Abstract
Aim:
Chronic high salt intake exaggerates renal injury and inflammation, especially with the loss of functional ETB receptors. Tauroursodeoxycholic acid (TUDCA) is a chemical chaperone and bile salt that is approved for the treatment of hepatic diseases. Our aim was to determine whether TUDCA is reno-protective in a model of ETB receptor deficiency with chronic high salt-induced renal injury and inflammation.
Methods:
ETB-deficient and transgenic control rats were placed on normal (0.8% NaCl) or high salt (8% NaCl) diet for 3 weeks, receiving TUDCA (400 mg/kg/day; i.p.) or vehicle. Histological and biochemical markers of kidney injury, renal cell death and renal inflammation were assessed.
Results:
In ETB-deficient rats, high salt diet significantly increased glomerular and proximal tubular histological injury, proteinuria, albuminuria, excretion of tubular injury markers KIM-1 and NGAL, renal cortical cell death and renal CD4+ T cell numbers. TUDCA treatment increased proximal tubule megalin expression as well as prevented high salt diet-induced glomerular and tubular damage in ETB-deficient rats, as indicated by reduced kidney injury markers, decreased glomerular permeability and proximal tubule brush border restoration, as well as reduced renal inflammation. However, TUDCA had no significant effect on blood pressure.
Conclusions:
TUDCA protects against the development of glomerular and proximal tubular damage, decreases renal cell death, and inflammation in the renal cortex in rats with ETB receptor dysfunction on a chronic high salt diet. These results highlight the potential use of TUDCA as a preventive tool against chronic high salt induced renal damage.
Keywords: high salt diet, ETB receptors, renal injury, cell death, CD4+ T cells
Introduction
Endothelin-1 (ET-1) is an endogenous 21 amino acid peptide that acts through two G protein-coupled receptors (ETA and ETB receptors) that bind ET-1 with the same affinity. Over-activation of ETA receptors promotes vasoconstriction and hypertension, renal inflammation, fibrosis and hypertrophy. ETB receptors in the pulmonary endothelium act as clearance receptors to maintain low circulating ET-1 levels. In the renal vasculature, the majority of ETB receptors are localized to the endothelial and smooth muscle cells of the efferent arteriole, whereas ETA receptors are predominant in afferent arteriole vascular smooth muscle cells and podocytes. ETB receptors are expressed by renal tubules, including proximal tubules, but especially in the renal collecting duct where they promote Na+ and water excretion by inhibiting tubular reabsorption via nitric oxide and prostaglandin production.1 Loss of ETB receptor function results in hypertension and increased susceptibility to renal injury.2
Immune cells are capable of responding to and producing ET-13–6. In particular, in vitro studies suggest that pharmacological blockade of the ETB receptor leads to increased T cell activation.3 Moreover, ETB receptor expression appears to be decreased in T cells of patients with autoimmune diseases like systemic sclerosis,3 compared to healthy individuals.
The endothelin system is one of the major pathways involved in salt-sensitive hypertension and the associated renal injury.1 Shindo et al.7 demonstrated that overexpression of the ET-1 gene (Edn1) in a mouse model leads to salt-dependent hypertension and proteinuria. Administration of ETA specific blockers is effective in attenuating salt-sensitive hypertension and kidney damage in experimental animal models.8–10 Further, circulating levels of ET-1 are elevated in patients with salt-dependent hypertension.11 High salt diet activates ET-1 production in both endothelial and collecting duct cells.12,13 Thus, in the absence of functional ETB receptors, high salt induces exaggerated activation of ETA receptors resulting in hypertension, renal injury and inflammation.2, 14 The ETB-deficient rat model lacks functional ETB receptors in all organs except neuronal tissues2 and has been utilized by our laboratory and others to demonstrate that over-activation of ETA receptors or loss of functional ETB receptors facilitate the progression of renal injury, while functional ETB receptors provide protection.14–17 Further reports have demonstrated oxidative stress-dependent dysfunction of ETB receptors in models of hypertension.18 Thus, the use of the ETB receptor-deficient rat model is a highly relevant model of hypertension and renal injury.
Tauroursodeoxycholic acid (TUDCA) is an endogenous hydrophilic bile salt that results from the chemical conjugation of the natural occurring uroursodeoxycholic acid (UDCA) with the amino acid taurine in the liver.19 Both UDCA and TUDCA are classified as chemical chaperones with cytoprotective properties, mostly due to their abilities to reduce the formation of reactive oxygen species,20 prevent mitochondrial dysfunction21 and decrease the cellular apoptotic threshold.22, 23 Circulating levels of UDCA/TUDCA in the body are normally extremely low; however, hibernating animals such as the black bear exhibit high plasma UDCA/TUDCA levels that are thought to help reduce cell death during long periods of low nutrient intake.24, 25 TUDCA is FDA-approved for treatment of liver diseases such as choleostasis,26, 27 cirrhosis28 and hepatitis.29 TUDCA has protective effects against apoptosis and/or mitochondrial dysfunction in experimental models of neurological disorders such as Parkinson’s,30–32 Huntington’s33 and Alzheimer’s34–37 diseases, as well as a rat model of myocardial infarction.38
TUDCA also appears to provide protection against the development of kidney disease. Compared with vehicle-treated animals, TUDCA treatment prior to renal ischemia/reperfusion results in lower blood urea nitrogen levels and decreased renal histological injury,39 as well as diminished necrosis and apoptosis.40 Short-term treatment with TUDCA was also recently shown to protect against folic acid nephropathy in a mouse model of the disease.41 More recent studies report beneficial effects of TUDCA treatment against aldosterone-infused renal injury42 and diabetic nephropathy.43, 44
In this study, we hypothesized that TUDCA treatment would prevent the development of proteinuria, albuminuria, and renal glomerular and/or tubular injury in a model of salt-sensitive hypertension, the ETB-deficient rat. We further examined whether TUDCA treatment would blunt renal apoptosis and inflammation in response to a high salt diet in the ETB-deficient rat model. The results demonstrate that TUDCA treatment prevents the development of high salt-induced proteinuria, albuminuria, and renal glomerular and cortical tubular injury, as well as reducing renal cortical cell death and CD4+ T cell infiltration, in the absence of functional ETB receptors and independent of lowering blood pressure.
Results
TUDCA treatment abolishes high salt-induced proteinuria and albuminuria
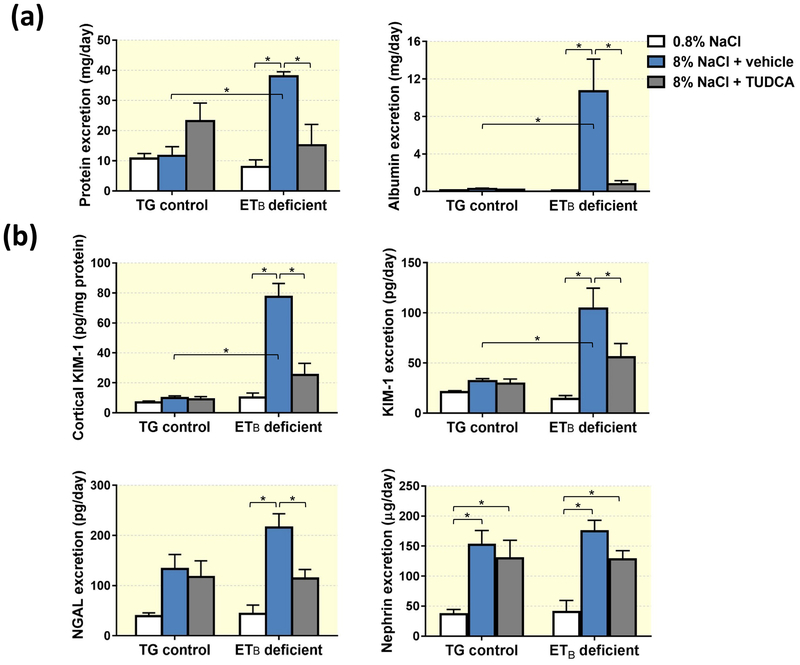
To assess if treatment with TUDCA protects against the development of salt-induced renal damage, ETB-deficient rats and their transgenic (TG) control littermates were fed a diet containing either normal (0.8% NaCl) or high (8% NaCl) salt content for 3 weeks. Animals fed the high salt diet received daily i.p. injections of either TUDCA or vehicle. TG control rats fed normal and high salt diets had similar urinary excretion of protein and albumin at the end of the 3-wk protocol, and TUDCA did not significantly alter those values (Figure 1a). In contrast, ETB-deficient rats fed the high salt diet developed kidney damage, as indicated by the significantly higher urinary excretion of protein and albumin compared to the same genotype fed the normal salt diet. TUDCA treatment significantly reduced urinary protein and albumin excretion by high salt-fed ETB-deficient rats (Figure 1a), indicating a renoprotective effect of TUDCA under these conditions.
Figure 1.
(a) Urinary excretion of protein and albumin in TG control and ETB-deficient rats fed normal salt (0.8% NaCl) or high salt (8% NaCl) diet and treated with vehicle or TUDCA. n = 5–6/group. (b) Renal cortical KIM-1 protein expression and urinary excretion of KIM-1, NGAL and nephrin in TG control and ETB-deficient rats fed normal salt (0.8% NaCl) or high salt (8% NaCl) diet and treated with vehicle or TUDCA. n = 5–6/group. *P < 0.05.
TUDCA treatment ameliorates excretion of KIM-1 and NGAL, but not nephrin
To further explore the specific nature of the high salt diet-induced kidney injury, urine collected at the end of the 3-wk protocol was assayed for markers of tubular damage, kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), and nephrin, a marker of podocyte damage. Similar to the increases seen in protein and albumin excretion, ETB-deficient rats fed a high salt diet displayed elevated urinary excretion of KIM-1 and NGAL compared to the same genotype fed a normal salt diet (Figure 1b), and this effect was attenuated by TUDCA treatment. KIM-1 content in renal cortex was also elevated in high salt-fed ETB-deficient rats and this effect was markedly blunted by co-treatment with TUDCA (Figure 1b). Dietary salt intake and TUDCA treatment did not alter these markers of proximal tubular damage in TG control rats. Both genotypes responded to the high salt feeding with similar levels of nephrinuria, and TUDCA failed to ameliorate this effect (Figure 1b).
