Introduction
Active surveillance has become a preferred management strategy for men with very low and low risk prostate cancers.1, 2 Active surveillance allows men to avoid or delay treatment and its undesirable side effects, and long term single center experiences have demonstrated excellent outcomes.3, 4 As a result, men with low risk prostate cancer are increasingly managed with initial active surveillance in selected clinical registries.5, 6
Despite the increasing acceptance of active surveillance, its real-world utilization beyond clinical registries is not well understood. There has not been a national population-based study of the use of active surveillance in the United States. Given the varied protocols described in large published series,3, 7, 8 it is essential to understand if and how active surveillance is being used outside of high-volume centers. Reports from regional clinical registries have suggested that the majority of men on active surveillance do not undergo recommended follow up testing.9, 10 Further, the use of active surveillance varies considerably, even among men with low-risk prostate cancer.11 To elucidate these findings, large population-based studies using claims data are needed. While clinical registries generally have high-quality data, they often include highly selected practices or those participating in quality improvement efforts. Claims data, on the other hand, offer a representative view of national utilization trends.
Historically, one challenge to determining national active surveillance trends has been the inability to identify active surveillance in administrative claims. Medicare claims data, specifically, includes a large, nationally representative, and diverse population of older men in whom active surveillance has not been studied. Therefore, we created algorithms to identify active surveillance using Medicare claims and validated them using linked data from a robust and validated clinical registry. Then, we applied these algorithms to national Medicare claims to describe population level trends in the use of active surveillance for prostate cancer and use of confirmatory prostate biopsies. By virtue of this approach, our findings will provide clinicians, policymakers, and patients with a better understanding of real-world, nationwide trends in the use of active surveillance.
Materials and Methods
Data Sources
We used data from a 100% sample of Medicare claims from the state of Michigan, provided by the Michigan Value Collaborative (a partnership between Michigan hospitals and Blue Cross Blue Shield of Michigan).12 These claims were linked with the clinical registry of the Michigan Urological Surgery Improvement Collaborative (MUSIC). Established in 2011 with support from Blue Cross Blue Shield of Michigan, MUSIC aims to improve the value of urological care in Michigan. Currently, the collaborative includes 44 urology practices and more than 250 urologists, comprising approximately 90% of urologists in the state. Trained abstractors at each site use medical records to prospectively enter clinical, pathological, and demographic data for each patient with prostate cancer into an electronic registry.6, 10 MUSIC’s registry undergoes regular and thorough data quality audits to ensure that registry data, such as treatment type is accurate.6 The most recent audit of newly diagnosed prostate cancer patients demonstrated 97% accuracy in initial treatment assignment. To describe national trends in the use of active surveillance, we used a 20% sample of national Medicare claims.
Study Population
We identified patients with a new diagnosis of prostate cancer between 2012 and 2014 in a 100% sample of Medicare patients in Michigan using established methods.13 Briefly, we identified men with at least two “Evaluation and Management” visit codes with an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code of “185” for “prostate cancer.” We required that all cases had a prostate biopsy within 180 days of the first visit associated with a diagnosis of prostate cancer. Finally, we excluded men with a claim in the preceding 12 months with an ICD-9 diagnosis code of “185” or “V10.46” (personal history of malignant neoplasm of prostate). This method has been validated using the Surveillance, Epidemiology, and End-Results (SEER) cancer registry and has a specificity of 99.8%.13
We excluded men without continuous Medicare Parts A and B coverage for at least 12 months before and after prostate cancer diagnosis and those with Medicare managed care plans. We used claims from the 12 months prior to diagnosis to determine a comorbidity score, using Klabunde’s modification of the Charlson comorbidity index.14
Michigan Medicare data was linked with MUSIC registry data using three factors: patient date of birth, prostate biopsy date, and urologist. We identified the urologist with the plurality of claims with a prostate cancer diagnosis code. Of 4317 men diagnosed from 2012–2014 with complete Medicare data, 1257 were matched with MUSIC data. We excluded duplicates and our final study cohort included 1186 men. Of these: 956 (80.6%) were matched based on all three factors and 230 (19.4%) based on two (date of birth and biopsy date).
