Abstract
The last two decades have witnessed remarkable advance in our understaning the role of D-amino acids in the mammalian nervous system: from the unknown, to known molecules with unknown functions, to potential central players in health and disease. D-Amino acids have emerged as an important class of signaling molecules. In particular, the exploration of the roles of D-serine in brain physiopathology is a vibrant field that is growing at an accelerating pace. However, disentangling the functions of a small chiral molecule in the complex chemical milieu of the brain requires to be familiar with distinct measurement approaches but also to currently work with delicate molecular tools which effects can be confounded. Thus, study of D-amino acids requires accurate methodologies and specific controls, and these have often been lacking. Here we outline best practices for D-amino acid research, with a special emphasis on D-serine. We hope these concepts help move the field to greater rigor and reproducibility, allowing the field to advance.
Keywords: D-Serine, NMDA receptors, analytical methods, rescue experiments, glia, neurons, serine racemase inhibitors, immunostainings
When exploring a new signaling pathway and moving to new measurements or methodologies outside of our expertise, it is easy to follow the minimum requirements as opposed to the most rigorous ones, resulting in false findings corrupting the scientific literature. Unfortunately, this has happened several times in the field of D-amino acid (D-AA) research. Along with our quest to understand how the brain works, research on the functions of the unusual neuromodulator D-serine requires distinct methodological approaches and controls which have been lacking in many studies. Here we inspect the recent literature and outline several measures to help counter issues of irreproducibility to strengthen our scientific conclusions.
From dark to the spotlight: where do problems originate?
The discovery by Nishikawa and colleagues1 that D-serine is present at high concentrations in the mammalian brain has not only challenged the long-cherished dogma that free D-AAs are uncommon in mammalian tissues but has also forced (neuro)biologists to reconsider the foundations of cell-to-cell signaling. The idea that D-AAs, originally considered toxic compounds, could serve important functions in mammals was quite radical and was initially dismissed since the authors had not identified pathways that produce them in the body. Considerable efforts from several groups worldwide have radically transformed this vision over the last 20 years, particularly with regard to D-serine. In the 90’s, Snyder’s laboratory developed specific stereoselective antibodies against D-serine to map its distribution in the brain2 and then established D-serine’s origin to the brain by purifying and cloning serine racemase (SR), the enzyme that produces D-serine3. At the same time, they also made a second conceptual breakthrough by demonstrating that D-serine serves as the endogenous co-agonist for the synaptic N-methyl-D-aspartate subtype of glutamate receptors (NMDARs) in the hippocampus4, a role initially thought to be played by glycine alone5,6. These two discoveries have dramatically transformed our understanding of the mechanisms regulating the activity of NMDARs. According to ISI Thomson, since the first report by Nishikawa and colleagues on the presence of free D-serine in the brain there have been approximately 1500 articles published on D-serine from 1992 until 2018, and publications on this topic are appearing at an accelerating pace. As a consequence, the field has grown fast but its novelty has created an Achilles’ heel.
In the brain, D-serine is synthesized from L-serine by SR, and is degraded by both SR and the peroxisome associated D-amino acid oxidase (DAAO) enzyme7, and its levels in the synaptic cleft are controlled by the alanine-serine-cysteine-1 (ASC-1) and ASCT-1 transporters8–10. Glycine synaptic availability is essentially regulated by glycine transporters (GlyT)9,11 but can also be controlled by ASC-18,9. Although D-serine can also bind to the δ2 glutamate receptor (GluD2, Grid2)12 to regulate long-term depression (LTD) of synaptic communication in the cerebellum13, it is mostly recognized as the primary co-agonist of NMDARs at synapses of the forebrain where it regulates many functions of these receptors in synaptic plasticity, neurodevelopment and neurodegeneration14,15. The D-serine/NMDAR signaling pathway is an appealing route with already clear implications in the pathophysiology and treatment of human diseases as highlighted by: i) the growing literature related to its role in schizophrenia (SCZ), depression, amyotrophic lateral sclerosis, drug abuse and Alzheimer’s disease (AD); ii) the expanding number of clinical trials targeting D-serine signaling to treat patients; and iii) the increasing interest in the development of new compounds that selectively target its metabolism or transport systems8,16.
The recognition that D-serine is the endogenous NMDAR co-agonist in forebrain, thus supporting crucial cerebral functions, has spurred its intensive study. However, because the L- and D-enantiomers share the same physical properties, its measurement is challenging. For example, quantifying D-serine using a simple migration match via liquid chromatography (LC) may not be sufficient, and yet is a common practice; there are too many moieties in the brain to allow simple migration matching to be robust. We note that many publications, even in high-impact journals, lack appropriate controls or use non-validated approaches for detecting D-serine and defining its function, thus leading to misinterpretation of experimental data as detailed below. Such publications propagate misinformation, biasing the field by introducing ‘misconceptions’.
The D-serine issue
Although the functions of D-serine have not been questioned, its cellular origin has spawned debate17–19. D-serine was long considered a prototypical “gliotransmitter”15 but recent works by several laboratories have challenged this view. They have shown that under physiological conditions, D-serine is primarily synthesized and released by neurons in the healthy brain to modulate NMDAR functions20–23 and will only substantially derive from reactive glia in pathological conditions24. It appears that these past reports that D-serine can be formed in primary cultured astrocytes follows from studies under such pathological conditions.
Some recent studies published in high-profile journals continue in nourishing the D-serine gliotransmission hypothesis or argue for specific functions of the co-agonist based on experimental evidence that may be questionable. In particular, some studies fail to properly detect and quantify D-serine levels by selective immunostainings or analytical methods, in addition to the use of unspecific pharmacological or genetic interventions. Consequently, their conclusions may appear speculative due to the absence of essential controls. Therefore, as specialists in the field of D-AAs with different and complementary expertise in analytical chemistry, biochemistry, cell biology, neurophysiology and animal models, we write this essay to advocate for good practices in exploring the mechanisms and functions of D-serine in the brain, guidelines that should be used when researching additional D-AAs such as D-alanine and D-aspartate. In addition to presenting a critical review of some aspects of the D-serine literature, we hope that this perspective encourages colleagues entering the field to use good practices in their investigations and evaluations of manuscripts and grants.
Detecting or not detecting D-serine properly with analytical methods: that is the question
The characterization of D-serine as a cell-cell signaling molecule often requires as a first step the ability to detect and quantify the D-AA levels under the different experimental conditions. Such a measurement needs careful experimental design, appropriate analytical techniques and proper execution in order to be confident in differentiating D-serine from other amino acids and from its L-enantiomer in biological samples. Analytical methods have matured, and we now have an array of techniques ranging from biosensors25–27, high pressure liquid chromatography (HPLC)28–30 and LC/LC31 to LC/mass spectrometry32,33 or capillary electrophoresis-laser-induced fluorescence (CE-LIF)34–36 with well described protocols to detect and measure D-AAs (including D-serine) even at very low levels in complex matrices like biological samples. Despite the availability of multiple approaches to confirm D-serine, analysis of recent publications suggests that this D-AA was not always quantified with the controls that are outlined in Box 1 and Figure 1.
Box 1 : Best practices in exploring D-AA function.
Characterization approaches
Detecting and quantifying one particular compound in brain or microdialysate samples require validated methodologies and technical training. The possibility of false positives becomes larger when measuring new categories of compounds, such as the D- from L-AAs in complex matrices. Matching the retention time of a peak in a chromatogram to a spiked standard is not sufficient to ‘prove’ the presence of the D-AA. Even with the much higher peak capacity of multidimensional (tandem) LC and CE, in the most convincing studies, the detection of the D-AA should be validated using secondary approaches such as enzyme degradation37, stable or radioisotope formation experiments38, removal of the peak via an antibody complexation39, or other orthogonal approaches. This validation remains important when one considers the presence of literally thousands of metabolites within mammalian tissue and the large differences in levels of the L- and D-enantiomers, making peak confirmations important. Such orthogonal confirmations become even more important when unusual levels of D-AA are reported that do not follow expected levels31. Lastly, we implore authors to show chromatograms/ electropherograms for each D-AA detected or each brain region that was studied in the supplemental information; showing one or two example traces is not enough.
