Abstract
Locoregional therapies are commonly used to treat patients with hepatocellular carcinoma. It has been noted for many years that locoregional therapies may have additional systemic effects other than simple tumor elimination. Immunological “side effects” have been described in response to locoregional therapies in animal studies and also in patients. With the advent of immunotherapy for hepatocellular carcinoma, there is an increasing interest in understanding how immunotherapy may be best combined with locoregional therapies. Here we provide a compact summary of answered and unanswered questions in this field including: What animal model is best suited to test combined immune-locoregional treatments? How does tumor cell death affect immune responses? What type of immune responses have been observed in patients treated with different types of locoregional therapies? What can be surmised from results of a first study testing the combination of locoregional therapy with immune checkpoint blockade? Finally, we provide an outlook of research issues and needs in order to advance this rapidly growing field bridging interventional radiology and cancer immunology.
Keywords: tumor immunology, T cell, cell death, locoregional therapy, radiofrequency ablation
Lay summary
Locoregional therapies are minimally invasive procedures like thermal ablation or intra-arterial particle delivery that directly treat a tumor. These commonly used therapies for primary liver cancer suggested to cause not only tumor cell death but also initiate an immune response against tumor cells. This review highlights what is already known in this field that bridges locoregional therapies and cancer immunology, and discusses research issues and needs in order to advance this rapidly growing field.
Animal models to study locoregional therapies
Animal models enable preclinical optimization and delivery of novel experimental drugs and immunomodulators prior to human trials. Local delivery, intra-arterial or intra-tumoral, of new agents with potentially lethal toxicity when administered systemically, should be first tested in a pre-clinical paradigm. In pre- clinical survival models, longitudinal and multiple surrogate and mechanistic biomarkers can be sampled, including tumor, blood, and repeated imaging over time, without radiation exposure concerns.
Multiple methods exist to induce tumor formation in mice, including genetically engineered mouse models, chemotaxis agents, intrahepatic or intra-splenic injection of tumor cells and xenograft approaches. Additionally, as HCC generally develops in the context of a diseased liver, methods exist to induce liver disease in mice to mimic viral hepatitis, fatty liver disease, fibrosis, alcohol-induced liver disease and cholestasis (1). Furthermore, humanized mice may potentially mimic the human immune response, however this technique is challenging and this approach has not been adopted by the HCC immunotherapy field so far.
Most of our understanding of the mechanism of the immune system in liver cancer was drawn from experiments on murine models. The mouse model has throughput advantages allowing treatment of a large number of animals with easy handling, housing and lower costs. However, the size becomes a geometric disadvantage for investigation of local therapies and drug delivery, due to the disparities in scale. Researchers are limited in their ability to model and apply locoregional techniques. Ablations of subcutaneous tumors can only be performed on rather small and spherical shaped tumors in order to prevent skin burn and damage to surrounding tissues such as nerves which can compromise the animals. Intra-arterial drug delivery is highly challenging in murine and rat models due to small vessel caliber. Both access vessels and target vessels with in the liver are smaller than standard clinical devices and invasive imaging equipment. Specific microsphere or bead sizes may have very different biologic effects in such small vessels, compared to human or large animal vessels, due to pure geometry and scale. Furthermore, contrast agents that can be used in real time to guide intra-arterial procedure in mice models are lacking. Contrast agents used in humans are nonionic and water soluble and therefore cleared from mice blood too rapidly to be used intra-procedurally with high resolution imaging. IV ultrasound contrast agents have not met the need for arterial phase information.
At least three animals’ models are of a sufficient size for modeling locoregional therapy and are used for investigation of the effects of interventional oncology tools on liver cancer. The rabbit VX2 model has been widely used for various ablation techniques and intra-arterial drug delivery to hepatic tumors (2–4). However, this model has a few major limitations for immunological investigations. First, VX2 is a cell line classified as leporine anaplastic squamous cell carcinoma induced by papilloma virus and does not resemble most human HCC subtypes histologically and genetically (5). The VX2 model is an orthotopic animal model with technical and resource challenges. Cells are propagated in the muscle of one donor animal, monitored for subsequent sufficient tumor volume, followed by tumor excision, where the necrotic part is removed, and only the viable tumor is transplanted into a recipient animal. These VX2 cells can be implanted in various locations such as flank, liver, lung and kidney (6–8). The transplantation technique leads to the second limitation of this model for immunology investigations. The donor and the recipient are not genetically identical; thus, the tumor can be considered an allograft, rather than autograft. Therefore, it is possible that the recipient animal recognizes the tumor as non-self and expresses rejection or alters the immune responses. Third, the removal of the necrotic part before implantation might confound the investigation of the immune response, or delete antigens or immune elements. Lastly, implanted VX2 tumors are unlikely to recapitulate the human HCC microenvironment. VX2 hepatic tumors evolve and grow with peripheral vascularity, while the central zone likely outgrows its blood supply or for other unknown reasons, develops central or heterogeneous necrosis. This might hamper antigen recognition or presentation, or subsequent influx of immune cells to the tumor. This may confound not only drug efficacy investigation but also limit understanding of the immunomodulation after local or regional therapy. The heterogeneity of VX2 tumors, unlike human tumors, is somewhat limited and as a result, activation or escape immune pathways may be absent, poorly modeled, or overlooked. Two autochthonous HCC models, rat and woodchuck, were introduced in recent years for investigation of interventional oncology paradigms (9, 10). HCC is induced in the former by the toxin diethylnitrosamine (DEN) and in the latter by woodchuck hepatitis virus. In both models, as in most patients, the tumors develop spontaneously in the liver on the background of liver disease: fibrotic liver or chronic inflammation, respectively. The degree of recapitulation of associated cirrhosis may vary. Autochthonous models might be superior to orthotopic models as they take into account the immunological particularities of the liver, an organ where both induction of tolerance and effective response against pathogens, probiotics and food-derived antigens must take place (11). A meta-analysis study, even though performed on colon cancer models and tissuses, found that among animal models, carcinogen induced tumors had the best correlation with clinical responses (12). Among these two carcinogen induced HCC models, it is possible that Woodchuck is more appropriate model for immune investigation as analysis of mutational landscape in different murine HCC has shown that DEN tumors were least similar to human disease, and almost universally carried the Braf V637E mutation, which is rarely found in human HCC (13).
