Abstract
PURPOSE OF REVIEW:
Both apoptotic and non-apoptotic cell extrusion preserve the barrier functions of epithelia. Live cell extrusion is the paradigm for homeostatic renewal of intestinal epithelial cells (IEC). By extension, because extruded cells are not apoptotic, this form of cell shedding is thought to be largely ignored by lamina propria phagocytes and without immune consequence.
RECENT FINDINGS:
Visualization of apoptotic IEC inside distinct subsets of intestinal phagocytes during homeostasis has highlighted apoptosis as a normal component of the natural turnover of the intestinal epithelium. Analysis of phagocytes with or without apoptotic IEC corpses has shown how apoptotic IEC constrain inflammatory pathways within phagocytes and induce immunosuppressive regulatory CD4 T cell differentiation. Many of the genes involved overlap with susceptibility genes for inflammatory bowel disease (IBD).
SUMMARY:
Excessive IEC death and loss of barrier function is characteristic of IBD. Because regulatory and tolerogenic mechanisms are broken in IBD, a molecular understanding of the precise triggers and modes of IEC death as well as their consequences on intestinal inflammation is necessary. This characterization should guide new therapies that restore homeostatic apoptosis, along with its associated programs of immune tolerance and immunosuppression, to achieve mucosal healing and long-term remission.
Keywords: Cell death, TNF-α, inflammatory bowel disease, intestinal epithelium, intestinal mononuclear phagocyte
Introduction
A convergence of genetic susceptibility factors, the enteric microbiota and injury to the intestinal epithelium underlies the pathogenesis of inflammatory bowel disease (IBD) (1, 2). Damage of the intestinal epithelium manifests in a notable increase in the death of intestinal epithelial cells (IEC) (3–9). High levels of cell death termed ‘apoptosis’ were noted in the epithelium of patients with ulcerative colitis (9–11). An association between apoptosis and IBD has also been found in epithelial cell proteome studies in both IBD patients and IBD mouse models (12, 13). IEC necroptosis is thought to underlie the microerosions and epithelial gaps observed in mice and humans by in vivo imaging (14, 15), and may serve as a pathophysiological mechanism that precipitates barrier dysfunction leading to inflammation. In all these cases, the most obvious effect of increased IEC death is disruption of the IEC barrier and consequent loss of its protective and antimicrobial activities leading to dysbiosis and microbial translocation into the sterile intestinal lamina propria. These events drive further inflammation and more damage to the intestinal epithelium, making it difficult to distinguish cause from effect. This review examines the different modes of cell death that have been reported in the intestinal epithelium and the conditions under which they occur. It also highlights apoptosis as the physiological form of cell death that can occur during intestinal epithelial turnover. The consequences of innate recognition of apoptotic IEC on intestinal tolerance and homeostasis are discussed and their relevance to IBD.
Apoptosis during homeostatic turnover of the intestinal epithelium
Apoptosis is the preferred mode of cell death during both embryonic development and adult tissue turnover, and its balance with cell division maintains proper tissue size and function (16). Within the intestine, continuous turnover of the intestinal epithelium is critical for ensuring effective barrier function against digested food and the commensal microbiota. IEC arising from stem cells at the base of the crypts travel towards the villi tips in the small intestine or luminal face of the crypts in the large intestine (17). This process takes 4–5 days at the end of which IEC are shed into the lumen through mechanisms debated to involve either apoptosis or live extrusion by upwardly moving cells (18). Basolateral contraction of actin and myosin during IEC extrusion precedes the appearance of the characteristic readouts of apoptosis, caspase-3 cleavage and phosphatidylserine exposure (19, 20). Thus, a commitment to apoptosis can signal extrusion of IEC that have not yet exhibited the hallmarks of apoptosis.
On the other hand, IEC-specific deletion of caspase-8, a critical orchestrator of apoptosis, did not lead to discernible abnormalities in the intestinal epithelium in mice, thereby undermining the role of apoptosis in the cycle of epithelium turnover (8, 21). Caspase-8-deficiency led to IEC death by inflammatory necroptosis and precipitated spontaneous terminal ileitis, Paneth cell loss, and high susceptibility of mice to dextran sulfate sodium (DSS)-induced colitis (21). Similarly, IEC-specific deletion of FAS-associated death domain protein (FADD), an adaptor that conveys signals from tumor necrosis factor (TNF) receptor 1 (TNFR1) or FAS to caspase-8, leads to spontaneous IEC necroptosis with loss of Paneth cells and both small and large intestinal inflammation (22). Disruption of the non-inflammatory process of apoptosis drove necroptosis concomitant with increased expression of the central kinase for necroptosis, receptor-interacting serine/threonine protein kinase 3 (RIPK3) whose levels were increased in the terminal ileum of patients with Crohn’s disease (21). While live IEC extrusion is likely unaffected by caspase-8 or FADD deficiency, the findings demonstrate a commitment to death at least in some IEC, either as an end to a short lifespan or in response to a specific signal. IEC destined to die will undergo death and if not by apoptosis then by necroptosis. By extension, if such a commitment to death is made under homeostatic conditions, it stands to reason that the favored mode of cell death would be non-inflammatory apoptosis.