Accordingly, here we have outlined several best practice suggestions for D-serine: i) identify the D-serine peak via the retention time of standard D-serine; eliminate the corresponding peak by treating the sample with a purified recombinant-specific degrading enzyme such as DAAO or DsdA (as described in the text). These practices can be adapted to other D-AA studies. For some, such as D-alanine, the same degrading enzymes can be used, and for others, such as D-aspartate or D-glutamate, D-aspartate oxidase would be an appropriate degrading enzyme. Besides peak identification, from the change in the peak area before and after enzymatic treatment, the D-AA can be quantified even if the two peaks are not completely resolved. ii) Add greater information such as with mass spectrometry and/or additional dimensions of a separation (LC/LC or even LC3). iii) Verify the yield of D-serine recovery from the starting sample: introduce a known amount of D-serine (or even better, an isotope of D-serine, and differentiate the endogenous and labeled D-serine via MS) in the starting sample and verify the recovery.
Amperometric- or photonic- derived DAAO based biosensor analysis of D-serine should be used in combination with a more selective assay, such as HPLC or CE separations or measurements made in SR knock-out mice as controls. Other controls should also include the absence of signal originating from the non specific oxidation of any drug (ie agonist, antagonists, inhibitors) used in the experiments.
Antibody-based methods to detect D-serine and its enzymes are robust techniques that do not require expensive equipment but rely on the highly specific and sensitive reaction of the antibody with its target, and still require several precautions. Regardless of the company from which the D-serine antibody is purchased, the use of the antibody requires that: i) the paraformaldehyde-based fixative should contain glutaraldehyde to obtain immunoreactivity in tissue/cell preparations; ii) incubation with the primary antibody should include inclusion of sufficiently high concentrations of L-serine glutaraldehyde conjugate to prevent cross-reaction of the antibody with endogenous L-serine present in the tissue/cell preparations (notably, astrocytes contain quite high concentrations of L-serine as they are the primary site of its synthesis in brain); iii) specific controls are used to pre-absorb the anti-serine antibody with D-serine glutaraldehyde conjugate in order to abolish the immunoreactivity in the biological samples46; and when possible, iv) use the same tissue area from SR knock-out mice, which have < 10% of wild-type levels of D-serine, treated in the same manner as the experimental tissue17,20–23. The specificity of the antibody for SR or DAAO should be also validated by western blot or immunostaining on tissues from SR−/− and DAAO−/− mice, respectively, since most if not all commercial antibodies are not specific for their claimed target.
Pharmacological rescue experiments
Here, we refer to experiments where a specific signaling pathway has been first pharmacologically or genetically invalidated and then D-serine is added to rescue physiological or behavioral outcomes. In many cases the pharmacological or genetical interventions are not specific to D-serine and could be rescued not only by D-serine but also by any agonist acting at the glycine site of NMDAR (i.e. glycine, D-alanine) but also by L-serine. Therefore, it is mandatory to: 1) show that the defective pathway (FAC, IP3R, and glial SNARE as examples) specifically affects the levels of D-serine over other amino acids (L-serine, glutamate, glycine) by using accurate separation and detection methods (see above), and then 2) perform rescue experiments with any agonist of the glycine site of NMDARs or L-serine.
Pharmacological inhibition of D-serine function and metabolic pathway
A first issue comes from the use of commercial preparations for DAAO (especially the enzyme from pig kidney) when probing the function of D-serine in physiological experiments. Indeed, these preparations show low activity (frequently because of inactive apoprotein production following enzyme dilution), are not pure, and thus could display off-target effects due to contamination by other enzymes like D-aspartate oxidase, which degrades glutamate, and NMDA or D-aspartic acid, which also activate and regulate NMDARs (see supplementary Fig. 1 in74). If commercial sources for DAAO have to be used (even when a home-made recombinant enzyme is used, i.e. RgDAAO), it is necessary to carry out control experiments in parallel, measuring the impact of enzymatic treatment on the levels of amino acids other than D-serine and especially on L-serine, as done in several reports, or to use SR−/− mice to ascertain the specificity of the enzyme. Application of DAAO on preparations obtained from SR−/− mice should have no additional inhibitory effects on the responses investigators are measuring.
A second source of false positives comes from the use of pharmacological tools to inhibit SR or DAAO. The actual real specificity of these compounds remains totally unknown and could display unwanted off-target effects as illustrated for OHAsp, PES and PM, which have been largely used to inhibit SR52,56,81–85. Therefore, the specificity of a putative inhibitor has to be first validated in SR−/− or DAAO−/− mice, depending on the target of the compound.
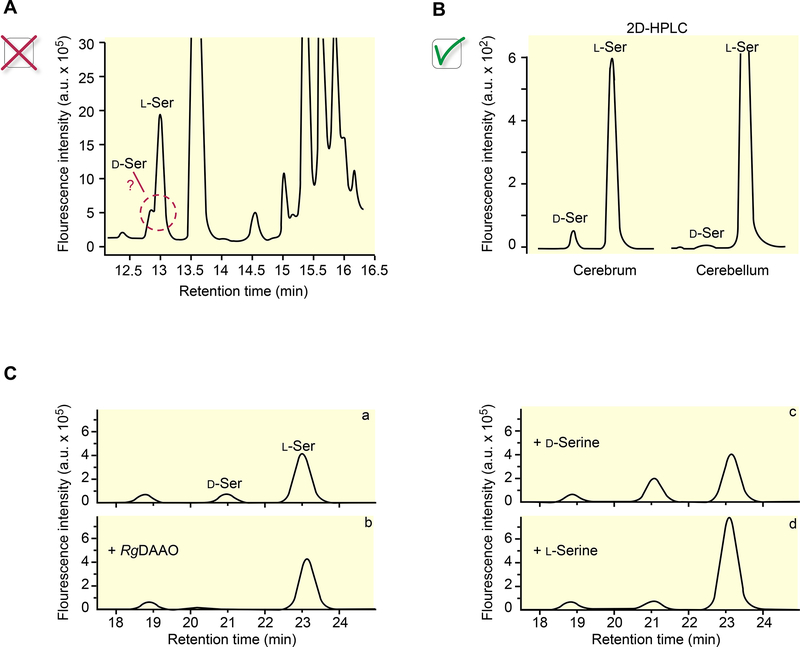
Figure 1: The bad and good practices in the detection of D-serine in biological samples.
The good checklist when detecting and measuring selected amino acids includes: a) well-resolved peaks for each amino acid (as illustrated in panels B & C, trace a). Traces displayed in B are from30 (modified with permission). A shoulder on a peak as illustrated in panel A from43 (modified with permission) is the sign of poorly resolved peaks, not allowing a conclusion. If such a shoulder is present, the analytical procedure showed be further optimized. b) Peak identification requires careful controls: spiking with the exogenous amino acid (panel C, traces c, d) and removing the peak with recombinant enzymes that specifically degrade the D-AA of interest (as illustrated in panel C, trace b).
A first example is represented by a mix-up of the analytical methods used. We specifically refer a case40 where it is stated that extracellular levels of D-serine, glycine, glutamate and GABA were analyzed by HPLC, but then the Supplementary section reports that a CE-LIF system was used following a procedure published elsewhere41; moreover, no electropherograms or chromatograms are shown. While confusing the descriptions of the approaches used is rare, a more common issue is the absence of experimental details on replicates, as well as not presenting electropherograms (via CE) or chromatograms (via HPLC)42. Most journals offer authors the opportunity to publish chromatograms/electropherograms in the Supplementary information because without them, it becomes hard to determine what was done to validate the results. Reports on D-AA characterization need to include such details.
Another fairly common issue is the poor resolution of the amino acid enantiomers (Figure 1A). As a second example, by using a combination of both HPLC and a biosensor, D-serine levels were reported to oscillate in brain samples (i.e., mouse hippocampus)43. We support the use of multiple measurement approaches increasing the confidence of their conclusions. However, in this case, poorly resolved D-serine and L-serine peaks render the strong conclusions difficult to support. Our goal here is to not to advocate for a specific protocol, but we do expect that the authors validate the specificity of their detection by employing a variety of controls (see Box 1).