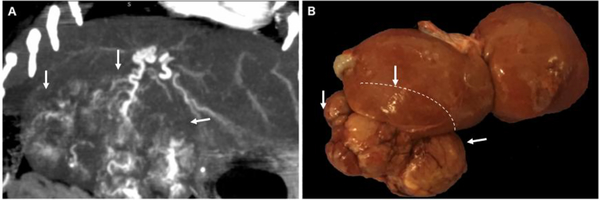
The gradual growth of tumors within the liver may result in better modeling with the development of adherent and hypertrophied arterial blood supply which mimics human HCC and may facilitate better interaction with the immune system. The woodchuck HCC model develops large, hyper-vascularized tumors that can be selectively targeted (Figure 1) and utilized for locoregional therapy investigation of new therapeutics and interventional oncology paradigms; tumor heterogeneity and genetic variation in this model should be further investigated but may offer the most appropriate model for novel therapeutic investigations for HCC.
Figure 1:
Spontaneous HCC developing in chronically hepatitis infected woodchucks. (A) Contrast-enhanced CT scan shows large heterogenous tumor with robust arterial blood supply (B). Gross pathology of liver and tumor (tumor edges demarcated by white arrows, margins of the tumor behind the liver demarcated by dashed line).
Cell death and anti-tumor immunity
The question how cell death shapes tumor immunity has been studied widely (14). There are three main immunological mechanisms affected by cell death: tumor antigens, antigen presenting cells, and effector cells. Preclinical studies have added a lot to our understanding on how the immune system may react to locoregional therapies and subsequent cell death. It is a well-known fact that the type of cell death has significant effects on anti-tumor immunity. Using a very clean, but artificial ex vivo model in which we killed tumor cells prior to injection using three freeze-thaw cycles (cryoablation) or gamma irradiation, we demonstrated that apoptotic, but not necrotic tumor cell death, induced tumor-specific immunity (15). Interestingly, pathogen associated molecular pattern analogs such as p(dI-dc) and/or unmethylated cpG DNA were able to induce immune responses even in response to tumor cell lysates (16). Additional molecular studies revealed that necrotic tumor cell death failed to induce anti-tumor immunity due to the activity of an oligo-peptidase in freeze-thawed cells (17). The presence and processing mechanisms of antigens to initiate the immune responses are likely governed by the type of cell death or cell injury, as well as supply of requisite molecular chaperones APC’s, regulatory T-cells, and proximity of nearby blood vessel supply chain. Such a complex interactive dance of dynamic molecular processes is challenging to characterize with simple linear cause and effect descriptive rules. Animal models fall short of recapitulation of the complex and dynamically evolving immunologic processes in human HCC that undergo pharmacologic immunomodulation.
Obviously, the situation is more complicated when cell death occurs in vivo. The group of Zitvogel and Kromer studied intensively the effect of chemotherapy induced cell death and its effect on anti-tumor immunity. They described that the initiating stimulus can cause an immunogenic or non-immunogenic cell death. Immunogenic cell death (ICD) involves release of calreticulin and other endoplasmic reticulum proteins at the cell surface, the secretion of ATP during the blebbing phase of apoptosis, and the cell death–associated release of the non-histone chromatin protein high-mobility group box 1 (HMGB1) facilitating recruitment and activation of dendritic cells into the tumor microenvironment, which will allow engulfment of tumor antigens from dying tumor cells and optimal antigen presentation to T cells (18). Chemotherapeutic reagents known to induce and potentiate ICD include doxorubicin and oxaliplatin, while cisplatin fails to induce immunogenic cell death (19). Doxorubicin is the most commonly administered drug in HCC chemoembolization (TACE or DEB TACE), which may have broad implications for its use in image guided regional therapies for HCC.
The type of cell death not only determines immune effects, but also the type of tumor in experimental mouse models. It was recently reported by the Zender lab that an apoptotic associated hepatic cytokine microenvironment determines HCC outgrowth from oncogenically transformed hepatocytes, whereas hepatocytes containing identical oncogenic drivers give rise to intrahepatic cholangiocarcinoma (ICC) if they face necroptosis microenvironment. Pharmacological or genetic suppression of necroptosis revert the necroptosis-dependent cytokine microenvironment and switches ICC to HCC (20).
