Abstract
Rationale and Objectives:
Healthy aging is associated with pervasive declines in cognitive, motor, and sensory functioning. There are, however, substantial individual differences in behavioral performance among older adults. Several lines of animal research link age-related reductions of gamma-aminobutyric acid (GABA), the brain’s primary inhibitory neurotransmitter, to age-related cognitive, motor, and sensory decline. Our study used proton magnetic resonance spectroscopy (MRS) at 3T to explore whether occipital GABA declines with age in humans and whether individual differences in occipital GABA are linked to individual differences in fluid processing ability.
Materials and Methods:
We used a MEGA-PRESS sequence that combines frequency spectral editing with a point-resolved spectroscopy sequence to quantify GABA. Spectra were obtained from a 30 × 30 × 25 mm voxel placed in the occipital cortex of 20 young adults (mean age 20.7 years) and 18 older adults (mean age 76.5 years). Participants also performed 11 fluid processing tasks outside the scanner, the results of which were z-scored and averaged to calculate a summary measure of fluid processing ability. Regression analysis was employed to determine the relationship between GABA concentrations in the occipital cortex and a summary measure of fluid processing ability.
Results:
Occipital GABA was significantly lower in older participants compared to the younger participants. We also observed a significant positive relationship between occipital GABA and fluid processing ability. In fact, higher GABA was associated with better task performance in 10 of the 11 tasks.
Conclusion:
These findings suggest that GABA levels decline with age in humans and are associated with declines in fluid processing ability.
Keywords: Magnetic resonance spectroscopy, Aging, MEGA-PRESS, Gamma-aminobutyric acid, Normal brain
INTRODUCTION
Healthy aging is associated with reduced fluid processing as indexed by impairments in working memory, long-term memory, and speed of processing. However, there are substantial individual differences in the effects of age on cognition. Some healthy older adults exhibit significant fluid processing impairments while others do not (13,15,39). Given the enormous personal and societal costs that are associated with age-related cognitive decline, it is important to determine factors that influence the severity of this phenomenon.
One factor may be age-related reductions of gamma-amino-butyric acid (GABA), the primary inhibitory neurotransmitter of the brain. Genes related to GABA-ergic function are down-regulated during normal aging in humans and rhesus macaques (20), and several lines of research in animals suggest that GABA function declines with age. For example, the number of GABA-immunoreactive neurons declines with age in the inferior colliculus (6), hippocampus (35), and striate visual cortex (17). There are also age-related reductions in baseline GABA, GABA release, and GABA receptor binding (6).
Furthermore, GABA function is linked with behavior in animals. For example, reduced hippocampal GABA(B) receptor expression is associated with spatial learning impairments in rats (22). In addition, apolipoprotein E4 knock-in mice exhibit age-related reductions in GABAergic interneurons that are associated with learning and memory impairments. However, those impairments are eliminated by daily injections of a GABA(A) receptor potentiator (1) and return if the injections are subsequently with-held (37). These findings raise the possibility that age-related declines of cognition are associated with age-related declines of GABA functioning.
Recently, it has become possible to study GABA in humans using magnetic resonance spectroscopy (MRS), a noninvasive technique that can estimate regional metabolite levels in vivo. Using this technique, researchers have revealed age-related declines in GABA in frontal, parietal, and posterior brain regions (11,12,27). MRS studies have also reported relationships between GABA measures and behavioral performance in a number of domains, including visual function (9,40), motor control (5), tactile function (14,28), and executive function (18,23,40).
These studies suggest that GABA levels tend to decline with age and that GABA function predicts behavior. Recent evidence also suggests that cognitive declines associated with aging may be related to age-related declines in GABA concentration, with declines in frontal GABA concentration associated with lower scores on the Montreal Cognitive Assessment, a widely used screening assessment for detecting cognitive impairment (27).
Here, we investigated whether age-related declines of GABA are associated with a very important aspect of age-related cognitive decline: reduced fluid processing ability (e.g., speed of processing and executive functioning). The aim of the present study was to test this hypothesis by combining MR spectroscopy with behavioral methods for assessing cognitive functioning.
We focused on the occipital cortex for three reasons. First, orientation and direction discrimination decline with age at both the neural (33) and behavioral (3) levels. Second, age-related declines in the orientation selectivity of visual cortex neurons can be reversed by the administration of GABA or a GABA agonist (19), thereby demonstrating that GABA plays a causal role in visual functioning. Third, prior findings from our group indicate that functional magnetic resonance imaging measures of heightened object selectivity in the human occipital cortex are associated with increased fluid processing ability (26).