Besides, the results obtained with biosensors should be interpreted as putative until secondary approaches validate their results. Indeed, several commercial (Sarissa LTD, UK) or home-made D-serine biosensors are based on the use of DAAO from the yeast Rhodotorula gracilis (RgDAAO) layered on an electroconductive support. In contrast to the enzyme isolated from other sources (and commercially available), RgDAAO shows a high specific activity, a stable interaction with the FAD cofactor, a high stability under experimental conditions and is not inhibited by L-amino acids44. RgDAAO catalyzes the oxidative deamination of neutral D-AAs44,45 and does not act either on glycine (Km = 160 mM, i.e. two to three orders of magnitude higher than the physiological concentration), or on D-aspartate and D-glutamate (kcat ≤ 1 s−1 vs. 61 s−1 for D-serine), known to act also on NMDAR. The experimental evidence shows that primary and secondary amines are not substrates or inhibitors of RgDAAO. However, current RgDAAO-based biosensors also detect most neutral D-AAs, including D-alanine, and might non-specifically oxidize other unexpected biological substances. Although D-alanine levels in the brain and in particular the hippocampus are much lower than those of D-serine29,31,32, the maximal activity and the catalytic efficiency of RgDAAO for D-alanine is 1.7- and 30 times higher than those for D-serine45, so it is conceivable that DAAO-based biosensors could also detect trace amounts of D-alanine which is also known to act as a co-agonist for NMDARs. Finally, it is also important to note that the commercial RgDAAO based biosensors are also sensitive to dopamine or to a series of structurally related agonists/antagonists of D1- and D2-like receptors (Dallerac & Mothet, personal communication).
D-Serine can be also measured using luminescent or fluorescent DAAO based assays3,24,45. But the response of these assays is nonlinear with D-serine concentration. We discourage their use which absolutely requires careful calibration for each concentration range used.
The good practice in detecting D-serine, SR and DAAO with antibodies
Another approach commonly used to visualize D-serine in tissues or cell preparations is immunocytochemistry. For D-serine, the technique was introduced first by Snyder and collaborators2 and has the remarkable advantage of revealing the distribution of the AA in entire cells or in cellular sub-compartments. Using this method with locally produced or commercial antibodies, investigators have reported that D-serine is present in many locations throughout the central nervous system (CNS)2,22,46,47. Immunostainings of AAs with antibodies at first glance could sound odd since antibodies require epitopes larger than an individual amino acid. Accordingly, raising anti-D-AA antibodies requires that the immunogen of the target D-AA be conjugated to a carrier protein by glutaraldehyde, which acts as an effective linker molecule. Many companies are selling polyclonal or monoclonal anti-D-serine antibodies. Antibody specificity is often validated with an ELISA test in competition experiments.
Despite well-described procedures and methods, some studies have reported the presence of D-serine by immunostainings using less-common methods (see Box 1 and Figure 2). This is the case where immunostainings to visualize D-serine were performed in tissues perfused with a fixative containing no glutaraldehyde while using a commercial rabbit polyclonal D-serine glutaraldehyde conjugated antibody48. To our best knowledge, commercially available monoclonal or polyclonal D-serine antibodies require glutaraldehyde in the fixative otherwise immunodetection of D-serine is less reliable. One study reported staining of D-serine with a paraformaldehyde-based fixative but the authors used an antibody optimized for formaldehyde fixation, which is not commercially available49.
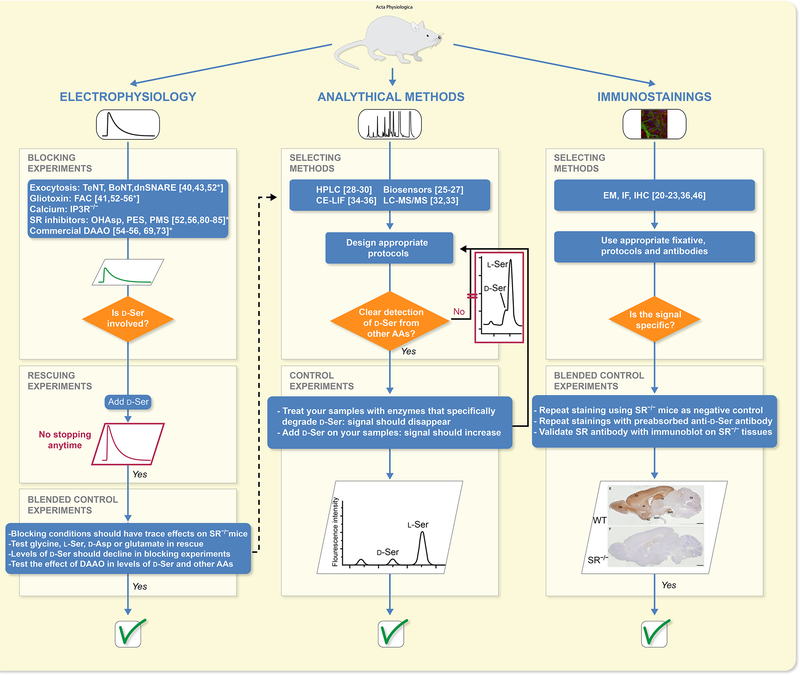
Figure 2: Chart for correct experimental strategy in exploring the function of D-serine.
Three main types of experimental methodologies are used to investigate the function of D-serine. In electrophysiological recordings, investigators use genetically or pharmacologically driven loss/gain-of functions of different pathways and perform rescue experiments. *References here are studies where problems arose in using at least one approach. Several well-described analytical methods can be used to detect and measure D-serine but all require careful management and optimization of the procedures in order to avoid confounding results, as displayed in the chromatogram extracted from43. Finally, immunodetection of D-serine or its metabolizing enzymes can be performed following different procedures (electron microscopy: EM; immunofluorescence: IF, and immunohistochemistry, IHC) but necessary controls should be run in order to ascertain the specificity of the signal as illustrated (modified with permission from21).
The same precaution should be also taken for detecting SR or DAAO by Western blotting or immunostaining analysis since most commercial antibodies are not specific and have not been validated in knock-out mice for the respective proteins. Indeed, the gold standard for immune specificity is the demonstration that the epitope is absent from tissues obtained from subjects in which the expression of the antigen has been genetically silenced. Miya et al21 were the first to apply this stringent requirement is studies of SR, establishing unequivocally that SR was expressed in neurons, primarily glutamatergic neurons but also GABAergic neurons, but not in astrocytes as was broadly accepted for a decade after their publication. The neuronal localization was confirmed by Balu et al23 in mouse and human brain utilizing antibodies validated against SR null-mutant mice. As SR is the primary source of D-serine, Balu et al.23 furthermore defined conditions for specific immune-staining of D-serine in tissue sections by showing that L-serine blocking concentrations had to be increased nearly a hundred-fold above reported conditions in order to prevent cross-reactivity (false positives) with the high concentrations of astrocytic L-serine.
Detection by analytical methods or visualization by immunohistochemistry of D-serine and its metabolic enzymes (i.e., SR and DAAO) is only one set of the experimental strategies used to interrogate whether D-serine has a function in their experimental model, a second set of experimental strategies relies on integrating pharmacological and genetic interventions in order to infer or support such functions. In the next sections, we present the different approaches commonly used but consider their limitation since correlation does not mean causation (Figure 2).