In 2003, one of the first studies focusing on the effect of locoregional therapies on anti-tumor immunity reported an activation of tumor-specific T cells upon radiofrequency ablation in a VX2 carcinoma models in rabbits (21). Radiofrequency and cryoablation have been shown to provide an antigen source for dendritic cells (DC) in vivo (22). In our own studies, subtotal radiofrequency ablation resulted in enhanced tumor-specific immune responses in vivo and an infiltration of tumors by dendritic cells in response to radiofrequency ablation and were able to confer immunity via adoptive transfer of splenocytes into naïve mice (23). These mechanisms function mainly by upregulating antigen-presentation. The effects of thermal ablation (percutaneous radiofrequency ablation, microwave ablation, cryoablation and irreversible electroporation) on immune responses are the subject of an insightful although somewhat speculative recent review (24).
Finally, different immune-based approaches have been evaluated in combination with locoregional therapies in animal models. Using a well-defined murine B16-OVA tumor model, the CTLA4-blocking antibody was one of the first compounds to be tested in this setting. The authors demonstrated not only a weak but detectable immune response directed against OVA, but also against a broader range of B16 antigens. The significance of this effect was confirmed in adoptive transfer experiments, in which splenocytes derived from mice after RFA ablation were transferred into naïve mice challenged with live tumor cells. Anti-CTLA treatment at the time of tumor destruction enhanced anti-tumor immunity (25). Similar results were observed in a preclinical murine prostate cancer model. Anti-CTLA4 treatment augmented the effect of cryoablation leading to impaired growth of secondary tumors (26). The combination of RFA and TLR9 stimulation was tested in a VX2 hepatoma model and the combination was shown to prevent subsequent tumor spread (27). Similar results have been obtained in a B16-Ova model. Combination treatment with cryoablation plus TLR9 stimulation via CpG-oligodeoxynucleotides was very effective in the eradication of local and systemic tumors due to enhanced DC maturation leading to more efficient cross-presentation in tumor-bearing mice (28).
Photodynamic therapy (PDT) uses non-toxic photosensitizers and harmless visible light in combination with oxygen to produce cytotoxic reactive oxygen species that selectively kill malignant cells by apoptosis and/or necrosis and shut down the tumor microvasculature and potentially spare normal tissue (29). It has been shown to cause acute inflammatory responses, expression of heat-shock proteins and immune cell infiltration, thereby inducing an immunogenic cell death. PDT has been shown to prevent outgrowth of distant untreated tumors and prevent mice from tumor rechallenge in a CD8+ T cell dependent manner. Anti-CTLA4 treatment significantly improved therapeutic efficacy and survival of mice (30). Similar results have been observed in combination with imiquimod (R837), a Toll-like-receptor-7 agonist, when co-encapsulated with a photothermal agent by poly(lactic-co-glycolic) acid (PLGA). Treatment of mice in combination with anti-CTLA4 generated strong immunological responses capable of inhibiting metastasis formation and leading to tumor shrinkage (31). IL-2 treatment in the form of a fusion protein bound to a tumor-specific antibody, was shown to enhance anti-tumor immunity against colon tumors treated with radiofrequency ablation (32).
Insufficient radiofrequency ablation has also been shown to promote angiogenesis of residual hepatocellular carcinoma (33) and promote tumor growth of non-small cell lung cancer cells (34) via HIF-1α/VEGFA. Subtotal ablation can also induce hepatic regeneration and tumorigenesis via IL6, c-met, or HGF-dependent pathways (35). Nonetheless modulation of RF heating parameters alone or in combination with adjuvant heat shock proteins inhibition, STAT3 inhibition, or simple Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs can mitigate or reduce unwanted, off-target systemic tumorigenic effects (36). Nevertheless, there may be a precarious and poorly understood balance between partial ablation or embolization inducing a tumorigenic response versus an immune regression. Tipping this balance via pharmaco-modulation may more fully recognize the pre-clinical promise and augmentation of HCC immunomodulation combined with local and regional image guided tools.
Immunological effects observed in patients treated with locoregional therapies
Locoregional therapy has been shown to induce immune responses in patients with HCC (37). No direct comparison between the different modes of ablation has been conducted, and reports primarily focus on individual modalities or at best two modalities. The effect of percutaneous microwave ablation (MWA) as a single therapy was one of the first to be investigated. Recently, immune responses (T cell activation and IL-12 release) 4 weeks after treatment have also been reported (38, 39). RFA is another ablation technique more commonly used to treat intrahepatic HCC. The hypothesis that ablation can cause tumor-specific immune responses was tested on tumor samples and PBMCs collected from patients before and after RFA (40). HCC specific T cell expansion was measured in vitro. It was shown that HCC specific T cell responses were induced by stimulation with the thermally ablated HCC extract. Using an antigen library, it was shown that up to 60% of the patients responded with T cell activation against at least one antigen after RFA in another study (41). It was found that a higher number of antigen specific CD8+ T cells after RFA correlated significantly with the length of HCC recurrence free survival. Another important question is whether and how locoregional therapies may influence the immunosuppressor mechanisms. Myeloid-derived suppressor cells (MDSCs) are known as key immune regulators in various human malignancies, and it was reported that CD14+HLA-DR−/low are increased in HCC patients and inhibit the function of T cells through the induction of regulatory T cells (42). In a trial aimed to investigate the ratio between MDSC and CD14 cells in HCC patients, it was found that this ratio significantly increased compared to healthy donor and to chronic hepatitis patients and the frequency of MDSC correlates with tumor progression (43). In patients who received RFA, the frequency of MDSCs was significantly decreased after treatment. However, in several patients the frequencies were increased after RFA and patients with high frequency of MDSCs after treatment relapsed. Similar results have been described by others (41). It was also suggested that ablation therapy alters the T-cell balance by increasing the systemic CTL/Treg, ratio. Heat-based ablation (RFA and MWA) might be a more effective approach than cryoablation to enhance systemic anti-tumor immunity (44). MDSCs and regulatory T cells in raw numbers may be epiphenomena of an immune response as well, possibly further complicating their predictive value however, as well as measurement in tumor, nodes or peripherally.