METHODS
Participants
Twenty older participant (8 males; aged, 59–87 years; mean = 76.5 years, SD = 8.73 years) and nineteen younger participants (9 males; aged 18–23 years, mean = 20.74 years, SD = 1.37 years) were recruited for this study. All participants provided informed consent following a protocol approved by the Institutional Review Board of the University of Michigan and were screened for magnetic resonance contraindications. Individuals were eligible to participate if they were fight handed and beleen 18 and 30 years old (young group) or over 59 (older group). Exclusion criteria included pregnancy, any history of neurological or psychiatric disorder, or any history of drug or alcohol abuse.
Participants attended a behavioral testing session, followed approximately two weeks later by an imaging session (intersession interval, 2–28 days, mean = 11.97 days, SD = 7.10 days). Participants were paid $10 per hour for the behavioral session, and $20 per hour for the imaging session.
Behavicral Testing
During the behavioral session, participants completed the Mini-Mental State Exam, the extended range vocabulary test, and 11 fluid processing tasks. Of the 11 tasks, seven measured perceptual processing (Contour detection, Digit-symbol coding, Pattern comparison, Cambridge Face Perception Test - Upright (CFPT upright), Cambridge Face Perception Test - Inverted (CFPT inverted), Dot speed and Dot coherence), three measured executive function (Controlled Oral Word Association test (COWAT), Trail making test A and Trail making test B) and one measured memory (Cambridge Face Memory Test (CFMT)). Of importance, performance on all of these tasks typically declines with age.
CFMT, (8):
Participants are introduced to six male target faces, and asked to learn each face in three views. Participants are presented with three faces and asked to choose the learned face from two distractors. For each target face, three test items contain views identical to those studied in the introduction, five present novel views and four present novel views with noise. Performance on the CFMT has been shown to decline with age (4).
Contour Detection:
Participants were presented with groups of lines and asked to determine whether some of the lines formed a circle or an ellipse. A staircase procedure was used to determine how much jitter in line orientation participants could tolerate, while still being able to make a correct judgment. Age-related impairments in contour integration have previously been observed (30).
Digit-Symbol Coding:
Participants viewed nine digit-symbol pairs followed by a list of digits, and were asked to write the symbol that corresponded to each digit as quickly as possible. The number of correct symbols participants produced within 90 seconds was measured. The task was taken from the Wechsler adult intelligence scale-third edition (38), and performance on this task shows a robust age related decline from around the age of 45 (16).
Pattern Comparison (31):
Participants were asked to determine if two patterns of lines were the same or different. Participants performed this task on three sets of 32 pattern pairs with 30 seconds to complete each set. The total number of correct judgments across all three sets was measured. Notably, performance on pattern comparison tasks declines with age (32).
COWAT, (2):
Participants were instructed to name as many words as they could that started with a target letter in 1 minute. The target letters were “F,” “A,” and “S.” The total number of words generated across all three letters was measured. Verbal fluency measures such as this have been observed to be sensitive to the effects ofaging (36).
CFPT upright (7):
Participants ranked six upright faces with respect to their similarity to a target face. Average deviation from the correct rank order was measured. Performance on the CFPT upright declines with age (4).
CFPT inverted (7):
Participants ranked six inverted faces with respect to their similarity to a target face. Average deviation from the correct rank order was measured. As with the CFPT upright, performance on the CFPT inverted demonstrates a marked decline with age (4).
Dot Speed:
Participants viewed two groups of moving dots and were asked to determine which group was moving faster. A staircase procedure was used to find individual thresholds. Participants completed two blocks of the task, each containing approximately 55 trials. An age-related loss of sensitivity to visual motion has been observed in older individuals (34).
Dot Coherence:
Participants viewed two groups of dots and determined which group moved more coherently. A staircase procedure was used to find individual thresholds. Participants completed two blocks of the task, each of which contained approximately 55 trials. An age-related deficit in motion coherence thresholds for slow speeds has been observed (34).
Trail Making Test A:
Participants were asked to draw lines connecting the numbers 1–25 in order. The numbers appeared in random positions on a sheet of paper. The amount of time it took to complete the task was measured. Significant age-related increases in completion time have been observed in this task (29).
Trail Making Test B:
Participants were asked to draw connecting lines between alternating numbers and letters in ascending order (e.g., 1, A, 2, B, 3, C, etc.). The characters appeared in random positions on a sheet of paper. The amount of time it took to complete the task was measured. As with the Trail Making Test A, significant age-related increases in completion time have been observed on the Trail Making Test B (29).
