Abstract
Transitional hypoglycemia is common in at-risk newborns, frequently resulting in therapeutic interference with bonding and breastfeeding; 40% dextrose gel massaged to the buccal mucosa has been shown to decrease hypoglycemia <2.6 mmol/L and NICU admissions. However, in the absence of a newborn-specific product, over-the-counter diabetes-care products with poorly documented composition are being used for neonates. We analyzed the carbohydrate content, and compared composition of the two commercially available gels in Canada, Dex4 and Insta-Glucose. We found that the glucose concentrations were significantly different from the expected 40% glucose, and that they contain artificial colorants, flavours and preservatives. In addition, we observed inconsistent concentration differences within each tube when aliquotes from the top, middle, or bottom were measured. There is a need for a custom made neonatal dextrose gel dispensed in unit dose vials, with a standardized concentration of glucose, and without chemical substances one would generally not recommend administering to newly born infants.
Keywords: Hypoglycemia, Newborn
Transitional hypoglycemia is common in at-risk newborns, affecting up to 47% of infants with risk factors such as being the infant of a diabetic mother (gestational, type 1, or type 2 diabetes), being late preterm (34 to 36 weeks’ gestation), being small (birthweight <10th centile or <2,500 g) or large (birthweight >90th centile or >4,500 g), or other reasons such as poor feeding (1). Maximizing skin-to-skin contact at birth, initiating breastfeeding soon after birth in healthy newborns, and screening at-risk newborns at 2 hours of age are recommended in order to avoid interference with bonding and breastfeeding, and over-diagnosing hypoglycemia (2).
In the Sugar Babies Study, Harris et al showed that supplementing breast feeding with 200 mg/kg (0·5 mL/kg) of a pharmacy compounded 40% dextrose gel massaged to the buccal mucosa in at-risk newborns with hypoglycemia is safe, and decreases hypoglycemia <2.6 mmol/L and NICU admissions for hypoglycemia (1,3,4). Thus, dextrose gel administration is becoming commonplace as the first line treatment of transitional hypoglycemia (4,5), and is being integrated into hospital practice to promote normality, and minimize the separation of mothers from their newborns. This supplementation of breastfeeding is in-line with the UNICEF Baby Friendly Initiative (6).
However, in the absence of a newborn-specific product, over-the-counter diabetes-care products are being used for neonates. These products are flavoured and coloured, and their composition, including their carbohydrate content, has not been analyzed and reported.
The goal of this evaluation was to determine the concentration of dextrose in the two brands of dextrose gel available in Canada: Insta-Glucose (Valeant Pharmaceuticals) and Dex4 (Perrigo Diabetes Care). We also attempted to identify the presence of other carbohydrates in these gels, and to report the types of additives they contain.
METHODS
Three different batches of Insta-Glucose and of Dex4 gel were analyzed, and the manufacturer’s literature about these products was reviewed. In each batch, 0.5 mL aliquots were taken from the top, middle, and bottom of the tube, to determine if dextrose was distributed evenly throughout the gel. We determined the dextrose content per gram of gel and also measured the density of each gel, as recommended dosing is in millilitres. Glucose concentrations were analyzed by dilution of the gel in water and by addition of a stable isotope labeled internal standard (U-13C-glucose, Cambridge isotopes #CLM-1396, Andover MD). High-pressure liquid chromatography tandem mass spectrometry (HPLC-MS/MS) and gas chromatography mass spectrometry (GCMS) were used to determine glucose concentrations and identify other carbohydrates, respectively. Lugol’s iodine test was performed on each of the gels to determine if any starches were present. Results are presented as means ± SD.
RESULTS
The HPLC-MS/MS analyses for glucose concentrations were performed in triplicates and yielded an average coefficient of variation of 6.1% and 9.3% for Insta-Glucose and for Dex4, respectively, with an average recovery of ~85% for both gel types. Calibration standard curves created with IV glucose solution (DIN 00037974, Hospira, Montreal, QC) were linear from 25 to 1,000 µg/mL, (r2=0.999).
The gels were highly viscous, and the mean ± SD density for Insta-Glucose was 1.53 ± 0.07 g/mL and for Dex4 was 1.24 ± 0.04 g/mL.