Apoptosis and necroptosis in intestinal inflammation
Unlike programmed apoptosis that preserves tissue function, excessive apoptosis in the intestinal epithelium compromises barrier integrity and leads to inflammation (18, 23). TNF-α, a critical molecule and therapeutic target in IBD (24), induces excessive IEC shedding (25, 26). Systemic and intestinal tissue levels of TNF-α are increased in IBD patients (27), and genome wide association studies (GWAS)-identified IBD risk alleles associated with TNF signaling (RELA, NFKB1, TNFAIP3), point to a critical role for TNF-α in IBD pathogenesis (28). Upon binding TNFR1, TNF-α can induce apoptosis upon deubiquitylation of the receptor-interacting serine/threonine protein kinase 1 (RIPK1) by ubiquitin modifying proteins such as A20 (TNFAIP3), but it can also induce necroptosis upon caspase-8 or A20 inhibition and the heterodimerization of RIPK1 with RIPK3 (29–31). TNF-α-induced IEC shedding is reported to be associated either with IEC apoptosis (14, 32), or with necroptosis where multiple IEC are shed and barrier integrity is lost driving inflammation (15, 26). Excessive TNF-α-induced IEC shedding is thus unlike the homeostatic IEC extrusion where rapid redistribution of tight junction proteins and replacement of extruded cells is observed (33, 34). Replacement of homeostatic IEC apoptosis with pathogenic inflammatory cell death may play a critical role in IBD pathogenesis (Figure 1).
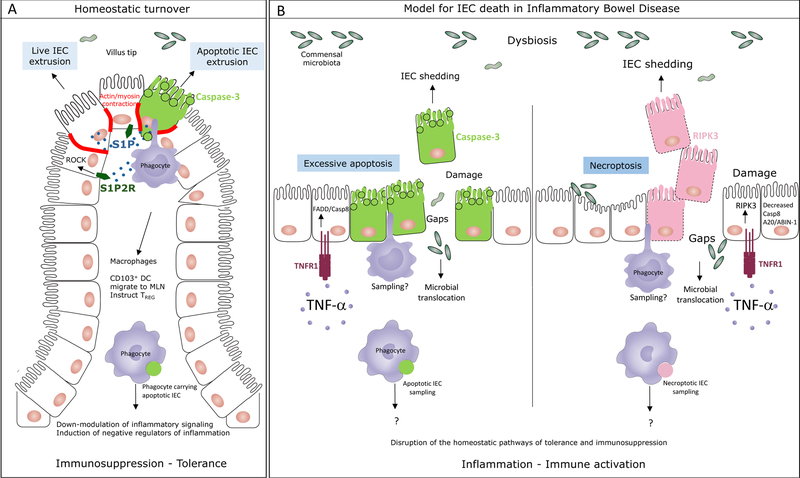
Figure 1. The mode of cell death in the intestinal epithelium impacts intestinal homeostasis.
A. Under homeostatic conditions, the intestinal epithelium undergoes natural turnover by continuous replacement of intestinal epithelial cells (IEC). Experimental evidence supports both apoptotic and non-apoptotic IEC extrusion at locations of overcrowding at the villi tips of the small intestine or luminal face of the crypts in the colon. Both of these processes require sphingosine 1-phosphate (S1P) signaling through S1P2 receptor (S1P2R) and resultant Rho associated kinase (ROCK) dependent actin/myosin contraction. ROCK can be cleaved and activated by caspase-3 during apoptosis to phosphorylate myosin light chain and induce apoptotic membrane blebbing. MHC class II+ CD11c+ intestinal lamina propria phagocytes, either macrophages or CD103+CD11b– dendritic cells (DC) sample the apoptotic IEC prior to their exclusion and respond by executing a broad program of immunosuppression. Apoptotic IEC carrying CD103+ DC also express negative regulators of inflammation such as the IBD susceptibility gene TNFAIP3 encoding A20. These cells express the lymph node homing receptor CCR7 and induce the emergence of regulatory CD4 T (TREG) cells. B. A proposed model for the types of IEC death that might occur during in inflammatory bowel disease. Increased levels of TNF-α in the intestinal lamina propria of patients with IBD induces IEC shedding that can be a result of excessive and unregulated apoptosis mediated by FADD and caspase-8 downstream of TNF receptor (TNFR1) engagement on IEC. Based on studies in mouse models, under conditions when caspase-8 or A20/ABIN-1 activity is impaired, TNF-α signaling through TNFR1 induces necroptosis mediated by the receptor interacting kinase 3 (RIPK3). Dying IEC are likely samples by intestinal phagocytes although formal proof of this process and its consequences is yet to come. In the absence of homeostatic apoptosis, it is expected that the immunosuppression and tolerance program in phagocytes, imparted by apoptotic IEC sampling, would be disrupted. Necroptosis is inflammatory in nature and would be expected to lead to the activation of intestinal phagocytes. Excessive apoptosis and necroptosis would compromise barrier integrity and lead to the translocation of the commensal microbiota into the sterile lamina propria leading to more inflammation.