Similar to ablation, trans arterial chemo-embolization (TACE) or drug-eluting bead TACE (DEB-TACE) causes local cell death. Doxorubicin is the preferred and most common chemo-agent used in TACE and it is considered an immunogenic drug, potentiating ICD (45). Tumors being treated by TACE are usually larger than those treated by ablation and it is not clear how that affects immune responses, nor is it known whether a subtotal treatment is ideal, so long as there is not overwhelming tumor burden and a vascularized route to remaining viable tumor, perhaps. TACE was shown to promote an immunogenic cell death (ICD) (46) and to induce tumor-associated antigen (TAA) specific responses (47). Glypican-3 (GPC3) is a carcinoembryonic antigen and is ideal target for anticancer immunotherapy against HCC (48). The induction of GPC3 specific T-cell-mediated immune response was investigated in HCC patients (49). It was shown that circulating GPC3-specific cytotoxic T lymphocytes (CTLs) were increased in 55% of HCC patients after RFA and in 44% of patients after TACE, but in only 11% of patients after surgical resection. To investigate GPC3 expression in HCC tissues, the researchers performed immunohistochemical staining for GPC3 in biopsy or resected specimens. Of note, all of the 7 patients with GPC3-expressing HCCs exhibited an increase in GPC3-specific CTLs after RFA or TACE, whereas none of the 7 patients with GPC3- expressing HCCs did after surgical resection. The difference in GPC3-specific CTLs activation between pre- and post-treatment in the RFA group was larger than that in the resection group (P=0.023). This difference in the TACE group was also larger than that in the resection group, but the difference was not statistically significant (P=0.096).
Radioembolization using intra-arterial delivery of Y90 is another locoregional approach for the treatment of HCC. The half-life of Y90 isotope is approximately 64 hours but maximal clinical response, and the effect on the surrounding liver, is only seen 3–6 months after treatment. Chew et al. presented in-depth immunophenotyping investigation at local and system level. The authors found higher immune activation in the tumor microenvironment of resected HCC after Y90 downstaging compared to naïve patients, in the Y90 treated patients analysis of PBMCs before and after treatment found increase in TNF-α on both CD4 and CD8 T cells and an increase in APC percentage. Furthermore, the authors offered response prediction model using systemic immune biomarkers pre-treatment (50). Conflicting data suggested that radioembolization cause lymphopenia, reduction of lymphocyte proliferation capacity, and reduction in their ability to produce inflammatory cytokines directly after treatment, these phenomena was observed as long as 1 month after treatment (51). Radioembolization can result in unwanted inflammation, and hypertrophy of non-treated hemiliver. Both Fernandez-Ros et al. and Seidensticker et al. showed increase in pro-inflammatory cytokines post Y90 treatment, the later also suggested that high level of IL-8 or IL-6 pre-treatment can predict decrease overall survival (52, 53). In summary, results from a number of studies suggest that locoregional therapies cause changes in tumor-specific and innate immune responses. It is very plausible that changes in the local cytokine and/or chemokine milieu, which are difficult to measure in patients, result from locoregional therapies and affect not only effector cell responses, but also immune suppressor mechanisms such as MDSCs. A smorgasbord of cytokine, chemokine, and inflammatory / DAMP / cell stress response markers have been described following virtually all of the various ablative modalities. A summary of studies in this topic and results for HCC patients are summarized in Table 1.
Table 1:
Changes in immune response observed in patients treated with locoregional therapies.
