Abstract
Store-operated calcium entry (SOCE) through Orai channels is triggered by receptor-stimulated depletion of Ca2+ from the ER. Orai1 is unique in terms of its activation mechanism, biophysical properties, and structure, and its precise regulation is essential for human health. Recent studies have begun to reveal the structural basis of the major steps in the SOCE pathway and how the system is reliably suppressed in resting cells but able to respond reliably to ER Ca2+ depletion. In this review we discuss current models describing the activation of ER Ca2+ sensor STIM1, its binding to Orai1, propagation of the binding signal from the channel periphery to the central pore, and the resulting conformational changes underlying opening of the highly Ca2+ selective Orai1 channel.
Introduction
Ca2+ release-activated Ca2+ (CRAC) channels are a unique and nearly ubiquitous class of store-operated Ca2+ channels that open in response to the loss of Ca2+ from the lumen of the ER [1]. They are activated by receptors that release Ca2+ from the ER, typically through the generation of inositol 1,4,5-trisphosphate, and are distinguished by an extremely high Ca2+ selectivity and low single-channel conductance. Their activity is essential for initiating the adaptive immune response, sustaining contractile activity in muscle, blood clotting by platelets, skin and tooth development and many other functions. Tight regulation of store-operated Ca2+ entry (SOCE) is critical, as loss-of-function and gain-of-function mutations in humans create serious health disorders, including severe combined immunodeficiency and autoimmunity, myopathy, ectodermal dysplasia, and Stormorken’s Syndrome [2].
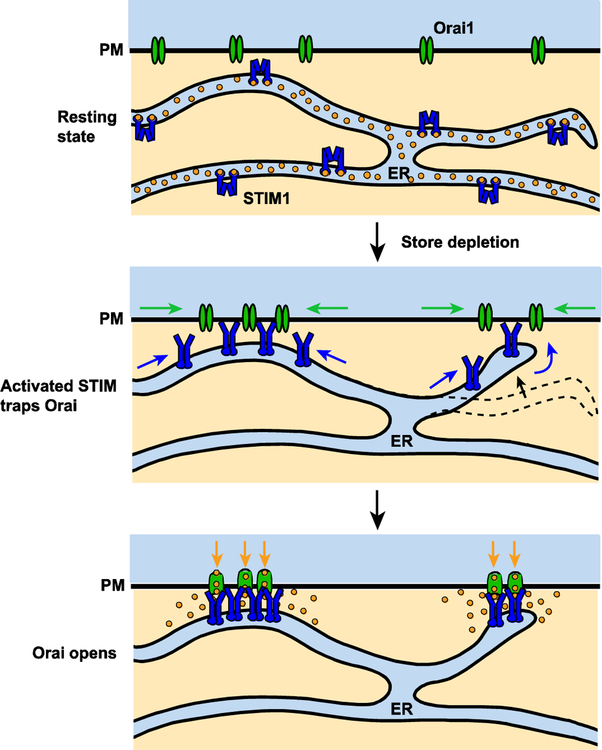
The essential components of SOCE are the STIM family of ER Ca2+ sensors (STIM1 and STIM2 in vertebrates) and the Orai pore-forming channel proteins (Orai1, 2, and 3 in vertebrates). In resting cells STIM1 and Orai1 diffuse independently in the ER and PM, but ER Ca2+ depletion activates STIM1, enabling it to oligomerize and accumulate at ER-plasma membrane junctions where it binds, traps and opens Orai1 channels (Figure 1). In this way, the core machinery of SOCE is assembled on demand through a self-organizing diffusion trap that provides a flexible means of targeting local Ca2+ signals to particular locations in the cell.
Figure 1. An overview of SOCE choreography.
In resting cells with high ER [Ca2+], STIM1 and Orai1 diffuse in the ER and PM, respectively (top). Upon ER Ca2+ depletion, STIM1 becomes activated and accumulates at ER-plasma membrane junctions to bind and trap Orai1 (middle). STIM1 binding opens Orai1 channels and allows extracellular Ca2+ to flow into the cell (bottom).
Recent work has provided new insights into the structural rearrangements of STIM1 that underlie activation, the relation between STIM1 binding and Orai1 channel opening, and how the channel pore may rearrange to allow ion conduction while conferring the extremely high Ca2+ selectivity characteristic of the native CRAC channel. This review emphasizes recent progress in understanding these and other structural aspects of STIM and Orai function. Comprehensive reviews provide more complete background information [1,3].