Despite the central role of RIPK1 in mediating apoptosis or necroptosis downstream of TNF-α/TNFR1 signaling, IEC-specific deletion of RIPK1 led to severe intestinal inflammation associated with IEC apoptosis mediated in part by TNFR1 but also by interferon and TLR signaling (35, 36). This unexpected phenotype revealed a scaffold function of RIPK1 that signals cell survival by preventing the degradation of pro-survival proteins, and is independent of its kinase activity that promotes apoptosis and necroptosis (31, 35–37). Indeed, mice harboring a mutant RIPK1 lacking kinase activity (kinase-dead) did not exhibit intestinal inflammation (36). These findings uncovered a different facet of RIPK1 that preserves rather than compromises IEC survival through poorly understood, kinase-independent scaffold functions. In fact, concomitant deletion of RIPK1 from FADD or caspase-8 deleted IEC (see above), rescued IEC and abolished the inflammatory phenotype in mice (35, 36). Interestingly, although RIPK3-mediated necroptosis was the culprit in the pathology of FADD or caspase-8 IEC deleted mice (21, 22), its concomitant deletion in FADD or caspase-8 deleted IEC had no effect on IEC death, intestinal pathology or inflammation demonstrating the unique role that RIPK1 plays in protecting the intestinal epithelium (35, 36).
Recent evidence shows that A20 and its binding partner ABIN-1 (TNIP-1) – single nucleotide polymorphisms in which correlate with susceptibility and severity of IBD (28, 38) – play a dual IEC-intrinsic role in protecting the intestinal epithelium from RIPK1-mediated apoptosis and necroptosis (39). Simultaneous IEC-specific deletion of A20 and ABIN-1 leads to IEC apoptosis, severe spontaneous enterocolitis, and mouse lethality (39). IEC-derived TNF-α was a prominent mediator, and A20 and ABIN-1 inhibited TNF-induced caspase-8 activation and RIPK1 activity. Neutralization of IEC-derived TNF-α is perhaps one mechanism underlying the decreased apoptosis and mucosal healing reported after anti-TNF therapy (40, 41). TNF-independent, MyD88-dependent mechanisms, functioning within the hematopoietic cell compartment, also mediated IEC apoptosis and appeared to be particularly controlled by ABIN-1 (39). Identification of these mechanisms could provide additional therapeutic targets for IBD patients who fail anti-TNF therapy. Collectively, the findings also point to the merit of identifying IBD patients with lower mucosal tissue levels of A20 and/or ABIN-1 (42–45), as these patients might particularly benefit from RIPK1 inhibitors presently in clinical trials on subjects with active ulcerative colitis clinical trials ((46, 47) and clinicaltrials.gov/ct2/show/NCT02903966). Reduced A20 expression has also been reported in monogenic early onset inflammatory diseases characterized by mucosal ulceration (48).
Apoptotic intestinal epithelial cells are sampled by intestinal lamina propria phagocytes
In 2000, apoptotic intestinal epithelial cell (IEC) DNA and cellular remnants were reported inside a distinct subset of rat intestinal lymph-borne dendritic cells (DC) originating from the lamina propria and Peyer’s patches and migrating to T cell areas of the Peyer’s patches and mesenteric lymph nodes (MLN) (49). Apoptotic IEC sampling by DC was also noted in the lamina propria and MLN of gnotobiotic rats suggesting that transport of apoptotic IEC to MLN was constitutive and independent of the presence of the microbiota (49). In mice, transgenic expression of the model antigen ovalbumin (OVA) specifically within the intestinal epithelium demonstrated the ability to induce tolerance of adoptively transferred OVA-specific CD8 T cells and precipitate intestinal tissue damage during infection with an OVA-expressing virus (50). These observations lend support to DC-dependent transport of apoptotic IEC antigens to the MLN for the induction of tolerance or immunity to epithelium derived antigens. In 2004, murine DC within the subepithelial dome underlying the Peyer’s patch follicle-associated epithelium were found to contain apoptotic epithelial cells both at steady state and during reovirus infection – where reovirus antigen from virus-infected apoptotic IEC was presented to CD4 T cells (51).