Analysis
We generated eight candidate algorithms to identify active surveillance in claims (Table 1) based on three principles. First, active surveillance entails the initial avoidance of prostate cancer treatment. Second, younger age and fewer comorbidities may help distinguish active surveillance from watchful waiting. Finally, additional testing (i.e., confirmatory prostate biopsy or prostate specific antigen [PSA] testing) could distinguish active surveillance from watchful waiting. We then identified beneficiaries meeting criteria for each algorithm in the 100% Michigan Medicare data. We used the linked MUSIC treatment assignment as the gold standard for all cases. We calculated the sensitivity, specificity, positive and negative predictive values for each algorithm.
Table 1.
Test characteristics of algorithms when applied to men with matched data in Michigan Medicare claims and MUSIC clinical registry. Bolded algorithms were selected to describe active surveillance trends in a national Medicare sample.
| Algorithm | Description | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| 1 | No treatment within 12 months of diagnosis | 88.24 | 93.48 | 73.77 | 97.45 |
| 2 | No treatment and 1 PSA within 12 months of diagnosis | 84.31 | 93.69 | 73.50 | 96.64 |
| 3 | No treatment and comorbidity score < 3 | 83.82 | 94.09 | 74.67 | 96.55 |
| 4 | No treatment and comorbidity score < 2 | 77.45 | 94.60 | 74.88 | 95.28 |
| 5 | No treatment and age < 75 | 65.20 | 96.33 | 78.70 | 93.02 |
| 6 | No treatment within 12 months, comorbidity score < 3, and age < 75 | 62.25 | 96.54 | 78.88 | 92.49 |
| 7 | No treatment, age < 75, and 1 PSA within 12 months of diagnosis | 61.76 | 96.44 | 78.26 | 92.39 |
| 8 | No treatment and ≥1 prostate biopsy between 1 and 12 months after diagnosis | 23.53 | 99.08 | 84.21 | 86.18 |
We applied three of these algorithms to a 20% sample of national Medicare claims from 2007 to 2014 to obtain national data on the use of active surveillance. Algorithm 1 (no active treatment in 12 months after diagnosis) was selected because it has the highest sensitivity and has previously been used to identify men undergoing “observation” (i.e., active surveillance and watchful waiting).15, 16 Algorithm 8 (no treatment and at least one prostate biopsy after diagnosis) was selected because it has the highest specificity. The repeat biopsy was required to be at least one month after, but within 12 months of, prostate cancer diagnosis to capture a true “confirmatory biopsy.”17 Finally, Algorithm 6 (no treatment, age less than 75 years, and comorbidity score less than 3) was selected because it has the second highest specificity while maintaining considerably more sensitivity than Algorithm 8.
We first examined differences among the three algorithms with respect to the use of confirmatory biopsy and the ability to differentiate active surveillance from watchful waiting. We described national trends in the use of active surveillance among men newly diagnosed with prostate cancer from 2007 to 2014. The use of active surveillance was first analyzed as a proportion of men newly diagnosed with prostate cancer. This method was used to determine changes in treatment patterns over time; however, it cannot account for underlying changes in screening and diagnosis during the study period.15 Therefore, we also determined the use of active surveillance as a population level “rate” using all men as the denominator. All analyses were performed using SAS 9.4 (Cary, NC). This study was deemed exempt from Institutional Review Board review.
Results
Of 1186 men with incident prostate cancer and linked records from the state of Michigan, 204 (17.2%) were managed with initial active surveillance, 60 (5.1%) with watchful waiting, and 922 (77.7%) with some form of treatment (Supplemental Table 1). Eight algorithms were tested with sensitivity ranging from 23.5% to 88.2% and specificity ranging from 93.5% to 99.1% (Table 1). As a sensitivity analysis, we report the performance of each algorithm to detect observation (i.e., active surveillance or watchful waiting) in Supplemental Table 2. As an additional sensitivity analysis, we analyzed an algorithm that required no treatment and at least one prostate biopsy in 18 months after diagnosis (Supplemental Tables 3 and 4).
Discrimination of active surveillance and watchful waiting
Of the 60 men managed with watchful waiting, 53 (88.3%) met criteria for Algorithm 1 while 7 (11.7%) had claims for a treatment. Algorithm 8 correctly excluded 53 (88.3%) patients who did not have a confirmatory prostate biopsy; however, 7 (11.7%) men who underwent watchful waiting had a repeat prostate biopsy. Algorithm 6 classified 27 (45%) men who received no treatment, were younger than 75, and had a comorbidity score less than 3 as undergoing active surveillance, while the reference MUSIC registry data indicated watchful waiting.