A bimodal switch selects the quiescent and active states of STIM1
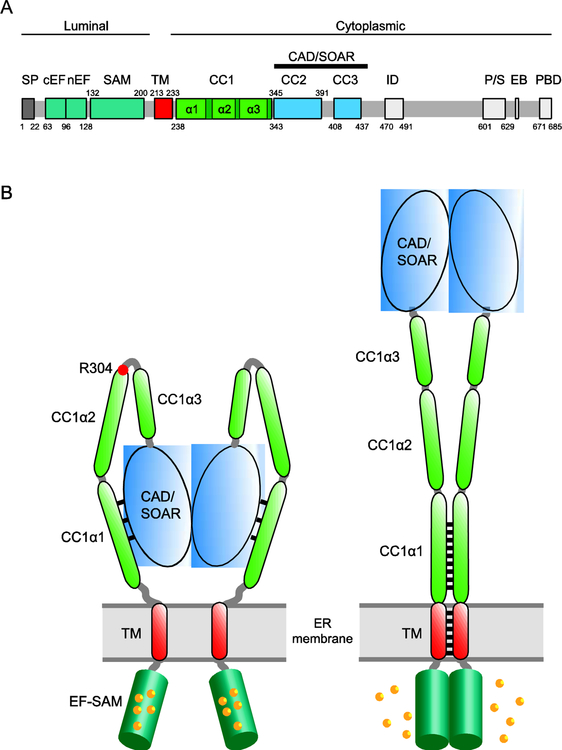
STIM1 activity is controlled primarily by regulating access to a cytosolic domain known as the CRAC activation domain (CAD; [4]) or STIM-Orai activation region (SOAR; [5]) to Orai1 (Figure 2A). In resting cells with ~400 μM [Ca2+]ER [6], Ca2+ is bound to the two luminal EF hands of the STIM1 dimer [7] and to 4–5 additional surface sites in each EF-SAM domain that are energetically coupled to the EF hand [8] (Figure 2B). Each EF hand envelops a helix of its adjacent SAM domain [7], separating the two EF-SAM domains [8,9]. In this state, the cytosolic domain adopts a compact structure in which the CC1 domain interacts with CAD/SOAR, effectively sequestering it near the ER membrane [10–14]. The ER-proximal CC1α1 helix appears to be most directly involved in binding CAD/SOAR, as suggested by the ability of a truncated STIM1 protein containing CC1α1 to capture soluble CAD/SOAR when ER [Ca2+] is high [12] and FRET between ER-anchored CC1α1 and CC3 fragments [15]. The binding interface is not yet defined, but several critical hydrophobic residues in CC1α1 (L248, L251. L258, and L261) and CC3 (L416, V419, and L423) have been proposed to interact through a coiled-coil based on mutagenesis studies [10,12] (Figure 2B). Mutations or deletions of the CC1α3 domain also cause constitutive STIM1 activation [14,16,17], and the amphipathic nature of the helix appears to be important for its ability to regulate STIM1 activity [18]. However, the underlying mechanism, in particular whether CC1α3 interacts directly with CAD/SOAR, is not clear [12,15].
Figure 2. The initial events of STIM1 activation.
A. Domain organization of STIM1. Colored regions indicate structural and functional domains (SP, signal peptide; cEF, canonical EF hand; nEF, noncanonical EF hand; SAM, sterile alpha motif; TM, transmembrane domain; CC1–3, putative coiled-coil domains 1–3; CAD, CRAC activation domain (aa 342–448) or SOAR, STIM-Orai activating region (aa 344–442); ID, inactivation domain; P/S, proline/serine-rich domain; EB, EB1 binding domain; PBD, polybasic domain. B. Cartoon showing STIM1 activation by ER Ca2+ depletion. In the resting state (left), the luminal EF-SAM domains are bound with 5–6 Ca2+ and separated. The cytosolic domain is in a compact conformation in which CC1α1 binds to CAD/SOAR and keeps it close to the ER membrane. Following store depletion (right), Ca2+ release from the EF-SAM domain triggers its dimerization which brings the TM domains together to form a coiled-coil. The rearrangement dissociates CC1α1 from CAD/SOAR to extend the coiled-coil (represented by black lines) and move CAD/SOAR towards the PM. The affinity and number of Ca2+ ions released may help explain the [Ca2+]ER sensitivity and high cooperativity of STIM1 and Orai1 activation in vivo (K1/2 ~200 μM, Hill coefficient of 4–8; [6,60]).
Following store depletion, Ca2+ unbinds from the EF hands and the additional luminal binding sites with an overall K1/2 of ~200 μM, allowing the two EF-SAM domains to dimerize [8,9,19] and bring together the two TM domains to form a coiled-coil [20] (Figure 2B). This rearrangement releases the two CC1α1 domains from CAD/SOAR, which then associate to extend the coiled-coil beyond the TM domains [20]. In this way, the CC1α1 domain serves a dual function: it pairs with CAD/SOAR to stabilize the quiescent state and dimerizes after store depletion to release and move it towards Orai1 in the active state. A critical requirement noted by Hirve et al. [20] is to balance the two conformations so that the quiescent and active forms are stable yet interchangeable. This balance may be established by an imperfect heptad repeat and several sentinel residues (N234, S237, D247) in the proximal part of CC1α1, which are thought to destabilize the coiled-coil enough to make it unfavorable under resting conditions when the TM domains are held apart, but allow it to form after the EF-SAM domains dimerize and the TM domains associate following Ca2+ release [20].