More than a decade later, development of a transgenic mouse model expressing green fluorescent protein (GFP) specifically within IEC enabled the tracking of apoptotic IEC-derived GFP into the complex network of mononuclear phagocytes present within the lamina propria of the small intestine (52, 53). These phagocytes sample luminal commensals and pathogens alike and orchestrate tolerance or the appropriate effector immune response (52, 53). Phagocytes within tissues can also internalize apoptotic tissue-derived cells (54). GFP-labeled apoptotic IEC were tracked into two subsets of intestinal lamina propria CD64+ macrophages, CD103–CD11b+ and CD103+CD11b+, as well as a distinct subset of CD24+CD103+CD11b– DC that migrated to the MLN and instructed the differentiation of regulatory CD4 T (TREG) cells (55). These findings revealed two important facets of the intestinal epithelium: (1) non-inflammatory apoptosis is a component of the natural life cycle of an IEC, and (2) apoptotic IEC are sampled by intestinal mononuclear phagocytes at steady state and homeostatic conditions.
Apoptotic IEC sampling orchestrates a broad program of immunosuppression
Visualization of the apoptotic IEC-derived GFP within intestinal lamina propria phagocytes enabled the isolation of phagocytes that carried or did not carry apoptotic IEC and a comparison of their transcriptional profile (55). Apoptotic IEC sampling was associated with a suppression of inflammation signature within engulfing phagocytes, although the specific genes and pathways involved varied between DC and macrophage reflecting specialized responses to apoptotic IEC. Interestingly, apoptotic IEC sampling was associated in all three phagocytes with a downmodulation of Itgb7 encoding integrin-β7 that mediates homing to gut-associated lymphoid tissue and serves as the target for IBD therapy with Vedolizumab and Etrolizumab (56). Intestinal lamina propria CD103+CD11b+ and CD103–CD11b+ macrophages that carried apoptotic IEC downmodulated genes encoding pattern recognition receptors such as Tlr2 and the C-type lectin receptors Clec4a, Clec4b1, Cd209a (55). These macrophages also downregulated Alox5ap encoding arachidonate 5-lipooxygenase (55), which facilitates inflammatory leukotriene biosynthesis (57). CD103+ DC, on the other hand, downregulated transcripts related to components of the inflammasome including Nlrp3, Casp1, and Il1a, as well as genes encoding MAPK/ERK signaling proteins including Lrrk2, Map3k4, and Fos.
Notably, several of the differentially expressed genes between apoptotic IEC bearing and non-bearing phagocytes overlapped with susceptibility genes associated with IBD (28). Leucine rich repeat kinase 2 (LRRK2), a multifunctional protein kinase known to be a prominent contributor to familial Parkinson’s disease (58), is a susceptibility gene for Crohn’s disease (28). LRRK2 transcript levels were increased in inflamed compared to non-inflamed biopsies from Crohn’s patients and the LRRK2 M2397T risk allele for Crohn’s disease impaired LRRK2 expression levels (59). LRRK2 M2397T was significantly associated with Paneth cell defects in Japanese Crohn’s disease patients instead of the autophagy gene ATG16L1 T300A polymorphism in North American Crohn’s disease (60). IL12b encoding the p40 subunit shared by IL-12 and IL-23 was also upregulated in apoptotic IEC bearing intestinal lamina propria CD103+ DC (55), and single nucleotide polymorphisms in IL12b are associated with Crohn’s disease in Korean populations (61)
A prominent transcriptional signature associated uniquely with the CD103+ DC that had sampled apoptotic IEC was the induction of negative regulators of inflammatory signaling (55). Within this category was Tnfaip3 encoding A20 (55). A20 inhibits NF-κB signaling (38), RIG-I signaling (62), and NLRP3 inflammasome activation (63, 64). Enhanced apoptotic cell engulfment, presentation of apoptotic cell antigens, and activation of self-reactive T cells has also been associated with A20 deficiency in DC (38). DC induction of A20 in response to apoptotic cells may thus serve not only to inhibit intestinal inflammation, but also mediate tolerance to IEC. Deletion of A20 in DC leads to the development of pathologies in mice similar to those seen in humans with IBD and systemic lupus erythematosus (SLE) including colitis, ankylosing spondylitis, autoantibody development, nephritis, and splenomegaly (38). Total deficiency in A20 in mice leads to severe systemic inflammation, cachexia, increased sensitivity to lipopolysaccharide and TNF-α, and premature death (38).