National trends
Using national Medicare data, we identified 83,514 men with incident prostate cancer from 2007 to 2014. Of these men, 14,756 (17.7%) had no treatment in the 12 months following diagnosis (Algorithm 1); 7,499 (9%) underwent no treatment, were younger than 75 years, and had a comorbidity score lower than 3 (Algorithm 6); and 2,572 (3.1%) underwent no treatment and had a repeat prostate biopsy (Algorithm 8). Using each algorithm, active surveillance use among all men diagnosed with incident prostate cancer increased over the study period (Figure 1). Despite the overall trend, the use of active surveillance decreased from 2013 to 2014 by each measure.
Figure 1.
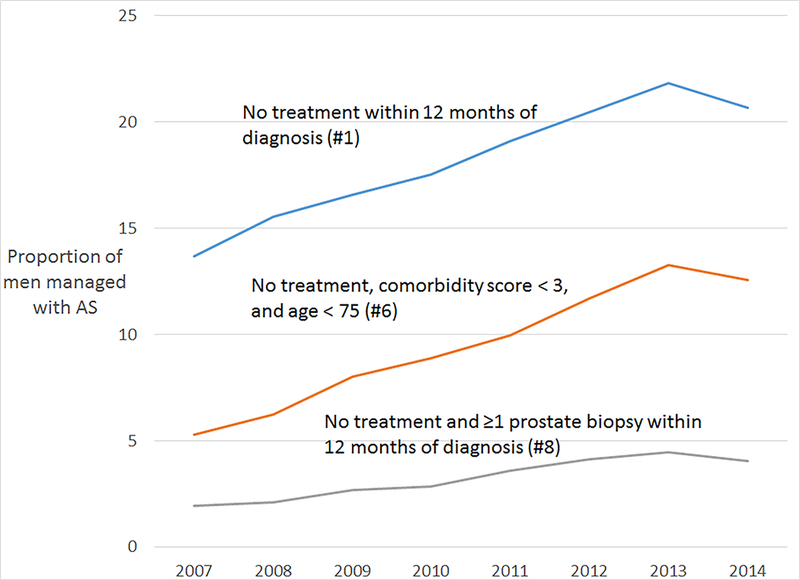
Proportion of men with a new diagnosis of prostate cancer who were managed with active surveillance.
On a population level, the rate of prostate cancer diagnosis decreased from 7.3 men per 1000 in 2007 to 4 men per 1000 in 2014 (Figure 2). The population rate of active surveillance increased from 2007 to 2014 as measured by algorithm 6 (OR 1.05 per year, 95% CI 1.02–1.08, P<0.001) and algorithm 8 (OR 1.04 per year, 95% CI 1.01–1.08, P=0.017). The rate of active surveillance using Algorithm 1 was unchanged over the study period (OR 0.98 per year, 95% CI 0.96–1.00, P=0.079).
Figure 2.
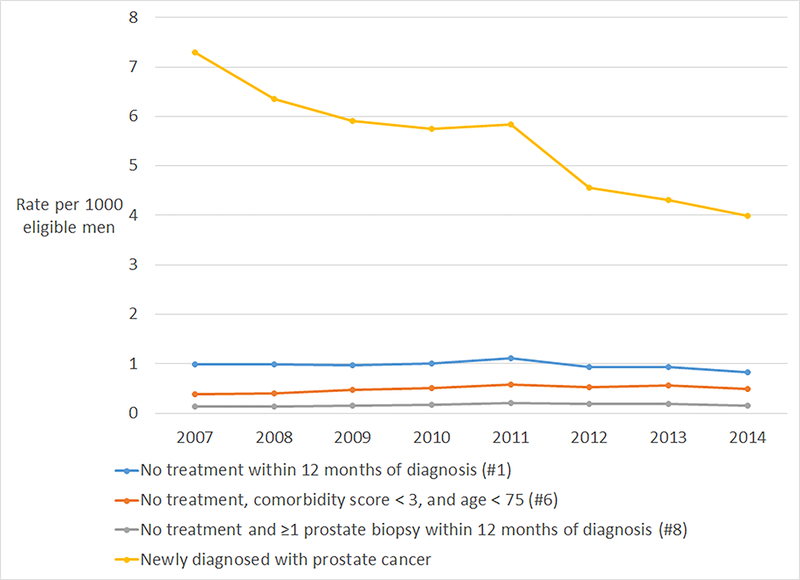
Population-based rate of men diagnosed with prostate cancer and treated with active surveillance over time
Use of confirmatory biopsy
Of the 14,756 total men with incident prostate cancer who underwent no treatment (Algorithm 1), 2572 (17.4%) had a repeat prostate biopsy within 12 months of diagnosis. Of the 7,499 men younger than 75 years with no treatment and a comorbidity score less than 3 (Algorithm 6), 1732 (23.1%) underwent a repeat prostate biopsy within 12 months of diagnosis (Supplemental Figure).
