth retardation is the clinical evaluation, with emphasis placed upon accurate serial measurements of height and determination of the height velocity’. (3)
The primary objective of this study was to determine the availability of previous measurements at time of referral for short stature. Secondary objectives were to characterize the paediatric endocrine diagnoses and delineate practical predictors of a PED.
METHODS
A retrospective chart review was performed on all referrals for short stature to a single paediatric endocrinologist between January 2008 and December 2014. The study was approved by the Human Research Ethics review board at Western University (Research Ethics Board File #: 105669). Those patients confirmed as having short stature, defined as either a height two SDs below the mean height for age and sex, or two SDs below the MPH were included in the study. Exclusion criteria included absence of short stature, short stature with a previously identified aetiology, and children already on growth hormone therapy.
At routine clinic visits, weight was measured using an electronic scale to the nearest 0.1 kg. Height was measured without shoes using a wall mounted stadiometer to the nearest 0.1 cm. Data were collected from a combination of the patient’s paper chart and electronic chart. All z-scores and percentiles for height and weight were calculated based on WHO GCs from 0 to 24 months and CDC for 2 to 20 years (Peditools.org). HV (cm/yr), MPH (cm) and HD were calculated as detailed in Table 1. As HV varies with age, a HV<25th percentile was considered the threshold for clinical significance on the basis of clinical guidelines and previous research studies (4,5). The 25th percentile was determined based on a graphic on Uptodate.com reproduced and using reference ranges on height velocities from North American children in a study by Tanner et al. (6). Bivariate analyses were conducted for continuous and categorical variables using t-tests and chi-squared tests, respectively. Multivariable logistic regression was used to determine significant predictors of PED status. All statistical analyses were conducted using SPSS v.24 (IBM Corp., Armonk, NY, USA), and P-values less than 0.05 were considered statistically significant.
RESULTS
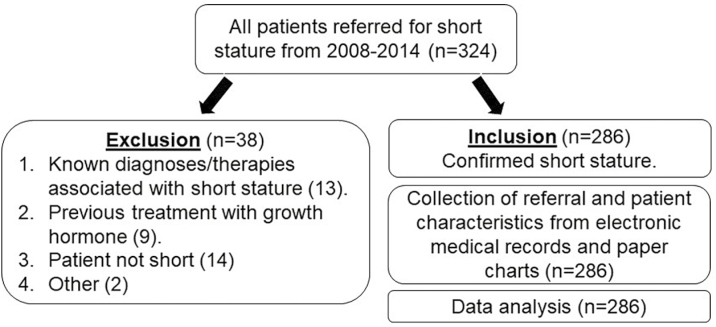
A total of 324 charts of patients aged 11 months to 18 years were reviewed, and 286 were eligible for inclusion (Figure 1). Males made up the majority of referrals (n=193, 67.5%). The mean (SD) age at referral was 9.46 (4.43) years with a mean (SD) age for males of 9.73 (4.43) years and 8.90 (4.41) years for females. The mean (SD) height for age z-score was −2.27 (1.12) overall, −2.37 (1.24) for males and −2.06 (0.78) for females.
Figure 1.
Flow chart for short stature referrals.
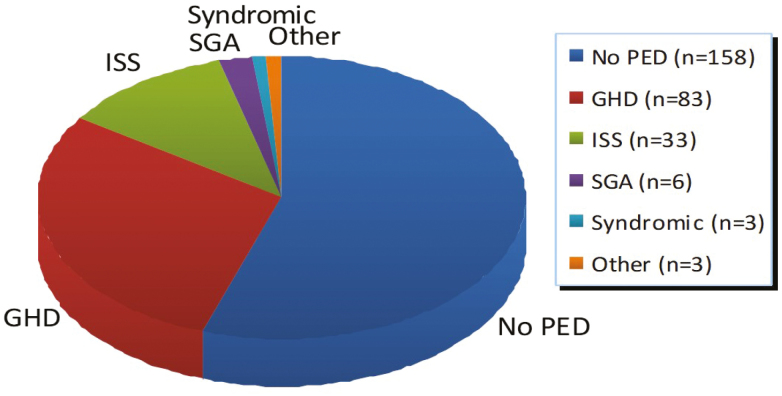
A GC with a minimum of 6 months of previous measurements was available for 207 (72.1%) of the 286 referrals included in the study. There was no significant difference in the availability of GCs between referrals from paediatricians (74.6%) versus family physicians (69.2%; chi-square P=0.329). One hundred and twenty-eight (44.8%; 98 males and 30 females) of the study population were found to have a PED for their short stature. Of those with a PED, GHD was the most common pathologic etiology (Figure 2). The prevalence of PED was comparable in the group of patients with available previous measurements (45.9%) and those without available previous measurements (41.8%; chi-square P=0.531).