Apoptotic IEC sampling mediates immune tolerance and induction of regulatory CD4 T cells
A distinct transcriptional signature uniquely associated with the apoptotic IEC carrying CD103+ DC was the activation of regulatory CD4 T (TREG) cells expressing the transcription factor Foxp3 (55). These CD103+ DC upregulate Aldh1a2 (55), which catalyzes the conversion of retinal to retinoic acid to generate TREG cells in the intestine (65). Also upregulated is Lrrc32 (55), which encodes LRRC32 (also known as GARP) associated with IBD susceptibility (66). The expression of GARP by DC in response to apoptotic IEC sampling may serve a role similar to that in TREG cells where LRRC32 associates with and retains latent TGF-β on the cell surface to promote TREG-mediated suppression (67).
Small intestinal lamina propria CD103+ DC that had sampled apoptotic IEC expressed the highest levels of the chemokine receptor CCR7 that mediates migration to lymph nodes (55). These cells upregulated the suppressive co-stimulatory molecule Cd274 (Programmed death ligand PD-L1) (55), which synergizes with TGF-β to promote the instruction of TREG cell differentiation by DC, and whose expression on TREG cells has been reported to promote their differentiation and maintain their function by enhancing Foxp3 expression (68). Also upregulated by these CD103+ DC in response to apoptotic IEC sampling are the chemokine encoding genes Ccl22 and Ccl17 that have been found to induce efficient chemotaxis of intestinal lamina propria TREG cells (69). Interestingly, CCL22 and CCL17 were reported to be selectively expressed by small intestinal lamina propria DCs at high levels, and that TREG cells were in close proximity to these DCs (69). The collective upregulation of these genes upon apoptotic IEC sampling by lamina propria CD103+ DC sets the stage for instructing differentiation of TREG cells (55), enabling DC to directly impact the generation of these critical mediators of intestinal tolerance and homeostasis.
Migratory CD103+ DC containing GFP-labeled apoptotic IEC were also detected at steady state within the MLN. These migratory DC shared a transcriptional profile with those carrying apoptotic IEC in the lamina propria (55). Several transcripts involved in TREG cell differentiation (Cd274, Cd40, Aldh1a2, Ccl22) were additionally increased in migratory MLN CD103+ DC relative to those that did not contain apoptotic IEC, as well as transcripts encoding indoleamine 2,3 dioxygenase-1 (IDO-1) and IL-10 (55). IDO-1 promotes immune tolerance through the suppression of effector T cell responses and inflammatory cytokine production by DC, as well as the induction of TREG cell differentiation (70), whereas IL-10 is a critical player in the induction of TREG cells by tolerogenic DC (71). Apoptotic IEC carrying CD103+ DC within the MLN were superior to their counterparts that did not contain apoptotic cells in the ability to induce Foxp3+ TREG cells ex-vivo, and notably without added TGF-β (55).
Conclusion
The natural cycle of IEC turnover by apoptosis has emerged as a critical component of the wide spectrum of innate, adaptive and microbiota dependent mechanisms that regulate intestinal homeostasis (52, 53, 72–74). GWAS have associated susceptibility to IBD with single nucleotide polymorphisms in genes involved in many of these pathways (28). Forty-five of the genes modulated in intestinal mononuclear phagocytes in response to the sampling of apoptotic IEC overlap with IBD susceptibility loci, the majority of which are in CD103+ DC (55). How allelic polymorphisms within these loci affect genes involved in intestinal homeostasis and tolerance is poorly understood. A molecular delineation of cell death within the intestinal epithelium during healthy and disease states is necessary. Excessive TNF-α mediated apoptosis and necroptosis is expected to undo the homeostatic programs directed by apoptotic IEC sampling at steady state. Consistent with this prediction, the induction of intestinal epithelial ulceration in mice reversed the induction of TREG cell activation genes in migratory MLN CD103+ DC, pointing to potential disruption of tolerogenic apoptotic IEC sampling during IBD (55). It will be important to examine whether mucosal healing in response anti-TNF therapy restores these homeostatic programs to achieve long-term clinical remission in patients with IBD. A broader understanding of the integration between apoptosis within the intestinal epithelium and other mediators of intestinal homeostasis is a necessary step towards designing successful therapies for IBD.
Key points.
Intestinal epithelial cells can undergo homeostatic apoptosis during natural turnover
Apoptotic intestinal epithelial cells are sampled by lamina propria mononuclear phagocytes
CD103+ DC carrying apoptotic intestinal epithelial cells migrate to the mesenteric lymph nodes
Apoptotic intestinal epithelial cell sampling mediates innate and adaptive immunosuppression
Acknowledgments
Our work on cell death within the intestinal epithelium has been supported by the US National Institutes of Health (NIH) grants DK072201, and DK111862. J.M.B. and her laboratory were supported by NIH grants AI095245, AI127658, AI080959, AI123284, AI073899, the Searle Scholars Award, the Burroughs Wellcome Fund, the American Cancer Society and the Leukemia and Lymphoma Society.