The case of astrocyte poisoning and pharmacological rescue experiments
Besides analytical measurements, pharmacological experiments require careful controls. One experimental design generally used by investigators consists of blocking a particular pathway with their favorite compound A and genetic intervention, and then evaluation of the rescue by compound B, in our case D-serine. An example is given by the use of fluoroacetate (FAC) to block metabolism of glial cells and then the pharmacological rescue by adding exogenous D-serine to restore normal function. FAC is the toxic constituent of the Dichapetalum plant family50. When brain tissues are exposed to FAC, it is converted into fluorocitrate, an inhibitor of aconitase, and hence impairs the tricarboxylic acid (TCA) cycle in astrocytes. FAC treatment results in the accumulation of citrate and in a reduction of glutamine51. Although the underlying mechanism for selectively affecting glia but not neurons remains elusive, FAC has been extensively used as a selective gliotoxin to uncover the contribution of astrocytes to synaptic transmission, functional plasticity and behavior51. Accordingly, studies have reported that FAC application to brain tissues impairs long-term potentiation (LTP) or long-term depression (LTD) expression through decreasing NMDAR currents but not α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor function41,52,53, and prevents allodynia54,55 or breathing response to CO2 levels in the brainstem56. These effects have been ascribed to the blockade of astrocytic D-serine release by FAC (but only based on the observation that adding exogenous D-serine rescues these NMDAR-dependent functions; see17,57). However, several lines of reasoning suggest that such an interpretation is speculative. First, it is also conceivable that FAC may indirectly affect the release of D-serine from neurons rather than directly block the release of the coagonist from glia17,28. Indeed, according to the “serine shuttle hypothesis” recently elaborated by Wolosker and colleagues58, L-serine is initially produced exclusively in astrocytes since only astrocytes express 3-phosphoglycerate dehydrogenase (3-PGDH), the key enzyme controlling L-serine synthesis. Then, L-serine is exported to neurons where it is converted into D-serine by neuronal SR. Once released by neurons, D-serine binds to the co-agonist site of NMDARs. Interestingly, FAC-induced impairments of LTP and NMDAR-mediated synaptic currents can be also fully reversed by L-serine28. Yet, the transport of glucose in astrocytes from blood vessels and the glycolytic pathway are two processes highly regulated by multiple pathways59. It has been shown that elevation of calcium and sodium increases the uptake and utilization of glucose by astrocytes. Although speculative, we could foresee that the activity of 3-PGDH in converting 3-phosphoglycerate into L-serine would be affected by all maneuvers (e.g., calcium clamp, FAC poisoning, …) that inhibit Ca2+ or Na+ waves in astrocytes.
Since L-serine does not affect NMDAR activity on its own, this result should support the view at first glance that FAC poisoning of astrocytes could affect the synthesis and/or the supply of L-serine to neurons. Restoring the glia-neuron shuttling by providing the D-serine precursor to neurons is thus able to fully overcome the astrocyte poisoning with FAC. However, rescue experiments with L-serine can also contribute to misleading interpretation since most commercial L-amino acids often contain D-AAs and vice versa as impurities at levels ranging from 0.1 to 5%. Therefore, when adding 1 mM L-serine, 1–50 μM of D-serine are added which would be sufficient to saturate the glycine site of NMDARs in most preparations. In some cases if high concentrations are used (≥1 mM) it might be necessary to purify the commercial sources of L- or D-serine with specific treatments (e.g., by enzymatic depletion) before using the amino acid for the purpose of physiological or biochemical assays. Also, the use of FAC to selectively impair astrocytes and not neurons is complicated since its glial selective action may depend on the duration of incubation and the concentration used. Indeed, FAC could also impair the metabolism of neurons themselves with prolonged exposure since neurons utilize the TCA cycle. Furthermore, FAC reduces glutamine synthesis in astrocytes and would likely impair glutamatergic synaptic transmission by reducing glutamine availability for glutamate synthesis in neurons. FAC also impairs the formation of glutamate from glucose by blocking the conversion of oxaloacetate into α-ketoglutaric acid. Finally, citrate is known to be an efficient chelator of many ions including Ca2+. Therefore, its accumulation in astrocytes may likely impair several calcium-dependent physiological processes such as constitutive exocytosis. Thus, FAC by interfering with the metabolism of astrocytes may impact neuronal activity by a number of different and complementary mechanisms.
In the same vein of artifact compromised methods is the use of genetical or pharmacological acute blockade of astrocytic inositol 1,4,5-trisphosphate (IP3) receptors with heparin, which is introduced specifically in the astrocytes, and which impairs NMDAR-dependent LTP at CA3-CA1 hippocampal synapses, a deficit rescued by exogenous D-serine60. Based on previous findings precisely demonstrating in vitro that glia may release D-serine through a Ca2+-regulated process61,62, it was claimed that astrocytic IP3 receptors contribute to the astrocyte release of D-serine thereby controlling functional plasticity at synapses. As stated above for FAC, it is also possible that blocking IP3 receptor function in astrocytes may have impacted the serine shuttle between glia and neurons, thus preventing the synthesis and release of L-serine from astrocytes and then the synthesis of D-serine by neurons. An alternative mechanism might be that IP3 receptor deletion or blockade may also impact the homeostasis of other gliotransmitters such as glutamate or ATP or most importantly glycine. Indeed, their release or uptake systems are known to be closely regulated by intracellular calcium57,61,62,64,65. Although highly debated for more than 20 years, astroglia have been shown to release glutamate or ATP through calcium-dependent mechanisms (putatively exocytosis) that requires intracellular calcium stores9,57,63–65. Therefore, impairments in synaptic plasticity (i.e., LTP) induced by genetic or pharmacological deletion of glial IP3 receptors is not specific to D-serine and would also involve these neuromodulators and potentially others (see Box 1 and Figure 2). Rescue experiments with L-serine or any other signaling molecule whose functions depend on astrocyte integrity (i.e., glycine, glutamate, ATP) have to be considered in order to draw a definitive conclusion. Similar possibilities may be relevant for studies in which exogenous D-serine was provided directly to the slice43 or through i.p. injection40 to rescue the respective NMDAR-mediated dysfunctions in mice where glial exocytosis has been genetically nulled. Since invalidation of IP3 receptors or glial exocytosis are not specific interventions restricted to D-serine’s function at NMDARs (validated only by the sole ability of exogenous D-serine to restore electrophysiological responses), we cannot exclude that the same inactivation would impact the release of glial glutamate, ATP, glycine and then their action on NMDARs as demonstrated by several groups at the same synapses using the same recording protocols in the same conditions, and at the same animal age63–65.
Adding any potentiating compounds acting on the glycine modulatory site (GMS) of NMDAR like glycine, D-alanine or D-cycloserine would have the same rescuing effect as observed with exogenous D-serine in all the above studies further undermining their conclusions (Figure 2). Rescuing NMDAR function with D-serine or any compounds just indicates that the GMS of NMDAR is involved. This is a pharmacological effect, as was the case during the initial investigations considering the identity of the co-agonist of NMDARs: adding D-serine, glycine, D-alanine or D-cycloserine and observing a potentiating action of such compounds on NMDAR functions does not say much on the precise implication of a specific endogenous ligand. Our message is therefore to encourage caution in the interpretation of experiments using pharmacological agents (i.e., D-serine) to rescue deficits since these experiments would only reveal a correlation but do not demonstrate causality.
Off-target effects of some genetic and pharmacological tools to explore the functions of D-serine
Two strategies have been commonly employed to investigate the roles played by D-serine in brain activity. First, in electrophysiological experiments performed in slice preparations, the role of the endogenous agonist can be selectively removed by the perfusion of slices with a purified catabolic enzyme that selectively degrades co-agonist such as D-serine4,66. Using this strategy, the Snyder laboratory was the first to provide evidence that application of purified DAAO reduces synaptic NMDARs function in cultured hippocampal neurons, hippocampal slices or in juvenile cerebellar slices4, an initial observation later confirmed by Papouin and colleagues67. Using this enzymatic approach, D-serine was shown to be the primary NMDAR co-agonist required for LTP at many CNS synapses28,41,67–69, or to be required for the LTD at synapses between parallel fibers and Purkinje cells in the immature cerebellum13. A second more selective approach at first glance has then been introduced by the Mori laboratory21 together with the Coyle laboratory70 by genetically silencing SR expression. SR−/− mice have a 90% decrease in brain D-serine and show reduced NMDAR currents, impaired synaptic plasticity, and learning deficits8,70,71. This genetic approach has been made more selective through conditional methods by using Cre-recombinase expression restricted to forebrain neurons with a promoter for the α-subunit of Ca2+/calmodulin-dependent kinase II or to astrocytes by a promoter glial fibrillary acidic protein to inactivated “floxed” SR gene72.