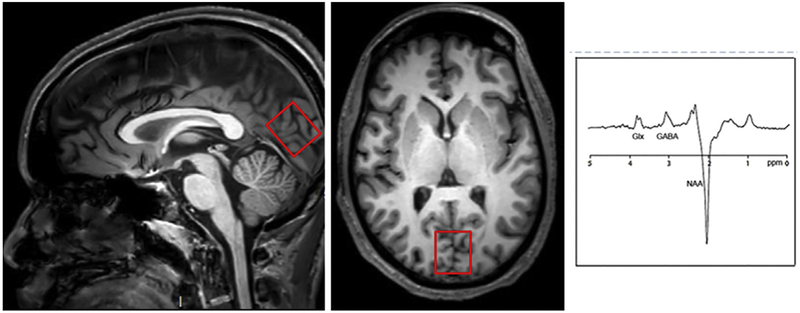
MR Spectroscopy
MR spectroscopy data were acquired using a 3T Phillips Ingenia system (Best, The Netherlands) with a 32-channel head coil. For each subject, we performed a T1 weighted 3D-MPRAGE sequence with 0.9 mm3 resolution for MRS voxel placement and subsequent tissue segmentation. Magnetic resonance spectroscopy spectra were collected from a 30 × 30 × 25 mm voxel placed in the primary visual cortex (Fig 1). Single-voxel point resolved spectroscopy spectra (PRESS) were collected using the following scanning parameters: TR/TE = 2000/35 ms and “VAPOR” water suppression with 32 averages (approximately 1 minute). A MEGAPRESS sequence combining frequency spectral editing with point-resolved spectroscopy was used to distinguish GABA from other metabolites. Details of this sequence were as follows: TE = 68 milliseconds (TE1 = 15 milliseconds, TE2 = 53 milliseconds); TR = 1.8 seconds; 256 transients of 2000 data points; spectral width = 2 kHz; frequency selective editing pulses (14 milliseconds) applied at 1.9 ppm (ON) and 7.46 ppm (OFF); total scan time of approximately 10 minutes per voxel; refocusing performed using an amplitude-modulated pulse “GTST1203” (length = 7 milliseconds, bandwidth = 1.2 kHz). The signal detected at 3.02 ppm using these experimental parameters is expected to contain contributions from both macromolecules and homocarnosine, and is therefore referred to as “GABA+.”
Figure 1.
Left: Axial and sagittal Tl-weighted images showing location of 1H-MRS voxel placements for the occipital cortex. Right: representative 1H-MRS MRS spectrum in the occipital cortex. Chemical shifts are indicated in parts per million (ppm). GABA is at 3.0 ppm.
DATA PREPROCESSING/ANALYSIS
Spectral Fitting
The PRESS spectra were analyzed using LCModel (http://s-provencher.com/lcmodel.shtml), which estimates the concentrations of glutamate and glutamine (Glx), N-acetyl aspartate (NAA), choline, and Myo-inositol (Myo). GANNET, a MATLAB toolbox (10), was used to preprocess and quantify the GABA+ signal in the MEGA-PRESS scans, using the default parameters, including frequency and phase correction of time-resolved data using spectral registration (24). The MEGA-PRESS scans are more vulnerable to field drifts and movement artifacts than typical PRESS scans because of the addition of an editing pulse and the subtraction of ON and OFF scans to obtain difference spectra, potentially leading to subtraction artifacts. Frequency correction can reduce quantification errors. All spectra were visually inspected for subtraction artifacts and noise levels. All metabolite concentrations were estimated relative to creatine as occipital creatine has been shown to be stable in the aging population (21). Metabolite concentrations were only used for statistical analysis from the LC model if the Cramer-Rao bounds were less than 20%.
Statistical Analysis
Statistical analyses were performed using the Statistical toolbox of MATLAB R2016a (MathWorks, Natick). To test the hypothesis that metabolite ratios in the visual cortex are reduced in older individuals compared to younger ones, onetailed, two-sample t tests were performed. Age-related differences in performance on behavioral tasks were also investigated using one-tailed, two-sample t tests, since lower performance was predicted in the older participants. To investigate the link between behavioral performance and GABA concentrations, a summary measure of fluid processing was extracted from the data. Performance on each of the eleven tasks was z-scored, resulting in eleven z-scores for each participant. Each of these z-scores reflected a participant’s performance on one of the 11 tasks relative to the performance of all other participants. The eleven z-scores for each participant were then averaged to obtain a single summary measure of fluid processing ability. As the 11 behavioral tasks could be classified into two groups—those in which better performance was indicated by higher scores (e.g., accuracy or number of items completed) and those in which better performance was indicated by lower scores (e.g., reaction time)—the sign was flipped (from negative to positive) for the z scores of the tasks in which lower scores are indicative of better performance. This ensured that positive z-scores would always reflect better performance.
Regression analyses were employed to determine the relationship between metabolite ratios in the occipital cortex and the summary measure of fluid processing ability. The main analyses investigated relationships between GABA and fluid processing ability, but relationships between other metabolites and fluid processing ability were also explored. Following the main analyses, correlations between GABA concentration and performance on each of the individual tasks were computed. Regressions were also performed separately for young and old participant groups, to explore age-specific relationships.
RESULTS
The demographic characteristics of the participants are summarized in Table 1. We found no significant age-related differences in the mean score of Mini-Mental State Exam. Older participants performed significantly better on the extended range vocabulary test.
