Abstract
Since first defined in 1998, paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) and its later, broader iteration, paediatric acute-onset neuropsychiatric syndrome (PANS), have garnered significant attention and controversy. The role of streptococcal infection in children with explosive onset obsessive-compulsive disorder and new onset tics, the natural history of this entity, and the role of symptomatic and disease-modifying therapies, including antibiotics, immunotherapy, and psychoactive drugs, are all issues that have yet to be definitively addressed. While definitive proof of the autoimmune hypothesis of PANDAS is lacking, given the heightened attention to this entity and apparent rise in use of this diagnostic category, addressing questions around diagnosis, treatment, and etiology is imperative. In this paper, we review current working definitions of PANDAS/PANS, discuss published evidence for interventions related to this entity, and propose a clinical approach to children presenting with acute symptoms satisfying criteria for PANDAS/PANS.
Keywords: Autoimmune, OCD, Paediatric, Psychiatric, Tics
Since first defined in 1998 (1), paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) and its later, broader iteration, paediatric acute-onset neuropsychiatric syndrome (PANS), have garnered significant attention and controversy. The role of streptococcal infection in children with explosive onset obsessive-compulsive disorder (OCD) and new onset tics, the natural history of this entity, and the role of symptomatic and disease-modifying therapies, including antibiotics, immunotherapy, and psychoactive drugs, are all issues that have yet to be addressed.
Because of these unresolved questions, the concept of PANDAS brings great challenges to clinicians, patients, and their families (2). This may be compounded by inconsistent application of PANDAS diagnostic criteria, potentially resulting in overly broad application of this diagnosis to children in whom immune-mediated symptoms are unlikely (3–5). Families who feel their children fit into this phenotype report fear, frustration, and feelings of not being heard (6) and stories of their struggles are reported in the news and other media. Advocacy groups, such as the PANDAS Network in the USA and PANDASHELP in Canada, have arisen and have attempted to influence policy at governmental levels.
Thus, while definitive proof of the autoimmune hypothesis of PANDAS is lacking, given the heightened attention to this entity and apparent rise in use of this diagnostic category, addressing questions around diagnosis, treatment, and etiology is imperative. In this paper, we review current working definitions of PANDAS/PANS, discuss published evidence for interventions related to this entity, and propose a clinical approach to children presenting with acute symptoms satisfying criteria for PANDAS/PANS.
PANDAS AND PANS: DEFINITIONS
The concept of PANDAS arose out of the hypothesis that their pathogenesis may be similar to that of Sydenham’s chorea (7). Diagnostic criteria for PANDAS were first proposed in 1998 (1) (Table 1).
Table 1.
1998 PANDAS criteria
| 1) Presence of diagnosis of OCD and/or tic disorder (must meet lifetime diagnostic criteria by DSM-III-R, DSM-IV, or DSM-IV-TR) |
| 2) Paediatric onset (symptoms first evident between age 3 and beginning of puberty) |
| 3) Episodic course (characterized by abrupt onset of symptoms or dramatic symptom exacerbations). |
| 4) Association with group A beta-hemolytic streptococcus infection. |
| 5) Association with neurologic abnormalities |
OCD Obsessive-compulsive disorder; PANDAS Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections.
A 2012 publication highlighted limitations of the PANDAS definition, and raised questions about the classification of patients who met all PANDAS criteria except for association with group A streptococcal (GAS) infection (8). New diagnostic criteria were proposed to define this group of patients and a new diagnostic label—PANS—was proposed (Table 2).
Table 2.
2012 PANS criteria
| 1) Abrupt, dramatic onset of obsessive-compulsive disorder or severely restricted food intake (<48 h). The obsessive-compulsive symptoms must be severe and frequent enough to meet DSM-IV criteria for OCD. |
| 2) Concurrent presence of additional neuropsychiatric symptoms, with similarly severe and acute-onset, from at least two of the following categories: |
| a. Anxiety |
| b. Emotional lability and/or depression |
| c. Irritability, aggression, and/or severely oppositional behaviours |
| d. Behavioural (developmental) regression |
| e. Deterioration in school performance |
| f. Sensory or motor difficulties |
| g. Somatic signs or symptoms, including sleep disturbances, enuresis, or urinary frequency. |
| 3) Symptoms are not better explained by a known neurologic or medical disorder, such as Sydenham chorea, systemic lupus erythematosus, Tourette syndrome, or others. |
OCD Obsessive-compulsive disorder; PANS Paediatric acute-onset neuropsychiatric syndrome.