These two approaches remain the gold standard methods to explore the functions of D-serine in brain slices or in living animals, but each have their own limitations (see Box 1). If the use of enzymatic scavengers represents an invaluable technique for neurophysiologists investigating the neuromodulation of the ‘glycine’ site of NMDARs in the CNS, it is important to keep in mind that the quality of the results is highly dependent on the source and purity of enzymes that the investigators are using. Important observations have been reported proposing that D-serine mediates the spread of gliogenic LTP in nociceptive pathways73, neuropathic pain depends upon D-serine co-activation of spinal NMDARs in rats54,55, D-serine released by astrocytes in the brainstem regulates breathing response to CO2 levels56, and glial D-serine controls synaptic metaplasticity in the hypothalamus69. One common factor in these studies was the use of commercial sources of DAAO. Trigonopsis variabilis DAAO from Boehringer Mannheim/Roche was active and relatively pure4,74, but this preparation is no longer commercially available and thus could not be used in the mentioned studies. So interpretation of the results from studies based on commercial DAAO should be made cautiously since these preparations show also strong activity against D-aspartate, glycine, D-glutamate but also NMDA. We recently reviewed one effective way to manipulate these enzymes during electrophysiological recordings in acute brain slices and highlighted several experimental tricks75 and asked investigators for prudence (Figure 2). An alternative to DAAO to eliminate D-serine is to use recombinant D-serine deaminase (DsdA) which displays a higher affinity and specificity for D-serine66,74,76. Enzymatic scavengers like Glycine Oxidase from Bacillus Subtilus (BsGO) have also been successfully used to probe the relative function of glycine at NMDARs28,41,66–69. But, a critical analysis regarding the use of enzymes to scavenge NMDAR coagonists may be useful here. Indeed, addition of DAAO (or DsdA) together with glycine oxidase (GlyOx)28,41,66–69, does not abolish NMDAR currents, possibly due to their insufficient activity and/or low affinity, thus preventing the complete removal of all of the co-agonists. BsGO is much less active than DAAO and is normally added at concentrations (in mg/ml) up to 10 times higher than DAAO. However, only the amount of enzyme that actually adsorbs to the slice surface would be active in removing local coagonists. Therefore, the BsGO local activity is likely much lower than DAAO, even when the former is added at higher concentrations (excess enzyme in the bath solution may not increase the amount of enzyme that adsorbs to the slice because of saturation of the binding sites). Perfusing tissues with medium containing an enzymatic scavenger system for extracellular D-serine or glycine does not guarantee by itself that the endogenous coagonist is fully eliminated: this must be verified by an analytical method as reported41,69. The considerations above suggest that quantitative estimates on the contribution of D-serine vs glycine achieved by the scavenging methods can be misleading knowing that in most case both enzymes when applied on slices will not fully remove the coagonists. This may explain in part the large variation and reproducibility problems regarding the relative effects of BsGO in decreasing NMDAR currents.
As an interesting and troubling possibility, perhaps SR−/− mice may also have their limitations since SR in addition to produce D-serine might be also involved in the synthesis of D-aspartate77. Genetic deletion of SR leads to reduced levels of D-serine but also of D-aspartate77,78. Therefore, one can imagine a potential scenario where deletion of SR induces loss of function of both D-serine and D-aspartate at NMDAR.
Another source of possible misinterpretation comes from the use of pharmacological tools to inhibit SR or DAAO (see Box 1). Studies aiming to explore the functions of D-serine in complex system (slices or behaving animals) through the pharmacological inhibition of SR offer a compelling case. Indeed, in vitro screenings have identified that L -erythro hydroxyaspartate (OHAsp), phenazine-ethosulfate (PES) and phenazine-methosulfate (PMS) act as relatively selective and low-potency inhibitors for SR79,80. Subsequently, investigators have used the agents for exploring the roles of D-serine in different physiological processes either in brain slices or in situ in living animals or some animal models of disease. Thus, it has been reported that OHAsp introduced in the astrocytes impaired LTP recorded in hippocampal slices although no rundown in glutamatergic responses was observed52 or reduced NMDA-excitatory postsynaptic currents (EPSCs)-EPSCs recorded at layer 5 pyramidal neurons81, effects that could be fully rescued by adding D-serine back to tissues. When OHAsp was bath applied, it increased locomotor-related activity recorded from the ventral roots of spinal cord preparations from neonatal mice82, an effect opposed to the application of DAAO (commercial source). Using bath application, PES was found to reduce light-evoked NMDAR response in the retina83, or the EPSCs and LTP in the visual cortex84. Application of PES and PMS to neonatal mouse cerebellum slices impedes neuronal migration85 and also decreases respiratory frequency when applied to brainstem slices56. In all these studies, the inhibitory effects of the compounds were counteracted by application of D-serine and were opposed to the enzymatic degradation of D-serine by applied DAAO, just apparently warranting the use of the SR inhibitors. In one case83, investigators also showed that PES decreased the extracellular levels of D-serine, a parameter that may be considered as at least the basic necessary control in pharmacological studies aiming at interfering with the metabolic enzyme of D-serine. Of course, these mentioned studies proposed a significant role of the NMDAR co-agonist despite that the actual real specificity of these compounds remains totally unknown since they were identified as prototype inhibitors of SR only from in silico and in vitro screening assays. However, a simple strategy to resolve this problem would have been to concomitantly test the compounds in SR−/− mice, thus used as negative controls. In an ongoing and yet unpublished work (Lecouflet, Potier, Dutar, Billard and Mothet, manuscript in preparation), we observe that all these compounds have unwanted off-target effects since they display similar dose-dependent inhibitory actions on NMDAR synaptic functions and LTP in both wild-type and SR−/− hippocampal slices whatever the mode of administration (bath applied or injected in a single astrocyte). Notably, application of exogenous D-serine partially rescues the inhibitory effect of the compounds in both slices from WT and SR−/− mice. Although we cannot exclude that different modes and times of application could cause to specific versus non-specific effects, our observations clearly suggest that the SR inhibitor compounds currently available cannot be used to determine the functions of D-serine in complex systems (i.e. brains slices, cells) other than cell-free preparations36 unless controls are performed with SR−/− mice (Figure 2). Here again, we call for a more cautious assessment of recent findings obtained using OHAsp and PES even if measurements on D-serine levels were made. Clearly, there is a need for the development of more specific SR inhibitors86,87. The same caution should be applied to compounds inhibiting DAAO. We encourage investigators to test their efficacity in DAAO−/− mice before going further in their studies. These controls seem to us absolutely necessary since inhibitors of DAAO are already used in pre-clinical studies to treat SCZ and affective disorders88 or for the early-phase of AD89.
Finally, another case of possible misinterpretation originates from the use of the iBot-Glast-CreERT2 and dnSNARE mouse lines65, which were initially generated with the ambition to control SNARE-dependent exocytosis in astrocytes. The first model, the iBot-Glast-CreERT2 mouse line, enables the tamoxifen-inducible, stable expression of the Clostridium botulinum toxin serotype B-light chain (boNT/B). Once expressed, boNT/B cleaves the vesicle-associated membrane synaptobrevin-2 (Sb2), a component of the SNARE complex, which is expressed in astrocytes43,52,57,61,62,65,90 thus impairing vesicular release. The second mouse model, the dnSNARE mouse line, expresses the dominant-negative SNARE domain of the Sb2 protein and GFP in astrocytes under the control of a tetoff tetracycline transactivator system controlled by doxycycline. These two models have been instrumental for studying gliotransmission and the role of glia in brain functions65. Exocytosis of gliotransmitters could be essential to normal brain function as well as in brain diseases but would require vesicles for transmitter storage, the presence of vesicular transporters for accumulating transmitters, and SNARE proteins for membrane fusion. Despite their elegancy, these tools are indirect and have limitations since they interfere with all VAMP3/Sb2-dependent fusion events including constitutive exocytosis which is important for the importation-exportation of proteins at the plasma membrane (i.e., receptors, transporters, exchangers…) and do not solely inhibit the release of gliotransmitters57. Inactivation of the exocytotic machinery in its whole, as achieved in the two mouse lines, most likely leads to off-target effects by rendering the astrocytes totally unable to detect or to adapt to any neuronal inputs. Therefore, astrocytes in these mice are “deaf”, i.e., unable to listen to neurons and in turn, unable to communicate with the latter, whatever the pathway being involved in this dialogue. As an example, cell surface expression and turnover of glycine transporters are regulated by calcium-dependent SNARE mechanisms and would most likely be altered in these transgenic mice91. Therefore, rescue seen with D-serine or any NMDAR ligands is a pure pharmacological effect with poor, if any, physiological significance on the identity of the molecule sustaining the glia-neuronal coupling. Interestingly, it has been reported that the D-serine levels measured with RgDAAO-based amperometric biosensors in hippocampal slices were not affected by astrocytic dnSNARE expression regardless of time of day43,92, an observation in conflict with a previous study40 that reported a clear decline in the levels of D-serine in the hippocampus of the same transgenic mice. Further studies are needed to solve these discrepancies.