| No. of reference | Locoregional Treatment (TRX) | Immune outcome measurements | Source of immune measurements tumor (T), peripheral blood (P) | Number of patients | Year of publication |
|---|---|---|---|---|---|
| (38) | MWA | Th17, CD3, CD4, CD8, Tregs | P | 30 HCC | 2018 |
| (39) | MWA | CD3, CD4, CD8, CD4+ CD25+ Tregs, and CD16+ CD56+ NK | P | 45 HCC | 2017 |
| (40) | RFA | APC maturation and function (in vitro assay for tissues before and after ablation) | T, P | 19 | 2008 |
| (41) | RFA | tumor-associated antigen (TAA)-derived peptides | P | 69 HCC | 2013 |
| (43) | RFA | MDSCs | P | 123 HCC patients, 33 received RFA | 2013 |
| (46) | TACE | Cell death sera markers: HMGB1, sRAGE, DNase | P | 50 HCC, 71 procedures | 2012 |
| (49) | RFA/TACE/Surgical resection | Glypican-3 -specific CTLs | P | 9 RFA, 9 TACE, 9 Surgical resections | 2012 |
| (54) | RFA | Th1 (IL-2, TNF-α, IFN-γ), Th2 (IL-4, IL-6, IL-10) | P | 26 HCC, 25 healthy | 2017 |
| (55) | RFA | Immune potentiating antigens in the serum, Ficolin-3 | P | 57 HCC | 2018 |
| (56) | TACE | IL12p70, IFN-γ, IL-17A, IL-2, IL-10, IL-9, IL-22, IL-6, IL-13, IL-4, IL-5, IL-1β, and TNF-α | P | 83 HCC, 33 healthy | 2013 |
| (57) | TACE | CD4 (Th1, Th17 and Treg cells), CD8, NK, NKT, IL-2, IL-4, IL-6, IL-10, IL17A, IFN-γ, and TNF-α | P | 28 stage I HCC, 51 stage III, 20 healthy | 2013 |
| (58) | TACE | CD4, CD8, Treg, IL-35 | P | 47 HCC, 15 healthy | 2015 |
| (59) | Bland embolization | Th1, Th2, Treg | P | 5 HCC | 2016 |
| (60) | Cryo+TACE | CD3/CD4, CD4/CD8, NK | P | 32 TACE, 31 Cryo, 35 Cryo+TACE | 2015 |
| (50) | Y90 | In depth phenotyping | T, P | 41 | 2018 |
| (51) | Y90 | IL10, IFN-γ, CD3, CD4, CD8, B, NK, cd45R0 | P | 25 | 2018 |
| (52) | Y90 | IL6,8, TNF-α, Nitrotyrosin, malondialdehyde | P | 14 | 2014 |
| (53) | Y90 | IL1,2,4,6,8, TNF-α, IFN-γ | P | 12 HCC (total of 34) | 2017 |
Combined immune checkpoint blockade plus locoregional therapies
Based on the preclinical and clinical studies described above, we decided to test the hypothesis that immune checkpoint blockade may enhance immune responses caused by locoregional therapies and therefore be good combination partners. Based on an earlier study by Sangro et al, in which he showed that tremelimumab (anti-CTLA4) may lead to clinical and immunological effects in HCC (61) we treated a total of 39 HCC patients with locoregional therapy plus tremelimumab. We initially enrolled patients who had progressed on sorafenib therapy. Tremelimumab was given every 4 weeks for a total 6 doses, followed by 3-monthly intravenous administrations until off-treatment criteria were met. On day 35, patients received locoregional therapy (ablation or DEB-TACE). An intentionally incomplete radiofrequency ablation or DEB-TACE was performed with the intent to induce and/or augment an anti-tumor immune response, and also to leave viable tumor tissue at the ablation-tumor junction, which may be a fertile and key site for cytokine, inflammatory and immune activity. We also enrolled a subgroup of patients with BCLC B HCC eligible for TACE. This design was chosen so that patients had recovered from potential tremelimumab related immune mediated side effects such transaminitis, which had initially been reported to occur in HCC patients treated with tremelimumab (61). The primary endpoint of this study feasibility and safety were met.
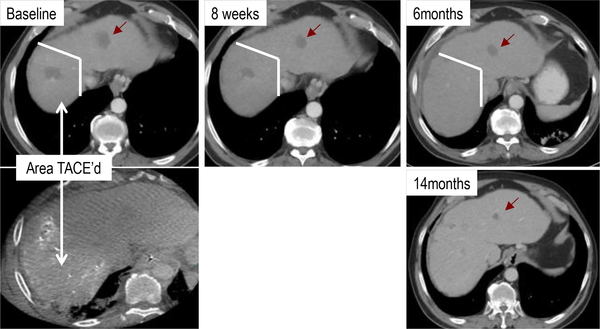
After a median follow-up of 36.6 months we observed a median overall survival of 10.9 months (95% CI 8.0–13.7 months). The overall median survival rates in the groups treated with TACE, RFA and cryoablation were 13.8 months (95% CI 10.2–17.4months, N=17), 9.2 months (95% CI 6.6–11.2 months, N=10), 15.0 months (95% CI 10.5–19.5 months, N=9), respectively. One complete response, 7 partial responses and 15 patients with stable disease were noted among 34 evaluable patients Aghdashian et al. submitted and (62). Of note, only lesions not treated by locoregional therapy were used to determine tumor responses (see Figure 3).
Figure 3:
Tumor response in HCC patient upon TACE + tremelimumab treatment in lesion not treated by TACE.
Treatment was well tolerated and no dose limiting toxicity was observed. The most common clinical toxicity was pruritus which was predominantly grade 1 and frequently accompanied by a rash consistent with immune-related dermatitis. Elevated transaminases were commonly observed. Other immune related adverse events included colitis, pneumonitis and endocrinopathies. A comprehensive study on immune correlatives in this patient population revealed an influx of T cells into tumors in patients responding to immunotherapy. Flowcytometry showed an increase in the frequency of CD4+-HLA-DR+, CD4+PD-1+, CD8+HLA-DR+, CD8+PD-1+, CD4+ICOS+ and CD8+ICOS+ T cells in the peripheral blood of the treated patients. Patients with higher CD4+PD1+ cell frequency at baseline were more likely to respond to tremelimumab therapy. Analysis of tumor-specific (AFP and survivin) T cell responses indicated PD-1 expression was increased upon tremelimumab treatment. An increase of tumor infiltrating CD3+ T cells was also seen in these patients. Immunosequencing of longitudinal PBMC showed that one cycle of tremelimumab significantly decreased peripheral clonality, while no additional effects were seen after locoregional therapy.