This definition no longer includes tic disorders as a primary criterion. The restriction of cases to prepubescent children was also removed. OCD and restrictive eating are emphasized. As stated by the authors of these criteria, the new working definition of PANS was created to more narrowly define this cohort and to improve standardization in research studies and comparability of cohorts.
It is unclear whether diagnostic criteria for PANS are sufficiently narrow to delineate a distinct clinical entity. However, inclusion of abrupt onset of psychiatric symptoms as a criterion seems to distinguish a subset of children from others referred for evaluation for PANS. Forty per cent (19 of 47) of patients evaluated in a specialized PANS clinic experienced acute-onset of symptoms (3 days or shorter), while the remainder met all criteria except for acuity of onset (9).
PATHOPHYSIOLOGICAL STUDIES OF PANDAS/PANS
Many studies have evaluated the relationship between infection and abrupt presentation of neuropsychiatric symptoms. Early reports suggested presentation of neuropsychiatric symptoms as long as 9 months after GAS infection (1), with subsequent case–control studies suggesting an increased odds ratio for previous strep infection in individuals with OCD and tic disorders compared to controls (OR: 2.22 to 13.6) (10). However, other studies have challenged this, and found no association between GAS and abrupt onset of neuropsychiatric symptoms (11) or exacerbations of these symptoms (12). More recently, two large cohort studies, one from Taiwan (13) and the other from Denmark (2) suggested a higher odds ratio of developing OCD, tics, and ‘mental disorders’ following streptococcal pharyngitis or other respiratory tract infections. In the Danish population-based cohort study that involved 1,067,743 children <18 years of age, the incidence rate ratio for any one of these disorders was higher in children who had a positive streptococcal test compared to those never tested (adjusted incidence rate ratio [IRR] 1.16, 95% confidence interval [CI] 1.14 to 1.20) and to a lesser extent in those who had a negative streptococcal test compared with those never tested (adjusted IRR 1.07, 95% CI 1.04 to 1.09).
While cohort studies suggest associations between infection and OCD and tics, whether ongoing inflammation drives symptoms in these children is unknown, and evidence supporting the presence of ongoing inflammation in biological samples in children satisfying PANDAS/PANS diagnostic criteria is lacking. OCD and tic disorders, however, have been studied with mixed, but generally negative, results. A systematic review of studies evaluating relationships between cytokines and OCD found only a reduction in IL-1β and no clear association between elevated cytokines (TNF-α, IL-6) and OCD, with potential confounding of comorbid illness, age, and medication (14). Fluctuations in serum cytokines during exacerbations in children with Tourette’s syndrome/tics were present in one study (15), but another small study found no cerebrospinal fluid (CSF) cytokine elevations in this cohort compared to healthy controls (16).
The role of antineuronal antibodies as a trigger for the PANDAS/PANS phenotype is unproven. Following descriptions of the presence of antibasal ganglia antibodies in a Sydenham’s chorea cohort (17) and later, by the same group, in a cohort of children with neuropsychiatric symptoms and movement disorders (17), these and other antibodies including those against dopamine receptors D1 and D2, β-tubulin, and lysoganglioside-GM1 (lyso-GM1) and calcium calmodulin-dependent kinase II activity have been investigated (CaMKII-activity) (18,19). While some of these studies have reported positive results, the findings have not been replicated in other cohorts (20–22). Together, these antibodies, named the ‘Cunningham’ panel, have been independently tested with no observed differences between patients satisfying PANS criteria and healthy children (23).