Whether the CC1α2 and CC1α3 domains also pair via coiled-coil interactions is not known, but they are likely to coordinate with CC1α1 during STIM1 activation. The R304W mutation activates STIM1 and causes Stormorken syndrome [2]. Located just before the linker region between CC1α2 and CC1α3, the R304W mutation appears to extend the helical domain through the linker, straightening this region and driving CC1α1 to unbind from CAD/SOAR [21] (Figure 2B). If a similar structural change occurs during activation of WT STIM1 by store depletion, extension of CC1α2 and CC1α3 would complement the pairing of CC1α1’s to promote CAD/SOAR release and could move it towards the PM by as much as ~15 nm, a truly dramatic conformational switch.
CRAC channel structure and STIM1-Orai1 binding
The first crystal structure of the Drosophila Orai (dOrai) channel was a critical breakthrough in the field, demonstrating a hexameric arrangement of 4-TM subunits [22] that countered the prevailing non-structural evidence for a tetramer (reviewed in [1,23]) (Figure 3A, B). The hexameric stoichiometry was tested functionally through electrophysiological studies of hexameric Orai1 concatemers [24,25]. Importantly, the pore properties of hexameric concatemers, including Ca2+ blocking affinity, unitary conductance, and the Cs+/Na+ permeability ratio all matched those of native CRAC channels [25]. Because these properties are determined by the local geometry of the pore helices (which would be quite different for tetrameric and hexameric configurations), the electrophysiological data strongly imply that the native CRAC channel functions as a hexamer of Orai1 subunits.
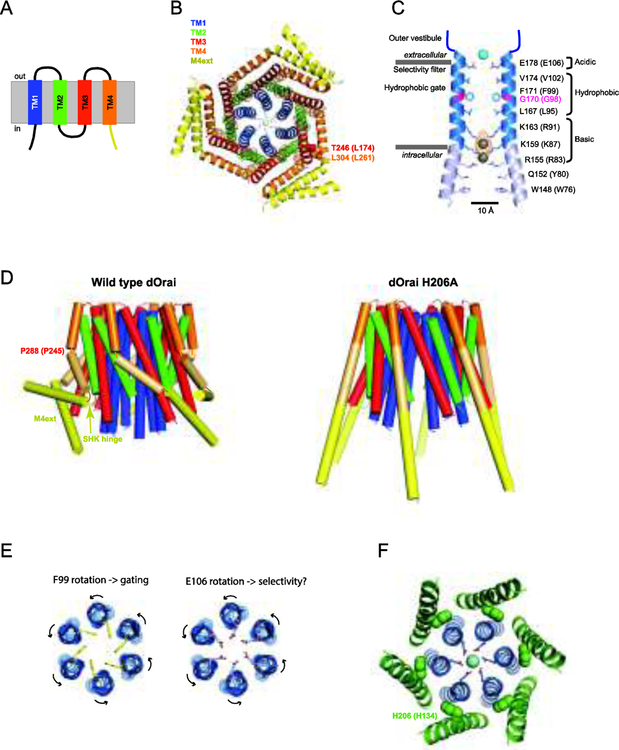
Figure 3. Structural aspects of Orai function.
A. Transmembrane topology of Orai1 showing 4 TM helices and the M4ext (yellow). B. Crystal structure of dOrai as viewed from the cytosolic side (4HKR.pdb). The closed channel shows six TM1 helices lining the pore, interlocking TM2 and TM3 helices, and peripheral TM4 helices ending in crossed M4ext pairs in the cytosol. C. A side view of two TM1 helices lining the dOrai pore. Pore-lining residues of dOrai are shown (human Orai1 equivalents in parentheses). Ba2+ is shown above the selectivity filter, and the anion density is shown in yellow with Fe atoms modeled into the structure of the inner pore. Adapted from [22]. D. Side view structures of WT and H206A dOrai channels. In the open mutant (right), the bends at P288 (human P245) and the SHK hinge straighten, separating the crossed M4ext pairs and allowing dilation of the inner pore (4HKR.pdb, 6BBF.pdb). E. (left) Top view of TM1 helices showing opening of the hydrophobic dOrai gate through rotation of F99 side chains out of the pore lumen. (right) A similar rotation of the selectivity filter at E106 may account for increased Ca2+ selectivity from STIM1 binding. F. Top view of dOrai TM1 (blue) and TM2 (green) helices displaying the close proximity of H206 (human H134) side chains in TM2 to the TM1 pore helix.
As predicted from earlier cysteine-scanning studies [26,27], the Orai channel structure shows that the six TM1s line the entire ion permeation pathway, comprising several domains in series: the selectivity filter, a 15 Å-long hydrophobic region and a 15 Å-long basic region (Figure 3C). The pore is surrounded by a shell of interlocking TM2 and TM3 helices and TM4s at the perimeter of the channel (Figure 3B). P245 creates a kink in TM4 which enables adjacent cytosolic M4 extensions (M4ext) to bend in opposite directions and interact through antiparallel coiled-coils, resulting in three crossed helical pairs (Figure 3B and3D, left). The M4 extensions are widely accepted to be the major binding site for STIM1, as they bind STIM1 in vitro, and deletion and mutagenesis show they are required for STIM1 binding, puncta formation, and Orai1 activation in vivo [1]. Human STIM1 does not bind the Orai1 2–3 loop in vitro, although binding has been detected in C. elegans which appears to utilize a somewhat different activation mechanism [28]. Binding to the N-terminus and its functional significance has been debated. The cytoplasmic extension of TM1 (aa 73–91) is required for STIM1-mediated CRAC channel activity and isolated fragments bind weakly to STIM1 and CAD/SOAR [4,29–31], but recent reports reveal it is also required to support constitutive activity of mutant Orai1 channels in the absence of STIM1 [32,33]. While these results do not rule out binding to STIM1, a simple interpretation is that channel opening requires the N terminus to interact with other parts of Orai1, possibly the 2–3 loop [34].