TABLE 1.
Demographic Characteristics of Young Adult and Older Adult Groups, and Group Differences As Determined by Two-Sample t Tests.
| Young Adults (n = 17) | Older Adults (n = 18) | Group Difference | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t37 | p | |
| Age | 20.74 | 1.37 | 76.50 | 8.73 | 27.50 | <0.001 |
| MMSE | 29.42 | 0.69 | 29.35 | 1.14 | 0.23 | 0.82 |
| ERVT | 19.71 | 6.17 | 31.35 | 10.29 | 4.26 | <0.001 |
EVRT, extended range vocabulary test; MMSE, Mini-Mental State Exam.
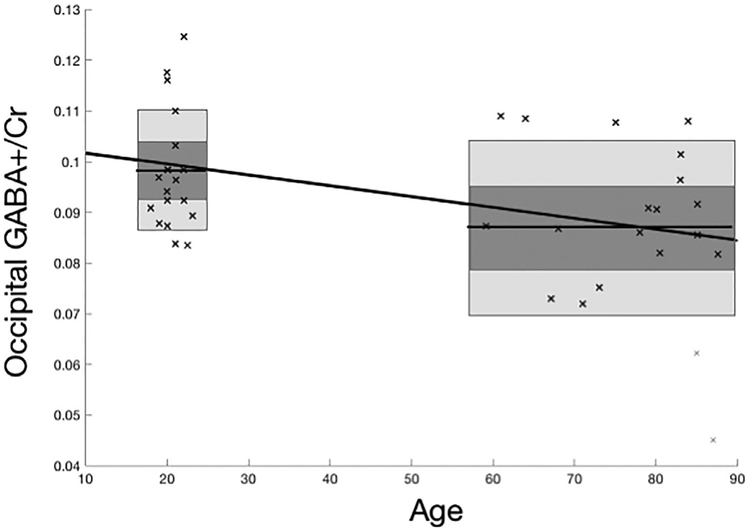
GABA+ spectra were unanalyzable for young and two old participants due to poor signal to noise, and PRESS spectra were unanalyzable for one old participant. Behavioral measures were collected from 18 young participants and 19 old participants. GABA+ concentrations are presented in Figure 2, and the results for all metabolites are provided in Table 2. A two-sample t test revealed that, as hypothesized, occipital GABA levels were significantly lower in older participants compared to younger ones (t33 = 2.22, p < 0.05, one-tailed). Additionally, occipital NAA and Myo concentrations were lower in the older group than in the younger group (NAA: t36 = 3.03, p < 01; Myo: t36 = 2.00, p < 05, one-tailed). Age differences for choline and Glx were not significant.
Figure 2.
Effect of age group on occipital GABA+/Cr ratios in occipital GABA concentrations in young (18–23 years old) and older (59–87 years old) participants. A lower GABA +/Cr ratio is seen in older participants. Horizontal line across the box marks the mean, dark gray area corresponds to 95% confidence interval, light gray area is standard deviation.
TABLE 2. Average Occipital Metabolite Ratios in Young Adult and Older Adult Groups, and Group Differences As Assessed by One-Tailed, Two-Sampled t Tests.
| Young Adults | Older Adults | Group Difference | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | df | t | p (one-tailed) | |
| GABA+/Cr | 0.10 | 0.01 | 0.09 | 0.01 | 33 | 2.22 | <0.05 |
| Myo/Cr | 0.60 | 0.06 | 0.53 | 0.13 | 36 | 2.00 | 0.05 |
| Cho/Cr | 0.16 | 0.01 | 0.16 | 0.02 | 36 | −0.42 | n.s.* |
| NAA/Cr | 1.49 | 0.11 | 1.39 | 0.09 | 36 | 3.03 | <0.01 |
| Glx/Cr | 1.61 | 0.18 | 1.52 | 0.23 | 36 | 1.45 | 0.07 |
Cho, choline; Cr, creatine; GABA, gamma-aminobutyric acid; Glx, glutamate and glutamine; Myo, myoinositol; NAA, N-acetylaspartic acid.
Nonsignificant. Direction of effect was opposite to that predicted, and thus a one-tailed t test was not conducted.
Performance on the behavioral tasks is summarized in Table 3. Consistent with prior findings (4,16,29–31,34,36), younger participants tended to perform better than older participants on these tasks. The age difference was statistically significant (p < 0.05, one-tailed) for eight of the tasks (CFPT upright, dot coherence, Trail Making A, Trail Making B, CFMT, contour detection, digit symbol, pattern comparison), was marginally significant for the CFPT inverted task (p < 0.1, one-tailed), and was not significant for the dot speed and letter fluency tasks (both p’s > 0.1).