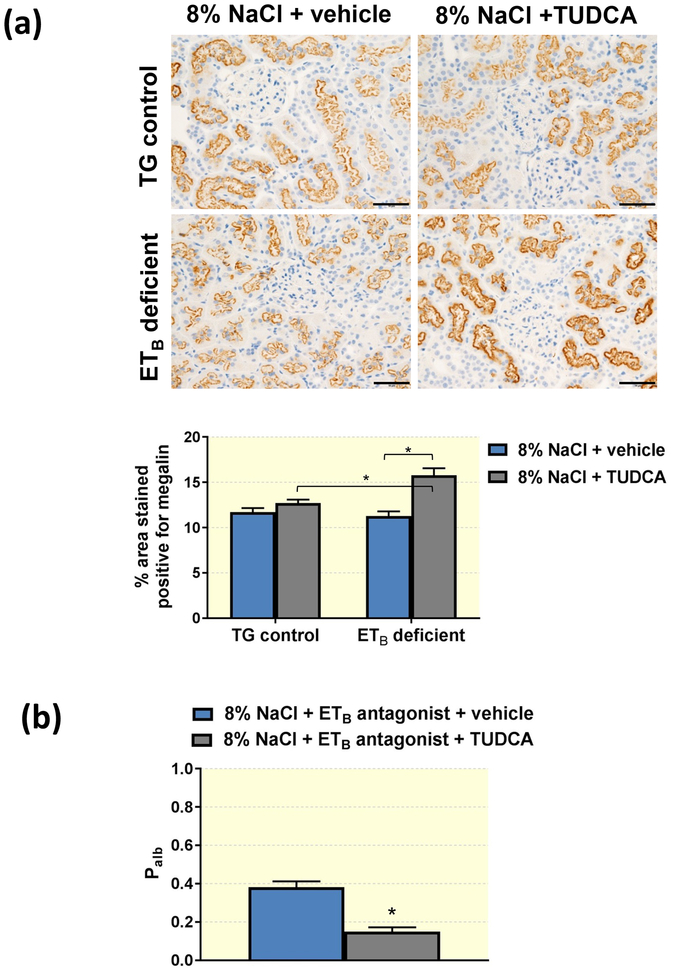
TUDCA upregulates megalin expression in the proximal tubule and decreases glomerular permeability to albumin
The proximal tubule has a key role in the reabsorption and processing of albumin filtered by the glomerulus45–47, and studies have reported that disruption of this process results in albuminuria and proteinuria48. To elucidate the effects of TUDCA on proximal tubule albumin reuptake mechanisms, immunohistochemical analysis was used to determine the expression of the endocytic receptor megalin. Although treatment with TUDCA did not result in any significant change in transgenic control rats, TUDCA led to a significantly elevated expression of megalin in proximal tubules of ETB-deficient rats (Figure 2a). These findings suggest that TUDCA may increase the reabsorption of filtered albumin by the proximal tubule.
Figure 2.
(a) Representative images and quantification of megalin in TG control and ETB-deficient rats fed high salt (8% NaCl) diet and treated with vehicle or TUDCA. n = 5–8/group. Scale bar = 50μm. *P < 0.05. (b) Average glomerular albumin permeability (Palb) in high salt-fed Wistar Kyoto rats treated with ETB antagonist in the drinking water and daily injections of vehicle or TUDCA for 3 weeks. *P < 0.05. n = 5/group.
To investigate the effects of TUDCA on glomerular function, Wistar Kyoto rats were fed high salt diet and treated with the ETB receptor antagonist A-192126 in drinking water for 3 weeks; rats also received daily i.p. injections of TUDCA or vehicle for the length of the high salt period. At the end of the 3 weeks, glomeruli were isolated and glomerular permeability to albumin (Palb) was compared between the groups. Pharmacological blockade of the ETB receptor during high salt consumption led to elevated Palb in the vehicle-treated rats (Figure 2b), indicating dysfunction of the glomerular filtration barrier. On the other hand, TUDCA-treated rats had lower Palb compared to vehicle-treated rats, suggesting that treatment with TUDCA minimizes the detrimental effects of high salt consumption on the glomerular filtration barrier.
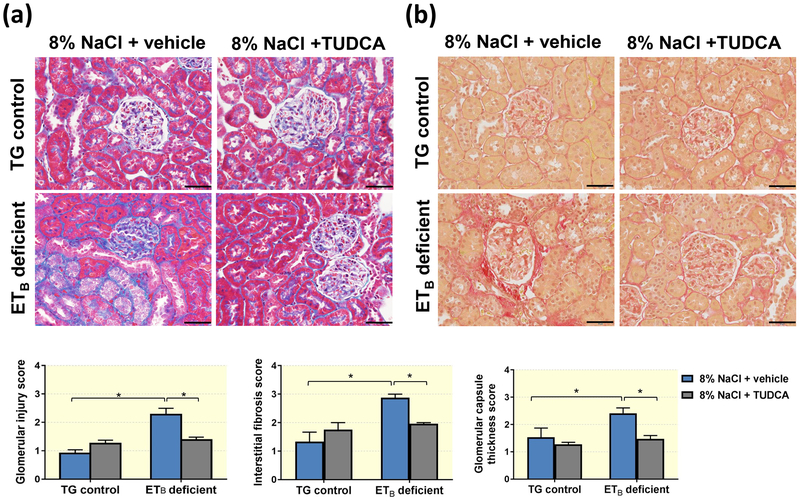
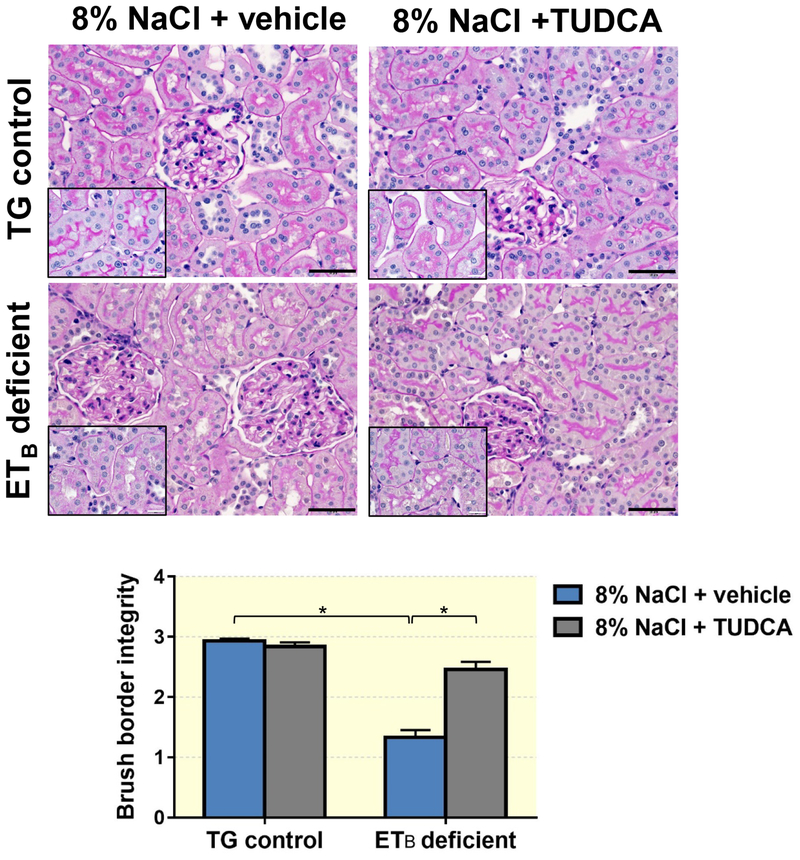
TUDCA ameliorates the development of glomerular and proximal tubule damage, as well as renal cortical cell death
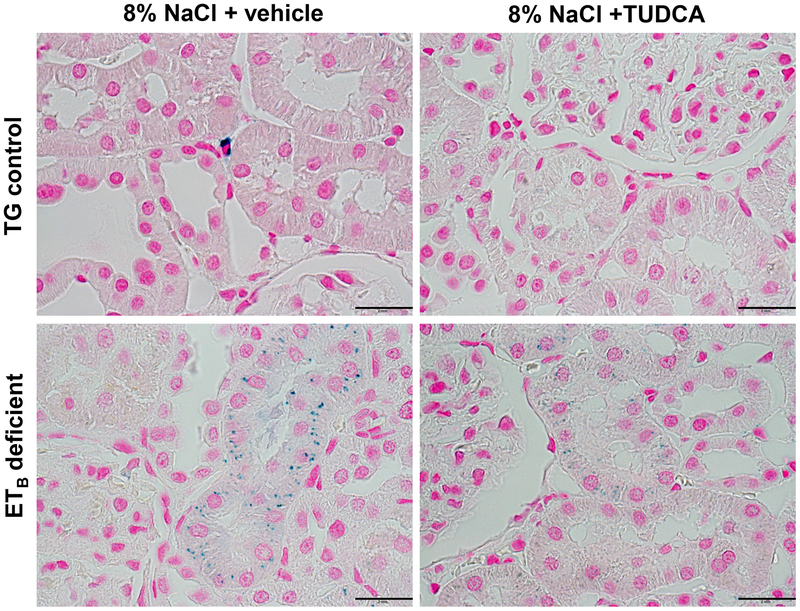
Renal histological assessment demonstrated that ETB-deficient rats fed a high salt diet develop exaggerated glomerular damage compared to TG controls on a high salt diet, as denoted by the presence of increased glomerulosclerosis, cortical interstitial fibrosis and exaggerated thickening of the glomerular capsule (Figure 3). The glomerular injury was accompanied by an apparent loss of brush border integrity in the proximal tubules (Figure 4) and accumulation of iron in proximal tubule cells (Figure 5) in the ETB-deficient rats. High salt feeding did not change the glomerular or proximal tubular morphology in TG control rats. On the other hand, treatment with TUDCA significantly prevented the development of glomerular injury, glomerular capsule thickening, brush border deterioration, cortical interstitial fibrosis and tubular iron deposition in the ETB-deficient rats (Figures 3–5), while not affecting the kidney morphology in TG control rats. The number of glomeruli and glomerular surface area were similar among the experimental groups (Table 1).
Figure 3.
Representative images and quantification of (a) glomerular injury, cortical interstitial fibrosis, and (b) thickness of the glomerular capsule in TG control and ETB-deficient rats fed high salt (8% NaCl) diet and treated with vehicle or TUDCA. n = 5–8/group. Scale bar = 50μm. *P < 0.05.
Figure 4.
Representative images and quantification of brush border integrity in TG control and ETB-deficient rats fed high salt (8% NaCl) diet and treated with vehicle or TUDCA. n = 5–8/group. Black bar = 50μm; white bar = 2mm. *P < 0.05.
Figure 5.
Representative images of iron deposition (blue dots) in proximal tubules of TG control and ETB-deficient rats fed high salt (8% NaCl) diet and treated with vehicle or TUDCA. n = 5–8/group. Scale bar = 2mm.
Table 1.