Footnotes
Conflict of interest statement
None
References and recommended reading
• Of special interest
•• Of outstanding interest
- 1.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389(10080):1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389(10080):1741–55. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. Journal of gastroenterology and hepatology 2000;15(2):109–20. [DOI] [PubMed] [Google Scholar]
- 4.Nunes T, Bernardazzi C, de Souza HS. Cell death and inflammatory bowel diseases: apoptosis, necrosis, and autophagy in the intestinal epithelium. BioMed research international 2014;2014:218493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Sabatino A, Ciccocioppo R, Luinetti O, Ricevuti L, Morera R, Cifone MG, et al. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Diseases of the colon and rectum 2003;46(11):1498–507. [DOI] [PubMed] [Google Scholar]
- 6.Dourmashkin RR, Davies H, Wells C, Shah D, Price A, O’Morain C, et al. Epithelial patchy necrosis in Crohn’s disease. Human pathology 1983;14(7):643–8. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Park SM, Turner JR, Peter ME. Cell death in the colonic epithelium during inflammatory bowel diseases: CD95/Fas and beyond. Inflamm Bowel Dis 2010;16(6):1071–6. [DOI] [PubMed] [Google Scholar]
- 8.Gunther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 2013;62(7):1062–71. [DOI] [PubMed] [Google Scholar]
- 9.Hagiwara C, Tanaka M, Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. Journal of gastroenterology and hepatology 2002;17(7):758–64. [DOI] [PubMed] [Google Scholar]
- 10.Souza HS, Tortori CJ, Castelo-Branco MT, Carvalho AT, Margallo VS, Delgado CF, et al. Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: evidence of altered expression of FasL and perforin cytotoxic pathways. International journal of colorectal disease 2005;20(3):277–86. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. The Journal of pathology 1996;180(2):152–9. [DOI] [PubMed] [Google Scholar]
- 12.Werner T, Shkoda A, Haller D. Intestinal epithelial cell proteome in IL-10 deficient mice and IL-10 receptor reconstituted epithelial cells: impact on chronic inflammation. Journal of proteome research 2007;6(9):3691–704. [DOI] [PubMed] [Google Scholar]
- 13.Shkoda A, Werner T, Daniel H, Gunckel M, Rogler G, Haller D. Differential protein expression profile in the intestinal epithelium from patients with inflammatory bowel disease. Journal of proteome research 2007;6(3):1114–25. [DOI] [PubMed] [Google Scholar]
- 14.Kiesslich R, Goetz M, Angus EM, Hu Q, Guan Y, Potten C, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 2007;133(6):1769–78. [DOI] [PubMed] [Google Scholar]
- 15.Watson AJ, Hughes KR. TNF-alpha-induced intestinal epithelial cell shedding: implications for intestinal barrier function. Ann N Y Acad Sci 2012;1258:1–8. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell 2011;147(4):742–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker N Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature reviews Molecular cell biology 2014;15(1):19–33. [DOI] [PubMed] [Google Scholar]
- 18.•• Blander JM. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. The FEBS journal 2016;283(14):2720–30.This review describes in detail the mechanisms behind extrusion of IEC from the intestinal epithelium during natural tunrover.
- 19.Penberthy KK, Ravichandran KS. Apoptotic cell recognition receptors and scavenger receptors. Immunol Rev 2016;269(1):44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.• Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Current biology : CB 2001;11(23):1847–57.This study shows that apoptosis initiates extrusion of cells within a simple epithelium by mediating the contraction of actin and mysoin within the apoptotic cell and its neighbors while maintaining barrier function. These events take place very early in the apoptotic process and well before the appearance of the classic markers of apoptosis (cleavage of caspase-3 and phosphatidyl serine exposure).
- 21.•• Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 2011;477(7364):335–9.Ref. [21••] along with Ref. [22••] show an important function for caspase-8 in regulating intestinal homeostasis by protecting IECs from TNF-a induced inflammatory necroptosis.
- 22.•• Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011;477(7364):330–4. [DOI] [PubMed] [Google Scholar]
- 23.•• Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol 2017;4(1):33–46.This review presents a summary of the cellular and molecular mediators of barrier function in the intestinal epithelium and how these mechanisms are disrupted during intestinal inflammation.
- 24.•• Levin AD, Wildenberg ME, van den Brink GR. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. Journal of Crohn’s & colitis 2016;10(8):989–97.This is a synopsis of the mechanisms through which anti-TNF-a is proposed to mediate clinical response, remission and mucosal healing.