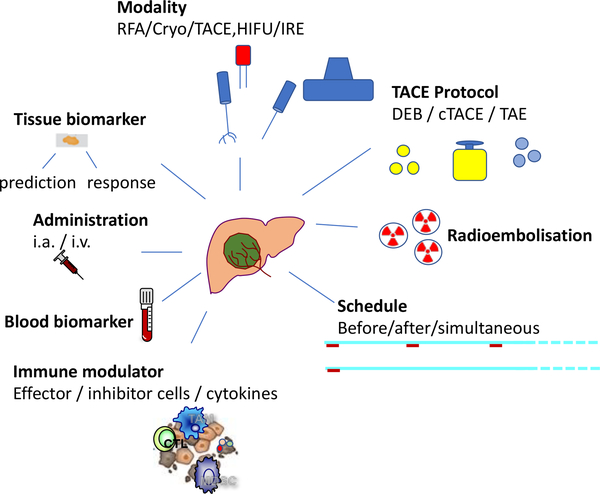
Based on positive results from this and other studies evaluating immune checkpoint inhibitors in HCC (63, 64) a number of studies have been initiated to test the combination of immune checkpoint blockade (and other types of immunotherapy) plus locoregional therapies. A list of such studies is provided in Table 2. However, many questions in this field remain unanswered, related to mechanisms, sequencing, timing, optimal loco regional therapy and optimal immunomodulation or combination of immunomodulation. The precise and distinct immunological effects of locoregional therapies are not clear. Which treatment modality is more immunogenic? What is the best timing for the immune checkpoint blockade? Before, during or after locoregional therapies/ What type of TACE is most immunogenic and how do we define biomarkers of response? Can these markers only be found in tumor biopsies or can we detect them in peripheral blood? What is the best delivery mode for immunotherapy and should we focus on effector cells or immune suppressor mechanisms in the microenvironment? Asking key questions may expedite and facilitate answers with major clinical implications. Unraveling this complex threaded knot will require true team science and interdisciplinary partnerships and co-education only a combined efforts between experts in tumor-immunology, hepatology and intervention oncology/ locoregional therapies will enable defining more optimal therapies for patients with hepatocellular carcinoma, who until very recently had few effective options. Indeed, an exciting time to have coffee with an expert with an another discipline.
Table 2:
Ongoing studies evaluating the combination of locoregional therapies and immunotherapy
| CLINICAL TRIAL | Number of patients | Locoregional therapy | Immunomodulator | |
|---|---|---|---|---|
| 1 | NCT03592706 | 60 | TACE | Immune killer cells |
| 2 | NCT03575806 | 60 | TACE | Central memory T cells |
| 3 | NCT03572582 | 49 | TACE | Nivolumab |
| 4 | NCT03397654 | 26 | TACE | Pembrolizumab |
| 5 | NCT03383458 | 530 | Ablation | Nivolumab |
| 6 | NCT03143270 | 14 | DEB-TACE | Nivolumab |
| 7 | NCT02856815 | 78 | TACE | Immuncell-LC |
| 8 | NCT03124498 | 55 | TACE, PEIT, RFA | Autologous cytokine induced killer cells (CIK) |
| 9 | NCT02821754 | 90 | TACE, RFA, Cryo | Durvalumab, Tremelimumab |
| 10 | NCT02568748 | 20 | TACE | CIK |
| 11 | NCT02487017 | 60 | TACE | DC-CIK |
| 12 | NCT02837029 | 35 | Y 90 Glass Microspheres | Nivolumab |
| 13 | NCT03380130 | 40 | Y90- Microspheres | Nivolumab |
| 14 | NCT03033446 | 40 | Y90 | Nivolumab |
| 15 | NCT03099564 | 30 | Y90 | Pembrolizumab |
| 16 | NCT03259867 | 80 | TATE | Nivolumab OR Pembrolizumab |
Figure 2:
Overview of considerations for future research studies combining immunotherapy with locoregional therapies
Acknowledgments
Financial Support: The authors work is supported by the intramural program of the National Institutes of Health and the National Cancer Institute.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 2.Duan XH, Li TF, Zhou GF, Han XW, Zheng CS, Chen PF, Feng GS. Transcatheter arterial embolization combined with radiofrequency ablation activates CD8(+) T-cell infiltration surrounding residual tumors in the rabbit VX2 liver tumors. Onco Targets Ther 2016;9:2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You Y, Wang Z, Ran H, Zheng Y, Wang D, Xu J, Wang Z, et al. Nanoparticle-enhanced synergistic HIFU ablation and transarterial chemoembolization for efficient cancer therapy. Nanoscale 2016;8:4324–4339. [DOI] [PubMed] [Google Scholar]
- 4.Deng G, Zhao DL, Li GC, Yu H, Teng GJ. Combination therapy of transcatheter arterial chemoembolization and arterial administration of antiangiogenesis on VX2 liver tumor. Cardiovasc Intervent Radiol 2011;34:824–832. [DOI] [PubMed] [Google Scholar]
- 5.Schlageter M, Terracciano LM, D’Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol 2014;20:15955–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parvinian A, Casadaban LC, Gaba RC. Development, growth, propagation, and angiographic utilization of the rabbit VX2 model of liver cancer: a pictorial primer and “how to” guide. Diagn Interv Radiol 2014;20:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueki A, Okuma T, Hamamoto S, Kageyama K, Murai K, Miki Y. Combination therapy involving radiofrequency ablation and targeted chemotherapy with bevacizumab plus paclitaxel and cisplatin in a rabbit VX2 lung tumor model. BMC Res Notes 2018;11:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bimonte S, Leongito M, Piccirillo M, Tamma ML, Vallifuoco M, Bracco A, Mancini A, et al. Induction of VX2 para-renal carcinoma in rabbits: generation of animal model for locoregional treatments of solid tumors. Infect Agent Cancer 2016;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gade TP, Hunt SJ, Harrison N, Nadolski GJ, Weber C, Pickup S, Furth EE, et al. Segmental Transarterial Embolization in a Translational Rat Model of Hepatocellular Carcinoma. J Vasc Interv Radiol 2015;26:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkins LR, Stone JR, Mata J, Hawrylack A, Kubicka E, Brautigan DL. The Use of the Woodchuck as an Animal Model for Evaluation of Transarterial Embolization. J Vasc Interv Radiol 2017;28:1467–1471. [DOI] [PubMed] [Google Scholar]