INTERVENTIONS IN PANDAS/PANS
Psychiatric interventions
Only a few small studies have examined psychiatric therapies in the context of the PANDAS/PANS phenotype. One study examined cognitive-behavioural therapy (CBT) in seven children with PANDAS OCD (six of seven taking concomitant selective serotonin reuptake inhibitors [SSRIs]) (24). Blinded assessments of symptoms suggested improvement of OCD symptoms immediately post-treatment and remission in three of six patients at 3 months. A second study (n=11) examined the effect of CBT together with antibiotics in children with PANS OCD with significant ongoing symptoms despite 4 weeks of antibiotics (25). All 8 of 11 who completed the treatment were classified as responders and remission was achieved in 6 of 8 subjects with variable follow-up (1 to 4 months). These findings are comparable to overall results of CBT in OCD in children (60 to 80% response) (26–28).
Although widely prescribed in these patients, randomized clinical trials of psychotropic medications in the PANDAS/PANS phenotype have not been completed. Fifty-four per cent of responders to an online survey (n=698) of caregivers of children diagnosed with PANS (including PANDAS) or adult PANS patients reported they had received psychotropic medication for psychiatric symptoms at some point (29). Approximately half of the responders reported SSRIs (44%) and medications for ADHD (e.g., methylphenidate) (43%) to be effective or somewhat effective (29).
Antibiotics
Acute antibiotic therapy
While appropriate treatment of GAS pharyngitis is important for the primary prevention of acute rheumatic fever (30,31), a role for antibiotics in preventing exacerbations of OCD symptoms or tics has not been established. There is only one published randomized, double-blind, placebo–control study that has examined the use of antibiotic therapy in youth satisfying PANDAS/PANS criteria (32). In this study, 31 children meeting PANS criteria with acute-onset or relapse of moderate-severe OCD symptoms within 6 months were randomized to receive azithromycin or placebo for 4 weeks. A greater reduction in the Clinical Global Impression-Severity Scale (CGI-S) for OCD symptoms was seen in the azithromycin group compared to the placebo group. However, other neuropsychiatric outcomes did not differ between the intervention and placebo groups.
Prophylaxis for neuropsychiatric symptoms
The use of long-term penicillin prophylaxis did not reduce exacerbation rate and tic and OCD severity in a randomized double-blind, placebo-controlled, cross-over trial (n=37) (33). Depression and anxiety scale ratings improved and more parents identified improvement in behaviour in the active phase compared to placebo. A second small non-placebo-controlled double-blind study randomized 23 children with PANDAS to azithromycin or penicillin for 12 months (34). Both groups had a significant reduction in GAS infections compared to the previous year (96% reduction overall), along with a 61% reduction in frequency of neuropsychiatric symptom exacerbations. As there was no control group, it is not clear whether the reduction in neuropsychiatric symptoms reflected natural history or medication effect.
Immunotherapy
Several case reports or case series have described improvement in core PANDAS symptoms using immunotherapy such as corticosteroids, NSAIDs, intravenous immunoglobulin (IVIG), or plasma exchange (35–37). However, the significance of these findings is difficult to interpret because of the retrospective and uncontrolled nature of these reports and because of the naturally waxing and waning course of PANDAS. There is no published evidence for use of rituximab or mycophenolate mofetil in children presenting with this symptom complex.
Corticosteroids
One retrospective study of consecutive patients meeting PANDAS/PANS criteria (n=98) examined the duration of ongoing symptoms after use of corticosteroids (prednisone 1 to 2 mg/kg/d for 5 days ± a taper of undefined duration) in the treatment of exacerbations (‘flares’) in youth satisfying PANDAS/PANS criteria (38). Subjects were those with a first episode or a relapsing/remitting course. Three hundred and eighteen flares were not treated with corticosteroids compared with 85 treated with corticosteroids. Corticosteroid treatment was associated with a reduction in symptom duration by 3.5 weeks after adjusting for other covariates. A longer duration of corticosteroid treatment was associated with a longer interval until the next symptom exacerbation. Limitations of this study include its retrospective nature, lack of randomization, potential biases in treatment decisions, absence of a placebo group, and the lack of validated outcome measures.