Average glomerular surface area and number of glomeruli in TG control and ETB-deficient rats after 3 weeks of high salt diet and daily treatment with vehicle or TUDCA. n = 6/group.
| GLOMERULAR SURFACE AREA (μm2) | |||
| TG control + 8% NaCl | ETB-deficient + 8% NaCl | ||
| Vehicle | TUDCA | Vehicle | TUDCA |
| 7761.7 ± 271.6 | 7167.2 ± 204.2 | 7782.2 ± 294.1 | 7254.1 ± 182.4 |
| NUMBER OF GLOMERULI (in 20 microscopy fields) | |||
| TG control + 8% NaCl | ETB-deficient + 8% NaCl | ||
| Vehicle | TUDCA | Vehicle | TUDCA |
| 83.0 ± 2.3 | 88.3 ± 2.9 | 98.0 ± 8.2 | 101.3 ± 5.8 |
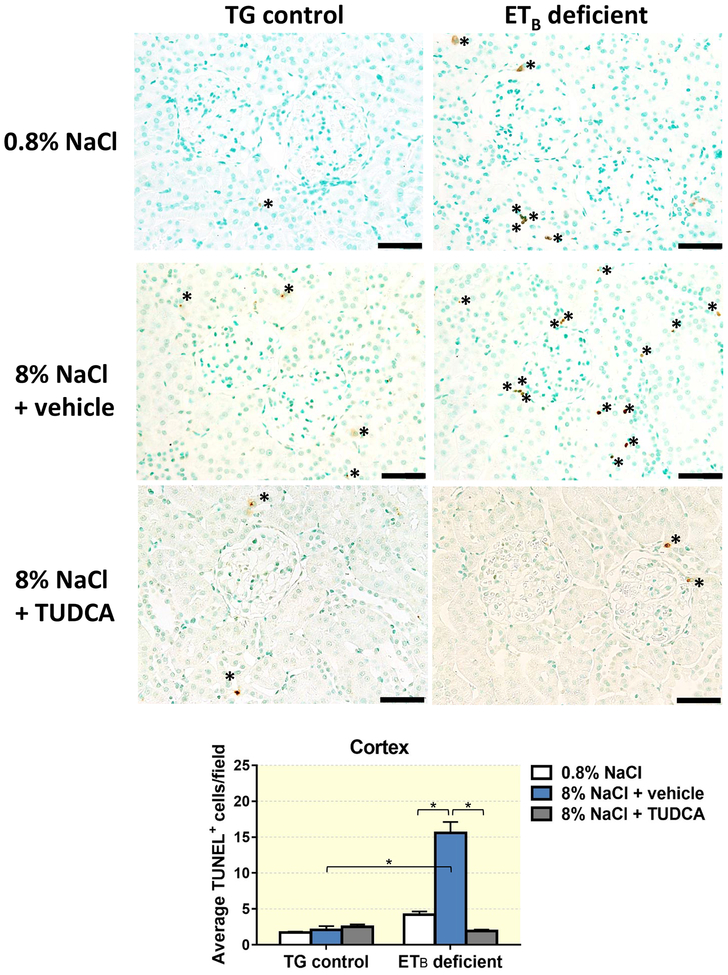
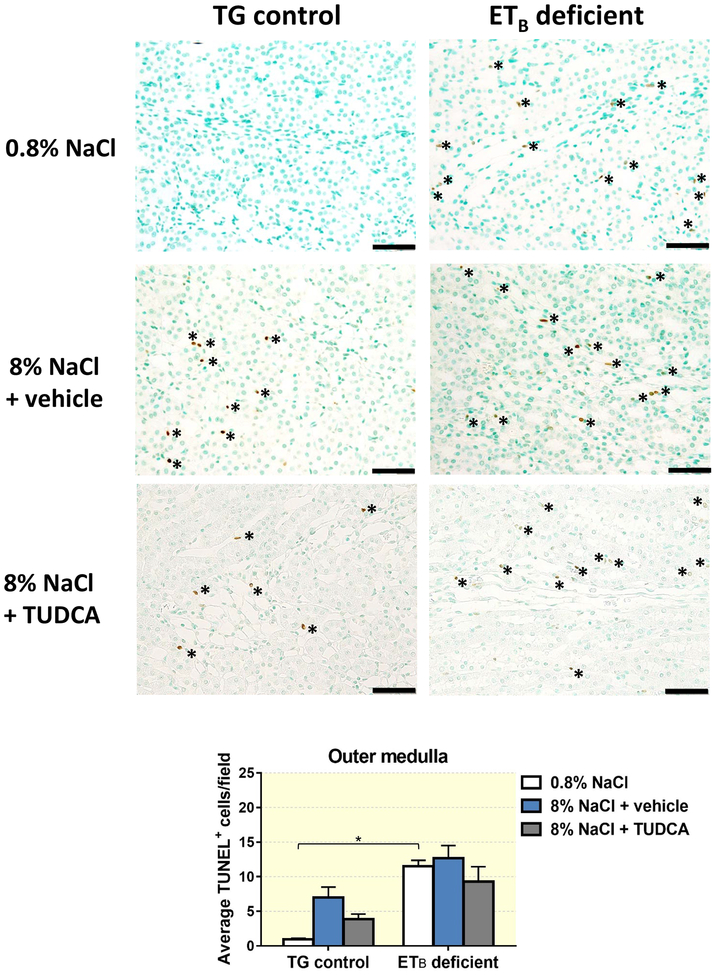
One of the major mechanisms through which TUDCA is reported to be protective against liver damage is by increasing the threshold for cell death.21 To determine if TUDCA protects against salt-induced kidney damage by decreasing cell death in the kidney, TUNEL-positive cells were counted in kidney sections from TG control and ETB-deficient rats fed normal or high salt and treated with vehicle or TUDCA. As seen in Figure 6, TG control and ETB-deficient rats presented with comparable numbers of TUNEL-positive cells when fed a normal salt diet, indicating similar instances of kidney cell death. In contrast, 3 weeks of high salt diet increased the number of TUNEL-positive cells (indicative of cell death) in the renal cortex of ETB-deficient rats, while no significant changes were observed in the cortex of TG control rats fed high salt. Co-treatment with TUDCA during the high salt diet period prevented the salt-induced cell death in the cortex of ETB-deficient rats, but did not induce any significant changes in the TG control rats (Figure 6). ETB-deficient rats fed high salt and treated with vehicle or TUDCA displayed similar numbers of TUNEL+ cells in the outer medulla to ETB-deficient rats fed normal salt diet (Figure 7). Interestingly, TUNEL+ cells in the cortex appear to be localized within the renal interstitium, and are non-tubular cells. The medullary TUNEL+ cells appear to be associated within the vasa recta bundles and vascular structures, although further in-depth analysis is needed to confirm the cell type(s) undergoing cell death in both regions.
Figure 6.
Representative images and quantification of cell death (TUNEL-positive cells) present in renal cortex of TG control and ETB-deficient rats fed normal salt (0.8% NaCl) or high salt (8% NaCl) diet and treated with vehicle or TUDCA for 3 weeks. n = 6/group. Bar = 50 μm. *P < 0.05.
Figure 7.
Representative images and quantification of cell death (TUNEL-positive cells) present in renal outer medulla of TG control and ETB-deficient rats fed normal salt (0.8% NaCl) or high salt (8% NaCl) diet and treated with vehicle or TUDCA for 3 weeks. n = 6/group. Bar = 50 μm. *P < 0.05.
TUDCA attenuates salt-induced CD4+ T lymphocyte accumulation in the renal cortex
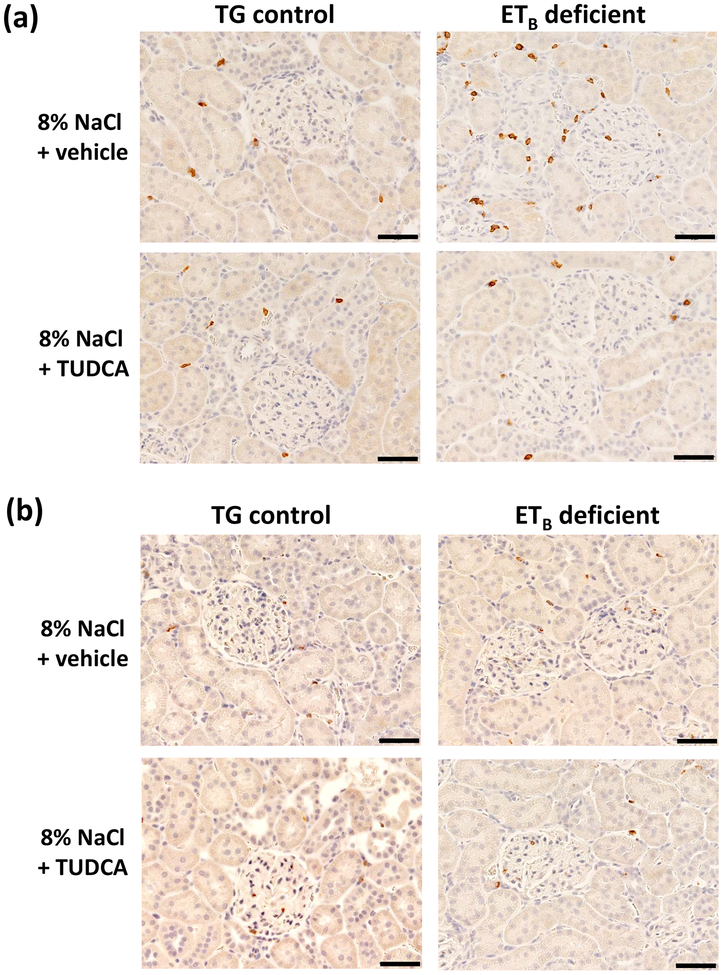
Because renal inflammation is closely associated with kidney injury, the effects of high salt diet on renal immune cell numbers were quantified in ETB-deficient and TG control rats. As seen in Figure 8a, high salt feeding induced greater T lymphocyte (CD3+ cells) infiltration in the renal cortex of ETB-deficient rats than in TG control rats. Moreover, treatment with TUDCA significantly decreased T cell numbers, normalizing the numbers of T cells in the cortex to baseline levels. TUDCA significantly reduced outer medullary T lymphocyte numbers to similar levels in both genotypes on a high salt diet (Table 2). In contrast, salt-induced macrophage (ED-1+ cells) numbers in the cortex did not differ among genotypes or treatments (Figure 8b). TUDCA did not affect macrophage (ED-1+ cells) numbers in the renal outer medulla (Table 2) of high salt-fed rats. In the renal inner medulla, T-cell and macrophage infiltration were unaltered (Table 2).
Figure 8.
Representative images of (a) T-lymphocyte (CD3+ cells) and (b) macrophage (ED-1+ cells) infiltration in renal cortex of TG control and ETB-deficient rats after 3 weeks of high salt diet and daily treatment with vehicle or TUDCA. n = 6/group. Bar = 50 μm.
Table 2.
Average numbers of T cells (CD3+ cells) and macrophages (ED-1+ cells) in renal cortex and outer and inner medulla of TG control and ETB-deficient rats after 3 weeks of high salt diet and daily treatment with vehicle or TUDCA. n = 6/group. *P < 0.05 vs. same genotype + vehicle; †P < 0.05 vs. TG control + vehicle
| CORTEX | ||||
| TG control + 8% NaCl | ETB-deficient + 8% NaCl | |||
| Vehicle | TUDCA | Vehicle | TUDCA | |
| CD3+ cells (cells/field) | 10.8 ± 0.5 | 9.1 ± 1.2 | 17.4 ± 4.5† | 10.8 ± 0.5* |
| ED-1+ cells (cells/field) | 6.9 ± 0.7 | 7.1 ± 1.2 | 10.5 ± 2.2 | 6.8 ± 0.6 |
| OUTER MEDULLA | ||||
| TG control + 8% NaCl | ETB-deficient + 8% NaCl | |||
| Vehicle | TUDCA | Vehicle | TUDCA | |
| CD3+ cells (cells/field) | 13.9 ± 1.1 | 9.1 ± 1.1* | 14.0 ± 0.6 | 9.6 ± 0.8* |
| ED-1+ cells (cells/field) | 4.1 ± 0.6 | 2.8 ± 0.7 | 4.0 ± 0.9 | 3.6 ± 0.5 |
| INNER MEDULLA | ||||
| TG control + 8% NaCl | ETB-deficient + 8% NaCl | |||
| Vehicle | TUDCA | Vehicle | TUDCA | |
| CD3+ cells (cells/field) | 9.8 ± 4.8 | 10.9 ± 3.7 | 10.5 ± 3.2 | 5.6 ± 1.6 |
| ED-1+ cells (cells/field) | 2.4 ± 0.5 | 1.7 ± 0.5 | 2.4 ± 0.7 | 2.3 ± 0.5 |
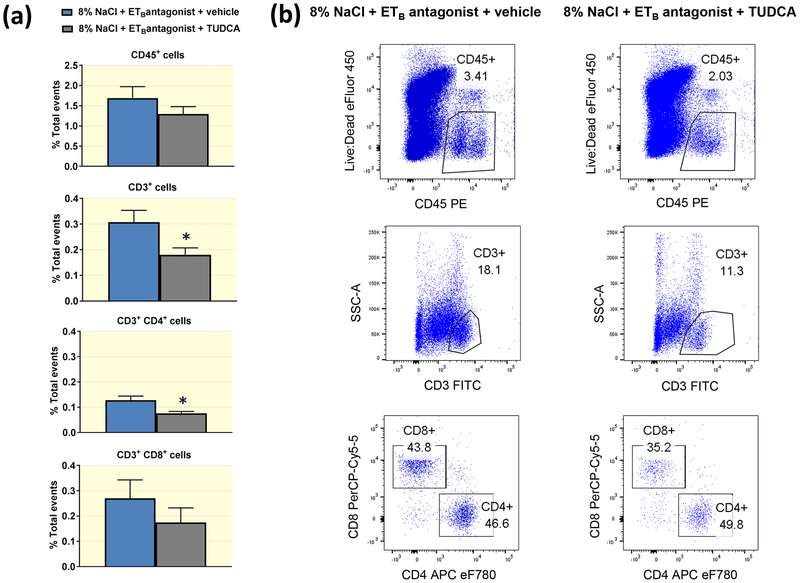
To further characterize the inflammatory cells present in the renal cortex, flow cytometry was performed in cortical immune cells isolated from high salt-fed Wistar Kyoto rats treated with A-192126 and daily i.p. injections of TUDCA or vehicle for 3 weeks. The flow cytometry data demonstrate that the numbers of cortical leukocytes (CD45+ cells) did not differ between vehicle- and TUDCA-treated rats (Figure 9). However, TUDCA-treated animals presented with significantly smaller percentages of CD3+ cells in the cortex compared to animals treated with vehicle. Further, the effects of TUDCA on the cortical T lymphocyte population were specific to CD4+ cells, as the percentages of CD3+CD8+ cells did not differ between the experimental groups (Figure 9).