- 11.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol 2005;26:512–517. [DOI] [PubMed] [Google Scholar]
- 12.Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. European Journal of Cancer 2005;41:1911–1922. [DOI] [PubMed] [Google Scholar]
- 13.Dow M, Pyke RM, Tsui BY, Alexandrov LB, Nakagawa H, Taniguchi K, Seki E, et al. Integrative genomic analysis of mouse and human hepatocellular carcinoma. Proc Natl Acad Sci U S A 2018;115:E9879–E9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamrekelashvili J, Greten TF, Korangy F. Immunogenicity of necrotic cell death. Cell Mol Life Sci 2015;72:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, Manns MP, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer 2003;103:205–211. [DOI] [PubMed] [Google Scholar]
- 16.Gamrekelashvili J, Ormandy LA, Heimesaat MM, Kirschning CJ, Manns MP, Korangy F, Greten TF. Primary sterile necrotic cells fail to cross-prime CD8(+) T cells. Oncoimmunology 2012;1:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamrekelashvili J, Kapanadze T, Han M, Wissing J, Ma C, Jaensch L, Manns MP, et al. Peptidases released by necrotic cells control CD8+ T cell cross-priming. J Clin Invest 2013;123:4755–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- 19.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015;28:690–714. [DOI] [PubMed] [Google Scholar]
- 20.Seehawer M, Heinzmann F, D’Artista L, Harbig J, Roux PF, Hoenicke L, Dang H, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 2018;562:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tha¨daus Till Wissniowski JHn, Neureiter Daniel, Frieser Markus, Schaber Stefan, Esslinger Birgit, Voll Reinhard, Strobel Deike, Hahn Eckhart G., and Schuppan Detlef. Activation of Tumor-specific T Lymphocytes by Radio-Frequency Ablation of the VX2 Hepatoma in Rabbits . CANCER RESEARCH;63:6496–6500. [PubMed] [Google Scholar]
- 22.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006;95:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dromi SA, Walsh MP, Herby S, Traughber B, Xie JW, Sharma KV, Sekhar KP, et al. Radiofrequency Ablation Induces Antigen-presenting Cell Infiltration and Amplification of Weak Tumor-induced Immunity. Radiology 2009;251:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14:199–208. [DOI] [PubMed] [Google Scholar]
- 25.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 2004;64:4024–4029. [DOI] [PubMed] [Google Scholar]
- 26.Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, Allison JP. Potent Induction of Tumor Immunity by Combining Tumor Cryoablation with Anti-CTLA-4 Therapy. Cancer Research 2011;72:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behm B, Di Fazio P, Michl P, Neureiter D, Kemmerling R, Hahn EG, Strobel D, et al. Additive antitumour response to the rabbit VX2 hepatoma by combined radio frequency ablation and toll like receptor 9 stimulation. Gut 2016;65:134–143. [DOI] [PubMed] [Google Scholar]
- 28.den Brok MHMGM, Sutmuller RPM, Nierkens S, Bennink EJ, Toonen LWJ, Figdor CG, Ruers TJM, et al. Synergy between In situ Cryoablation and TLR9 Stimulation Results in a Highly Effective In vivo Dendritic Cell Vaccine. Cancer Research 2006;66:7285–7292. [DOI] [PubMed] [Google Scholar]
- 29.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer 2006;6:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinovink JW, Fransen MF, Lowik CW, Ossendorp F. Photodynamic-Immune Checkpoint Therapy Eradicates Local and Distant Tumors by CD8(+) T Cells. Cancer Immunol Res 2017;5:832–838. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun 2016;7:13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson EE, Yamane BH, Buhtoiarov IN, Lum HD, Rakhmilevich AL, Mahvi DM, Gillies SD, et al. Radiofrequency ablation combined with KS-IL2 immunocytokine (EMD 273066) results in an enhanced antitumor effect against murine colon adenocarcinoma. Clin Cancer Res 2009;15:4875–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong J, Kong J, Pan B, Ke S, Dong S, Li X, Zhou A, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1alpha/VEGFA. PLoS One 2012;7:e37266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan J, Wu W, Chen Y, Kang N, Zhang R. Insufficient radiofrequency ablation promotes the growth of non-small cell lung cancer cells through PI3K/Akt/HIF-1alpha signals. Acta Biochim Biophys Sin (Shanghai) 2016;48:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nir Rozenblum PEZ Scaiewicz Viviana Bulvik Baruch Gourevitch Svetlana Yotvat Hagit, Eithan Galun M Goldberg Nahum. Oncogenesis: An “Off-Target” Effect of Radiofrequency Ablation. radiology 2015;276:426–432. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed M, Kumar G, Gourevitch S, Levchenko T, Galun E, Torchilin V, Goldberg SN. Radiofrequency ablation (RFA)-induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins. Int J Hyperthermia 2018;34:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunologic perspective. Clin Cancer Res 2013;19:6678–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Xu XL, Ding JM, Jing X, Wang FM, Wang YD, Wang P. Dynamic changes of T-cell subsets and their relation with tumor recurrence after microwave ablation in patients with hepatocellular carcinoma. Journal of Cancer Research and Therapeutics 2018;14:40–45. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Hou X, Cai H, Zhuang X. Effects of microwave ablation on T-cell subsets and cytokines of patients with hepatocellular carcinoma. Minim Invasive Ther Allied Technol 2017;26:207–211. [DOI] [PubMed] [Google Scholar]