TABLE 3. Performance on Behavioral Tasks, Group Differences As Assessed by One-Tailed, Two-Sample t Tests and Correlations Between Behavioral Performance and GABA Concentration.
| Young Adult Group (n = 17) | Older Adult Group (n = 18) | Group Difference | Correlation with GABA (Across All Participants) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p (one-tailed) | R | p (one-tailed) | ||
| Better performance higher | CFMT | 76.32 | 10.83 | 66.34 | 10.92 | 2.98 | <0.001 | 0.15 | 0.20 |
| Contour detection | 43.27 | 7.26 | 36.19 | 4.39 | 3.83 | <0.001 | 0.08 | 0.32 | |
| Digit symbol | 73.57 | 15.20 | 50.29 | 9.38 | 5.98 | <0.001 | 0.16 | 0.18 | |
| Pattern comparison | 22.62 | 3.10 | 14.60 | 2.58 | 9.11 | <0.001 | 0.27 | 0.06 | |
| COWAT | 44.76 | 9.85 | 42.29 | 13.66 | 0.67 | 0.25 | 0.49 | <0.01 | |
| Better performance lower | CFPT upright | 4.22 | 1.11 | 5.62 | 1.94 | −2.86 | <0.001 | −0.29 | <0.05 |
| CFPT inverted | 7.94 | 2.22 | 8.70 | 1.28 | −1.37 | 0.09 | 0.09 | n.s.* | |
| Dot Speed | 23.26 | 10.38 | 28.78 | 11.69 | −1.62 | 0.06 | −0.18 | 0.16 | |
| Dot Coherence | 38.23 | 12.68 | 48.48 | 23.09 | −1.78 | <0.05 | −0.30 | <0.05 | |
| Trail making A | 22.97 | 6.51 | 34.19 | 10.20 | −4.25 | <0.001 | −0.07 | 0.35 | |
| Trail making B | 50.34 | 12.85 | 82.96 | 38.09 | −3.72 | <0.001 | −0.30 | <0.05 | |
CFMT, Cambridge face memory test; CFPT, Cambridge face perception test; COWAT, controlled oral word association test; GABA, gamma-aminobutyric acid.
Nonsignificant. Relationship between task performance and GABA levels was opposite to that predicted, and so exact significance values were not calculated.
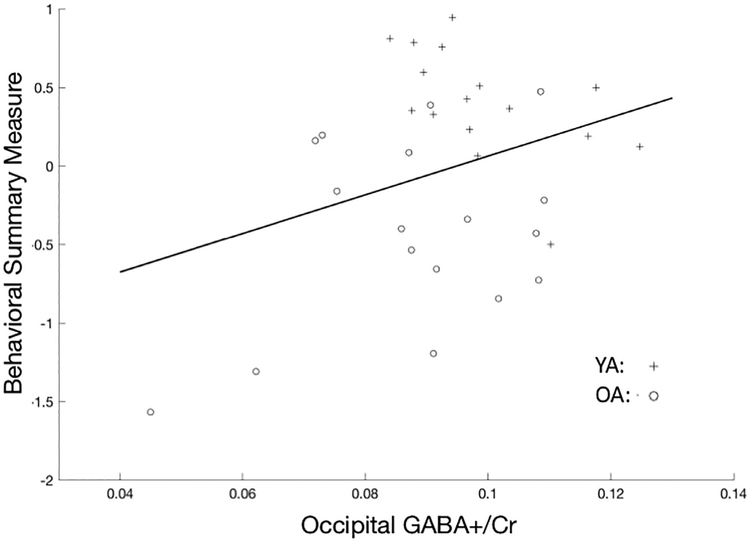
As predicted, higher GABA+ levels were associated with better task performance (i.e., enhanced fluid processing) as assessed by the summary measure of behavioral performance (t31= 1.84, p < 0.05, one-tailed; Fig 3, Table 4). Higher Myo, NAA, and Glx levels were also associated with better task performance as assessed by this summary measure (Myo: t34 = 2.70, p < 0.01, one-tailed; NAA: t34 = 1.87, p < 0.05, one-tailed; Glx: t34 = 3.36, p < 001, one-tailed).
Figure 3.
Relationship between occipital GABA+/Cr ratios (x axis) and scores on the summary measure extracted by z scoring and averaging behavioral performance on 11 tasks (y axis). Higher GABA+/Cr ratios are associated with better behavioral performance. OA, older adults; YA, young adults.
TABLE 4.