Intravenous immunoglobulin and plasma exchange
Two controlled trials involving the use of IVIG and/or plasma exchange (PLEX) in PANDAS have been published, both suggesting a 1-year improvement in behaviour regardless of whether placebo or immunotherapy was given at the outset. In the first (n=30), children satisfying PANDAS criteria, on prophylactic antibiotics, who were experiencing a symptom exacerbation were randomized to IVIG (1 g/kg/day for 2 days), plasma exchange (PLEX) (5 to 6 exchanges over 10 to 12 days), or placebo (39). Both active treatment groups showed significant improvement at 1 month in obsessive-compulsive symptoms, anxiety, depression, emotional lability, and global functioning. All children initially treated with placebo then received open-label active therapy (IVIG=2, PLEX=8) and eight subsequently showed improvement in neuropsychiatric symptoms on assessment 1 month later. At 1-year follow-up, three children had received additional immunotherapy and 82% of children showed a >50% improvement.
A second randomized, double-blind trial of IVIG (1 g/kg/daily for 2 days) versus placebo (40) included 35 children satisfying PANDAS criteria who experienced a first episode or first recurrence of symptoms, moderate-severe OCD symptoms, onset of symptoms within 6 months of study entry, and symptom onset within 6 to 8 weeks of GAS infection. All participants were placed on antibiotic prophylaxis. At the 6-week mark, no significant differences in response rate were seen between the groups. Subjects (n=24) considered to be nonresponders in the blinded phase received open-label IVIG. The improvement from weeks 6 to 12 was similar across all groups, including those who received placebo followed by IVIG (50% mean reduction in the Children’s Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) scores), a second dose of IVIG (55% mean reduction), and those who received no further therapy (46% mean reduction). When reassessed at 24 weeks, responder rate ranged from 67 to 80% across all groups.
In addition to the controlled trials of IVIG and PLEX, one retrospective study (n=35) reported the use of PLEX (three exchanges of 1.5 plasma volume over 3 to 5 days) in children satisfying PANDAS criteria, all of whom also received prophylactic antibiotics and psychiatric treatment (41). This study reported an average improvement in symptoms of 65% at 6 months and 78% on long-term follow-up. The retrospective nature of the study and lack of a comparator group limit the conclusions drawn from this study.
Tonsillectomy
Tonsillectomy (± adenoidectomy) has been examined as a potential treatment for PANDAS. Although case reports and case series have described improvement or resolution of PANDAS neuropsychiatric symptoms following tonsillectomy (42,43), this has not been supported by larger studies. One retrospective study assessed 20 PANDAS patients with a history of tonsillectomy/adenoidectomy compared to 23 PANDAS patients without and found no difference in OCD/tic severity or streptococcal antibody titres between the groups (44). Further, a prospective study involving 120 PANDAS patients, 56 of whom underwent surgery (in a nonrandomized fashion), failed to find a difference between those treated with surgery versus those who did not have surgery in the rate of neuropsychiatric symptom remission, relapse, severity, or streptococcal antibody titres over 2 years of follow-up (45).
Summary: Therapies
The studies outlined above have consistently reported a 60 to 80% reduction in symptoms over time. Notably, symptom remission rates in these studies appear to be similar to long-term remission rates reported for non-PANDAS paediatric OCD and may represent the natural history of these cases rather than true treatment effect.