Conclusions
There are several important lessons to take away from the example of D-serine (and the other D-AA). This D-AA has emerged as an important signaling molecule with high hopes for more efficient therapies in multiple chronic brain disorders that affect our societies. As a consequence, any scientific publication has the potential to propagate the enthusiasm of the scientific community but also to attract the interest of the general media and the lay public by offering new hopes for treatments. This is a high level of responsibility for each scientist to assess and becomes harder when exploring a new signaling pathway outside of their expertise.
The past years have shown exciting progress in understanding D-serine biology and the coming decade is expected to continue this trend with other D-AA. Thanks to the rapid development of new animal models and the refinement of technical procedures, our knowledge about the roles of D-AAs, and particularly D-serine, in brain function has increased incredibly over the past years. Because it is crucial for science to progress as fast as possible, and since it appears likely that D-AAs are involved in a wide range of neurological and psychiatric human disorders, we encourage the use of appropriate procedures with multiple controls before delivering a message perhaps “vendeur” at first glance but that might direct the community towards dead ends.
Funding:
JPM is supported by CNRS and by Fondation pour la Recherche Medicale team award (Equipe FRM DEQ20150331734). JMB is supported by INSERM and by Fondation France Alzheimer. LP is supported from Fondo di Ateneo per la Ricerca and by Fondazione Comunitaria del Varesotto, 2017. JTC received support from the National Institutes of Health grants R01MH05190 and P50MH0G0450. JVS is supported by the National Institute on Drug Abuse under Award No. P30 DA018310, and the National Institute of Neurological Disorders and Stroke under Award No. R01 NS031609. The funders had no role in the decision to publish or prepare the manuscript.
Abbreviations:
- AD
Alzheimer’s disease
- ASC-1
alanine-serine-cysteine-1
- CE-LIF
capillary electrophoresis with laser-induced fluorescence
- CNS
central nervous system
- DAA
D-amino acid
- DAAO
D-amino acid oxidase
- DsdA
recombinant D-serine deaminase enzyme
- EPSCs
excitatory postsynaptic currents
- FAC
fluoroacetate
- GlyT
glycine transporters
- GMS
glycine modulatory site
- HPLC
high pressure liquid chromatography
- LC
liquid chromatography
- LTD
long term depression
- LTP
long term potentiation
- NMDARs
NMethyl D-aspartate receptors
- OHAsp
L-erythro hydroxyaspartate
- PES
Phenazine-EthoSulfate
- PM
Phenazine-Methosulfate
- SCZ
schizophrenia
- SR
serine racemase
- TCA
tricarboxylic acid
- 3-PGDH
3-phosphoglycerate dehydrogenase
Footnotes
Publisher's Disclaimer: Disclaimer. The views expressed in this article are those of the authors and do not reflect the official policy or position of the University Paris-Saclay, ENS Paris-Saclay & CNRS, or INSERM & Caen University, Harvard University , University of Urbana-Champaign, University of Insubria.
Competing Interests: The authors declare that no competing interests exist.
References
- 1.Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, et al. The presence of free D-serine in rat brain. FEBS Letters. 1992;296: 33–36. doi: 10.1016/0014-5793(92)80397-Y [DOI] [PubMed] [Google Scholar]
- 2.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proceedings of the National Academy of Sciences. 1995;92: 3948–3952. doi: 10.1073/pnas.92.9.3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proceedings of the National Academy of Sciences of the United States of America. 1999;96: 13409–13414. doi: 10.1073/pnas.96.23.13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mothet J-P, Parent AT, Wolosker H, Brady RO, Linden DJ, Ferris CD, et al. D-Serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences. 2000;97: 4926–4931. doi: 10.1073/pnas.97.9.4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325: 529–531. doi: 10.1038/325529a0 [DOI] [PubMed] [Google Scholar]
- 6.Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA receptors expressed in xenopus oocytes. Science. 1988; doi: 10.1126/science.2841759 [DOI] [PubMed] [Google Scholar]
- 7.Pollegioni L, Sacchi S. Metabolism of the neuromodulator D-serine. Cellular and Molecular Life Sciences. 2010. pp. 2387–2404. doi: 10.1007/s00018-010-0307-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sason H, Billard JM, Smith GP, Safory H, Neame S, Kaplan E, et al. Asc-1 transporter regulation of synaptic activity via the tonic release of D-serine in the forebrain. Cerebral cortex (New York, NY : 1991). 2017;27: 1573–1587. doi: 10.1093/cercor/bhv350 [DOI] [PubMed] [Google Scholar]
- 9.Martineau M, Parpura V, Mothet JP. Cell-type specific mechanisms of D-serine uptake and release in the brain. Frontiers in Synaptic Neuroscience. 2014. doi: 10.3389/fnsyn.2014.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan E, Zubedat S, Radzishevsky I, Valenta AC, Rechnitz O, Sason H, et al. ASCT1 (Slc1a4) transporter is a physiologic regulator of brain D-serine and neurodevelopment. Proceedings of the National Academy of Sciences. 2018; doi: 10.1073/pnas.1722677115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, Schmidt CJ, et al. Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. Journal of Physiology. 2004;557: 489–500. doi: 10.1113/jphysiol.2004.063321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elegheert J, Kakegawa W, Clay JE, Shanks NF, Behiels E, Matsuda K, et al. Structural basis for integration of GluD receptors within synaptic organizer complexes. Science. 2016;353: 295–300. doi: 10.1126/science.aae0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, Kohda K, et al. D-Serine regulates cerebellar LTD and motor coordination through the δ2 glutamate receptor. Nature Neuroscience. 2011;14: 603–613. doi: 10.1038/nn.2791 [DOI] [PubMed] [Google Scholar]
- 14.Horn Van MR, Sild M, Ruthazer ES. D-serine as a gliotransmitter and its roles in brain development and disease. Frontiers in Cellular Neuroscience. 2013;7. doi: 10.3389/fncel.2013.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martineau M, Baux G, Mothet JP. D-Serine signalling in the brain: friend and foe. Trends in Neurosciences. 2006. pp. 481–491. doi: 10.1016/j.tins.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 16.Sacchi S, Rosini E, Pollegioni L, Molla G. D-amino acid oxidase inhibitors as a novel class of drugs for schizophrenia therapy. Curr Pharm Des. 2013;19: 2499–2511. doi:CPD-EPUB-20121031–1 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Wolosker H, Balu DT, Coyle JT. The rise and fall of the D-serine-mediated gliotransmission hypothesis. Trends in Neurosciences. 2016. pp. 712–721. doi: 10.1016/j.tins.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolosker H, Balu DT, Coyle JT. Astroglial versus neuronal D-serine: Check your controls! Trends in Neurosciences. 2017. pp. 520–522. doi: 10.1016/j.tins.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papouin T, Henneberger C, Rusakov DA, Oliet SHR. Astroglial versus neuronal D-serine: Fact checking. Trends in Neurosciences. 2017. pp. 517–520. doi: 10.1016/j.tins.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 20.Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281(20):14151–62. [DOI] [PubMed] [Google Scholar]
- 21.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, et al. Serine racemase is predominantly localized in neurons in mouse brain. Journal of Comparative Neurology. 2008;510: 641–654. doi: 10.1002/cne.21822 [DOI] [PubMed] [Google Scholar]
- 22.Ehmsen JT, Ma TM, Sason H, Rosenberg D, Ogo T, Furuya S, et al. D-Serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. Journal of Neuroscience. 2013;33: 12464–12469. doi: 10.1523/JNEUROSCI.4914-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balu DT, Takagi S, Puhl MD, Benneyworth MA, Coyle JT. D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cellular and Molecular Neurobiology. 2014;34: 419–435. doi: 10.1007/s10571-014-0027-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez EJ, Tapanes SA, Loris ZB, Balu DT, Sick TJ, Coyle JT, et al. Enhanced astrocytic D-serine underlies synaptic damage after traumatic brain injury. Journal of Clinical Investigation. 2017;127: 3114–3125. doi: 10.1172/JCI92300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pernot P, Mothet JP, Schuvailo O, Soldatkin A, Pollegioni L, Pilone M, et al. Characterization of a yeast D-amino acid oxidase microbiosensor for D-serine detection in the central nervous system. Analytical Chemistry. 2008;80: 1589–1597. doi: 10.1021/ac702230w [DOI] [PubMed] [Google Scholar]