Figure 9.
(a) Percentage of total events of leukocytes (CD45+), T lymphocytes (CD3+), T helper cells (CD3+CD4+) and T cytotoxic cells (CD3+CD8+) in renal cortical tissue of high salt-fed Wistar Kyoto rats treated with ETB antagonist in the drinking water and daily injections of vehicle or TUDCA for 3 weeks. *P < 0.05. n = 5/group. (b) Representative flow cytometry dot plots depicting the proportion of CD45+, CD3+, CD3+CD4+ and CD3+CD8+ cells in renal cortical tissue of high salt-fed Wistar Kyoto rats treated with ETB antagonist in the drinking water and daily injections of vehicle or TUDCA for 3 weeks.
In concordance with the immunohistochemistry observations in high salt- fed ETB-deficient rats, the flow cytometry results did not indicate an effect of TUDCA on the macrophage populations present in the renal cortex after three weeks of high salt feeding (% total events CD11b/c+ MHC-II+ cells, vehicle-treated vs. TUDCA-treated: 1.15 ± 0.3 vs. 0.85 ± 0.15; P > 0.05; n = 5/group).
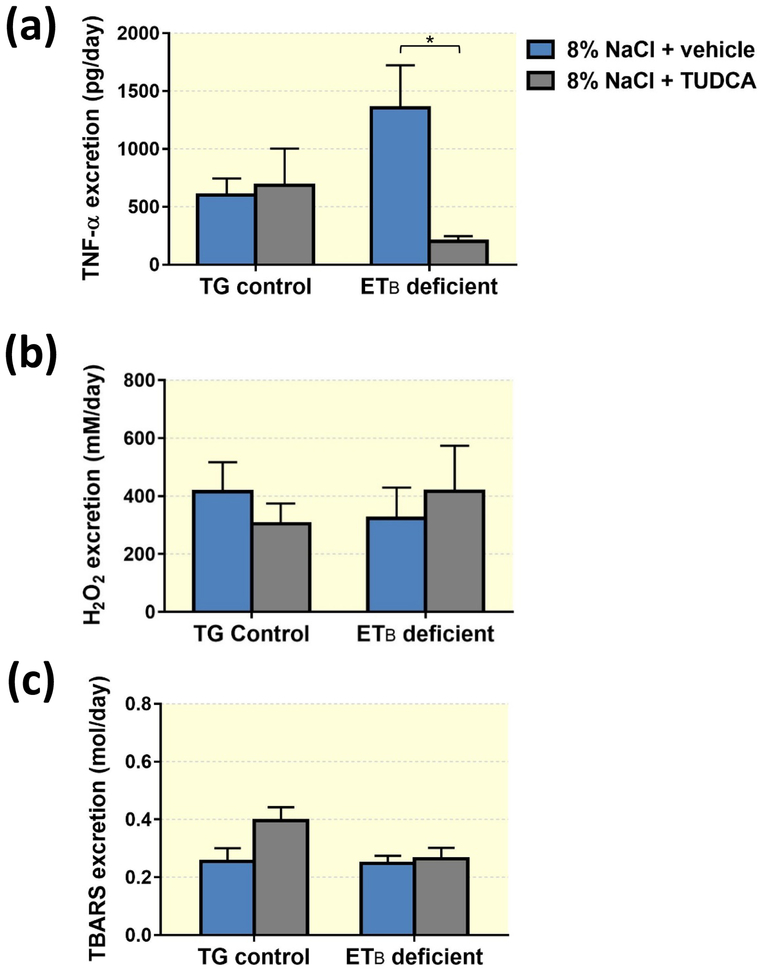
TUDCA decreases TNF-α excretion, but does not change urinary oxidative stress markers
In response to high salt feeding, ETB-deficient rats tended to increase urinary excretion of TNF-α, an indication of increased inflammation in the kidney. Further, treatment with TUDCA markedly suppressed TNF-α excretion by high salt-fed ETB-deficient rats, without evoking any significant changes in TNF-α excretion in the TG control rats (Figure 10a). To investigate whether TUDCA may work by reducing renal oxidative stress, H2O2 and thiobarbituric reactive substances (TBARS) urinary excretion were assessed in TG control and ETB-deficient rats after 3 weeks on the high salt diet and daily treatment with either TUDCA or vehicle. As shown in Figure 10b, urinary H2O2 excretion by TG control and ETB-deficient rats did not differ significantly, and was unaltered by TUDCA. Similarly, there were no significant differences in urinary TBARS excretion (Figure 10c) across genotypes regardless of TUDCA treatment.
Figure 10.
Excretion of TNF-α (a), hydrogen peroxide (b) and TBARS (c) in TG control and ETB-deficient rats after 3 weeks of high salt diet and daily treatment with vehicle or TUDCA. n = 5–6/group.
TUDCA treatment does not alter the renal ET system
To determine if treatment with TUDCA could be modifying the ET system in our animals, mRNA expression levels of pre-pro-ET-1 (Edn1), ETA receptors (Ednra) and ETB receptors (Ednrb) in renal cortex were determined by RT-PCR. As shown in Table 3, mRNA expression levels of these genes in renal cortex of rats fed high salt and treated with TUDCA were similar to those in rats fed high salt and treated with vehicle. Moreover, urinary excretion of ET-1, a marker of ET-1 production in the kidney, was unchanged by treatment with TUDCA in either genotype on high salt diet (TG control rats, vehicle vs. TUDCA: 17.9 ± 3.7 pg/day vs. 15.3 ± 2.2 pg/day; ETB-deficient rats, vehicle vs. TUDCA: 18.0 ± 2.0 pg/day vs. 14.7 ± 2.0 pg/day; P > 0.05; n = 5–6/group).
Table 3.
Relative mRNA expression of pre-pro-ET-1, ETA and ETB receptors, ER stress markers and toll-like receptors 2 and 4 in cortex of TG control and ETB-deficient rats after 3 weeks of high salt diet and daily treatment with vehicle or TUDCA. n = 6–9/group.
| CORTICAL mRNA EXPRESSION | ||||
|---|---|---|---|---|
| TG control + 8% NaCl | ETB-deficient + 8% NaCl | |||
| Vehicle | TUDCA | Vehicle | TUDCA | |
| Edn1 | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.5 ± 0.2 | 0.7 ± 0.3 |
| Ednra | 1.0 ± 0.5 | 2.0 ± 0.7 | 0.7 ± 0.4 | 0.9 ± 0.5 |
| Ednrb | 1.0 ± 0.4 | 1.3 ± 0.4 | 1.0 ± 0.5 | 0.9 ± 0.4 |
| GRP78 | 1.0 ± 0.4 | 1.26 ± 0.2 | 1.8 ± 0.8 | 1.3 ± 0.4 |
| ATF-6 | 1.0 ± 0.4 | 1.5 ± 0.5 | 1.1 ± 0.5 | 1.3 ± 0.5 |
| ATF-4 | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.1 ± 0.5 | 0.8 ± 0.3 |
| sXBP-1 | 1.0 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.5 | 1.5 ± 0.7 |
| CHOP | 1.0 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.4 | 1.9 ± 0.5 |
| Caspase-12 | 1.0 ± 0.4 | 0.9 ± 0.3 | 1.9 ± 1.1 | 1.2 ± 0.6 |
| Tlr2 | 1.0 ± 0.2 | 0.9 ± 0.2 | 1.7 ± 0.3 | 1.0 ± 0.2 |
| Tlr4 | 1.0 ± 0.2 | 1.2 ± 0.4 | 3.4 ± 1.2 | 1.9 ± 0.4 |
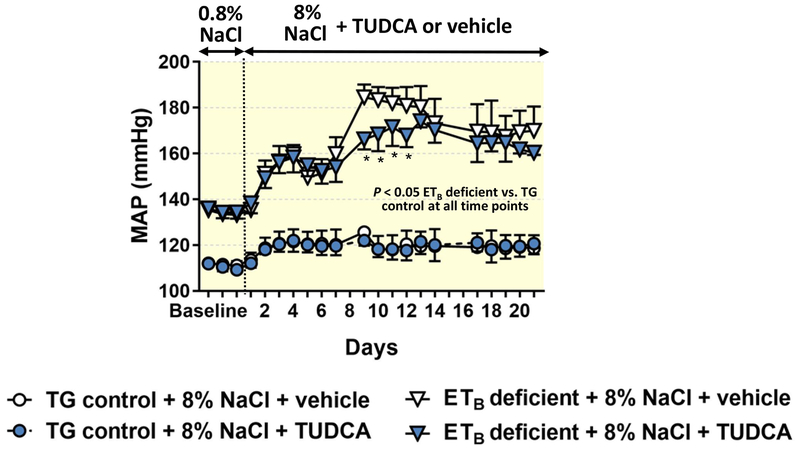
TUDCA treatment does not decrease mean arterial pressure in high salt-fed ETB-deficient rats
To determine if the protective effects of TUDCA against high salt-induced renal damage were due to decreases in blood pressure, 24-hr mean arterial pressure (MAP) was measured by telemetry in ETB-deficient and TG control rats. Similar to previous observations,2 ETB-deficient rats fed a normal salt diet had elevated MAP when compared to TG controls (Figure 11). Upon initiation of the high salt diet, the MAP of ETB-deficient rats further increased to a maximum of ~180 mmHg, but remained unchanged in the TG control rats. Treatment with TUDCA did not change MAP in either TG control or ETB-deficient rats red high salt diet. During the first week of high salt, no differences in MAP were observed between ETB-deficient rats receiving vehicle and those receiving TUDCA; however, treatment with TUDCA significantly reduced MAP on days 9–12 of high salt feeding, although this difference in blood pressure between the groups was not sustained from day 13 to day 21.