Orai1 opening is a remarkably steep function of STIM1 binding. Inhibition of STIM1 binding to just a single M4ext carrying an L273D mutation reduces the open probability to <10% of the WT channel [35]. In addition, incomplete STIM1 binding alters the pore properties [36]; the single L273D mutation triples the unitary conductance while reducing Ca2+ block affinity and selectivity for Na+ over Cs+ [35]. These dramatic effects imply that STIM1 binding to all six Orai1 subunits is required not only to effectively open the channel gate but also to properly configure the pore of the native CRAC channel to achieve its normal conduction and selectivity.
Two general models have been proposed to describe STIM1 binding to the Orai1 C terminus. In the dimeric model, each STIM1 dimer engages a pair of adjacent M4 extensions; this proposal was originally based on the NMR solution structure of a complex of STIM1 and Orai1 fragments, in which the CC2 domains of the CAD/SOAR dimer fold to create a pair of binding pockets for two crossed, antiparallel M4 extensions [37]. That STIM1 interacts with pairs of Orai1 subunits is supported to some extent by the ability of a mutant L273D Orai1 subunit deficient in STIM1 binding to enhance STIM1-Orai1 FRET when located next to a WT subunit [35]. However, there is as yet no direct evidence for simultaneous binding of STIM1 or CAD/SOAR dimers to adjacent Orai1 subunits, and a recent open-channel dOrai structure [38] (discussed below) is not easily reconciled with the antiparallel M4 extensions of the dimeric STIM-Orai complex described by NMR [37].
Other studies support a monomeric binding model in which each STIM1 dimer engages only one Orai1 C terminus, through one of its two subunits. This model is based on the ability of CAD/SOAR dimers with one non-binding F394H subunit to bind and activate Orai1 to the same extent as WT CAD/SOAR [39]. Interestingly, the free CAD/SOAR subunit would help explain how CAD/SOAR expression crosslinks channels into clusters and slows Orai1 diffusion in the PM [4,40]. Crosslinking could also account for the preferred ~15-nm spacing of Orai1 particles at ER-PM junctions [41], approximating the dimensions of two channels separated by a CAD/SOAR dimer [40]. It is worth noting that the monomeric and dimeric models need not be mutually exclusive, if they represent different stages of the activation process (as in a sequential binding mechanism [42]), or if the NMR structure [37] engages two channels rather than adjacent subunits on the same channel. A full resolution to the question of how STIM1 binds to Orai1 awaits a structural description of the full-length STIM1-Orai1 complex, a particularly challenging goal given that the proteins are flexible, reside in separate membranes, and interact with low affinity.
From STIM1 binding to Orai1 opening
Mutagenesis and the closed dOrai structure have offered important new insights into how STIM1 binding at the channel periphery is allosterically transmitted through the TM2-TM3 shell to the TM1 pore and gate. Orai1 mutations at over 20 locations, predominantly in TM2-TM4, constitutively activate the channel [32,33,36,43–47]. These findings suggest that the resting closed state is stabilized by multiple interactions between the TM domains and that the energy barrier to opening is quite low [32]. While most of constitutively active mutants are non-selective, several mutations produce highly Ca2+-selective channels without STIM1 by interfering with interhelical contacts. Replacing 261LVSHK265 with ANSGA in the M4ext is likely to disrupt hydrophobic contacts between M4 and M3 [33] (Figure 3B), while P245L in TM4 [44] may break the same contacts by straightening a kink in the TM4 helix and disrupting the coiled-coil arrangement of M4ext helices [38] (Figure 3D). H134 mutations in TM2 that activate Orai1 [32,47] disrupt a contact between TM2 and TM1 within a region of alternating stripes of interacting polar and hydrophobic residues that appears critical for gating and selectivity of Orai1 [32] (Figure 3F). Above this location and lateral to the hydrophobic pore region, a tightly packed hydrophobic stack of interacting TM1, TM2 and TM3 residues is thought to transmit force from the TM2/TM3 ring to the pore gate, based on the inhibitory effects of alanine substitutions [32]. In addition, as noted above the cytoplasmic extension of TM1 is likely to interact with other parts of Orai1 during channel activation. The ways in which the forces from STIM1 binding are funnelled from the periphery to the pore are clearly multifaceted and an exciting focus for further research.