RECOMMENDATIONS FOR PRIMARY CARE PROVIDERS
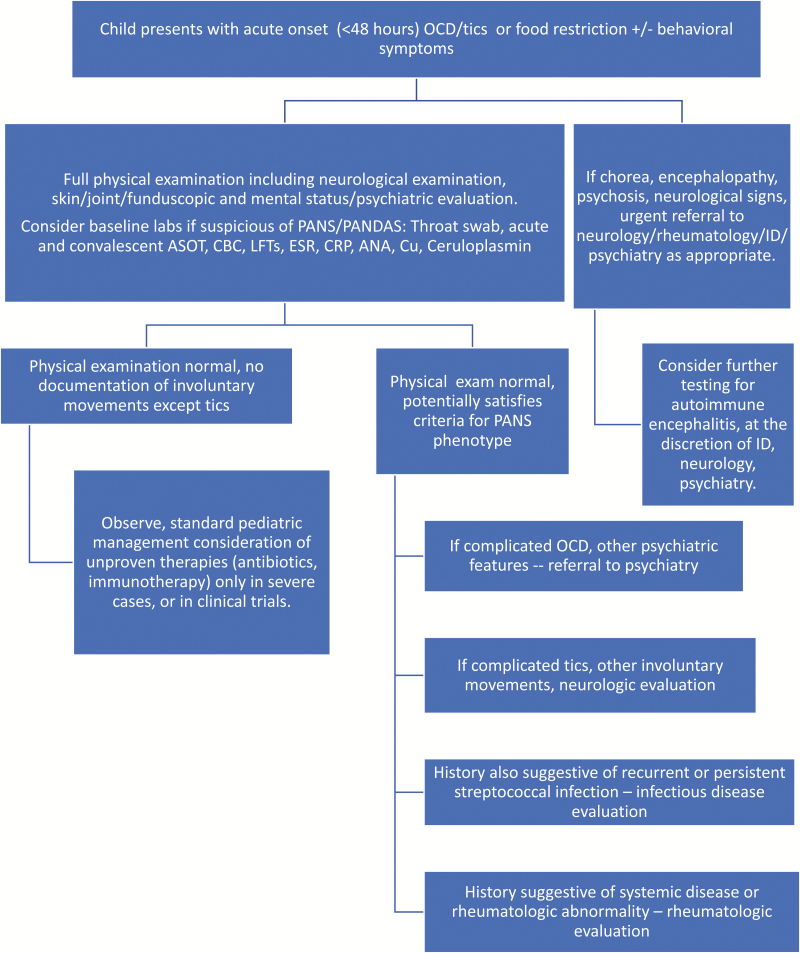
The abrupt onset of neuropsychiatric symptoms in a previously well child warrants close and careful assessment, including a detailed history, examination, and investigations to rule out underlying aetiologies. As the need to understand this group of children and standardize the clinical approach to this population is great, we provide an algorithmic approach to evaluation of a child suspected of having PANDAS/PANS (Figure 1).
Figure 1.
Suggested algorithm for the evaluation of the child presenting with possible PANDAS/PANS. ANA Antinuclear antibody; ASOT Antistreptolysin O titre; CBC Complete blood count; CRP C-reactive protein; ESR Erythrocyte sedimentation rate; LFTs Liver function tests; OCD Obsessive/compulsive disorder; PANDAS Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections; PANS Paediatric acute-onset neuropsychiatric syndrome.
With regards to treatment, given the limitations of published literature focused on therapy for youth presenting with the PANDAS/PANS phenotype, we suggest the following: (1) Children satisfying PANDAS/PANS criteria should receive treatment with psychiatric medications and interventions (such as CBT or SSRIs) known to be effective in childhood OCD. (2) Referral of patients with persistent and disabling symptoms should be made to physicians with expertise in paediatric OCD, neurology, infectious diseases, and inflammatory disorders as appropriate based on symptom complex. (3) Neither tonsillectomy nor antibiotic prophylaxis are recommended. When acute GAS infection is identified, treatment (following current guidelines) is recommended. (4) Treatment of children with immunotherapy should be undertaken only with subspecialist guidance, and preferably in the context of a clinical trial.
CONCLUSION
Significant attention has recently focused on the PANDAS/PANS syndrome. Cohort studies have shown associations between infection and emergence of psychiatric symptoms, especially OCD, in children. Notably, however, to date, biological studies have failed to show children satisfying PANDAS/PANS criteria to have a clear immune basis. Moreover, strong evidence for treatment with antimicrobials or immunotherapy is lacking. Response rates to psychiatric intervention appear to be similar to those of non-PANDAS/PANS associated OCD. Studies of immunotherapy have produced inconsistent results: the only RCTs performed suggest little difference in outcome between placebo, IVIG, and PLEX. Studies of antibiotic prophylaxis and tonsillectomy do not support use of these modalities for this clinical indication.