Figure 11.
Mean arterial pressure (24-hr averages) in TG control and ETB-deficient rats, monitored by telemetry. Rats were fed normal salt (0.8% NaCl) diet during the first 3 days of recordings and then switched to high salt (8% NaCl) diet for a period of 3 weeks. During the high salt period, rats received daily i.p. injections of vehicle or TUDCA. n = 4/group. *P < 0.05 vs. ETB-deficient treated with vehicle. At all time points, ETB-deficient rats had higher mean arterial pressure than TG control rats.
TUDCA does not alter ER stress in the renal cortex
TUDCA is widely known as an inhibitor of the endoplasmic reticulum (ER) stress pathway.49 To determine if TUDCA decreases salt-induced kidney damage by inhibiting ER stress, mRNA expression of ER stress markers (GRP78, ATF-4, ATF-6, sXBP-1, CHOP and caspase-12) was assessed in renal cortex of TG control and ETB-deficient rats fed a high salt diet for 3 weeks and treated with TUDCA or vehicle for the same period of time. Table 3 shows that the expression levels of these markers of ER stress were similar across all groups. We conclude that TUDCA does not alter the development of salt-induced ER stress in the renal cortex.
Toll-like receptor pathways in the kidney are not altered by treatment with TUDCA
TUDCA has been reported to protect the liver against steatosis during transplantation by increasing expression of TLR4.50 To determine if the renoprotective effects of TUDCA were due to changes in toll-like receptor pathways in this organ, Tlr4 and Tlr2 mRNA expression were assessed in renal cortex of TG control and ETB-deficient rats after 3 weeks of high salt feeding and treatment with vehicle or TUDCA. Although ETB-deficient rats tended to present slightly elevated expression of these genes when compared to high salt-fed TG control rats, statistical significance was not achieved (Table 3). Likewise, treatment with TUDCA did not significantly change the expression of these genes in renal cortex of ETB-deficient rats (Table 3).
Discussion
The major findings of this study demonstrate that treatment with the endogenous hydrophilic bile salt TUDCA prevents the high salt-induced renal damage that is associated with ETB receptor dysfunction, and that the renoprotective effects of TUDCA are independent of sustained blood pressure lowering. TUDCA also decreased high salt-induced cell death and T cell accumulation in the renal cortex of ETB-deficient rats. In a model of pharmacological blockade of ETB receptors, TUDCA explicitly lessened the cortical numbers of CD3+ CD4+ cells and ameliorated the glomerular filtration dysfunction associated with high salt consumption. Taken together, these results demonstrate that loss of ETB function contributes to high-salt induced renal injury independent of hypertension, and that TUDCA or related therapeutics may be a potential approach for reducing the risk of chronic kidney injury.
Evidence in the literature demonstrates that ETB receptors are reduced or dysfunctional in human hypertension,51 and that animal models of salt-sensitive hypertension like the Dahl rat also demonstrate dysfunction of this receptor.52 Using the ETB-deficient rat as an animal model of salt-dependent renal damage,2, 14 and given the beneficial effects of TUDCA reported in the liver26–29 and other organs,30–33, 38 we tested if TUDCA would also be protective against the development of salt-induced renal damage. Recent studies demonstrated that treatment with TUDCA significantly decreases renal tubular injury in a mouse model of type 2 diabetes53 and attenuates kidney damage mediated by increased aldosterone,42 suggesting a protective effect of this chemical chaperone in chronic kidney disease and specifically for renal tubules. We now provide evidence that TUDCA also protects against the development of the kidney damage associated with ETB receptor dysfunction after continued high salt intake.
Importantly, TUDCA treatment led to an elevated expression of megalin in the proximal tubules of high salt-fed ETB deficient rats. Megalin is an endocytic receptor expressed in tubular epithelial cells, specifically in the brush border,54, 55 and it is involved in the proximal tubule reuptake of filtered proteins such as albumin.55 A kidney-specific megalin knockout mouse exhibits albuminuria,56, 57 and biopsies obtained from patients of non-proteinuric renal diseases display elevated expression of megalin in proximal tubule when compared to patients of proteinuric diseases.54 Surprisingly, TUDCA did not significantly diminish the urinary nephrin excretion nor did TUDCA normalize the glomerular permeability, since the Palb was still about twice that in a normal rat,58 supporting the notion that podocyte injury is still present, most likely due to the persistent hypertension. Thus, we propose that despite the remaining high salt-mediated podocyte damage in this model, proteinuria and albuminuria were ameliorated by a TUDCA-mediated compensation in the proximal tubule: upregulation of megalin in this segment of the nephron results in increased reabsorption of filtered protein and albumin, thereby, reducing excretion of protein and albumin and less subsequent injury to the tubules.
Prior studies have clearly demonstrated a role for ET-1 and ETA receptor activity in contributing to glomerular injury in a variety of models such as diabetes and sickle cell disease.59, 60 Saleh et al.61 provided in vivo evidence that ET-1 directly increases glomerular permeability to albumin via the ETA receptor, but that albuminuria is prevented by increased proximal tubule albumin uptake in the rat. Our results reveal that high salt induces proximal tubular cell damage in ETB-deficient rats, suggesting that high salt-induced ET-1 may directly activate proximal tubular ETB receptors to facilitate protein and albumin uptake. Further work is necessary to fully understand the role of the ET system in proximal tubular function and the albumin uptake mechanism.
Although reports in the literature indicate that TUDCA reduces inflammation in liver,62 intestine,63 vasculature64 and perhaps other organs, through downregulation of the ER stress response, TUDCA treatment in the present study did not decrease measures of ER stress in the cortex after high salt. This implies that the anti-inflammatory effects of TUDCA most likely are mediated by a different mechanism. For instance, it is possible that TUDCA could directly block the inflammasome pathway, which is involved in the development of several kidney diseases.65–67 Decreased renal expression of members of the NLRP3 inflammasome and decreased renal fibrosis have been described after treatment with TUDCA in a model of aldosterone-infusion kidney disease.42 Further studies are needed to test the possible effects of TUDCA on the renal inflammasome in the high salt fed ETB-deficient rats.
Endogenous bile acids like TUDCA and UDCA reportedly modulate cell death by regulating death and survival transduction pathways in hepatocytes, neurons, lymphocytes and cardiomyocytes,21, 68, 69 among other cell types. TUDCA is thought to reduce the apoptotic threshold by modulating the glutamate-mediated activation of caspases and cytochrome c release.23, 70 We demonstrate that loss of ETB function is associated with exaggerated high salt-mediated cell death in the renal cortex. TNF-〈 is one of the most potent inducers of cell death71 and plays a role in the development of salt-sensitive kidney disease.72, 73 TUDCA significantly attenuated the excretion of TNF-〈 and also decreased cortical cell death in ETB-deficient rats fed a high salt diet. Interestingly, TUDCA failed to decrease the numbers of TUNEL-positive cells in the outer medulla of ETB-deficient rats. We speculate that TUDCA blunts TNF-〈-dependent mechanisms that contribute to salt-mediated cortical cell death. Because TUDCA also decreased the CD4+ populations of T cells in the kidney of rats with ETB dysfunction fed high salt, and CD4+ T cells produce TNF-〈 when activated,74 TUDCA may be influencing TNF-〈 excretion through its inhibitory effects on the renal cortical CD4+ T cell populations. Our laboratory previously provided evidence that ETA receptors contribute to renal T cell and macrophage accumulation in the angiotensin II-induced hypertensive mouse model.75 Similar to the ETB deficient rat, this model displays increased ET-1,76 and down-regulation of renal ETB receptors,77 thus leading to over-activation of ETA receptors. Further, ETA selective blockade blunts angiotensin II-induced hypertension.75, 78–82 We also reported that T cell accumulation in the renal cortex of angiotensin II-induced hypertensive mice was mediated by ETA receptors independent of blood pressure, while T cell accumulation in the medulla was blood pressure dependent.75
We found similar numbers of TUNEL-positive cells in the outer medulla of ETB-deficient rats regardless of salt content in the diet or TUDCA treatment. The outer medullary region is known to express ETB receptors more abundantly than ETA receptors.83, 84 Our group recently reported the critical role of ETA receptor over-activation in promoting kidney cell death in a rat model of tunicamycin-induced acute kidney injury.85 The increased numbers of TUNEL+ cells in the present studies indicate that the ETB receptor opposes the actions of the ETA receptor and, in the ETB-deficient rats, where the ETB receptor is dysfunctional, unopposed activation of the ETA receptor leads to exaggerated cell death response especially in this area of the kidney. We propose that the mechanism of cell death in the renal medulla in this model of ETB deficiency and high salt diet is distinct from that in the renal cortex and is hypertension-dependent.
ETB-deficient rats fed a normal salt diet display elevated MAP when compared to TG controls, an effect attributed to elevated circulating ET-1 levels and ETA activation.2 Moreover, ETB-deficient rats develop even further increases in MAP when fed a high salt diet, with further exaggerated circulating ET-1 levels.2, 14 Interestingly, the protective effects of TUDCA against kidney damage seen in the present studies are blood pressure-independent, as TUDCA did not cause a sustained decrease on MAP during the period of high salt intake. Loss of ET-1 or ETB receptor in the nephron results in exaggerated salt-sensitive hypertension through negative effects on Na+ transport resulting in decreased natriuresis.86, 87 In addition, ETB blockade in renal microvessels leads to exaggerated afferent arteriole vasoconstriction after high salt intake.88 The unchanged arterial blood pressure that we observed in our experimental animals suggests that treatment with TUDCA does not affect Na+ transport or afferent arteriole sensitivity. Similarly, the protective effects of TUDCA cannot be attributed to modifications of the ET system given that the cortical expression of pre-pro-ET-1, ETA and ETB did not differ among genotypes or treatments. Furthermore, urinary ET-1 excretion, a well-known marker of renal ET-1 production, was similarly unaltered by TUDCA treatment. In addition, we found that ER stress and urinary oxidative stress-related parameters were unaltered by TUDCA in this model. However, we recognize that the ER stress and oxidative stress measurements were limited and not performed at the cellular level; hence, further analysis is required to monitor ER stress and oxidative stress in isolated proximal tubules and/or T cells with ETB deficiency under high salt conditions.
Perspectives
Western diets, especially in the United States, contain substantial amounts of salt. Many cardiovascular and renal diseases, especially with high fat diets and hypertension, lead to a loss of ETB receptor function, which increases risk of salt dependent complications. ETB receptors are reduced or dysfunctional in human hypertension51 and animal models of salt-sensitive hypertension like the Dahl rat.52 ETB receptor deficiency and chronic high salt diet results in glomerular and proximal tubular injury, increased proteinuria and albuminuria, exaggerated renal cortical cell death, increased renal cortical T cell accumulation, and salt-sensitive hypertension. The loss of functional ETB receptors may “prime” renal cells for high salt-induced injury possibly via over-activation of ETA receptors. These studies indicate that high salt plus ETB dysfunction promotes glomerular and proximal tubular injury leading to exaggerated albuminuria. This increase in albumin excretion with higher interstitial sodium may alone provide the stimulus for renal interstitial cell death and/or T cell activation. However, we cannot exclude the possibility that loss of ETB receptors on interstitial cells and/or T cells promotes cell death and activation, respectively.