Aliquots from different locations (top, middle, and bottom of each tube) at room temperature (~20°C) showed that both gels were nonhomogeneous, and had large, random differences in glucose concentration per gram of gel (Table 1). Maximum differences in glucose concentration were observed at 81% for Insta-Glucose and at 43% and for Dex4.
Table 1.
Measured dextrose content (in grams per gram of gel) and calculated concentration (in grams per mL) in two commercial gels
|
Insta-Glucose Dextrose Content
1 (grams of dextrose/gram of gel) |
Dex4 Dextrose Content
1 (grams of dextrose/gram of gel) |
||||||
|---|---|---|---|---|---|---|---|
| Batch # | Top | Middle | Bottom | Batch # | Top | Middle | Bottom |
| 8074168 | 0.139 | 0.144 | 0.139 | 6366863 | 0.397 | 0.415 | 0.385 |
| 8095170 | 0.158 | 0.174 | 0.169 | 5453584 | 0.445 | 0.399 | 0.518 |
| 8077743 | 0.116 | 0.096 | 0.096 | 5453583 | 0.361 | 0.441 | 0.376 |
| Median: 0.139 (21.3%)2 | Median: 0.399 (49.5%)2 | ||||||
| Max: 0.174 (26.6%)2 | Max: 0.518 (64.2%)2 | ||||||
| Min: 0.096 (14.7%)2 | Min: 0.361 (44.8%)2 | ||||||
| Max-Min difference3: 81% | Max-Min difference3: 43% | ||||||
1Dextrose content was measured using LC-MS/MS.
2Dextrose concentration in % = dextrose content (grams of dextrose/gram of gel) × 100 × gel density (grams of gel/mL of gel). Insta-Glucose density: 1.53 ± 0.07 g/mL. Dex4 density: 1.24 ± 0.04 g/mL.
3Max-min difference (in %) = (Max – Min) × 100/Min, where Max is the highest and Min is the lowest dextrose content value among three aliquots (top, middle, and bottom) of three random batches of each product.
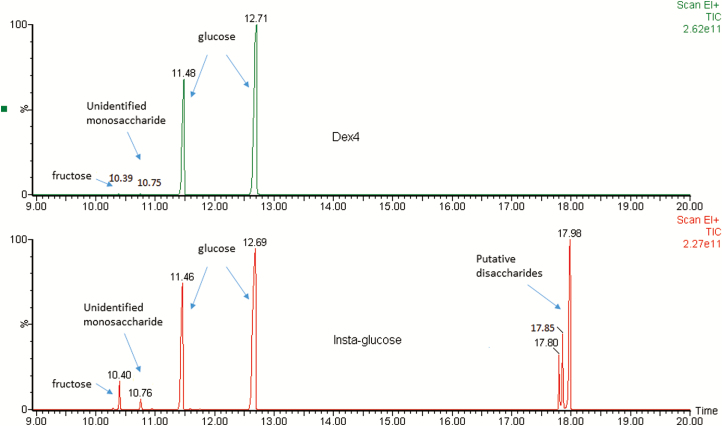
When analyzed for presence of other carbohydrates, Insta-Glucose gel had dextrose, fructose, and other unidentified carbohydrates, that are likely to be disaccharides. The putative disaccharides in Insta-Glucose did not match the GCMS retention times for sucrose or lactose (data not shown). In Dex 4 gel the carbohydrate content was almost all dextrose, with trace amounts of fructose and an unidentified carbohydrate (Figure 1).
Figure 1.
GCMS chromatograms of carbohydrates in commercial gels. The top panel shows the chromatogram of Dex4 and the bottom panel the chromatogram of Insta-Glucose. Retention time of peaks is in minutes. Peaks were identified using authentic standards of glucose, fructose, sucrose and lactose. Glucose appears as two peaks due to interconversion of stereoisomers.
Reviewing the product label indicated that other ingredients in Insta-Glucose gel were water, artificial cherry flavour, methylparaben, potassium sorbate, propylparaben, and sodium benzoate. Other ingredients In Dex4 gel included water, xantham gum, glycerin, citric acid, sorbic acid, natural and artificial flavour, sodium benzoate, FD&C Red#40, FD&C Blue #1.