Two regions have been implicated in Orai gating: the hydrophobic region near the extracellular end of the pore [29,36,43], and the more interior polybasic region [22] (Figure 3C). Several models have recently been proposed for the Orai1 open state that involve conformational changes in these regions [38,43,47]. In the pore rotation model, hydrophobic side chains of V102 and F99 extending into the pore near the outer mouth of the channel create a barrier to water entry and ion permeation in the closed state [36,43]. STIM1 binding changes the pore accessibility of cysteines introduced at F99 or G98 in opposite ways, consistent with a local ~20o rotation of TM1 helices that moves the F99 side chains out of the lumen [43] (Figure 3E, left). Molecular dynamics simulations illustrate how this reduction of pore hydrophobicity would lower the energy barrier for water entry to allow Ca2+ to enter the pore [43]. Interestingly, a coupled rotation of F99 and the selectivity filter at E106 (two helical turns above F99) could serve to configure the Ca2+ binding site to optimize selectivity (Figure 3E, right), helping to explain the tight coupling of gating and ion selectivity in CRAC channels.
A second gating model based on dilation of the polybasic pore region is based on the crystal structure of a constitutively active dOrai mutant. As discussed above, the H134A mutation in human Orai1 opens the channel while retaining almost normal Ca2+ selectivity in the absence of STIM1 [32,47]. Comparison of the homologous dOrai H206A structure with that of the closed WT dOrai revealed two major changes. First, the M4ext helices are fully extended rather than crossed in paired coiled-coil interactions (Figure 3D). Second, the inner pore of the H206A channel is highly dilated (by ~ 10 Å) due to a rigid body rotation of all four TM helices away from the pore axis, which would remove a conduction block imposed by anion binding seen between the three rings of closely apposed basic residues in the closed state (Figure 3C, D). Iodide localized in the open pore structure suggests that free intracellular anions may ease the passage of Ca2+ through this region by screening positive charges lining the inner pore [38,48]. A second closed-state structure with straightened M4 extensions showed that breaking the M4ext coiled-coils is not sufficient for opening, but importantly is required to allow enough room for the TM helices to move outward and reach the open state. Hou et al [38] suggest that the crossed M4ext helices serve as latches and enable M4-M3 interactions to stabilize the closed state, and these must be released before STIM1 can bind and open the channel. Interestingly, the latched configuration requires the helical bend at P288 (human P245) and the SHK hinge (Figure 3D), which may help to explain the activating effects of mutations at these locations [38] (see above). A third gating model based on MD simulations and cysteine crosslinking of the H134A Orai1 mutant suggests that gating involves a more modest dilation of both the hydrophobic and basic sections of the pore, and a rotation of R91 side chains toward the pore perimeter [47].
How can these several gating models be reconciled? While the dOrai H206A structure lacks sufficient resolution to localize side chains and permit detailed comparisons, some aspects of the models are compatible. For example, Yeung et al [32] reported rotation of F99 out of the pore in the H134A mutant hOrai1 channel, along with a small (~2 A) pore dilation in this region, and Hou et al [38] observed a downward shift in the location of Ba2+ bound to the selectivity filter in the open channel, which could result from dilation and/or rotation at this site. However, the large pore dilation observed in the dOrai H206A structure is unique, and it is important to consider whether it accurately mimics the physiological STIM1-bound open state. Multiple factors could exaggerate the dilation, including the absence of bound STIM1 combined with stabilization of fully extended M4ext helices by contacts in the crystal lattice. An atomic-resolution structure of the full length STIM1-Orai1 complex will be indispensable in fully resolving the native open state of Orai1.
Beyond activation: negative regulation of SOCE
CRAC channels are also negatively regulated in several significant ways to control the extent of Ca2+ influx. Alternative splicing of STIM2 inserts 8 residues into the CC2 of CAD/SOAR, transforming STIM2 from an activator into an inhibitor of Orai1 [49,50]. Biochemical studies thus far show that the insert reduces the helicity and enhances the exposed hydrophobicity of CAD/SOAR, which may underlie its inhibitory action [51]. Redox regulation of Orai1 has been traced to oxidation of C195 at the extracellular end of TM3 [52], which has been proposed to hydrogen bond with S239 at the top of TM4 to prevent channel opening [53]. Fast Ca2+-dependent inactivation (CDI) of Orai1 depends on functional interactions of an acidic inactivation domain in STIM1 [54–56] with W76 in the cytosolic extension of the pore [57], raising the possibility of STIM1-TM1 interaction. For slow CDI [58], recent evidence suggests that Ca2+-calmodulin binds to the C-terminal end of CC2 in CAD (L390, F391), to displace STIM1 from Orai1 and allow channel closure [59]. These several modes of CRAC channel regulation have not been as extensively studied as activation, and direct structural studies will undoubtedly help clarify their mechanisms.