Regressions Between Behavioral Summary Measure of Fluid Processing and Occipital Metabolite Ratios.
| Full Sample | Young Adult Group | Older Adult Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | T |
p (one tailed) |
df | T |
p (two tailed) |
df | T |
p (two tailed) |
|
| GABA+/Cr | 31 | 1.84 | 0.07 | 14 | −2.45 | <0.05 | 15 | 1.47 | 0.16 |
| Myo/Cr | 34 | 2.70 | 0.01 | 16 | 0.69 | 0.85 | 16 | 1.28 | 0.22 |
| Cho/Cr | 34 | −0.16 | n.s.* | 16 | −1.52 | 0.15 | 16 | 0.86 | 0.40 |
| NAA/Cr | 34 | 1.87 | 0.07 | 16 | −0.08 | 0.94 | 16 | −0.04 | 0.97 |
| Glx/Cr | 34 | 3.36 | <0.01 | 16 | 0.27 | 0.79 | 16 | 3.34 | <0.01 |
Cho, choline; Cr, creatine; GABA, gamma-aminobutyric acid; Glx, glutamate and glutamine; Myo, myoinositol; NAA, N-acetylaspartic acid.
Nonsignificant. Relationship between task performance and metabolite ratios was opposite to that predicted, and so exact significance values were not calculated.
NAA levels are thought to reflect neuronal integrity, and given the correlation between NAA and behavior, we wondered if the association between GABA+ and behavior was due to individual differences in NAA. After controlling for NAA, the relationship between GABA+ and behavioral performance was reduced, although it was still marginally significant (partial correlation r = .25, p = 0.09, one-tailed).
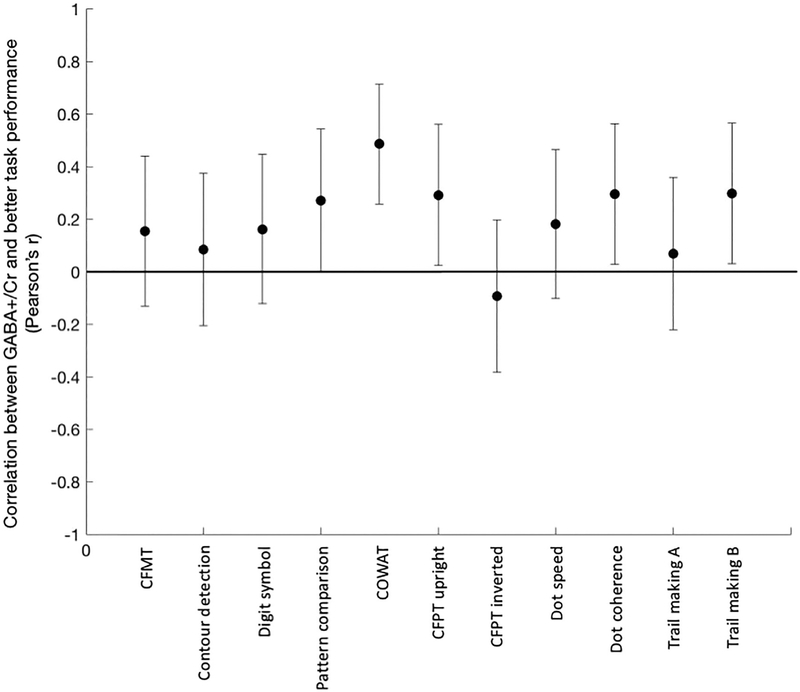
We also explored relationships between GABA+ concentration and performance on individual tasks. GABA+ levels were associated with better performance in 10 of 11 tasks (Fig 4, Table 2). This relafionship was statistically significant (p < 0.05, one-tailed) in four of the tasks (CFPT upright, dot coherence, trail making B, and COWAT), marginally significant (p < 0.1, one-tailed) in one task (pattern comparison), and did not reach statistical significance (p > 0.1) in the other six tasks.
Figure 4.
Correlations between occipital GABA+/Cr ratios and performance (y axis) on each of 11 behavioral tasks (x axis). Correlations on tasks in which better performance is associated with lower scores are flipped, so that for all tasks, a positive correlation indicates better performance is associated with higher GABA+/Cr ratios. Positive correlations were found for ten out of 11 tasks. CFMT, Cambridge face memory test; CFPT, Cambridge face perception test; COWAT, controlled oral word association test.
Relationships between metabolite ratios and performance were examined in the two age groups separately, revealing that they were driven by the older group (Table 3). The relationship between GABA+ concentration and the behavioral summary measure approached significance in the older group (t16 = 1,48, p < 0.08, one-tailed), but not the younger group. Additionally, the relationship between behavior and Glx was significant in the older participants, but not in the young participants (GlxO = t16 = 3.34, p < 0.001, one-tailed; GlxY: t16 = 0.27, p = ns).
DISCUSSION
In the present study, we found that GABA+ concentrations in the human occipital cortex decline with age, and that lower GABA+ levels predict worse fluid processing ability in older adults. These findings are consistent with the hypothesis that age-related declines of GABA functioning contribute to age-related declines of fluid processing ability. Thus, they may help to explain why some healthy older adults exhibit significant fluid processing impairments while others do not (13,15,39).