The chemical chaperone bile salt, TUDCA, may be a valuable therapeutic tool for chronic cardiovascular and renal diseases, which are exacerbated with high salt diets. TUDCA treatment abolished the proteinuria, albuminuria, and glomerular and cortical tubular injury but not the podocyte injury in the salt-induced ETB-deficient rat model. Further, TUDCA treatment ameliorated the renal cortical cell death and T cell accumulation observed in the ETB-deficient rat model independent of sustained changes in the salt-induced hypertension.
Materials and methods
Animal studies
All protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Ten-to-twelve week old male ETB-deficient rats (specifically, DBH-ETB;ETBsl/sl rats) and their DBH-ETB;ETB+/+ transgenic littermates (TG control rats) were used in these studies. Both ETB-deficient rats and TG control rats carry the transgene for the ETB receptor driven by the dopamine β-hydroxylase promoter. This promoter rescues ETB-deficient rats from a lethal phenotype by expressing a functional ETB receptor in adrenergic tissues;2 however, ETB-deficient rats lack functional ETB receptors in nonadrenergic tissues such as vascular endothelium and renal tubular epithelium. All animals were housed under constant temperature and humidity conditions, in a 12-h dark:12-h light cycle, with food and water available ad libitum.
Rats were randomly assigned to stay on either a normal salt diet (0.8% NaCl; LabDiet 5L3Z,) or a high salt diet (8% NaCl; Teklad 8604) for 3 weeks. The group of rats receiving high salt diet was further divided in 2 subgroups receiving intraperitoneal injections of tauroursodeoxycholic acid (TUDCA, 400 mg/kg/day; EMD Millipore, Billerica, MA) or vehicle (isotonic saline) daily throughout the 3-wk period. Urine was collected on the last day of the study (after one day acclimatization to metabolic cages), a plasma sample was obtained, the animals were sacrificed, and the kidneys were harvested. In some cases, kidneys were divided into renal cortex and outer medulla, which were snap frozen in liquid nitrogen and kept at −80 °C until further analysis. Other kidneys were fixed overnight in formalin and preserved in paraffin for immunohistochemistry studies. Because preliminary studies revealed no changes in the outer medulla, the present studies focused on the renal cortex. In a second set of animals, PA-C40 transmitters (Data Sciences International, Duluth, MN) were implanted in the abdominal aorta to allow monitoring of blood pressure in the conscious state, as previously described.12 In brief, rats were anesthetized with 2–3% isoflurane, the abdominal aorta exposed and the telemeter implanted and held in place with tissue glue (Vetbond, 3M, St. Paul, MN). Animals recovered from surgery for one week prior to the beginning of the 3-wk protocol detailed above, during which mean arterial pressure (MAP) was recorded at 10-sec intervals every 10 min throughout the protocol.
In a second set of experiments, ten week-old Wistar Kyoto male rats received 8% NaCl diet and the selective ETB receptor antagonist A-192621 (10mg/Kg/day in the drinking water, AbbVie Inc., North Chicago, IL) for 3 weeks, and were randomly assigned to receive daily i.p. injections of vehicle or TUDCA for the entirety of the high salt diet period.
Quantitative RT-PCR
RNA was extracted from renal cortex using RNeasy mini kit (Qiagen, Valencia, CA) and quantified by spectrophotometric analysis (NanoDrop ND-1000, Thermo Scientific, Waltham, MA). RNA reverse transcription was performed using Quantitect Reverse Transcription kit (Qiagen) following manufacturer’s instructions. Primers for components of the ET system were purchased from QuantiTect (Qiagen; Edn1: QT00371308; Ednra: QT00182546 and Ednrb: QT01084454) and GAPDH was used as housekeeping gene (Qiagen, QT01658692). ER stress and toll-like receptor (TLR) primers were synthesized by Integrated DNA Technologies (IDT, Coralville, IA; primer sequences are indicated in Table 4). RNA expression was detected with Quantitect SYBR green kit (Qiagen) and using a CFX96 Touch RT-PCR detection system (Bio-Rad, Hercules, CA).
Table 4.
Sequences for ER stress and TLR primers used for RT-PCR.
| Gene name | Sequence |
|---|---|
| GRP78 | F: AAC CCA GAT GAG GCT GTA GCA93 R: ACA TCA AGC AGA ACC AGG TCA C |
| ATF-4 | F: TAT GGA TGG GTT GGT CAG TG94 R: CTC ATC TGG CAT GGT TTC C |
| ATF-6 | F: GAT TTG ATG CCT TGG GAG TC94 R: GGA CCG AGG AGA AGA GAC AG |
| sXBP-1 | F: CTG AGT CCG AAT CAG GTG CAG95 R: GGT CTT GTA GAA GGG TAC CTA |
| CHOP | F: CCA GCA GAG GTC ACA AGC AC93 R: CGC ACT GAC CAC TCT GTT TC |
| Caspase 12 | F: CAC TGC TGA TAC AGA TGA GG94 R: CCT TCC ATC CGT TCT CAC C |
| TLR4 | F: CCA GAG CCG TTG GTG TAT CT96 R: TCA AGG CTT TTC CAT CCA AC |
| TLR2 | F: GGA GAC TCT GGA AGC AGG TG96 R: CGC CTA AGA GCA GGA TCA AC |
Histological assessment of renal damage
Gomori’s Blue Trichrome was used to visualize renal structures using bright-field microscopy (Olympus BX40; Olympus America, Melville, NY). Photographs were obtained with a digital camera (Olympus DP12; Olympus America). In a blinded manner, 20 glomeruli per slide were examined and given a glomerulosclerosis score of 0 = 0%, 1 = 25%, 2 = 50%, 3 = 75%, or 4 = 100%. The 20 glomerulosclerosis scores per kidney were then averaged. Presence of interstitial fibrosis was also scored in blue trichrome-stained kidney slices, using a scale from 0 to 3, with 0 = no fibrosis present and 3 = extensive fibrosis.
Thickness of the glomerular capsule was evaluated by using picrosirius red staining. 20 glomeruli per animal were scored in a blinded manner using a scale of 1 = normal, 2 = moderately thick, 3 = very thick and 4 = extremely thick. The data is reported as the average score per experimental group.
For examination of proximal tubule brush border integrity, periodic acid-Schiff (PASH) stain was utilized. 20 fields per kidney were examined, each receiving a score for integrity (0 = no brush border, 1 = poor integrity, 2 = moderate integrity, and 3 = excellent integrity). The scores of the 20 fields/kidney were averaged and reported.
To evaluate possible differences in the number of glomeruli and glomerular surface area among the groups, a total of 20 microscopy fields were assessed per animal in hematoxylin and eosin (H&E)-stained kidney slices. The number of glomeruli per field was recorded and the average per animal was calculated. The glomerular surface area was measured for all whole glomeruli within a microscopy field using CellSens software (Olympus America).
Iron deposition in the renal cortex was assessed using standard Prussian blue histology staining protocol.59
Immunohistochemical analysis
Kidneys were fixed overnight in 4% buffered formalin solution at room temperature, transferred to 70% ethanol for 24 h, and paraffin-embedded. Tissues were cut longitudinally into 4 μm-thick sections and mounted on Superfrost slides. Tissue sections were stained with primary antibodies specific for CD3 (1:400; Abcam, Cambridge, MA), ED-1 (1:100; Bio-Rad, Hercules, CA), and megalin (1:50,000; Abcam) and detected with polymer conjugated secondary antibody (Biocare Medical, Concord, CA). Quantification of renal T-lymphocyte (CD3+ cells) and macrophage (ED-1+ cells) infiltration was performed by blindly counting 10 microscopic fields (400 × 400 μm, 200× magnification) in each renal cortex. Infiltrating cell numbers are reported as average of the counts in the 10 fields per renal cortex.
For evaluation of megalin expression, 20 cortical images were taken from each animal at 400× magnification. The percentage of cortical area stained positive for megalin was quantified in each image using MetaMorph software (Molecular Devices LLC., San Jose, CA) and the average megalin expression per animal was calculated. The data is reported as the average % area stained positive for megalin per experimental group.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
Following manufacturer’s directions, the Apoptag® Plus Peroxidase In Situ Apoptosis Kit (MP Biomedicals, Santa Ana, CA) was used to detect dead cells in renal tissue slides. TUNEL-positive cells in renal cortex were counted in 10 microscopic fields (400 × 400 μm, 200× magnification). TUNEL-positive cell counts are reported as average of the counts in the 10 fields per kidney cortical region.
Renal injury marker determination
Albumin and the proximal tubular injury marker NGAL were determined in urine by ELISA (GenWay Biotech, Inc, San Diego, CA, and Abcam, respectively) and urinary excretion calculated. Renal cortical expression and excretion of KIM-1 were also determined by ELISA (R&D Systems, Minneapolis, MN). Protein excretion by the experimental animals was determined by a Bradford assay (Bio-Rad). Excretion rates were calculated using gravimetrically determined values for 24-hr urine volume collected in metabolic cages at the beginning of the study (normal salt) and at the end of the 3 week period of on high salt diet.
Assessment of ET-1, TNF-α, H2O2 and TBARS excretion
ET-1 levels in undiluted urine samples were determined by a chemiluminescent assay (Human ET-1 QuantiGlo kit; R&D Systems). Urinary TNF-α concentration was determined by ELISA (R&D Systems), and H2O2 concentration was determined using the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (Thermo Fisher Scientific; Waltham, MA). Urinary concentration of Thiobarbituric Acid Reactive Substances (TBARS) was also assessed by ELISA (Cayman Chemical; Ann Harbor, MI). Excretion rates were determined from 24-hr urine volume collected in metabolic cages at the end of the 3 week period of on high salt diet.
Measurement of glomerular albumin permeability (Palb)
Glomeruli were isolated by consecutively passing the isolated renal cortex through a series of filters of decreasing pore size (180, 100 and 75 μm), as previously described;89, 90 the isolation was performed on ice. This technique results in intact and decapsulated glomeruli suspended in ice-cold Hanks balanced salt solution (pH 7.4, Corning, Corning, NY). The freshly isolated glomeruli were affixed to poly-L-lysine-covered wells in a 6 well-plate. Changes in glomerular volume were recorded in response to an oncotic gradient created by changing the concentrations of albumin in the medium. The consequent changes in glomerular volume were measured using CellSens software (Olympus America). Glomerular permeability to albumin, expressed as convectional Palb, was calculated as Palb = 1 - albumin reflection coefficient, as previously described.91
Isolation of cortical immune cells and flow cytometry
Immune cells were isolated from renal cortical tissue as previously described with minor modifications.92 In brief, rats were anesthetized with isoflurane and a catheter was implanted in the abdominal aorta to perfuse the kidneys with saline. The kidneys were then collected and the renal cortex was isolated from the left kidney, minced and digested with collagenase in DMEM for 30 min and 37°C in a shaking water bath. The digestate was then passed 3 times through a 40μm filter (ThermoFisher, Waltham, MA) and washed with PEB buffer. Red blood cells were lysis with ACK lysis buffer for 3 min at room temperature and then washed with PBS.