Conclusions/Future Perspectives
Precise regulation of STIM1 and Orai1 activity is critical for human health, and the bistability of STIM1 activation, multi-site stabilization of the Orai1 closed state, nonlinear activation of Orai by STIM1 binding, and negative regulation all contribute to the fine control of SOCE. While the physical bases of these mechanisms are beginning to emerge, there are as yet very few atomic-level structures of STIM1 and Orai1 in their various states individually or in combination, and definitive solutions to many of these mechanistic questions will ultimately require more extensive and direct structural information. A central goal is to delineate the complete sequence of conformational changes that drive STIM1 activation as well as its binding and activation of Orai1. In this regard, structures of the full-length STIM1-Orai1 complex will be particularly informative, in describing the clustered arrangement of STIM1 around the channel, its binding interface with Orai1, and the physiologically relevant open state of the channel. One additional hope is that detailed structural information will reveal intermediate states as potential druggable targets for treating a variety of SOCE-associated diseases.
Acknowledgements
Work in the authors’ laboratory is supported by the National Institutes of Health [grant R37 GM45374].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The author declares no conflict of interest.
References and recommended reading
- 1.Prakriya M, Lewis RS: Store-operated calcium channels. Physiol Rev 2015, 95:1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacruz RS, Feske S: Diseases caused by mutations in ORAI1 and STIM1. Ann N Y Acad Sci 2015, 1356:45–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen NT, Han W, Cao W-M, Wang Y, Wen S, Huang Y, Li M, Du L, Zhou Y: Store-operated calcium entry mediated by ORAI and STIM. Compr Physiol 2018, 8:981–1002. [DOI] [PubMed] [Google Scholar]
- 4.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS: STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 2009, 136:876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan J, Zeng W, Dorwart M, Choi Y, Worley P, Muallem S: SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 2009, 11:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS: Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 2008, 454:538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stathopulos PB, Zheng L, Li G-Y, Plevin MJ, Ikura M: Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 2008, 135:110–122. [DOI] [PubMed] [Google Scholar]
- *8.Gudlur A, Zeraik AE, Hirve N, Rajanikanth V, Bobkov AA, Ma G, Zheng S, Wang Y, Zhou Y, Komives EA, et al. : Calcium sensing by the STIM1 ERluminal domain. Nat Commun 2018, 9:4536.The STIM1 EF-SAM domain was attached to the dimerizing GrpE protein to allow the first studies of the EF-SAM domain in a dimeric form. The authors discovered additional Ca2+ binding sites in the STIM1 luminal domain that are energetically coupled to the EF hand and may explain the low overall Ca2+ affinity of the luminal domain and the highly cooperative dependence of STIM1 activation on [Ca2+]ER [6,60]
- 9.Stathopulos P, Li G, Plevin M, Ames J, Ikura M: Stored Ca2+ depletion-induced oligomerization of STIM1 via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem 2006, 281:35855–35862. [DOI] [PubMed] [Google Scholar]
- 10.Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, et al. : STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J 2011, 30:1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos PB, Ikura M, Rao A, Hogan PG: Initial activation of STIM1, the regulator of store-operated calcium entry. Nat Struct Mol Biol 2013, 20:973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma G, Wei M, He L, Liu C, Wu B, Zhang SL, Jing J, Liang X, Senes A, Tan P, et al. : Inside-out Ca2+ signalling prompted by STIM1 conformational switch. Nat Commun 2015, 6:7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma G, Zheng S, Ke Y, Zhou L, He L, Huang Y, Wang Y, Zhou Y: Molecular determinants for STIM1 activation during store-operated Ca2+ entry. Curr Mol Med 2017, 17:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korzeniowski MK, Manjarrés IM, Varnai P, Balla T: Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal 2010, 3:ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahrner M, Muik M, Schindl R, Butorac C, Stathopulos P, Zheng L, Jardin I, Ikura M, Romanin C: A coiled-coil clamp controls both conformation and clustering of stromal interaction molecule 1 (STIM1). J Biol Chem 2014, 289:33231–33244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Jin H, Cai X, Li S, Shen Y: Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci U S A 2012, 109:5657–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Zhang H, Zhang M, Deng Y, Wang H, Lu J, Xu T, Xu P: An aromatic amino acid in the coiled-coil 1 domain plays a crucial role in the auto-inhibitory mechanism of STIM1. Biochem J 2013, 454:401–409. [DOI] [PubMed] [Google Scholar]
- 18.Yu F, Sun L, Hubrack S, Selvaraj S, Machaca K: Intramolecular shielding maintains the ER Ca2+ sensor STIM1 in an inactive conformation. J Cell Sci 2013, 126:2401–2410. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa Y, Teraguchi S, Ikegami T, Dagliyan O, Jin L, Hall D, Dokholyan NV, Namba K, Akira S, Kurosaki T, et al. : Intrinsic disorder mediates cooperative signal transduction in STIM1. J Mol Biol 2014, 426:2082–2097. [DOI] [PubMed] [Google Scholar]
- **20.Hirve N, Rajanikanth V, Hogan PG, Gudlur A: Coiled-coil formation conveys a STIM1 signal from ER lumen to cytoplasm. Cell Rep 2018, 22:72–83.This study describes a critical early event in STIM activation in which the TM domains associate and adopt a coiled-coil structure which propagates through the ER-proximal CC1α domain of STIM1. Conserved structural features (imperfect heptad repeat and sentinel residues in CC1) help explain the readily switchable CC1 ‘brake’ on STIM1 activation.