- 25.Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012;61(8):1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppkes M, Roulis M, Neurath MF, Kollias G, Becker C. Pleiotropic functions of TNF-alpha in the regulation of the intestinal epithelial response to inflammation. Int Immunol 2014;26(9):509–15. [DOI] [PubMed] [Google Scholar]
- 27.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14(5):329–42. [DOI] [PubMed] [Google Scholar]
- 28.de Lange KM, Barrett JC. Understanding inflammatory bowel disease via immunogenetics. Journal of autoimmunity 2015;64:91–100. [DOI] [PubMed] [Google Scholar]
- 29.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol 2015;15(6):362–74. [DOI] [PubMed] [Google Scholar]
- 30.Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol 2015;16(6):618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.• Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nature reviews Molecular cell biology 2017;18(2):127–36.This is a timeline article of the key discoveries pertaining to receptor-interacting serine/threonine kinases (RIPKs) in cell death, inflammation and disease.
- 32.Guan Y, Watson AJ, Marchiando AM, Bradford E, Shen L, Turner JR, et al. Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am J Physiol Cell Physiol 2011;300(6):C1404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. The Journal of membrane biology 1990;116(2):177–84. [DOI] [PubMed] [Google Scholar]
- 34.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 2011;140(4):1208–18 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.•• Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014;513(7516):90–4.Ref. [34••] along with Ref. [35••] reveal the surprising discovery that RIPK1, a central mediator of cell death pathways, can also function to preserve IEC survival independently of its kinase activity and through a poorly understood scaffolding function that maintains the levels and activity of pro-survival proteins.
- 36.•• Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 2014;513(7516):95–9. [DOI] [PubMed] [Google Scholar]
- 37.• Newton K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol 2015;25(6):347–53.This review highlights the complex regulation of apoptosis and necroptosis by both RIPK1 and RIPK3. It highlights the unexpected pro-survival role of RIPK1 in the intestinal epithlium and the induction of apoptosis upon inhibiting the activity of the necroptosis orchestrator RIPK3.
- 38.Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol 2014;35(1):22–31. [DOI] [PubMed] [Google Scholar]
- 39.•• Kattah MG, Shao L, Rosli YY, Shimizu H, Whang MI, Advincula R, et al. A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival. J Exp Med 2018;215(7):1839–52.This study shows how A20 and ABIN-1, two IBD susceptibility proteins, protect IEC from TNF-a dependent apoptosis by inhibiting caspase-8 activation and RIPK1 kinase activity. The study also reveals an epistatic relationship between these susceptibility genes in a murine model of intestinal inflammation.
- 40.Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut 2004;53(9):1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. The American journal of gastroenterology 2002;97(8):2000–4. [DOI] [PubMed] [Google Scholar]
- 42.Zaidi D, Huynh HQ, Carroll MW, Baksh S, Wine E. Tumor necrosis factor alpha-induced protein 3 (A20) is dysregulated in pediatric Crohn disease. Clin Exp Gastroenterol 2018;11:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majumdar I, Ahuja V, Paul J. Altered expression of Tumor Necrosis Factor Alpha-Induced Protein 3 correlates with disease severity in Ulcerative Colitis. Sci Rep 2017;7(1):9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruno ME, Rogier EW, Arsenescu RI, Flomenhoft DR, Kurkjian CJ, Ellis GI, et al. Correlation of Biomarker Expression in Colonic Mucosa with Disease Phenotype in Crohn’s Disease and Ulcerative Colitis. Dig Dis Sci 2015;60(10):2976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arsenescu R, Bruno ME, Rogier EW, Stefka AT, McMahan AE, Wright TB, et al. Signature biomarkers in Crohn’s disease: toward a molecular classification. Mucosal immunology 2008;1(5):399–411. [DOI] [PubMed] [Google Scholar]
- 46.• Weisel K, Scott NE, Tompson DJ, Votta BJ, Madhavan S, Povey K, et al. Randomized clinical study of safety, pharmacokinetics, and pharmacodynamics of RIPK1 inhibitor GSK2982772 in healthy volunteers. Pharmacol Res Perspect 2017;5(6).This report describes the results from Phase-I of the first small molecule inhibitor of RIPK1 and neroptosis, GSK2982772, to advance into human clinical trials. GSK2982772 was administered orally to healthy volunteers, and was deemed safe and well tolerated.