It is worth noting that the relationship between occipital GABA+ and behavior was not restricted to purely visual tasks. In fact, some of the strongest relationships were with Letter Fluency and Trail Making B, which are typically associated with executive function rather than with vision. One possible interpretation is that age-related declines in occipital GABA+ impair aspects of visual processing that support higher cognition. Another is that age-related declines in occipital GABA+ reflect declines in GABA+ throughout the brain including prefrontal cortex. Future studies could explore if either (or both) of these potential mechanisms can explain the relationship that we have observed.
When we investigated the relationship between GABA+ concentrations and fluid processing while controlling for individual differences in NAA levels, the relationship was weaker, although still marginally significant. This trend suggests that the relationship between GABA+ and behavior may be related to declines in neuronal integrity, but cannot be completely explained by them.
More broadly, the present findings are consistent with prior work involving nonhuman primates that has related reductions of occipital GABA to age-related declines of visual processing. For example, Leventhal et al., (19) found that the receptive fields of neurons in the visual cortex were less direction and orientation specific in old macaques, relative to young macaques. Interestingly, the administration of GABA, or a GABA agonist, to old neurons restored their specificity to youth-like levels, while the administration of a GABA antagonist to neurons in young monkeys reduced their specificity to that of neurons in older monkeys. This prior work suggests that GABA influences behavior by affecting the specificity of neural representations. Specifically, as GABA declines with age, neural representations may become less specific or distinct, thereby undermining behavioral performance.
A growing body of functional MRI findings in humans is consistent with this hypothesis. For example, Park et al. (25) found that the neural responses elicited by faces, places and words were less specialized in older adults compared to younger adults. Further, reduced neural distinctiveness is associated with reduced fluid processing ability in older adults (26). These findings, along with the present results, suggest the possibility that reductions of GABA in old age lead to declines in neural distinctiveness, which, in turn, contribute to age-related cognitive decline. Of course, the present study was purely correlational. We are therefore unable to infer a causal relationship between reduced GABA levels and fluid processing impairments. One potential way to overcome this limitation would be to manipulate GABA levels pharmacologically via the administration of drugs that affect the GABA system (e.g., benzodiazepines). We are currently pursuing such a line ofresearch.
In conclusion, our findings indicate that human occipital GABA+ concentrations decline with age, and that individual differences in GABA are related to individual differences in fluid processing ability. These findings are consistent with the hypothesis that age-related declines in GABA may contribute to some of the age-related cognitive impairments that characterize healthy aging.
ACKNOWLEDGMENTS
This work was supported by a pilot grant from the University of Michigan Geriatrics Center to Daniel H Weissman.
Abbreviations
- CFMT
Cambridge Face Memory Test
- CFPT inverted
Cambridge Face Perception Test - inverted
- CFPT upright
Cambridge Face Perception Test -upright
- Cho
Choline
- COWAT
Controlled Oral Word Association Test
- Cr
Creatine
- fMRI
functional magnetic resonance imaging
- GABA
gamma-aminobutyric acid
- Glx
Glutamate and Glutamine
- Myo
Myo-inositol
- NAA
N-Acetyl Aspartate
Footnotes
Confiicts of interest: The authors declare no competing financial interests.
REFERENCES
- 1.Andrews-Zwilling Y, Bien-Ly N, Xu Q, et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci 2010; 30:1370713717. 10.1523/JNEUR0SCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton AL, Hamsher Kde. Multilingual aphasia examination. Iowa City, IA: University of Iowa, 1976. [Google Scholar]
- 3.Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision Res 2007; 47:1769–1780. 10.1016/j.visres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Bowles DC, McKone E, Dawel A, et al. Diagnosing prosopagnosia: effects of ageing, sex, and participant–stimulus ethnic match on the Cambridge Face Memory Test and Cambridge Face Perception Test. Cognit Neuropsychol 2009; 26:423–455. 10.1080/02643290903343149. [DOI] [PubMed] [Google Scholar]
- 5.Boy F, Evans CJ, Edden RAE, et al. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol 2010; 20:1779–1785. https://doi.org/10.1016Zj.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol 1995; 30(3):349–360. 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- 7.Duchaine B, Germine L, Nakayama K. Family resemblance: ten family members with prosopagnosia and within-class object agnosia. Cognit Neuropsychol 2007; 24:419–430. 10.1080/02643290701380491. [DOI] [PubMed] [Google Scholar]
- 8.Duchaine B, Nakayama K. The Cambridge Face Memory Test: results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia 2006; 44:576–585. 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Edden RAE, Muthukumaraswamy SD, Freeman TCA, et al. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 2009; 29:15721–15726. 10.1523/JNEUR0-SCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edden RAE, Puts NAJ, Harris AD, et al. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging 2014; 40:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao F, Edden RA, Li M, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 2013; 78:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grachev ID, Apkarian AV. Chemical network of the living human brain: Evidence of reorganization with agin. Cognitive brain research 2001; 11 (2):185–197. [DOI] [PubMed] [Google Scholar]