Figure 2.
Distribution of pediatric endocrine diagnoses.
There was a significant relation between HV<25th percentile and a PED (P<0.0001), as well as, between HD and a PED (P<0.0001). Logistic regression analysis showed that a HV<25th percentile and a HD>2 SDs, significantly increased the odds of a PED (Table 2).
Table 2.
Summary of logistic regression analyses
| Outcome | Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|
| Paediatric endocrine diagnosis | Height velocity < 25th percentile | 5.148 | 2.941, 9.012 | <0.0001 |
| Height deficit > 2 standard deviations | 1.385 | 1.101, 1.742 | <0.005 |
CI Confidence interval.
DISCUSSION
In our study, we found a high frequency of GC availability (72%) on initial assessment for short stature. Routine utilization of GCs by primary care providers as well as paediatricians facilitates calculation of HV and can guide referrals. In addition, the availability of a completed GC at referral to a paediatric endocrinologist is an essential component of the initial assessment of a child with short stature and impacts on the timing and direction of investigations. We have shown that HV below the 25th percentile was associated with a five times greater likelihood of the child having a PED. Early calculation of HV may not only prevent delay in diagnosis, but more importantly it may prevent delay in treatment which may have a critical impact on both a child’s physical and emotional health.
Our study population demographics with respect to mean age (9.5 years), gender (67.5% males) and height for age z-score (−2.72) at referral are consistent with other reports, apart from our finding of an earlier age at referral for girls. In an observational study of over 21,000 children referred for short stature from 237 institutions and practices, the mean age at referral was 8.6 years, 69% were male, and the mean height SD was −2.1 (7). Others have reported increased referral rates for males ranging from 61% to 65% (8–10). In our population, females were referred earlier than males despite the finding that a greater proportion of males had a PED (50.8% versus 32.3% females). This finding could reflect an under-referral of males, an over-referral of females, or may have been due to sampling error. Reported HDs at referral are very similar, with mean HDs of −2.0 (10). In a cross sectional study in an academic centre of 371 patients referred for short stature, the mean age at referral was 9.8 years, with 61% males and a mean SD of −2.4; there were no significant differences between males and females in age and growth parameters at the time of referral (9).
This retrospective chart review showed that there was a high frequency of GCs at initial assessment of patients referred to our centre for short stature: 72% compared to reported rates of 41 to 59% (8,9). This was likely attributable to our standard clinical practice, wherein following receipt of a referral for short stature, repeated requests were made to the referring physician for previous measurements. In a review of all referrals to The Children’s Hospital of Philadelphia for short stature or poor growth during a one year period prior GCs were available in only 41% of the 278 referrals and the percentage of GCs provided for boys and girls was similar (8). Another study of 371 children referred for short stature reported significant deficiencies in the referral letters: 14% of referrals did not include current height measurements, parental height data were reported in only 26.7% of the referrals and previous GCs were available in only 54% of the referrals (9). Prior to introducing a fax system for referrals in an Academic Canadian paediatric endocrinology clinic GCs were provided for only 47% of referrals for short stature (11). With the implementation of a fax system GCs were received for 96% of referrals compared to a persistent low rate of 41% for those still not using a fax system.
The relatively higher overall prevalence of PEDs in our population (44.8%) is likely due to the fact that our population was from a paediatric endocrine clinic, and thus, more likely to have pathologic disease relative to the general population (12). In addition, generalizability may be limited because our sample was from a paediatric endocrinologist clinic and our referral base was confined to Southwestern Ontario. However, apart from an increased prevalence of PED, our population shared many characteristics with patients noted in other studies. In an observational study of 214 patients at two tertiary care hospitals in Pakistan, GHD was the most common identified etiology at 6.1%, followed by hypothyroidism at 5.5% (12). It is probable that hypothyroid did not make up a large proportion of our population due to routine newborn screening. In the Utah Growth Study, Lindsay and colleagues measured 123,948 children and idiopathic short stature was the most common cause of short stature at 4.9% followed by GHD at 2.9% (13). Ahmed and colleagues in the Oxford Growth Study measured 20,338 children with GHD as the most common etiology at 1.3% (5).