Given the severe and debilitating nature of the symptoms and clear effects on family and child functioning, identification of evidence-based, effective therapies and understanding the incidence, prevalence, and biological basis of this syndrome are necessary. These goals can only be achieved by cross-institutional collaboration, deep clinical phenotyping from prospectively collected data through collaborative registries, and well-conducted investigations of underlying biological mechanisms in this cohort.
What’s New
Youth satisfying criteria for PANDAS/PANS should receive treatment with psychotropic medications and can be expected to show improvement rates similar to youth with OCD not satisfying PANDAS/PANS criteria.
The evidence for use of antimicrobial and immune-based therapies in youth satisfying criteria for PANDAS/PANS is of low quality.
Future research on biological markers that can help to distinguish this entity from others is needed.
Potential Conflicts of Interest
EAY has served on a scientific advisory board for Juno Therapeutics and has provided a one-time consultation to Novartis. Teva provided unrestricted funds to the Hospital for Sick Children Foundation for a symposium she organized. RML serves as a consultant Sobi, Novartis, Eli Lilly, and Sanofi. SK, CW, DL, MS, AB, and WJL have no relevant disclosures to report. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author Contributions
CW: drafting and revision of manuscript, analysis and interpretation, paper concept/design, and supervision. DL: review and revision of manuscript for important intellectual content. RML: review and revision of manuscript for important intellectual content. MS: review and revision of manuscript for important intellectual content. AB: review and revision of manuscript for important intellectual content. SK: review and revision of manuscript for important intellectual content. WJL: review and revision of manuscript for important intellectual content. EAY: drafting and revision of manuscript, analysis and interpretation, paper concept/design, and supervision.
References
- 1. Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am J Psychiatry 1998;155(2):264–71. [DOI] [PubMed] [Google Scholar]
- 2. Orlovska S, Vestergaard CH, Bech BH, Nordentoft M, Vestergaard M, Benros ME. Association of streptococcal throat infection with mental disorders: Testing key aspects of the PANDAS hypothesis in a nationwide study. JAMA Psychiatry 2017;74(7):740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gabbay V, Coffey BJ, Babb JS, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcus: Comparison of diagnosis and treatment in the community and at a specialty clinic. Pediatrics 2008;122(2):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helm CE, Blackwood RA. Pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS): Experience at a tertiary referral center. Tremor Other Hyperkinet Mov (N Y) 2015;5:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swedo SE, Seidlitz J, Kovacevic M, et al. Clinical presentation of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections in research and community settings. J Child Adolesc Psychopharmacol 2015;25(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McClelland M, Crombez MM, Crombez C, et al. Implications for advanced practice nurses when pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) is suspected: A qualitative study. J Pediatr Health Care 2015;29(5):442–52. [DOI] [PubMed] [Google Scholar]
- 7. Swedo SE. Sydenham’s chorea. A model for childhood autoimmune neuropsychiatric disorders. JAMA 1994;272(22):1788–91. [DOI] [PubMed] [Google Scholar]
- 8. Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Therapeut 2012;2:113. [Google Scholar]
- 9. Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K, Chang K. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: Presenting characteristics of the first 47 consecutive patients. J Child Adolesc Psychopharmacol 2015;25(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mell LK, Davis RL, Owens D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics 2005;116(1):56–60. [DOI] [PubMed] [Google Scholar]