- 26.Campos-Beltrán D, Konradsson-Geuken Å, Quintero JE, Marshall L. Amperometric self-referencing ceramic based microelectrode arrays for D-serine detection. Biosensors. 2018;8. doi: 10.3390/bios8010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Pietrantonio F, Benetti M, Cannatà D, Verona E, Girasole M, Fosca M, et al. A Shear horizontal surface acoustic wave biosensor for a rapid and specific detection of D-serine. Sensors and Actuators, B: Chemical. 2016;226: 1–6. doi: 10.1016/j.snb.2015.11.099 [DOI] [Google Scholar]
- 28.Le Bail M, Martineau M, Sacchi S, Yatsenko N, Radzishevsky I, Conrod S, et al. Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proceedings of the National Academy of Sciences. 2015;112: E204–E213. doi: 10.1073/pnas.1416668112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyoshi Y, Hamase K, Okamura T, Konno R, Kasai N, Tojo Y, et al. Simultaneous two-dimensional HPLC determination of free D-serine and D-alanine in the brain and periphery of mutant rats lacking D-amino-acid oxidase. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2011;879: 3184–3189. doi: 10.1016/j.jchromb.2010.08.024 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Imanishi N, Mita M, Hamase K, Aiso S, Sasabe J. Heterogeneity of D-serine distribution in the human central nervous system. ASN Neuro. 2017;9. doi: 10.1177/1759091417713905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weatherly CA, Du S, Parpia C, Santos PT, Hartman AL, Armstrong DW. D -Amino Acid Levels in Perfused Mouse Brain Tissue and Blood: A Comparative Study. ACS Chemical Neuroscience. 2017;8. doi: 10.1021/acschemneuro.6b00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karakawa S, Shimbo K, Yamada N, Mizukoshi T, Miyano H, Mita M, et al. Simultaneous analysis of D-alanine, D-aspartic acid, and D-serine using chiral high-performance liquid chromatography-tandem mass spectrometry and its application to the rat plasma and tissues. Journal of Pharmaceutical and Biomedical Analysis. 2015;115: 123–129. doi: 10.1016/j.jpba.2015.05.024 [DOI] [PubMed] [Google Scholar]
- 33.Visser WF, Verhoeven-Duif NM, Ophoff R, Bakker S, Klomp LW, Berger R, et al. A sensitive and simple ultra-high-performance-liquid chromatography-tandem mass spectrometry based method for the quantification of D-amino acids in body fluids. Journal of Chromatography A. 2011;1218: 7130–7136. doi: 10.1016/j.chroma.2011.07.087 [DOI] [PubMed] [Google Scholar]
- 34.Singh NS, Paul RK, Sichler M, Moaddel R, Bernier M, Wainer IW. Capillary electrophoresis-laser-induced fluorescence (CE-LIF) assay for measurement of intracellular D-serine and serine racemase activity. Analytical Biochemistry. 2012;421: 460–466. doi: 10.1016/j.ab.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciriacks CM, Bowser MT. Monitoring D-serine dynamics in the rat brain using online microdialysis-capillary electrophoresis. Analytical Chemistry. 2004;76: 6582–6587. doi: 10.1021/ac0490651 [DOI] [PubMed] [Google Scholar]
- 36.Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, et al. Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. Journal of Neuroscience. 2013;33: 3413–3423. doi: 10.1523/JNEUROSCI.3497-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel AV, Kawai T, Wang L, Rubakhin SS, Sweedler JV. Chiral measurement of aspartate and glutamate in single neurons by large-volume sample stacking capillary electrophoresis. Analytical Chemistry. 2017;89: 12375–12382. doi: 10.1021/acs.analchem.7b03435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scanlan C, Shi T, Hatcher NG, Rubakhin SS, Sweedler JV. Synthesis, accumulation, and release of D-aspartate in the Aplysia californica CNS. Journal of Neurochemistry. 2010;115: 1234–1244. doi: 10.1111/j.1471-4159.2010.07020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miao H, Rubakhin SS, Sweedler JV. Confirmation of peak assignments in capillary electrophoresis using immunoprecipitation: Application to D-aspartate measurements in neurons. Journal of Chromatography A. 2006;1106: 56–60. doi: 10.1016/j.chroma.2005.09.037 [DOI] [PubMed] [Google Scholar]
- 40.Sultan S, Li L, Moss J, Petrelli F, Cassé F, Gebara E, et al. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron. 2015;88: 957–972. doi: 10.1016/j.neuron.2015.10.037 [DOI] [PubMed] [Google Scholar]
- 41.Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, et al. Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cerebral Cortex. 2012;22: 595–606. doi: 10.1093/cercor/bhr130 [DOI] [PubMed] [Google Scholar]
- 42.Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A, et al. Astroglial CB1 receptors determine synaptic D-serine availability to enable recognition memory. Neuron. 2018; doi: 10.1016/j.neuron.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 43.Papouin T, Dunphy JM, Tolman M, Dineley KT, Haydon PG. Septal Cholinergic Neuromodulation Tunes the Astrocyte-Dependent Gating of Hippocampal NMDA Receptors to Wakefulness. Neuron. 2017;94: 840–854.e7. doi: 10.1016/j.neuron.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G. Physiological functions of D-amino acid oxidases: From yeast to humans. Cellular and Molecular Life Sciences. 2007. pp. 1373–1394. doi: 10.1007/s00018-007-6558-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frattini L, Rosini E, Pollegioni L, Pilone MS. Analyzing the D-amino acid content in biological samples by engineered enzymes. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2011;879: 3235–3239. doi: 10.1016/j.jchromb.2011.02.036 [DOI] [PubMed] [Google Scholar]
- 46.Puyal J, Martineau M, Mothet J-P, Nicolas M-T, Raymond J. Changes in D-serine levels and localization during postnatal development of the rat vestibular nuclei. Journal of Comparative Neurology. 2006;497. doi: 10.1002/cne.21016 [DOI] [PubMed] [Google Scholar]
- 47.Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, et al. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100: 6789–94. doi: 10.1073/pnas.1237052100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryu HJ, Kim JE, Yeo SI, Kim DS, Kwon OS, Choi SY, et al. Potential roles of D-serine and serine racemase in experimental temporal lobe epilepsy. Journal of Neuroscience Research. 2010;88: 2469–2482. doi: 10.1002/jnr.22415 [DOI] [PubMed] [Google Scholar]
- 49.Williams SM, Diaz CM, Macnab LT, Sullivan RKP, Pow DV. Immunocytochemical analysis of D-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. GLIA. 2006;53: 401–411. doi: 10.1002/glia.20300 [DOI] [PubMed] [Google Scholar]
- 50.Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. GLIA. 1997;21: 106–113. doi: [DOI] [PubMed] [Google Scholar]
- 51.Peña-Ortega F, Rivera-Angulo AJ, Lorea-Hernández JJ. Pharmacological tools to study the role of astrocytes in neural network functions. Glial cells in health and disease of the CNS Advances in Experimental Medicine and Biology. 2016. pp. 47–66. doi: 10.1007/978-3-319-40764-7 [DOI] [PubMed] [Google Scholar]
- 52.Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463: 232–236. doi: 10.1038/nature08673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrade-Talavera Y, Duque-Feria P, Paulsen O, Rodríguez-Moreno A. Presynaptic spike timing-dependent long-term depression in the mouse hippocampus. Cerebral Cortex. 2016;26: 3637–3654. doi: 10.1093/cercor/bhw172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lefèvre Y, Amadio A, Vincent P, Descheemaeker A, Oliet SHR, Dallel R, et al. Neuropathic pain depends upon D-serine co-activation of spinal NMDA receptors in rats. Neuroscience Letters. 2015;603: 42–47. doi: 10.1016/j.neulet.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 55.Miraucourt LS, Peirs C, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through astrocyte-derived D-serine. Pain. 2011;152: 1340–1348. doi: 10.1016/j.pain.2011.02.021 [DOI] [PubMed] [Google Scholar]
- 56.Beltrán-Castillo S, Olivares MJ, Contreras RA, Zúñiga G, Llona I, Von Bernhardi R, et al. D-serine released by astrocytes in brainstem regulates breathing response to CO2 levels. Nature Communications. 2017;8. doi: 10.1038/s41467-017-00960-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nature Reviews Neuroscience. 2010. pp. 227–238. doi: 10.1038/nrn2803 [DOI] [PubMed] [Google Scholar]