- 21.Fahrner M, Stadlbauer M, Muik M, Rathner P, Stathopulos P, Ikura M, Müller N, Romanin C: A dual mechanism promotes switching of the Stormorken STIM1 R304W mutant into the activated state. Nat Commun 2018, 9:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou X, Pedi L, Diver MM, Long SB: Crystal structure of the calcium release-activated calcium channel Orai. Science (80- ) 2012, 338:1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen M, Lewis RS: Numbers count: how STIM and Orai stoichiometry affect store-operated calcium entry. Cell Calcium [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai X, Zhou Y, Nwokonko RM, Loktionova NA, Wang X, Xin P, Trebak M, Wang Y, Gill DL: The Orai1 store-operated calcium channel functions as a hexamer. J Biol Chem 2016, 291:25764–25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen M, Lokteva LA, Lewis RS: Functional analysis of Orai1 concatemers supports a hexameric stoichiometry for the CRAC channel. Biophys J 2016, 111:1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNally BA, Yamashita M, Engh A, Prakriya M: Structural determinants of ion permeation in CRAC channels. Proc Natl Acad Sci U S A 2009, 106:22516–22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Ramachandran S, Oh-hora M, Rao A, Hogan PG: Pore architecture of the ORAI1 store-operated calcium channel. Proc Natl Acad Sci USA 2010, 107:4896–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KM, Wijerathne T, Hur J-H, Kang UJ, Kim IH, Kweon YC, Lee AR, Jeong SJ, Lee SK, Lee YY, et al. : Distinct gating mechanism of SOC channel involving STIM-Orai coupling and an intramolecular interaction of Orai in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2018, 115:E4623–E4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudlur A, Quintana A, Zhou Y, Hirve N, Mahapatra S, Hogan PG: STIM1 triggers a gating rearrangement at the extracellular mouth of the ORAI1 channel. Nat Commun 2014, 5:5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lis A, Zierler S, Peinelt C, Fleig A, Penner R: A single lysine in the N-terminal region of store-operated channels is critical for STIM1-mediated gating. J Gen Physiol 2010, 136:673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derler I, Plenk P, Fahrner M, Muik M, Jardin I, Schindl R, Gruber HJ, Groschner K, Romanin C: The extended transmembrane Orai1 N-terminal (ETON) region combines binding interface and gate for Orai1 activation by STIM1. J Biol Chem 2013, 288:29025–29034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Yeung PS-W, Yamashita M, Ing CE, Pomès R, Freymann DM, Prakriya M: Mapping the functional anatomy of Orai1 transmembrane domains for CRAC channel gating. Proc Natl Acad Sci U S A 2018, 115:E5193–E5202.Through extensive structure-function studies the authors identify multiple interactions between TM helices that keep Orai1 closed in the resting state, and sets of hydrophobic and alternating ridges of polar and hydrophobic residues that are involved in transmitting the STIM1 gating signal to the pore. The also show that H134S opens the pore in a similar manner as STIM1 binding (through pore rotation), and that it may function as a steric brake to prevent activation in the absence of STIM1.
- *33.Zhou Y, Cai X, Loktionova NA, Wang X, Nwokonko RM, Wang X, Wang Y, Rothberg BS, Trebak M, Gill DL: The STIM1-binding site nexus remotely controls Orai1 channel gating. Nat Commun 2016, 7:13725.This study of a constitutively active Orai1 ANSGA mutant provides functional evidence for a physical link between TM4 and TM3 in the region of the SHK hinge that are likely to couple STIM1 binding to conformational changes in the channel ultimately leading to activation gating. The authors also show a STIM1-independent role of the N terminus in channel opening, indicating a structural role for the N terminus in gating.
- 34.Fahrner M, Pandey SK, Muik M, Traxler L, Butorac C, Stadlbauer M, Zayats V, Krizova A, Plenk P, Frischauf I, et al. : Communication between N terminus and loop2 tunes Orai activation. J Biol Chem 2018, 293:1271–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Yen M, Lewis RS: Physiological CRAC channel activation and pore properties require STIM1 binding to all six Orai1 subunits. J Gen Physiol 2018, 150:1373–1385.Electrophysiological analysis of L273D mutations introduced into a hexameric Orai1 concatemer revealed an unexpectedly high sensitivity of Orai1 channel activation and ion permeation properties to the number of functional STIM1 binding sites. A single mutatation reduces the open probability by ~90%, while greatly altering permeation properties. The results imply that STIM1 normally binds to all six subunits of native CRAC channels to activate the channel and confer its characteristic pore properties.
- 36.McNally BA, Somasundaram A, Yamashita M, Prakriya M: Gated regulation of CRAC channel ion selectivity by STIM1. Nature 2012, 482:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi-Seabrook GM, Muik M, Romanin C, Ikura M: STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat Commun 2013, 4:2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **38.Hou X, Burstein SR, Long SB: Structures reveal opening of the store-operated calcium channel Orai. Elife 2018, 7.This paper presents the first open pore structure of Orai using the constitutively active H206A dOrai mutant (equivalent to human H134A). The open channel shows a surprisingly large dilation of the inner pore that is only possible if the M4ext helices are not crossed. They also show that the extended M4 structure is not sufficient for opening, and propose a novel activation mechanism in which the crossed M4ext’s serve as latches to keep the resting channel closed, and must uncross to allow STIM1 binding and pore opening.