Discussion
The use of active surveillance among men diagnosed with prostate cancer increased from 2007 to 2014, but changed little on a population-level. This suggests that active surveillance has gained acceptance nation-wide and its use among men with newly diagnosed prostate cancer has increased during the study period. Simultaneously, the rate of prostate cancer diagnosis has decreased among Medicare beneficiaries, potentially as a result of concern about overdiagnosis and inappropriate screening.
To identify active surveillance in Medicare claims, we created and validated 8 algorithms. The simplest algorithm (1) identifies all men who do not undergo curative therapy within 12 months of diagnosis and was the most sensitive and least specific. Despite its inability to distinguish active surveillance and watchful waiting, algorithm 1 had a specificity of over 93% due to the low use of watchful waiting in our validation cohort. Algorithm 6 identified men younger than 75 with a comorbidity score less than 3 who did not undergo any treatment. This algorithm had excellent specificity but was considerably less sensitive than algorithm 1. Almost half of men in MUSIC who chose watchful waiting were both younger than 75 and had a comorbidity score less than 3. Finally, the most specific algorithm (8) identified men who underwent at least one repeat prostate biopsy after diagnosis. This algorithm had excellent specificity but very poor sensitivity, reflecting the low use of confirmatory biopsy in our validation cohort.
The increasing use of observation (active surveillance or watchful waiting) for men with prostate cancer has been described using clinical registries in the US and Sweden.5, 18–21 While clinical registry data can accurately determine treatment, it has historically been more difficult to distinguish active surveillance and watchful waiting in claims-based analyses. Our study is the first to quantify the performance of various algorithms to identify active surveillance and to describe its utilization in the United States in a population-based cohort. Further, using Medicare data to evaluate active surveillance among both men with incident prostate cancer and among the entire population of men, we are able to account for changing national trends in the screening and diagnosis of prostate cancer.15 We demonstrate the increasing use of active surveillance among men diagnosed with prostate cancer and the stable or increasing rate of active surveillance at a population level, depending on the algorithm used. Few men underwent confirmatory biopsies.
While the proportion of newly diagnosed men who underwent active surveillance increased in each year from 2007 to 2013, the relative use of active surveillance decreased from 2013 to 2014. The overall increasing use of active surveillance has been described in other cohorts, but this is the first study to our knowledge to describe a relative decrease in the use of active surveillance. We cannot determine the reasons for this decrease using our data. However, we speculate that decreased screening and prostate cancer diagnosis may have led to a disproportionate reduction in the diagnosis of low-risk prostate cancers and therefore a lower relative use of surveillance. Alternatively, more men with low-risk prostate cancer may be undergoing treatment in 2014, perhaps as a consequence of the availability of novel focal therapy options. Additional studies are needed to confirm this trend and examine reasons for a recent decrease in the relative use of active surveillance.
In addition to evaluating the frequency of active surveillance, there is a need to characterize the intensity and specific testing used in real-world practice. Active surveillance in the community setting differs considerably from protocols published by high volume centers.9, 10, 22 In MUSIC, fewer than one-third of men on active surveillance underwent testing frequency concordant with National Comprehensive Cancer Network (NCCN) guidelines.10 In men on active surveillance in SEER regions, surveillance intensity very rarely (5%−11.1%) achieved that described by published cohorts.9 However, this study required confirmatory biopsy for inclusion. As we have shown, the use of confirmatory biopsy is rare and its use as an inclusion criterion will fail to identify a large majority of men undergoing active surveillance.
This is the first national, population-based study to demonstrate the low rate of confirmatory biopsy among older men on active surveillance. The consequences of omitting confirmatory biopsy are unknown but may increase the chances of missing higher risk disease present at initial diagnosis. While the PIVOT23 and ProtecT24 studies have demonstrated acceptable prostate-cancer specific survival with less rigorous surveillance, it is not clear if these outcomes are applicable to the U.S. population or will persist with longer duration follow-up. Future work should characterize the consequences of avoiding confirmatory biopsy and the potential of other tests such as MRI and/or genomic testing as replacements for confirmatory biopsy.25 Additionally, future work should consider the shift in the intensity of observation over time. Active surveillance and watchful waiting can be thought of as two ends of a spectrum, with watchful waiting representing the least “active” surveillance and strict re-biopsy and MRI being used in a more intense surveillance regimen. Real-world claims data may be essential to parsing these trends, as registry data and single-center cohorts may not capture the variation in practice patterns.