- 11. Leckman JF, King RA, Gilbert DL, et al. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: A prospective longitudinal study. J Am Acad Child Adolesc Psychiatry 2011;50(2):108–18 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo F, Leckman JF, Katsovich L, et al. Prospective longitudinal study of children with tic disorders and/or obsessive-compulsive disorder: Relationship of symptom exacerbations to newly acquired streptococcal infections. Pediatrics 2004;113(6):e578–85. [DOI] [PubMed] [Google Scholar]
- 13. Wang HC, Lau CI, Lin CC, Chang A, Kao CH. Group A streptococcal infections are associated with increased risk of pediatric neuropsychiatric disorders: A Taiwanese population-based cohort study. J Clin Psychiatry 2016;77(7):e848–54. [DOI] [PubMed] [Google Scholar]
- 14. Gray SM, Bloch MH. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr Psychiatry Rep 2012;14(3):220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parker-Athill EC, Ehrhart J, Tan J, Murphy TK. Cytokine correlations in youth with tic disorders. J Child Adolesc Psychopharmacol 2015;25(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pranzatelli MR, Tate ED, Allison TJ. Case-control, exploratory study of cerebrospinal fluid chemokines/cytokines and lymphocyte subsets in childhood Tourette syndrome with positive streptococcal markers. Cytokine 2017;96:49–53. [DOI] [PubMed] [Google Scholar]
- 17. Church AJ, Cardoso F, Dale RC, Lees AJ, Thompson EJ, Giovannoni G. Anti-basal ganglia antibodies in acute and persistent Sydenham’s chorea. Neurology 2002;59(2):227–31. [DOI] [PubMed] [Google Scholar]
- 18. Pavone P, Bianchini R, Parano E, et al. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr Neurol 2004;30(2):107–10. [DOI] [PubMed] [Google Scholar]
- 19. Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol 2006;179(1–2):173–9. [DOI] [PubMed] [Google Scholar]
- 20. Singer HS, Hong JJ, Yoon DY, Williams PN. Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology 2005;65(11):1701–7. [DOI] [PubMed] [Google Scholar]
- 21. Brilot F, Merheb V, Ding A, Murphy T, Dale RC. Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology 2011;76(17):1508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris-Berry CM, Pollard M, Gao S, Thompson C, Singer HS; Tourette Syndrome Study Group Anti-streptococcal, tubulin, and dopamine receptor 2 antibodies in children with PANDAS and Tourette syndrome: Single-point and longitudinal assessments. J Neuroimmunol 2013;264(1–2):106–13. [DOI] [PubMed] [Google Scholar]
- 23. Hesselmark E, Bejerot S. Biomarkers for diagnosis of pediatric acute neuropsychiatric syndrome (PANS) - sensitivity and specificity of the Cunningham panel. J Neuroimmunol 2017;312:31–7. [DOI] [PubMed] [Google Scholar]
- 24. Storch EA, Murphy TK, Geffken GR, et al. Cognitive-behavioral therapy for PANDAS-related obsessive-compulsive disorder: Findings from a preliminary waitlist controlled open trial. J Am Acad Child Adolesc Psychiatry 2006;45(10):1171–8. [DOI] [PubMed] [Google Scholar]
- 25. Nadeau JM, Jordan C, Selles RR, et al. A pilot trial of cognitive-behavioral therapy augmentation of antibiotic treatment in youth with pediatric acute-onset neuropsychiatric syndrome-related obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 2015;25(4):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Højgaard DRMA, Hybel KA, Ivarsson T, et al. One-year outcome for responders of cognitive-behavioral therapy for pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 2017;56(11):940–947.e1. [DOI] [PubMed] [Google Scholar]
- 27. Torp NC, Dahl K, Skarphedinsson G, et al. Effectiveness of cognitive behavior treatment for pediatric obsessive-compulsive disorder: Acute outcomes from the Nordic long-term OCD treatment study (NordLOTS). Behav Res Ther 2015;64:15–23. [DOI] [PubMed] [Google Scholar]
- 28. Arnold P, Kronenberg S. Biological models and treatments for obsessive-compulsive and related disorders for children and adolescents. In: Storch EA, Abramowitz JS, McKay D, eds. The Wiley Handbook of Obsessive Compulsive Disorders, Vol. 2, 1st edn Chichester, UK; Hoboken, NJ: John Wiley and Sons, 2017:1073–80. [Google Scholar]