GCs are essential to identify short stature as well as poor growth velocity. In the absence of measurements plotted on a GC it is unclear how referring physicians can assess accurately a child as being short. It is evident that a significant proportion of children referred for short stature are determined by the paediatric endocrine centre as not being short. Of 526 children evaluated for short stature at a general paediatric endocrine clinic over 30% of referrals were not short, defined as height z-score ≥−2 SD and height z-score within 2 SD of parental target height (10). Accurate measurement and use of GCs by family doctors and paediatricians could reduce unnecessary referrals as well as the associated healthcare costs. In our study, 4.32% of patients did not satisfy criteria to be considered short. Measurement of growth is an essential component of the health assessment of children and adolescents and the time required for this can be minimal. A study of Utah elementary school children included trained assessors to measure height using a stadiometer and found that children could be accurately measured and the results could be recorded in less than 30 seconds (13).
Though the clinical significance of HV is widely agreed to be important in assessment of short stature, using logistic regression analysis we were able to show a significant relation between HV<25th percentile and a PED. These results will need to be replicated to confirm their clinical significance and possible future application. However, our quantification of the relationship suggests that a HV<25th percentile increases the likelihood of an eventual diagnosis of PED by approximately fivefold.
CONCLUSION
A GC is an essential component in the process of evaluation of growth. Maintainence of a GC has a significant clinical impact because in the absence of serial measurements, calculation of HV is delayed by a minimum of 6 months. Though our high rate of previous measurement availability (72%) is likely due to our effective referral screening protocol, a significant proportion of referrals for short stature still had insufficient data to calculate HV. We found that HV<25% was a good predictor of a PED with an associated five times greater likelihood of having a PED. This study quantifies the valuable information provided by HV and highlights the importance of maintaining GCs to facilitate calculation of HV. With routinely calculated HV, delay in investigation, diagnosis, and treatment of a PED can be attenuated.
References
- 1. Marchand V. Promoting Optimal Monitoring of Child Growth in Canada: Using the New World Health Organization Growth Charts. A Collaborative Statement of Dietitians of Canada, Canadian Paediatric Society, The College of Family Physicians of Canada, and Community Health Nurses of Canada. Canadian Journal of Dietetic Practice and Research - Vol 71 No 1, Spring 2010. [DOI] [PubMed]
- 2. Rosenfeld RG, Albertsson-Wikland K, Cassorla F, et al. Diagnostic controversy: The diagnosis of childhood growth hormone deficiency revisited. J Clin Endocrinol Metab 1995;80(5):1532–40. [DOI] [PubMed] [Google Scholar]
- 3. Cohen P, Rogol AD, Deal CL, et al. ; 2007 ISS Consensus Workshop participants. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: A summary of the growth hormone research society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab 2008;93(11):4210–7. [DOI] [PubMed] [Google Scholar]
- 4. Nwosu BU, Lee MM. Evaluation of short and tall stature in children. Am Fam Physician 2008;78(5):597–604. [PubMed] [Google Scholar]
- 5. Ahmed ML, Allen AD, Sharma A, Macfarlane JA, Dunger DB. Evaluation of a district growth screening programme: The Oxford growth study. Arch Dis Child 1993;69(3):361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr 1985;107(3):317–29. [DOI] [PubMed] [Google Scholar]
- 7. Wyatt D, Parker KL, Kemp SF, Chiang J, Davis DA. The evaluation and follow up of children referred to pediatric endocrinologists for short stature. Int J Pediatr Endocrinol 2010;2010:652013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimberg A, Kutikov JK, Cucchiara AJ. Sex differences in patients referred for evaluation of poor growth. J Pediatr 2005;146(2):212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yardeni D, Loewenthal N, Limony Y, Hershkovitz E. Ethnic and gender inequities in the evaluation of referred short children. Horm Res Paediatr 2011;76(1):50–5. [DOI] [PubMed] [Google Scholar]
- 10. Lee JM, Davis MM, Clark SJ, Kemper AR. Threshold of evaluation for short stature in a pediatric endocrine clinic: Differences between boys versus girls?J Pediatr Endocrinol Metab 2007;20(1):21–6. [DOI] [PubMed] [Google Scholar]
- 11. Chiniara L, Perry RJ, Van Vliet G, Huot C, Deal C. Quality of referral of short children to the paediatric endocrinologist and impact of a fax communication system. Paediatr Child Health 2013;18(10):533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sultan M, Afzal M, Qureshi SM, et al. Etiology of short stature in children. J Coll Physicians Surg Pak 2008;18(8):493–7. [PubMed] [Google Scholar]
- 13. Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah growth study: Growth standards and the prevalence of growth hormone deficiency. J Pediatr 1994;125(1):29–35. [DOI] [PubMed] [Google Scholar]