DISCUSSION
We studied and analyzed two over-the-counter diabetes-care glucose gels that are available in Canada and found that their dextrose content can vary by up to 81% by batch and in aliquots from the same tube. These products are also considerably different in their composition from the gel used in the Sugar Babies study (Table 2) (1). Of the two products studied, the one with the most different and variable dextrose concentration content is Insta-Glucose.
Table 2.
Formulation of the ‘Sugar Babies’ D40% glucose gel1
| Glucose Anhydrous BP 40 g |
| Carmellose sodium BP 2 g |
| 2Methylhydroxybenzoate BP 0.09% |
| 2Propylhydroxybenzoate BP 0.01% |
| Water to 100 mL |
1Personal communication to Dr. Eddie Kwan from Dr. Jane Harding, PI of the Sugar Babies Trial.
2Methylhydroxybenzoate and propylhydroxybenzoate are added as preservatives.
Both gels contain colorants, flavour, preservatives, and other substances that are not necessary to make them acceptable to newborns. The demonstrated benefits of implementing baby-friendly protocols using dextrose gels are clear. Based on our findings, we strongly suggest that the next natural step is to follow the format of commercially made 24% sucrose solutions formulated specifically for the management of procedural pain in newborns. TootSweet by Natus Medical Inc and SweetUms by Sandbox Medical are available in 1 and 2 mL single use vials, and only contain preservatives and buffers as additives. Future development of a colour- and flavour-free commercial 40% glucose gel formulated for newborns and dispensed in small vials would not only facilitate dosing but also eliminate the concern of internal variations in its concentration due to viscosity.
AUTHORSHIP AND ACKNOWLEDGEMENTS
Funding/Support: This study was funded in part by the BC Children’s Hospital Research Institute (Solimano).
Potential Conflicts of Interest: All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions: AS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AS, EK, HO, RE. Acquisition of data: RE, RD, EK. Analysis and interpretation of data: AS, EK, HO, RE, RD. Drafting of the manuscript: AS, HO, RE. Critical revision of the manuscript for important intellectual content: AS, EK, HO, RE, RD. Statistical analysis: AS, RE. Obtained funding: AS. Administrative, technical, or material support: AS, RE. Study supervision: AS, RE.
Internal review and feedback: Dr. Daniel Metzger, Division of Endocrinology, Department of Pediatrics, University of British Columbia, Vancouver.
References
- 1. Harris DL, Weston PJ, Signal M, Chase JG, Harding JE. Dextrose gel for neonatal hypoglycaemia (the sugar babies study): A randomised, double-blind, placebo-controlled trial. Lancet 2013;382(9910):2077–83. [DOI] [PubMed] [Google Scholar]
- 2. Solimano A. ACoRN – Acute of at-Risk Newborns. Vancouver, BC: ACoRN Neonatal Society, 2012. [Google Scholar]
- 3. Harris DL, Alsweiler JM, Ansell JM et al. ; Children with Hypoglycaemia and their Later Development (CHYLD) Study Team Outcome at 2 years after dextrose gel treatment for neonatal hypoglycemia: Follow-up of a randomized trial. J Pediatr 2016;170:54–9.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weston PJ, Harris DL, Battin M, Brown J, Hegarty JE, Harding JE. Oral dextrose gel for the treatment of hypoglycaemia in newborn infants. Cochrane Database Syst Rev 2016;5:CD011027. [DOI] [PubMed] [Google Scholar]
- 5.Stewart CE, Sage ELM, Reynolds P. Supporting ‘Baby Friendly’: A quality improvement initiative for the management of transitional neonatal hypoglycaemia. Arch Dis Child Fetal Neonatal Ed 2016;101:F344–F347. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Baby-Friendly Hospital Initiative: Revised, Updated and Expanded for Integrated Care. Geneva: World Health Organization, 2009. <https://www.ncbi.nlm.nih.gov/books/NBK153471/>. [PubMed] [Google Scholar]