- 58.Wolosker H Serine racemase and the serine shuttle between neurons and astrocytes. Biochimica et Biophysica Acta - Proteins and Proteomics. 2011. pp. 1558–1566. doi: 10.1016/j.bbapap.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 59.Bolaños JP. Bioenergetics and redox adaptations of astrocytes to neuronal activity. Journal of Neurochemistry. 2016;139: 115–125. doi: 10.1111/jnc.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherwood MW, Arizono M, Hisatsune C, Bannai H, Ebisui E, Sherwood JL, et al. Astrocytic IP3Rs: Contribution to Ca2+ signalling and hippocampal LTP. GLIA. 2017;65: 502–513. doi: 10.1002/glia.23107 [DOI] [PubMed] [Google Scholar]
- 61.Mothet J-P, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proceedings of the National Academy of Sciences. 2005;102: 5606–5611. doi: 10.1073/pnas.0408483102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martineau M, Galli T, Baux G, Mothet JP. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. GLIA. 2008;56: 1271–1284. doi: 10.1002/glia.20696 [DOI] [PubMed] [Google Scholar]
- 63.Fiacco TA, McCarthy KD. Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. The Journal of Neuroscience. 2018;38: 3–13. doi: 10.1523/JNEUROSCI.0016-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savtchouk I, Volterra A. Gliotransmission: beyond black-and-white. The Journal of Neuroscience. 2018;38: 14–25. doi: 10.1523/JNEUROSCI.0017-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahlender DA, Savtchouk I, Volterra A. What do we know about gliotransmitter release from astrocytes? Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369: 20130592–20130592. doi: 10.1098/rstb.2013.0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, et al. Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. Journal of Neuroscience. 2013;33: 3533–3544. doi: 10.1523/JNEUROSCI.3836-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150: 633–646. doi: 10.1016/j.cell.2012.06.029 [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Sacchi S, Pollegioni L, Basu AC, Coyle JT, Bolshakov VY. Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nature Communications. 2013;4. doi: 10.1038/ncomms2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125: 775–784. doi: 10.1016/j.cell.2006.02.051 [DOI] [PubMed] [Google Scholar]
- 70.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Molecular Psychiatry. 2009;14: 719–727. doi: 10.1038/mp.2008.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeVito LM, Balu DT, Kanter BR, Lykken C, Basu AC, Coyle JT, et al. Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes, Brain and Behavior. 2011;10: 210–222. doi: 10.1111/j.1601-183X.2010.00656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT. Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol. 2012;32(4):613–24. doi: 10.1007/s10571-012-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kronschläger MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkühler J. Gliogenic LTP spreads widely in nociceptive pathways. Science. 2016;354: 1144–1148. doi: 10.1126/science.aah5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shleper M, Kartvelishvily E, Wolosker H. D-Serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. Journal of Neuroscience. 2005;25: 9413–9417. doi: 10.1523/JNEUROSCI.3190-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turpin F, Dallérac G, Mothet JP. Electrophysiological analysis of the modulation of NMDA-receptors function by D-serine and glycine in the central nervous system. Methods in Molecular Biology. 2012;794: 299–312. doi: 10.1007/978-1-61779-331-8_20 [DOI] [PubMed] [Google Scholar]
- 76.Gustafson EG, Stevens ES, Miller RF. Dynamic regulation of D-serine release in the vertebrate retina. Journal of Physiology. 2015;593: 843–856. doi: 10.1113/jphysiol.2014.283432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ito T, Hayashida M, Kobayashi S, Muto N, Hayashi A, Yoshimura T, et al. Serine racemase is involved in D-aspartate biosynthesis. Journal of Biochemistry. 2016;160: 345–353. doi: 10.1093/jb/mvw043 [DOI] [PubMed] [Google Scholar]
- 78.Horio M, Ishima T, Fujita Y, Inoue R, Mori H, Hashimoto K. Decreased levels of free D-aspartic acid in the forebrain of serine racemase (Srr) knock-out mice. Neurochemistry International. 2013;62: 843–847. doi: 10.1016/j.neuint.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 79.Strisovsky K, Jiráskova J, Mikulová A, Rulíšek L, Konvalinka J. Dual substrate and reaction specificity in mouse serine racemase: Identification of high-affinity dicarboxylate substrate and inhibitors and analysis of the β-eliminase activity. Biochemistry. 2005;44: 13091–13100. doi: 10.1021/bi051201o [DOI] [PubMed] [Google Scholar]
- 80.De Miranda J, Panizzutti R, Foltyn VN, Wolosker H. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proceedings of the National Academy of Sciences of the United States of America. 2002;99: 14542–14547. doi: 10.1073/pnas.222421299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meunier C, Wang N, Yi C, Dallerac G, Ezan P, Koulakoff A, et al. Contribution of astroglial Cx43 hemichannels to the modulation of glutamatergic currents by D-serine in the mouse prefrontal cortex. The Journal of Neuroscience. 2017; 2204–16. doi: 10.1523/JNEUROSCI.2204-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Acton D, Miles GB. Differential regulation of NMDA receptors by D-serine and glycine in mammalian spinal locomotor networks. Journal of Neurophysiology. 2017;117: 1877–1893. doi: 10.1152/jn.00810.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stevens ER, Gustafson EC, Sullivan SJ, Esguerra M, Miller RF. Light-evoked NMDA receptor-mediated currents are reduced by blocking D-serine synthesis in the salamander retina. NeuroReport. 2010;21: 239–244. doi: 10.1097/WNR.0b013e32833313b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meunier CNJ, Dallérac G, Le Roux N, Sacchi S, Levasseur G, Amar M, et al. D-Serine and glycine differentially control neurotransmission during visual cortex critical period. PLoS ONE. 2016;11. doi: 10.1371/journal.pone.0151233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, et al. Serine racemase: Activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proceedings of the National Academy of Sciences. 2005;102: 2105–2110. doi: 10.1073/pnas.0409723102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beato C, Pecchini C, Cocconcelli C, Campanini B, Marchetti M, Pieroni M, et al. Cyclopropane derivatives as potential human serine racemase inhibitors: Unveiling novel insights into a difficult target. Journal of Enzyme Inhibition and Medicinal Chemistry. 2016;31: 645–652. doi: 10.3109/14756366.2015.1057720 [DOI] [PubMed] [Google Scholar]
- 87.Jiraskova-Vanickova J, Ettrich R, Vorlova B, Hoffman HE., Lepsik M, Jansa P, et al. Inhibition of human serine racemase, an emerging target for medicinal chemistry. Current Drug Targets. 2011;12: 1037–1055. doi: 10.2174/138945011795677755 [DOI] [PubMed] [Google Scholar]
- 88.Lane H, Lin C, Green M, Hellemann G, Huang C, Chen P, et al. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA psychiatry. 2013. pp. 1267–1275. doi: 10.1001/jamapsychiatry.2013.2159 [DOI] [PubMed] [Google Scholar]
- 89.Lin C-H, Chen P-K, Chang Y-C, Chuo L-J, Chen Y-S, Tsai GE, et al. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase alzheimer disease: A randomized, double-blind, placebo-controlled trial. Biological Psychiatry. 2014;75: 678–685. doi: 10.1016/j.biopsych.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 90.Verkhratsky A, Matteoli M, Parpura V, Mothet J-P, Zorec R. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. The EMBO Journal. 2016;35: 239–257. doi: 10.15252/embj.201592705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geerlings A, López-Corcuera B, Aragón C. Characterization of the interactions between the glycine transporters GLYT1 and GLYT2 and the SNARE protein syntaxin 1A. FEBS Lett. 2000; 470(1):51–54. [DOI] [PubMed] [Google Scholar]
- 92.Clasadonte J, Dong J, Hines DJ, Haydon PG. Astrocyte control of synaptic NMDA receptors contributes to the progressive development of temporal lobe epilepsy. Proceedings of the National Academy of Sciences. 2013;110: 17540–17545. doi: 10.1073/pnas.1311967110 [DOI] [PMC free article] [PubMed] [Google Scholar]