- 47.Berger SB, Harris P, Nagilla R, Kasparcova V, Hoffman S, Swift B, et al. Characterization of GSK’963: a structurally distinct, potent and selective inhibitor of RIP1 kinase. Cell Death Discov 2015;1:15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nature genetics 2016;48(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.• Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med 2000;191(3):435–44.The first report of the presence of IEC remnants within a distinct population of dendritic cells in the mesenteric lymph nodes of rats. The findings had important implications to the trafficking of apoptotic IEC to the mesenteric lymph nodes for the induction of immune tolerance to IEC antigens.
- 50.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity 2000;12(5):505–14. [DOI] [PubMed] [Google Scholar]
- 51.Fleeton MN, Contractor N, Leon F, Wetzel JD, Dermody TS, Kelsall BL. Peyer’s patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J Exp Med 2004;200(2):235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joeris T, Muller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal immunology 2017;10(4):845–64. [DOI] [PubMed] [Google Scholar]
- 53.Sanders TJ, Yrlid U, Maloy KJ. Intestinal Mononuclear Phagocytes in Health and Disease. Microbiol Spectr 2017;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fond AM, Ravichandran KS. Clearance of Dying Cells by Phagocytes: Mechanisms and Implications for Disease Pathogenesis. Adv Exp Med Biol 2016;930:25–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.•• Cummings RJ, Barbet G, Bongers G, Hartmann BM, Gettler K, Muniz L, et al. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 2016;539(7630):565–9.A novel mouse model where IEC-specific expression of a green fluorescent protein fused to the diptheria toxin receptor enables induction of apoptosis with low doses of diphtheria toxin and visualization of apoptotic GFP labeled IEC within small intestinal lamina propria and mesenteric lymph node phagocytes. The phagocytes that sample apoptotic IEC display an immunosuppressive and tolerogenic profile that orchestrates intestinal homeostasis.
- 56.Mosli MH, Rivera-Nieves J, Feagan BG. T-cell trafficking and anti-adhesion strategies in inflammatory bowel disease: current and future prospects. Drugs 2014;74(3):297–311. [DOI] [PubMed] [Google Scholar]
- 57.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta 2015;1851(4):331–9. [DOI] [PubMed] [Google Scholar]
- 58.Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 2016;539(7628):207–16. [DOI] [PubMed] [Google Scholar]
- 59.Bae JR, Lee BD. Function and dysfunction of leucine-rich repeat kinase 2 (LRRK2): Parkinson’s disease and beyond. BMB Rep 2015;48(5):243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu TC, Naito T, Liu Z, VanDussen KL, Haritunians T, Li D, et al. LRRK2 but not ATG16L1 is associated with Paneth cell defect in Japanese Crohn’s disease patients. JCI Insight 2017;2(6):e91917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moon CM, Shin DJ, Son NH, Shin ES, Hong SP, Kim TI, et al. Genetic variants in the IL12B gene are associated with inflammatory bowel diseases in the Korean population. Journal of gastroenterology and hepatology 2013;28(10):1588–94. [DOI] [PubMed] [Google Scholar]
- 62.Feng W, Sun X, Shi N, Zhang M, Guan Z, Duan M. Influenza a virus NS1 protein induced A20 contributes to viral replication by suppressing interferon-induced antiviral response. Biochem Biophys Res Commun 2017;482(4):1107–13. [DOI] [PubMed] [Google Scholar]
- 63.Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 2014;512(7512):69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duong BH, Onizawa M, Oses-Prieto JA, Advincula R, Burlingame A, Malynn BA, et al. A20 restricts ubiquitination of pro-interleukin-1beta protein complexes and suppresses NLRP3 inflammasome activity. Immunity 2015;42(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu ZM, Wang KP, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol Immunol 2015;12(5):553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America 2009;106(32):13445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206(13):3015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Z, Jang MH, Otani K, Bai Z, Umemoto E, Matsumoto M, et al. CD4+CD25+ regulatory T cells in the small intestinal lamina propria show an effector/memory phenotype. Int Immunol 2008;20(3):307–15. [DOI] [PubMed] [Google Scholar]
- 70.Munn DH. Indoleamine 2,3-dioxygenase, Tregs and cancer. Curr Med Chem 2011;18(15):2240–6. [DOI] [PubMed] [Google Scholar]
- 71.Zhu L, Shi T, Zhong C, Wang Y, Chang M, Liu X. IL-10 and IL-10 Receptor Mutations in Very Early Onset Inflammatory Bowel Disease. Gastroenterology Res 2017;10(2):65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Penny HA, Hodge SH, Hepworth MR. Orchestration of intestinal homeostasis and tolerance by group 3 innate lymphoid cells. Semin Immunopathol 2018;40(4):357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol 2017;18(8):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma A, Rudra D. Emerging Functions of Regulatory T Cells in Tissue Homeostasis. Frontiers in immunology 2018;9:883. [DOI] [PMC free article] [PubMed] [Google Scholar]