- 39.Zhou Y, Wang X, Wang X, Loktionova NA, Cai X, Nwokonko RM, Vrana E, Wang Y, Rothberg BS, Gill DL: STIM1 dimers undergo unimolecular coupling to activate Orai1 channels. Nat Commun 2015, 6:8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Nwokonko RM, Cai X, Loktionova NA, Abdulqadir R, Xin P, Niemeyer BA, Wang Y, Trebak M, Gill DL: Cross-linking of Orai1 channels by STIM proteins. Proc Natl Acad Sci U S A 2018, 115:E3398–E3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perni S, Dynes JL, Yeromin AV, Cahalan MD, Franzini-Armstrong C: Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry. Proc Natl Acad Sci U S A 2015, 112:E5533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palty R, Fu Z, Isacoff EY: Sequential steps of CRAC channel activation. Cell Rep 2017, 19:1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.Yamashita M, Yeung PS-W, Ing CE, McNally BA, Pomès R, Prakriya M: STIM1 activates CRAC channels through rotation of the pore helix to open a hydrophobic gate. Nat Commun 2017, 8:14512.Cysteine accessibility and molecular dynamics were used to describe a hydrophobic gate in Orai1, in which a ~20o rotation of the outer pore helix moves F99 side chains out of the pore to allow water entry and Ca2+ permeation. This mechanism has important implications for how STIM1 not only opens Orai1 but also establishes its signature high Ca2+ selectivity.
- 44.Palty R, Stanley C, Isacoff EY: Critical role for Orai1 C-terminal domain and TM4 in CRAC channel gating. Cell Res 2015, 25:963–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang SL, Yeromin AV, Hu J, Amcheslavsky A, Zheng H, Cahalan MD: Mutations in Orai1 transmembrane segment 1 cause STIM1-independent activation of Orai1 channels at glycine 98 and channel closure at arginine 91. Proc Natl Acad Sci U S A 2011, 108:17838–17843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srikanth S, Yee M-KW, Gwack Y, Ribalet B: The third transmembrane segment of Orai1 protein modulates Ca2+ release-activated Ca2+ (CRAC) channel gating and permeation properties. J Biol Chem 2011, 286:35318–35328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frischauf I, Litviňuková M, Schober R, Zayats V, Svobodová B, Bonhenry D, Lunz V, Cappello S, Tociu L, Reha D, et al. : Transmembrane helix connectivity in Orai1 controls two gates for calcium-dependent transcription. Sci Signal 2017, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong H, Fiorin G, Carnevale V, Treptow W, Klein ML: Pore waters regulate ion permeation in a calcium release-activated calcium channel. Proc Natl Acad Sci U S A 2013, 110:17332–17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miederer A-M, Alansary D, Schwär G, Lee P-H, Jung M, Helms V, Niemeyer BA: A STIM2 splice variant negatively regulates store-operated calcium entry. Nat Commun 2015, 6:6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rana A, Yen M, Sadaghiani AM, Malmersjö S, Park CY, Dolmetsch RE, Lewis RS: Alternative splicing converts STIM2 from an activator to an inhibitor of store-operated calcium channels. J Cell Biol 2015, 209:653–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung S, Zhang M, Stathopulos PB: The 2β splice variation alters the structure and function of the stromal interaction molecule coiled-coil domains. Int J Mol Sci 2018, 19:3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, et al. : Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci Signal 2010, 3:ra24. [DOI] [PubMed] [Google Scholar]
- 53.Alansary D, Schmidt B, Dörr K, Bogeski I, Rieger H, Kless A, Niemeyer BA: Thiol dependent intramolecular locking of Orai1 channels. Sci Rep 2016, 6:33347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mullins FM, Park CY, Dolmetsch RE, Lewis RS: STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci 2009, 106:15495–15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S: Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc Natl Acad Sci USA 2009, 106:14687–14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derler I, Fahrner M, Muik M, Lackner B, Schindl R, Groschner K, Romanin C: A Ca2+ release-activated Ca2+ (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2+-dependent inactivation of ORAI1 channels. J Biol Chem 2009, 284:24933–24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullins FM, Lewis RS: The inactivation domain of STIM1 is functionally coupled with the Orai1 pore to enable Ca2+-dependent inactivation. J Gen Physiol 2016, 147:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zweifach A, Lewis RS: Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and -independent mechanisms. J Biol Chem 1995, 270:14445–14451. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Wu G, Yang Y, Fu S, Liu X, Kang H, Yang X, Su X-C, Shen Y: Calmodulin dissociates the STIM1-Orai1 complex and STIM1 oligomers. Nat Commun 2017, 8:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandman O, Liou J, Park W, Meyer T: STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 2007, 131:1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]