- 29. Calaprice D, Tona J, Murphy TK. Treatment of pediatric acute-onset neuropsychiatric disorder in a large survey population. J Child Adolesc Psychopharmacol 2018;28(2):92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: A scientific statement from the American heart association rheumatic fever, endocarditis, and Kawasaki disease committee of the council on cardiovascular disease in the young, the interdisciplinary council on functional genomics and translational biology, and the interdisciplinary council on quality of care and outcomes research: Endorsed by the American academy of pediatrics. Circulation 2009;119(11):1541–51. [DOI] [PubMed] [Google Scholar]
- 31. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the infectious diseases society of America. Clin Infect Dis 2012;55(10):1279–82. [DOI] [PubMed] [Google Scholar]
- 32. Murphy TK, Brennan EM, Johnco C, Parker-Athill EC, Miladinovic B, Storch EA, Lewin AB. A double-blind randomized placebo-controlled pilot study of azithromycin in youth with acute-onset obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 2017;27(7):640–51. [DOI] [PubMed] [Google Scholar]
- 33. Garvey MA, Perlmutter SJ, Allen AJ, et al. A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biol Psychiatry 1999;45(12):1564–71. [DOI] [PubMed] [Google Scholar]
- 34. Snider LA, Lougee L, Slattery M, Grant P, Swedo SE. Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol Psychiatry 2005;57(7):788–92. [DOI] [PubMed] [Google Scholar]
- 35. Frankovich J, Thienemann M, Rana S, Chang K. Five youth with pediatric acute-onset neuropsychiatric syndrome of differing etiologies. J Child Adolesc Psychopharmacol 2015;25(1):31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kovacevic M, Grant P, Swedo SE. Use of intravenous immunoglobulin in the treatment of twelve youths with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Child Adolesc Psychopharmacol 2015;25(1):65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown KD, Farmer C, Freeman GM Jr, et al. Effect of early and prophylactic nonsteroidal anti-inflammatory drugs on flare duration in pediatric acute-onset neuropsychiatric syndrome: An observational study of patients followed by an academic community-based pediatric acute-onset neuropsychiatric syndrome clinic. J Child Adolesc Psychopharmacol 2017;27(7):619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown K, Farmer C, Farhadian B, Hernandez J, Thienemann M, Frankovich J. Pediatric acute-onset neuropsychiatric syndrome response to oral corticosteroid bursts: An observational study of patients in an academic community-based PANS clinic. J Child Adolesc Psychopharmacol 2017;27(7):629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999;354(9185):1153–8. [DOI] [PubMed] [Google Scholar]
- 40. Williams KA, Swedo SE, Farmer CA, et al. Randomized, controlled trial of intravenous immunoglobulin for pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Am Acad Child Adolesc Psychiatry 2016;55(10):860–867.e2. [DOI] [PubMed] [Google Scholar]
- 41. Latimer ME, L’Etoile N, Seidlitz J, Swedo SE. Therapeutic plasma apheresis as a treatment for 35 severely ill children and adolescents with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Child Adolesc Psychopharmacol 2015;25(1):70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alexander AA, Patel NJ, Southammakosane CA, Mortensen MM. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): An indication for tonsillectomy. Int J Pediatr Otorhinolaryngol 2011;75(6):872–3. [DOI] [PubMed] [Google Scholar]
- 43. Demesh D, Virbalas JM, Bent JP. The role of tonsillectomy in the treatment of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). JAMA Otolaryngol Head Neck Surg 2015; 141(3):272–5. [DOI] [PubMed] [Google Scholar]
- 44. Murphy TK, Lewin AB, Parker-Athill EC, Storch EA, Mutch PJ. Tonsillectomies and adenoidectomies do not prevent the onset of pediatric autoimmune neuropsychiatric disorder associated with group A streptococcus. Pediatr Infect Dis J 2013;32(8):834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pavone P, Rapisarda V, Serra A, et al. Pediatric autoimmune neuropsychiatric disorder associated with group a streptococcal infection: The role of surgical treatment. Int J Immunopathol Pharmacol 2014;27(3):371–8. [DOI] [PubMed] [Google Scholar]