Our findings should be considered in the context of several limitations. First, the performance of the algorithms we have described are based on the gold standard of MUSIC’s validated clinical registry data. The most common false-positive result for these algorithms is due to the use of watchful waiting. If the utilization of watchful waiting in other populations differs considerably from its use in MUSIC, the performance of these algorithms may vary. Second, the present study uses Medicare claims data and therefore includes only men age 66 and older. These results may not be generalizable to younger patients with prostate cancer. However, approximately 57% of incident prostate cancer cases occur in this age group26 and Medicare aged patients are often included in other active surveillance studies.9, 11 Additionally, as Medicare data does not include cancer grade or stage, our analyses are presented in the context of all men with newly diagnosed prostate cancer and among all men without pre-existing prostate cancer; we cannot comment on active surveillance use in specific risk categories.
Notwithstanding these limitations, this study provides important national trends and a foundation for additional claims-based evaluation of active surveillance. Clinicians can use this to better understand the use of active surveillance on a national level. Researchers can select one of the algorithms in this manuscript for future research, depending on the goals of their studies. Policymakers can use these findings to identify discrepancies between guidelines and active surveillance practice. As the acceptance of active surveillance increases, it will be essential to understand variation in the frequency of its use and the specific testing employed in real-world practice.
Conclusions
The use of active surveillance in real-world practice is heterogeneous and differs from the strict protocols described in published cohort studies. A better understanding of how claims data perform in identifying patients on active surveillance is necessary to study the use of observation practices in the United States. In a national population-based sample, we demonstrate the increased use of active surveillance among men with incident prostate cancer. We also provide a set of algorithms validated against a robust clinical registry for use in claims data. Finally, we confirm the low utilization of confirmatory biopsy extends to a national sample of older men undergoing active surveillance for prostate cancer. Future studies should evaluate reasons for and consequences of these trends.
Supplementary Material
Populations of men undergoing active surveillance identified using 3 algorithms
Table 2.
Percentages may not sum to 100 because of rounding.
| Algorithm 1 (n=14,756) | Algorithm 6 (n=7,499) | Algorithm 8 (n=2,572) | |
|---|---|---|---|
| Age, n (%) | |||
| 66–69 | 3595 (24.4) | 3371 (45.0) | 900 (35.0) |
| 70–74 | 4485 (30.4) | 4128 (55.1) | 942 (36.6) |
| 75–79 | 3809 (25.8) | excluded# | 511 (19.9) |
| 80–84 | 2071 (14.0) | excluded# | 162 (6.3) |
| 85+ | 796 (5.4) | excluded# | 57 (2.2) |
| Race, n (%) | |||
| White | 13063 (88.8) | 6554 (87.6) | 2291 (89.4) |
| Black | 1220 (8.3) | 663 (8.9) | 197 (7.7) |
| Asian | 88 (0.6) | 40 (0.5) | 12 (0.5) |
| Hispanic/Other/Unknown* | 334 (2.3) | 221 (2.9) | 62 (2.5) |
| Socioeconomic status**, n (%) | |||
| Low | 4440 (30.1) | 2215 (29.5) | 654 (25.4) |
| Medium | 5133 (34.8) | 2550 (34) | 828 (32.2) |
| High | 5183 (35.1) | 2734 (36.5) | 1090 (42.4) |
| Comorbidity score***, n (%) | |||
| 0 | 8652 (58.6) | 5019 (66.9) | 1626 (63.2) |
| 1 | 3270 (22.2) | 1717 (22.9) | 521 (20.3) |
| 2 | 1568 (10.6) | 763 (10.2) | 237 (9.2) |
| 3 or higher | 1266 (8.6) | excluded# | 188 (7.3) |
Categories combined due to small cell sizes
Socioeconomic status is defined by the median income in a ZIP code and divided into tertiles.
Comorbidity score of 0 indicates no comorbidities, while a score of 3 or more indicates severe comorbidities.
Excluded according to the criteria in the algorithm.