- 40.Zerbini A, Pilli M, Fagnoni F, Pelosi G, Pizzi MG, Schivazappa S, Laccabue D, et al. Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J Immunother 2008;31:271–282. [DOI] [PubMed] [Google Scholar]
- 41.Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013;57:1448–1457. [DOI] [PubMed] [Google Scholar]
- 42.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008;135:234–243. [DOI] [PubMed] [Google Scholar]
- 43.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, et al. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 2013;62:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takaki H, Imai N, Thomas CT, Yamakado K, Yarmohammadi H, Ziv E, Srimathveeravalli G, et al. Changes in peripheral blood T-cell balance after percutaneous tumor ablation. Minim Invasive Ther Allied Technol 2017;26:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apetoh L, Mignot G, Panaretakis T, Kroemer G, Zitvogel L. Immunogenicity of anthracyclines: moving towards more personalized medicine. Trends Mol Med 2008;14:141–151. [DOI] [PubMed] [Google Scholar]
- 46.Kohles N, Nagel D, Jungst D, Stieber P, Holdenrieder S. Predictive value of immunogenic cell death biomarkers HMGB1, sRAGE, and DNase in liver cancer patients receiving transarterial chemoembolization therapy. Tumour Biol 2012;33:2401–2409. [DOI] [PubMed] [Google Scholar]
- 47.Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, Burroughs AK, et al. Unmasking of -Fetoprotein-Specific CD4+ T Cell Responses in Hepatocellular Carcinoma Patients Undergoing Embolization. The Journal of Immunology 2007;178:1914–1922. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Yao M, Pan L-H, Qian Q, Yao D-F. Glypican-3 is a biomarker and a therapeutic target of hepatocellular carcinoma. Hepatobiliary & Pancreatic Diseases International 2015;14:361–366. [DOI] [PubMed] [Google Scholar]
- 49.Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, Nakachi K, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol 2012;40:63–70. [DOI] [PubMed] [Google Scholar]
- 50.Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, Lai L, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domouchtsidou A, Barsegian V, Mueller SP, Best J, Ertle J, Bedreli S, Horn PA, et al. Impaired lymphocyte function in patients with hepatic malignancies after selective internal radiotherapy. Cancer Immunol Immunother 2018;67:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Ros N, Inarrairaegui M, Paramo JA, Berasain C, Avila MA, Chopitea A, Varo N, et al. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int 2015;35:1590–1596. [DOI] [PubMed] [Google Scholar]
- 53.Seidensticker M, Powerski M, Seidensticker R, Damm R, Mohnike K, Garlipp B, Klopffleisch M, et al. Cytokines and (90)Y-Radioembolization: Relation to Liver Function and Overall Survival. Cardiovasc Intervent Radiol 2017;40:1185–1195. [DOI] [PubMed] [Google Scholar]
- 54.Ji LL, Gu J, Chen L, Miao DL. Changes of Th1/Th2 cytokines in patients with primary hepatocellular carcinoma after ultrasound-guided ablation. International Journal of Clinical and Experimental Pathology 2017;10:8715–8720. [PMC free article] [PubMed] [Google Scholar]
- 55.Shen S, Peng H, Wang Y, Xu M, Lin M, Xie X, Peng B, et al. Screening for immune-potentiating antigens from hepatocellular carcinoma patients after radiofrequency ablation by serum proteomic analysis. BMC Cancer 2018;18:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim MJ, Jang JW, Oh BS, Kwon JH, Chung KW, Jung HS, Jekarl DW, et al. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine 2013;64:516–522. [DOI] [PubMed] [Google Scholar]
- 57.Liao Y, Wang B, Huang ZL, Shi M, Yu XJ, Zheng L, Li S, et al. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: a prospective study. PLoS One 2013;8:e60444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao J, Xiao J, Zhou Y, Liu Z, Wang C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep 2015;12:6065–6071. [DOI] [PubMed] [Google Scholar]
- 59.Takaki H, Imai N, Contessa TT, Srimathveeravalli G, Covey AM, Getrajdman GI, Brown KT, et al. Peripheral Blood Regulatory T-Cell and Type 1 Helper T-Cell Population Decrease after Hepatic Artery Embolization. J Vasc Interv Radiol 2016;27:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang M, Wang X, Bin H. Effect of Transcatheter Arterial Chemoembolization Combined with Argon-Helium Cryosurgery System on the Changes of NK Cells and T Cell Subsets in Peripheral Blood of Hepatocellular Carcinoma Patients. Cell Biochem Biophys 2015;73:787–792. [DOI] [PubMed] [Google Scholar]
- 61.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81–88. [DOI] [PubMed] [Google Scholar]
- 62.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]