The isolated cortical immune cells were then washed with staining buffer and incubated on ice for 30 minutes with the following mouse-anti rat antibodies: phycoerythrin-conjugated anti-CD45 (CD45-PE, clone OX-1), fluorescein isothiocyanate-conjugated anti-CD3 (CD3- FITC, clone G4.18), allophycocyanin-conjugated anti-CD4 (CD4-APC, clone OX-35), peridinin chlorophyll protein complex-conjugated anti-CD8 (CD8- PerCP, clone OX-8), PE-cyanine 7 anti-CD11b/c (CD11b/c- PE-Cy7, clone OX-42), and peridinin chlorophyll protein complex-conjugated anti-MHC-II (MHC-II- PerCP, clone OX-17). To block the unspecific binding to the Fcγ3 receptors, cells were also incubated with mouse anti-rat CD16/32. E450-conjugated fixable viability stain was also added to the samples. All antibodies were purchased from BD Biosciences (San Jose, CA), except MHC-II- PerCP that was purchased from eBiosciences (San Diego, CA). Data acquisition was performed using a LSRII flow cytometer (BD Biosciences) and FACSDiva software (BD Biosciences). Results were analyzed with FlowJo software (Tree Star Inc, Ashland, OR).
Statistical analysis
All data are expressed as mean ± SEM. Differences between genotypes and treatments were analyzed by two-way analysis of variance with a Tukey’s post hoc test. MAP was analyzed by two-way repeated measures ANOVA. A P value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using GraphPad Prism 6 (GraphPad Software; La Jolla, CA).
Acknowledgements
The authors would like to thank Xiaofen Liu for her expertise in histology. This work was funded in part by the National Institutes of Health (T32 DK007545 and P01 HL136267–01S1 to C.D.M.; F31 DK111067 to R.S.S.; F31 DK115169 and T32 GM008361 to J.M.L.; P01 HL136267, P01 HL69999, U01 HL117684 grants and 15SFRN2390002 to J.S.P. and D.M.P.) and the American Society of Nephrology Joseph A. Carlucci Research Fellowship to M.K.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kohan D, Inscho EW, Wesson D, and Pollock DM: Physiology of endothelin and the kidney. Compr Physiol, 1: 883–919, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M: Salt-sensitive hypertension in endothelin-B receptor–deficient rats. The Journal of clinical investigation, 105: 925–933, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elisa T, Antonio P, Giuseppe P, Alessandro B, Giuseppe A, Federico C, Marzia D, Ruggero B, Giacomo M, Andrea O, Daniela R, Mariaelisa R, Claudio L: Endothelin Receptors Expressed by Immune Cells Are Involved in Modulation of Inflammation and in Fibrosis: Relevance to the Pathogenesis of Systemic Sclerosis. Journal of immunology research, 2015: 147616, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guruli G, Pflug BR, Pecher S, Makarenkova V, Shurin MR, Nelson JB: Function and survival of dendritic cells depend on endothelin-1 and endothelin receptor autocrine loops. Blood, 104 2107–2115, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Juergens UR, Racke K, Uen S, Haag S, Lamyel F, Stober M, Gillissen A, Novak N, Vetter H: Inflammatory responses after endothelin B (ETB) receptor activation in human monocytes: new evidence for beneficial anti-inflammatory potency of ETB-receptor antagonism. Pulmonary pharmacology & therapeutics, 21: 533–539, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Mencarelli M, Pecorelli A, Carbotti P, Valacchi G, Grasso G, Muscettola M: Endothelin receptor A expression in human inflammatory cells. Regulatory peptides, 158: 1–5, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Shindo T, Kurihara H, Maemura K, Kurihara Y, Ueda O, Suzuki H, Kuwaki T, Ju KH, Wang Y, Ebihara A, Nishimatsu H, Moriyama N, Fukuda M, Akimoto Y, Hirano H, Morita H, Kumada M, Yazaki Y, Nagai R, Kimura K: Renal damage and salt-dependent hypertension in aged transgenic mice overexpressing endothelin-1. Journal of molecular medicine (Berlin, Germany), 80: 105–116, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Kassab S, Miller MT, Novak J, Reckelhoff J, Clower B, Granger JP: Endothelin-A receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertension (Dallas, Tex : 1979), 31: 397–402, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Vaneckova I, Kramer HJ, Backer A, Vernerova Z, Opocensky M, Cervenka L: Early endothelin-A receptor blockade decreases blood pressure and ameliorates end-organ damage in homozygous Ren-2 rats. Hypertension (Dallas, Tex : 1979), 46: 969–974, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Samad MA, Kim UK, Kang JJ, Ke Q, Kang PM: Endothelin A receptor antagonist, atrasentan, attenuates renal and cardiac dysfunction in Dahl salt-hypertensive rats in a blood pressure independent manner. PloS one, 10: e0121664, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elijovich F, Laffer CL, Amador E, Gavras H, Bresnahan MR, Schiffrin EL: Regulation of plasma endothelin by salt in salt-sensitive hypertension. Circulation, 103: 263–268, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Pollock DM, Pollock JS: Evidence for endothelin involvement in the response to high salt. American Journal of Physiology - Renal Physiology, 281: F144–F150, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hyndman KA, Dugas C, Arguello AM, Goodchild TT, Buckley KM, Burch M, Yanagisawa M, Pollock JS: High salt induces autocrine actions of ET-1 on inner medullary collecting duct NO production via upregulated ETB receptor expression. American journal of physiology Regulatory, integrative and comparative physiology, 311: R263–271, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor TA, Gariepy CE, Pollock DM, Pollock JS: Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension (Dallas, Tex : 1979), 41: 657–662, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Tazawa N, Okada Y, Nakata M, Izumoto H, Takasu M, Takaoka M, Gariepy CE, Yanagisawa M, Matsumura Y: Exaggerated vascular and renal pathology in endothelin-B-receptor-deficient rats with subtotal nephrectomy. Journal of cardiovascular pharmacology, 44 Suppl 1: S467–470, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Okada Y, Nakata M, Izumoto H, Takasu M, Tazawa N, Takaoka M, Gariepy CE, Yanagisawa M, Matsumura Y: Role of endothelin ETB receptor in partial ablation-induced chronic renal failure in rats. European journal of pharmacology, 494: 63–71, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hocher B, Dembowski C, Slowinski T, Friese ST, Schwarz A, Siren AL, Neumayer HH, Thone-Reineke C, Bauer C, Nafz B, Ehrenreich H: Impaired sodium excretion, decreased glomerular filtration rate and elevated blood pressure in endothelin receptor type B deficient rats. Journal of molecular medicine (Berlin, Germany), 78: 633–641, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA: Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation, 126: 963–974, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortez L, Sim V: The therapeutic potential of chemical chaperones in protein folding diseases. Prion, 8: 197–202, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ: Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Molecular medicine (Cambridge, Mass), 4: 165–178, 1998. [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ: A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. The Journal of clinical investigation, 101: 2790–2799, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, Rodrigues CM: miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. Journal of hepatology, 58: 119–125, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Castro RE, Sola S, Ramalho RM, Steer CJ, Rodrigues CM: The bile acid tauroursodeoxycholic acid modulates phosphorylation and translocation of bad via phosphatidylinositol 3-kinase in glutamate-induced apoptosis of rat cortical neurons. The Journal of pharmacology and experimental therapeutics, 311: 845–852, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hagey LR, Crombie DL, Espinosa E, Carey MC, Igimi H, Hofmann AF: Ursodeoxycholic acid in the Ursidae: biliary bile acids of bears, pandas, and related carnivores. Journal of lipid research, 34: 1911–1917, 1993. [PubMed] [Google Scholar]
- 25.Sola S, Garshelis DL, Amaral JD, Noyce KV, Coy PL, Steer CJ, Iaizzo PA, Rodrigues CM: Plasma levels of ursodeoxycholic acid in black bears, Ursus americanus: seasonal changes. Comparative biochemistry and physiology Toxicology & pharmacology : CBP, 143: 204–208, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Festi D, Montagnani M, Azzaroli F, Lodato F, Mazzella G, Roda A, Di Biase AR, Roda E, Simoni P, Colecchia A: Clinical efficacy and effectiveness of ursodeoxycholic acid in cholestatic liver diseases. Current clinical pharmacology, 2: 155–177, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Paumgartner G, Beuers U: Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology (Baltimore, Md), 36: 525–531, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR: Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology, 136: 1281–1287, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Omata M, Yoshida H, Toyota J, Tomita E, Nishiguchi S, Hayashi N, Iino S, Makino I, Okita K, Toda G, Tanikawa K, Kumada H: A large-scale, multicentre, double-blind trial of ursodeoxycholic acid in patients with chronic hepatitis C. Gut, 56: 1747–1753, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan WM, Rodrigues CM, Zhao LR, Steer CJ, Low WC: Tauroursodeoxycholic acid improves the survival and function of nigral transplants in a rat model of Parkinson’s disease. Cell transplantation, 11: 195–205, 2002. [PubMed] [Google Scholar]
- 31.Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, Liu L, Przedborski S, Wolozin B: Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. The Journal of biological chemistry, 280: 42655–42668, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro-Caldas M, Carvalho AN, Rodrigues E, Henderson CJ, Wolf CR, Rodrigues CM, Gama MJ: Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Molecular neurobiology, 46: 475–486, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC: Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proceedings of the National Academy of Sciences of the United States of America, 99: 10671–10676, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viana RJ, Nunes AF, Castro RE, Ramalho RM, Meyerson J, Fossati S, Ghiso J, Rostagno A, Rodrigues CM: Tauroursodeoxycholic acid prevents E22Q Alzheimer’s Abeta toxicity in human cerebral endothelial cells. Cellular and molecular life sciences : CMLS, 66: 1094–1104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dionisio PA, Amaral JD, Ribeiro MF, Lo AC, D’Hooge R, Rodrigues CM: Amyloid-beta pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiology of aging, 36: 228–240, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Lo AC, Callaerts-Vegh Z, Nunes AF, Rodrigues CM, D’Hooge R: Tauroursodeoxycholic acid (TUDCA) supplementation prevents cognitive impairment and amyloid deposition in APP/PS1 mice. Neurobiology of disease, 50: 21–29, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Nunes AF, Amaral JD, Lo AC, Fonseca MB, Viana RJ, Callaerts-Vegh Z, D’Hooge R, Rodrigues CM: TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-beta deposition in APP/PS1 mice. Molecular neurobiology, 45: 440–454, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Rivard AL, Steer CJ, Kren BT, Rodrigues CM, Castro RE, Bianco RW, Low WC: Administration of tauroursodeoxycholic acid (TUDCA) reduces apoptosis following myocardial infarction in rat. The American journal of Chinese medicine, 35: 279–295, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Li S, Abedin MJ, Noppakun K, Wang L, Kaur T, Najafian B, Rodrigues CM, Steer CJ: Prevention of acute kidney injury by tauroursodeoxycholic acid in rat and cell culture models. PloS one, 7: e48950, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao X, Fu L, Xiao M, Xu C, Sun L, Zhang T, Zheng F, Mei C: The nephroprotective effect of tauroursodeoxycholic acid on ischaemia/reperfusion-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Basic & clinical pharmacology & toxicology, 111: 14–23, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Fan Y, Xiao W, Lee K, Salem F, Wen J, He L, Zhang J, Fei Y, Cheng D, Bao H, Liu Y, Lin F, Jiang G, Guo Z, Wang N, He JC: Inhibition of Reticulon-1A-Mediated Endoplasmic Reticulum Stress in Early AKI Attenuates Renal Fibrosis Development. J Am Soc Nephrol, 28: 2007–2021, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo H, Li H, Ling L, Gu Y, Ding W: Endoplasmic Reticulum