Acknowledgments
Funding: Funding for the Michigan Urological Surgery Improvement Collaborative (MUSIC) and the Michigan Value Collaborative (MVC) is provided by Blue Cross Blue Shield of Michigan (BCBSM) as part of the Blue Cross Blue Shield of Michigan Value Partnerships program; however, the opinions, beliefs and viewpoints expressed by the authors do not necessarily reflect those of Blue Cross Blue Shield of Michigan or any of its employees. Additional support from NCI T32CA180984 (PM), AHRQ R01HS257007 (BH), NCI R01CA168691 (VS). JMD receives salary support from Blue Cross Blue Shield of Michigan (BCBSM) for his roles in the Michigan Urological Surgery Improvement Collaborative and Michigan Value Collaborative. The views expressed in this article do not reflect the views of the federal government.
References
- 1.Chen RC, Rumble RB, Loblaw DA et al. : Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol, 34: 2182, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Sanda MG, Cadeddu JA, Kirkby E et al. : Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol, 2017 [DOI] [PubMed]
- 3.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol, 33: 272, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Tosoian JJ, Mamawala M, Epstein JI et al. : Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol, 33: 3379, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Carroll PR: Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA, 314: 80, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Womble PR, Montie JE, Ye Z et al. : Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol, 67: 44, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Bul M, Zhu X, Valdagni R et al. : Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol, 63: 597, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Carter HB, Kettermann A, Warlick C et al. : Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol, 178: 2359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeb S, Walter D, Curnyn C et al. : How Active is Active Surveillance? Intensity of Followup during Active Surveillance for Prostate Cancer in the United States. J Urol, 196: 721, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luckenbaugh AN, Auffenberg GB, Hawken SR et al. : Variation in Guideline Concordant Active Surveillance Followup in Diverse Urology Practices. J Urol, 197: 621, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loppenberg B, Friedlander DF, Krasnova A et al. : Variation in the use of active surveillance for low-risk prostate cancer. Cancer, 124: 55, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Ellimoottil C, Syrjamaki JD, Voit B et al. : Validation of a claims-based algorithm to characterize episodes of care. Am J Manag Care, 23: e382, 2017 [PubMed] [Google Scholar]
- 13.Hollenbeck BK, Bierlein MJ, Kaufman SR et al. : Implications of evolving delivery system reforms for prostate cancer care. Am J Manag Care, 22: 569, 2016 [PMC free article] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol, 53: 1258, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Borza T, Kaufman SR, Shahinian VB et al. : Sharp Decline In Prostate Cancer Treatment Among Men In The General Population, But Not Among Diagnosed Men. Health Aff (Millwood), 36: 108, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Tyson MD, Graves AJ, O’Neil B et al. : Urologist-Level Correlation in the Use of Observation for Low- and High-Risk Prostate Cancer. JAMA Surg, 152: 27, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Kovac E, Lieser G, Elshafei A et al. : Outcomes of Active Surveillance after Initial Surveillance Prostate Biopsy. J Urol, 197: 84, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Ingimarsson JP, Celaya MO, Laviolette M et al. : Trends in initial management of prostate cancer in New Hampshire. Cancer Causes Control, 26: 923, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Loeb S, Berglund A, Stattin P: Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol, 190: 1742, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Ritch CR, Graves AJ, Keegan KA et al. : Increasing use of observation among men at low risk for prostate cancer mortality. J Urol, 193: 801, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh RR, Kim S, Stein MN et al. : Trends in active surveillance for very low-risk prostate cancer: do guidelines influence modern practice? Cancer Med, 6: 2410, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loeb S, Folkvaljon Y, Curnyn C et al. : Uptake of Active Surveillance for Very-Low-Risk Prostate Cancer in Sweden. JAMA Oncol, 3: 1393, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilt TJ, Jones KM, Barry MJ et al. : Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl J Med, 377: 132, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Hamdy FC, Donovan JL, Lane JA et al. : 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med, 375: 1415, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Hamoen EHJ, Hoeks CMA, Somford DM et al. : Value of Serial Multiparametric Magnetic Resonance Imaging and Magnetic Resonance Imaging-guided Biopsies in Men with Low-risk Prostate Cancer on Active Surveillance After 1 Yr Follow-up. Eur Urol Focus, 2018 [DOI] [PubMed]
- 26.Herget KA, Patel DP, Hanson HA et al. : Recent decline in prostate cancer incidence in the United States, by age, stage, and Gleason score. Cancer Med, 5: 136, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Populations of men undergoing active surveillance identified using 3 algorithms