- 13.Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing 2011; 40:684–689. 10.1093/ageing/afr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heba S, Puts NAJ, Kalisch T, et al. Local GABA concentration predicts perceptual improvements after repetitive sensory stimulation in humans. Cereb Cortex 2016; 26:1295–1301. 10.1093/cercor/bhv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci 2004; 5:87–96. 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 16.Hoyer WJ, Stawski RS, Wasylyshyn C, et al. Adult age and digit symbol substitution performance: a meta-analysis. Psychol Aging 2004; 19:211214 10.1037/0882-7974.19.1.211. [DOI] [PubMed] [Google Scholar]
- 17.Hua T, Kao C, Sun Q, Li X, Zhou Y. Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex. Brain research bulletin 2008; 75(1):119–125. [DOI] [PubMed] [Google Scholar]
- 18.Kihara K, Kondo HM, Kawahara JI. Differential contributions of GABA concentration in frontal and parietal regions to individual differences in attentional blink. J Neurosci 2016; 36:8895–8901. 10.1523/JNEUR0SCI.0764-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leventhal AG, Wang Y, Pu M, et al. GABA and its agonists improved visual cortical function in senescent monkeys. Science 2003; 300:812–815. 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- 20.Loerch PM, Lu T, Dakin KA, et al. Evolution of the aging brain transcriptome and synaptic regulation. PLoS One 2008; 3:e3329 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marjahska M, McCarten JR, Hodges J, et al. Region-specific aging of the human brain as evidenced by neurochemical profiles measured noninvasively in the posterior cingulate cortex and the occipital lobe using 1H magnetic resonance spectroscopy at 7 T. Neuroscience 2017; 354:168–177. 10.1016/j.neuroscience.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQuail JA, Banuelos C, LaSarge CL, et al. GABA B receptor GTP-binding is decreased in the prefrontal cortex but not the hippocampus of aged rats. Neurobiol Aging 2012; 331124–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakai T, Okanoya K. Neural basis of the cross-domain syntactic priming between language and mathematics. Int J Psychol 2016; 51:880–881. [Google Scholar]
- 24.Near J, Edden R, Evans CJ, et al. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med 2015; 73:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences 2004; 101(35):13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J, Carp J, Hebrank A, Park DC, Polk TA. Neural specificity predicts fluid processing ability in older adults. Journal of Neuroscience 2010; 30 (27):9253–9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, Cohen RA. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging 2017; 2(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puts NAJ, Edden RAE, Evans CJ, et al. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci 2011; 31:16556–16560. 10.1523/JNEUR0-SCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmusson XD, Zonderman AB, Kawas C, et al. Effects of age and dementia on the trail making test. Clin Neuropsychol 1998; 12:169–178. 10.1076/clin.12.2.169.2005. [DOI] [Google Scholar]
- 30.Roudaia E, Bennett PJ, Sekuler AB. Contour integration and aging: the effects of element spacing, orientation alignment and stimulus duration. Front Psychol 2013; 4 10.3389/fpsyg.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salthouse TA. Aging associations: influence of speed on adult age differences in associative learning. J Exp Psychol Learn Mem Cogn 1994; 20:1486–1503. [DOI] [PubMed] [Google Scholar]
- 32.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 1996; 103:403–428. [DOI] [PubMed] [Google Scholar]
- 33.Schmolesky MT, Wang Y, Pu M, et al. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci 2000; 3:384–390. 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- 34.Snowden RJ, Kavanagh E. Motion perception in the ageing visual system: minimum motion, motion coherence, and speed discrimination thresholds. Perception 2006; 35:9–24. 10.1068/p5399. [DOI] [PubMed] [Google Scholar]
- 35.Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J Neurochem 2004;89:204–216. 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 36.Tombaugh TN, Kozak J, et al. Normative data stratified by age and education for two measures of verbal fluency. Arch Clin Neuropsychol 1999; 14:167–177 10.1016/S0887-6177(97)00095-4. [DOI] [PubMed] [Google Scholar]
- 37.Tong LM, Yoon SY, Andrews-Zwilling Y, et al. Enhancing GABA signaling during middle adulthood prevents age-dependent gabaergic interneuron decline and learning and memory deficits in ApoE4 Mice. J Neurosci 2016; 36:2316–2322. 10.1523/JNEUR0SCI.3815-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wechsler D WAIS-III: Wechsler adult intelligence scale. Psychological Corporation, 1997. [Google Scholar]
- 39.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002; 17:179–193. 10.1037/0882-7974.17.2.179. [DOI] [PubMed] [Google Scholar]
- 40.Yoon JH, Grandelis A, Maddock RJ. Dorsolateral prefrontal cortex GABA concentration in humans predicts working memory load processing capacity. J Neurosci 2016;36:11788–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]