Chaperon Tauroursodeoxycholic Acid Attenuates Aldosterone-Infused Renal Injury. Mediators of inflammation, 2016: 4387031, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Fan Y, Zeng C, He L, Wang N: Tauroursodeoxycholic Acid Attenuates Renal Tubular Injury in a Mouse Model of Type 2 Diabetes. Nutrients, 8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marquardt A, Al-Dabet MM, Ghosh S, Kohli S, Manoharan J, ElWakiel A, Gadi I, Bock F, Nazir S, Wang H, Lindquist JA, Nawroth PP, Madhusudhan T, Mertens PR, Shahzad K, Isermann B: Farnesoid X Receptor Agonism Protects against Diabetic Tubulopathy: Potential Add-On Therapy for Diabetic Nephropathy. J Am Soc Nephrol, 28: 3182–3189, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen EI, Birn H: Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. American journal of physiology Renal physiology, 280: F562–573, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, Kaldenbach M, Boor P, Fuss A, Uhlig S, Lanzmich R, Willemsen B, Dijkman H, Grepl M, Wild K, Kriz W, Smeets B, Floege J, Moeller MJ: Albumin is recycled from the primary urine by tubular transcytosis. J Am Soc Nephrol, 24: 1966–1980, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickson LE, Wagner MC, Sandoval RM, Molitoris BA: The Proximal Tubule and Albuminuria: Really! Journal of the American Society of Nephrology : JASN, 25: 443–453, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int, 71: 504–513, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B: Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology (Baltimore, Md), 36: 592–601, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez-Castro MB, Elias-Miro M, Mendes-Braz M, Lemoine A, Rimola A, Rodes J, Casillas-Ramirez A, Peralta C: Tauroursodeoxycholic acid affects PPARgamma and TLR4 in Steatotic liver transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 12: 3257–3271, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Ergul A, Tackett RL, Puett D: Distribution of endothelin receptors in saphenous veins of African Americans: implications of racial differences. Journal of cardiovascular pharmacology, 34: 327–332, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Speed JS, LaMarca B, Berry H, Cockrell K, George EM, Granger JP: Renal medullary endothelin-1 is decreased in Dahl salt-sensitive rats. American journal of physiology Regulatory, integrative and comparative physiology, 301: R519–523, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Fan Y, Zeng C, He L, Wang N: Tauroursodeoxycholic Acid Attenuates Renal Tubular Injury in a Mouse Model of Type 2 Diabetes. Nutrients, 8: 589, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun J, Hultenby K, Axelsson J, Nordstrom J, He B, Wernerson A, Lindstrom K: Proximal Tubular Expression Patterns of Megalin and Cubilin in Proteinuric Nephropathies. Kidney international reports, 2: 721–732, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen R, Christensen EI, Birn H: Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int, 89: 58–67, 2016. [DOI] [PubMed] [Google Scholar]
- 56.Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Muller I, Andreassen TT, Wolf E, Bachmann S, Nykjaer A, Willnow TE: Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 17: 247–249, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE: An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell, 96: 507–515, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Kasztan M, Piwkowska A, Kreft E, Rogacka D, Audzeyenka I, Szczepanska-Konkel M, Jankowski M: Extracellular purines’ action on glomerular albumin permeability in isolated rat glomeruli: insights into the pathogenesis of albuminuria. American journal of physiology Renal physiology, 311: F103–111, 2016. [DOI] [PubMed] [Google Scholar]
- 59.Kasztan M, F B, Speed JS, De Miguel C, Gohar EY, Townes TM, Kutlar A, Pollock JS, Pollock DM: Long-Term Endothelin-A Receptor Antagonism Provides Robust Renal Protection in Humanized Sickle Cell Disease Mice. J Am Soc Nephrol, 28: 2443–2458, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasser JM, S J, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS: Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol, 18: 143–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saleh MA, Sandoval RM, Rhodes GJ, Campos-Bilderback SB, Molitoris BA, Pollock DM: Chronic endothelin-1 infusion elevates glomerular sieving coefficient and proximal tubular albumin reuptake in the rat. Life sciences, 91: 634–637, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aslan M, Kirac E, Yilmaz O, Unal B, Konuk EK, Ozcan F, Tuzcu H: Effect of tauroursodeoxycholic acid on PUFA levels and inflammation in an animal and cell model of hepatic endoplasmic reticulum stress. Human & experimental toxicology: 960327117734621, 2017. [DOI] [PubMed] [Google Scholar]
- 63.Wang W, Zhao J, Gui W, Sun D, Dai H, Xiao L, Chu H, Du F, Zhu Q, Schnabl B, Huang K, Yang L, Hou X: Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in NAFLD mice. British journal of pharmacology, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amin A, Choi SK, Galan M, Kassan M, Partyka M, Kadowitz P, Henrion D, Trebak M, Belmadani S, Matrougui K: Chronic inhibition of endoplasmic reticulum stress and inflammation prevents ischaemia-induced vascular pathology in type II diabetic mice. The Journal of pathology, 227: 165–174, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang A, Ko K, Clark MR: The Emerging Role of the Inflammasome in Kidney Diseases. Current opinion in nephrology and hypertension, 23: 204–210, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hutton HL, Ooi JD, Holdsworth SR, Kitching AR: The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton, Vic), 21: 736–744, 2016. [DOI] [PubMed] [Google Scholar]
- 67.Masood H, Che R, Zhang A: Inflammasomes in the Pathophysiology of Kidney Diseases. Kidney Diseases, 1: 187–193, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodrigues CM, Stieers CL, Keene CD, Ma X, Kren BT, Low WC, Steer CJ: Tauroursodeoxycholic acid partially prevents apoptosis induced by 3-nitropropionic acid: evidence for a mitochondrial pathway independent of the permeability transition. Journal of neurochemistry, 75: 2368–2379, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Doerflinger M, Glab J, Nedeva C, Jose I, Lin A, O’Reilly L, Allison C, Pellegrini M, Hotchkiss RS, Puthalakath H: Chemical chaperone TUDCA prevents apoptosis and improves survival during polymicrobial sepsis in mice. Scientific reports, 6: 34702, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM: Bile acids: regulation of apoptosis by ursodeoxycholic acid. Journal of lipid research, 50: 1721–1734, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rath PC, Aggarwal BB: TNF-Induced Signaling in Apoptosis. Journal of Clinical Immunology, 19: 350–364, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM: TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. American journal of physiology Regulatory, integrative and comparative physiology, 294: R76–83, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elmarakby AA, Quigley JE, Pollock DM, Imig JD: Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension (Dallas, Tex : 1979), 47: 557–562, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Hildner K, Finotto S, Becker C, Schlaak J, Schirmacher P, Galle PR, Marker-Hermann E, Neurath MF: Tumour necrosis factor (TNF) production by T cell receptor-primed T lymphocytes is a target for low dose methotrexate in rheumatoid arthritis. Clinical and experimental immunology, 118: 137–146, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boesen EI, Krishnan KR, Pollock JS, Pollock DM: ET(A) Activation Mediates Angiotensin II-Induced Infiltration of Renal Cortical T Cells. Journal of the American Society of Nephrology : JASN, 22: 2187–2192, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sasser JM, Pollock JS, Pollock DM: Renal endothelin in chronic angiotensin II hypertension. American journal of physiology Regulatory, integrative and comparative physiology, 283: R243–248, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Kittikulsuth W, Pollock JS, Pollock DM: Loss of renal medullary endothelin B receptor function during salt deprivation is regulated by angiotensin II. American journal of physiology Renal physiology, 303: F659–666, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ballew JR, Fink GD: Role of ET(A) receptors in experimental ANG II-induced hypertension in rats. American journal of physiology Regulatory, integrative and comparative physiology, 281: R150–154, 2001. [DOI] [PubMed] [Google Scholar]
- 79.Barton M, d’Uscio LV, Shaw S, Meyer P, Moreau P, Luscher TF: ET(A) receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension (Dallas, Tex : 1979), 31: 499–504, 1998. [DOI] [PubMed] [Google Scholar]
- 80.d’Uscio LV, Moreau P, Shaw S, Takase H, Barton M, Luscher TF: Effects of chronic ETA-receptor blockade in angiotensin II-induced hypertension. Hypertension (Dallas, Tex : 1979), 29: 435–441, 1997. [DOI] [PubMed] [Google Scholar]
- 81.Moreau P, d’Uscio LV, Shaw S, Takase H, Barton M, Luscher TF: Angiotensin II increases tissue endothelin and induces vascular hypertrophy: reversal by ET(A)-receptor antagonist. Circulation, 96: 1593–1597, 1997. [DOI] [PubMed] [Google Scholar]
- 82.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG: Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension (Dallas, Tex : 1979), 30: 29–34, 1997. [DOI] [PubMed] [Google Scholar]
- 83.Gellai M, DeWolf R, Pullen M and Nambi P: Distribution and functional role of renal ET receptor subtypes in normotensive and hypertensive rats. Kidney Int, 46: 1287–1294, 1994. [DOI] [PubMed] [Google Scholar]
- 84.Taylor TA, Gariepy CE, Pollock DM and Pollock JS: Unique endothelin receptor binding in kidneys of ETB receptor deficient rats. American journal of physiology Regulatory, integrative and comparative physiology, 284: R674–681, 2003. [DOI] [PubMed] [Google Scholar]
- 85.De Miguel C, Hamrick WC, Hobbs JL, Pollock DM, Carmines PK, Pollock JS: Endothelin receptor-specific control of endoplasmic reticulum stress and apoptosis in the kidney. Scientific reports, 7: 43152, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE: Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. The Journal of clinical investigation, 114: 504–511, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE: Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. American journal of physiology Renal physiology, 291: F1274–1280, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Schneider MP, Inscho EW, Pollock DM: Attenuated vasoconstrictor responses to endothelin in afferent arterioles during a high-salt diet. American journal of physiology Renal physiology, 292: F1208–1214, 2007. [DOI] [PubMed] [Google Scholar]
- 89.Misra RP: Isolation of Glomeruli from Mammalian Kidneys by Graded Sieving. American Journal of Clinical Pathology, 58: 135–139, 1972. [DOI] [PubMed] [Google Scholar]
- 90.Piwkowska A, Rogacka D, Kasztan M, Angielski S, Jankowski M: Insulin increases glomerular filtration barrier permeability through dimerization of protein kinase G type Iα subunits. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1832: 791–804, 2013. [DOI] [PubMed] [Google Scholar]
- 91.Savin VJ, Sharma R, Lovell HB, Welling DJ: Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol, 3: 1260–1269, 1992. [DOI] [PubMed] [Google Scholar]
- 92.Hull TD, Bolisetty S, DeAlmeida AC, Litovsky SH, Prabhu SD, Agarwal A, George JF: Heme oxygenase-1 expression protects the heart from acute injury caused by inducible Cre recombinase. Laboratory investigation; a journal of technical methods and pathology, 93: 868–879, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z: Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochemical and biophysical research communications, 370: 651–656, 2008. [DOI] [PubMed] [Google Scholar]
- 94.Lim MP, Devi LA, Rozenfeld R: Cannabidiol causes activated hepatic stellate cell death through a mechanism of endoplasmic reticulum stress-induced apoptosis. Cell death & disease, 2: e170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lipson KL, Ghosh R, Urano F: The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells. PloS one, 3: e1648, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ren MY, Wu XY: Toll-like receptor 4 signalling pathway activation in a rat model of Acanthamoeba Keratitis. Parasite immunology, 33: 25–33, 2011. [DOI] [PubMed] [Google Scholar]